Disruption of dystrophin or the sarcoglycans within the dystrophin protein complex leads to muscular dystrophy. The dystrophin complex is enriched in myofibers and concentrated into costameres, rib-like structures in the plasma membrane. In the absence of dystrophin, the plasma membrane becomes fragile with reduced stiffness and increased leakiness, and exposing dystrophin-deficient muscle to hypoosmotic conditions produces membrane blebbing. These features are associated with an increase in intracellular calcium, reactive oxygen species (ROS), and activation of a protease cascade. The ensuing loss of myofibers, largely mediated by a necrotic cell death process, is associated with progressive replacement of the myofibers by fibrosis. Although membrane repair mechanisms may be activated, apparently repaired myofibers may exhibit defective function from the intracellular consequences of membrane disruption. Additionally, remodeling of the extracellular matrix for those surviving myofibers may also adversely affect muscle function. Thus, the causes of muscle weakness in muscular dystrophy are multifold and encompass the matrix, membrane and intracellular elements of muscle identifying multiple target pathways for therapeutic intervention.
Introduction
The muscular dystrophies are a group of genetic diseases characterized by muscle weakness. The most common form, Duchenne muscular dystrophy (DMD), results from loss-of-function mutations in dystrophin. Dystrophin encodes a 427-kD protein that resides below the sarcolemma. Together with its associated proteins, dystroglycan and the sarcoglycans, dystrophin participates in a mechanically strong link from the matrix to the underlying cytoskeleton (Rybakova et al., 2000). Loss-of-function mutations in the genes encoding γ, α, β, or δ sarcoglycan result in autosomal recessive muscular dystrophies that phenotypically resemble DMD. Mutations in the genes encoding dystroglycan-glycosylating enzymes are linked to congenital forms of muscular dystrophy, emphasizing the importance of dystroglycan’s connection to the extracellular matrix.
Boys with DMD are born with normal muscle function; however, as they age, they become progressively weaker. Muscle biopsies from young ambulatory children with DMD show a mixed picture of degenerating myofibers alongside regenerating myofibers. Ongoing regeneration occurs in muscular dystrophy from activation of satellite cells, or muscle stem cells. Satellite cells divide and differentiate into myoblasts that may fuse with each other or to damaged myofibers. Muscle biopsies taken from older DMD patients with less mobility show fewer myofibers with far more replacement of the muscle tissue by adipose and connective tissue, referred to as fatty-fibrous infiltrate. The role of exercise in promoting muscle injury in muscular dystrophy remains controversial. The diaphragm muscle is in constant use and often displays the most severe pathology, especially in animal models of muscular dystrophy, leading many to conclude that exercise exacerbates degeneration. The heart, also under constant activity and also affected by the loss of dystrophin and sarcoglycans, has a lagging pathology compared with skeletal muscle, where patients and animal models show signs of cardiac dysfunction later in life.
Without question, much of muscle dysfunction in later-stage muscle dystrophy arises from an absence of functional myofibers. However, the degree to which intact muscle has normal function in muscular dystrophy remains in question. In intact muscle, dystrophin is required for protection from contraction-induced injury. Muscle that lacks a functional dystrophin complex is mechanically weak, such that when it contracts, it damages the membrane (Cox et al., 1993; Petrof et al., 1993). Loss of membrane integrity likely contributes to dysfunction of the affected myofiber through multiple pathways. Disruption of the plasma membrane triggers a cascade of events, some of which are reparative such as satellite cell activation and intracellular resealing events. However, some intracellular consequences such as Ca2+ influx and increased ROS may have direct deleterious consequences for sarcomere function.
Membrane fragility
Dystrophin, and its associated proteins, are found at the sarcolemma in register with the sarcomeric Z disc in structures known as costameres (Fig. 1). Costameres reside over the Z disc within the plasma membrane perpendicular to the long axis of the myofiber. This intracellular position, and the capacity of dystrophin to bind actin, argues for a role in transmitting force from the sarcomeres to the extracellular matrix and neighboring cells. Rybakova et al. (2000) demonstrated that peeled membrane from normal myofibers retains cytoskeletal actin on its inner cytoplasmic face, underscoring the tight linkage of dystrophin and actin. The link between dystrophin and actin is not only mediated by the calponin binding domain at dystrophin’s amino terminus, but also through the basic residues in spectrin repeats 11–14 in a lateral association (Amann et al., 1998). Peeled membrane from the myofibers of the mdx mouse that lacks dystrophin was not found to retain actin. Peeled membrane from the laminin α2-deficient dy mice retained both dystrophin and actin, indicating a specific role for dystrophin in the actin-binding interface. Recently, it was shown that dystrophin directly binds to tubulin and organizes the microtubule network (Prins et al., 2009). Because microtubules not only provide cell structure, but also scaffold the Golgi network and direct intracellular traffic, loss of this dystrophin–microtubule interaction is a plausible mechanism for the altered localization of many proteins in dystrophin-mutant animals.
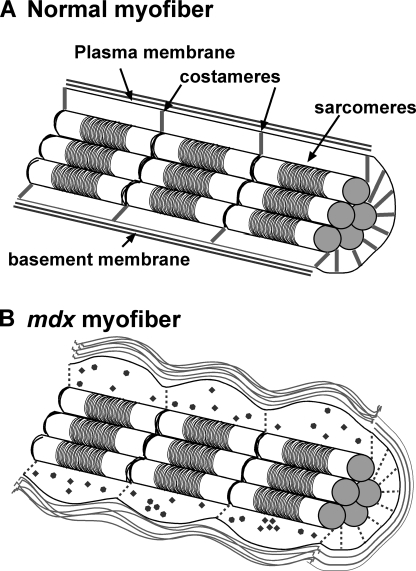
Figure 1.
Effect of dystrophin loss of myofiber function and organization. (A) Normal myofiber and (B) dystrophin-deficient mdx myofiber. Dystrophin and associated proteins are normally found at the rib-like costameres linking sarcomeric Z bands with the plasma membrane. Loss of dystrophin results in disorganized costameres and enhanced membrane leak. Increased edema and inappropriate cytosolic Ca2+ and ROS generation (filled circles and diamonds) contribute further to muscle dysfunction. Increased extracellular matrix deposition surrounding myofibers also occurs and perturbs membrane integrity. This interaction is particularly important during muscle contraction.
Because of dystrophin’s role as a scaffolding protein integral to membrane integrity, multiple means of inducing stress and strain on myofibers have been tested. When isolated myofibers from mdx mice were placed in hypoosmotic medium, the membrane was observed to separate from the cytoskeleton in “blebs,” and the cells hypercontracted resulting in cell death (Menke and Jockusch, 1991). The difference between normal and dystrophin-deficient muscle disappeared when the actin cytoskeleton was first disrupted with cytochalasin D, demonstrating the necessity of actin for the osmotic response. Curiously, sarcoglycan-deficient myofibers also developed blebs when subjected to hypoosmotic shock, despite the presence of dystrophin (Iwata et al., 2005). In support of this structural role for dystrophin, mdx-derived myotubes were found to be less stiff than myotubes from normal mice when probed with a glass stylus (Pasternak et al., 1995). The degree to which reduced stiffness derives from the specific loss of dystrophin and/or the disruption of the sarcoglycan–dystroglycan complex is not clear. However, these abnormal membrane properties translate to defective membrane integrity in vivo.
Mechanical properties of dystrophic muscle
Most commonly, ex vivo studies have been used to determine active and passive properties of the dystrophin-deficient muscle. Active properties of mdx muscle, such as twitch and tetanic force, are normal in mdx muscle, as are passive mechanical properties (Wolff et al., 2006). However, other studies have supported a decrease in specific twitch force in the mdx diaphragm muscle (Stedman et al., 1991). The decrease in specific force was also observed in limb skeletal muscle from animals lacking α sarcoglycan (Sgca-null mice) (Duclos et al., 1998). More specifically, twitch force is preserved, but the cross-sectional area is increased, thus reducing specific force. Fiber cross-sectional area is calculated based on the mass and length of the muscle, and the increase in area in Sgca-null muscle is due to increased mass. It is possible that increase in mass is accounted for by increases in water content and extracellular matrix deposition rather than sarcomere content (Consolino et al., 2005). Increased water content or edema could be due to increased intracellular ions, primarily Na+, which may enter via membrane holes, the Na+/Ca2+ exchanger, or through nonselective transient receptor potential channels. This finding is somewhat complicated by histological studies of these mice that show that fibers are more numerous and of the same median size in Sgca-null mice. This could be explained by loss of the responsible ions during tissue processing (Consolino et al., 2005).
Another series of studies has focused on force deficits after eccentric contraction or lengthening contraction experiments. In this ex vivo protocol, isolated muscles are lengthened and stimulated to maximally contract, thus mimicking in vivo exercise such as downhill walking. The decrement in maximum force achieved after five repeated contractions is measured. With such protocols, normal muscle exhibits ∼10% reduction in force from the first to the fifth contraction. In contrast, mdx muscle shows a much larger decrease (Petrof et al., 1993), and this decrease is attributed to the loss of dystrophin’s normal scaffolding and support properties for the plasma membrane of muscle. Enhanced contraction-induced damage is also present in limb and diaphragm muscles from δ sarcoglycan–deficient mice, but it is less than what is observed in mdx muscle (Hack et al., 2000). Curiously, γ sarcoglycan–null mice do not have contraction-induced damage, yet they display membrane leak and phenotypic features similar to mdx (Hack et al., 1999). This finding suggests that the dystrophin complex has a role beyond simple scaffolding and mechanical support. In sarcoglycan-mutant mice, dystrophin is still present, but only the sarcoglycan complex is disrupted, whereas in dystrophin-deficient muscle, both dystrophin and the sarcoglycan complex are disrupted. The sarcoglycan complex has been proposed to stabilize the interaction between the dystroglycan subunits, and thereby modify attachment to the matrix via dystroglycan. The extracellular components of the sarcoglycan complex contain modified cysteine-rich epidermal-like growth factor repeats and may themselves mediate interaction with matrix elements. The cysteine-rich units in sarcoglycan are essential because point mutations affecting only these residues result in muscular dystrophy. Most ex vivo muscle mechanical experiments have used muscle from younger animals (6–20 wk of age) where the muscle has a measurable increase in fibrosis, but this muscle does not carry the heavy fibrotic burden seen in older muscle. Progressive deposition of matrix around myofibers may also contribute to muscle dysfunction (see below).
Membrane leak and rupture
One noted feature of DMD and limb girdle muscular dystrophies is the release of muscle enzymes, generally assayed as creatine kinase, into the circulation. However, other proteins also leak into the serum, including lactate dehydrogenase and aldolase, and these serum proteins are effective as biomarkers of disease. It is unclear whether the leak of these myo-enzymes contributes to muscle dysfunction in muscular dystrophy. Disrupted membrane integrity also promotes entry of small molecules into the myofiber syncytium. The availability of Ca2+-sensitive dyes has been used effectively to trace calcium entry into muscle cells (Wang et al., 2005). The vital tracer Evans blue dye is a small molecule that gains entry into muscle lacking dystrophin, where normal fibers are typically impermeable (Matsuda et al., 1995). In vivo, Evans blue dye circulates in the vasculature, complexes with albumin, and then extravasates into the muscle. The albumin–dye complex is visualized in subsets of myofibers, indicating membrane breakdown of those fibers. Ex vivo, incubation of dye alone with muscle sections on a slide also marks the subset of dye-positive fibers and reflects an accumulation of albumin that occurred in vivo in dystrophic fibers. Both in vivo and ex vivo, dye uptake occurs focally throughout the muscle where clusters of myofibers are dye-positive and the surrounding myofibers remain dye-negative. Evans blue dye-positive fibers may also be positive for apoptotic markers (Matsuda et al., 1995), although quite clearly only a minority of dye-positive fibers are undergoing apoptosis (Hack et al., 1998).
The mechanism to account for focal disruption of membrane integrity is not well understood but could relate to biomechanical weakness in muscle where certain aspects of the muscle are more prone to damage. Alternative mechanisms such as regional vascular spasm have been used to explain patchy involvement of groups of myofibers. The entry of albumin–dye complexes implies that myofibers develop holes large enough to admit an ∼70-kD protein and survive, at least temporarily. It may also have implications for drug studies in that disrupted or damaged cells may develop a much higher intracellular concentration, or any fiber with a disrupted dystrophin complex may be more permeable to drugs. During stimulated ex vivo contraction, uptake of the dye Procion orange can be readily detected in mdx muscle, although this dye usually indicates cell necrosis (Petrof et al., 1993).
Calcium and its sequelae
The prospect of large holes allowing entry of extracellular molecules, including generous amounts of Ca2+, is reminiscent of classical excitotoxicity models of ischemic damage in which high intracellular calcium swells cells, activates intracellular proteases, deranges signaling, and quenches the mitochondrial membrane potential. Considering the large spike and fall in intracellular [Ca2+] that occurs in contracting striated muscle, it makes sense to consider the significance of Ca2+ in a larger context. There are varying reports on resting Ca2+ in isolated mdx fibers (Turner et al., 1988, 1991; Fong et al., 1990; Hopf et al., 1996). In vitro studies of isolated myofibers consistently report an increase in Ca2+ sparks in mdx muscle, especially when subjected to stress, such as hypoosmolar conditions (Wang et al., 2005). Sparks are also detected in myofibers isolated from mdx mice that were exercised, even mildly; however, Ca2+ sparks only appear in normal myofibers if the fibers were exercised to fatigue (Wang et al., 2005). Interestingly, calcium sparks and the adverse hypoosmotic response are not dependent on exogenous calcium, implying that an alternative intracellular source, such as the sarcoplasmic reticulum, may be critical. Sparks can be inhibited by the alkaloid ryanodine, which has focused attention specifically on RYR1 (Wang et al., 2005; Bellinger et al., 2009). In muscular dystrophy, Ca2+ release by RYR is thought to become uncoupled from excitation (Bellinger et al., 2009). This is thought to be due to dissociation of FKBP12 from the RYR complex. This dissociation can be inhibited by introduction of the small molecule S-107. Systemic application of S-107 in mdx mice, either by continuous infusion pumps or in drinking water, reduced the severity of the mdx phenotype by several measures. These measures included reduction in calcium spark frequency in isolated fibers, decreased numbers of fibers with central nuclei, and increased grip strength (Bellinger et al., 2009). These findings support a model in which calcium dysregulation causes increased myocyte death and gross muscle dysfunction. Surprisingly, S-107 treatment also reduced serum creatine kinase and Evans blue dye uptake (Bellinger et al., 2009). The mechanism by which RYR stabilization results in increased membrane integrity is unclear.
An alternative proposed source of Ca2+ in dystrophic fibers is the transient receptor potential canonical (TRPC) channels. These have been variously proposed as sources of mechanosensitive, leak, sarcoplasmic reticulum Ca2+ depletion–mediated (store-operated), or calcium-induced Ca2+ currents. This confusion owes to the redundancy of channels and the lack of specificity of current transient receptor potential inhibitory drugs. Overexpression of TRPC3 increases Ca2+ uptake into muscles and was sufficient to cause some aspects of muscular dystrophy (Millay et al., 2009). These include central nuclei, fibro-fatty infiltrate, and muscle wasting with age. However, it is not clear that this approach reproduces the sarcolemmal fragility such as that seen with disruption of the dystrophin complex. Overall, this model is consistent with the role of elevated Ca2+ entry as an intermediate event in muscular dystrophy. The introduction of a dominant-negative TRPC6 reduced calcium uptake in δ sarcoglycan (Sgcd)–null muscle fibers and fibrosis (Millay et al., 2009).
Multiple mechanisms have been proposed for Ca2+-mediated muscle dysfunction. One promising explanation is that Ca2+ induces mitochondrial production of ROS via NADPH oxidase (Whitehead et al., 2008). Reduction in ROS using the membrane-permeant antioxidant N-acetylcysteine reduced the force decrement in mdx muscle after eccentric contraction and decreased central nuclei when it was provided in the drinking water. ROS are hypothesized to activate signaling via nuclear factor κB or ROS-sensitive kinases, leading to inflammation and altered cytoskeleton. ROS may also contribute to muscle dysfunction by directly oxidizing contractile proteins or sarcolemmal lipids.
The extracellular matrix in muscle pathology in muscular dystrophy
Patients with muscular dystrophy experience an absolute decrease in muscle force, not only relative to muscle size and not only after traumatic stretch. So, at best, even late stages of the mouse phenotype represent an early stage of the human disease. There are several potential explanations for the difference in human and mouse phenotypes, including larger muscle size and increased number of slow-twitch fibers, and these are difficult to investigate. In addition to species differences in disease course, there are also striking differences within the human muscular dystrophy population. When comparing patients with the identical gene mutations, there may be substantial differences in the amount and progression of muscle weakness. These differences may arise from environmental factors such as size of muscle mass and exercise and diet. The range of muscle phenotype may also be due to difference in other genes that alter the function of the dystrophin complex or the response of muscle to injury or regeneration. These genetic modifiers are easier to study in mouse models of muscular dystrophy, despite limitations of the small animal model.
Using a mouse model of limb girdle muscular dystrophy lacking γ sarcoglycan, a genome-wide scan was undertaken to identify genes that modify the outcome in muscular dystrophy. The approach identified Ltbp4, a gene that encodes the latent transforming growth factor (TGF)-β–binding protein, as a modifier of muscular dystrophy (Heydemann et al., 2009). This protein resides in the extracellular matrix, where it binds to the cytokine TGF-β. Proteolytic cleavage of LTBP4 results in release of TGF-β, making it available to bind to TGF-β receptors on myofibers and other cells within the muscle. The protease-sensitive domain of LTBP4 was found to vary among mouse strains, and this difference correlated with the amount of Evans blue dye uptake into muscle and the amount of fibrosis in dystrophic muscle. This variation in LTBP4 did not affect normal muscle. The correlation of TGF-β activity with fibrosis is not so surprising, given the well-known role of TGF-β family members in mediating fibrosis in many different cell and tissue types. What is more surprising is that the amount of TGF-β activity and Ltbp4 gene variants strongly correlated with the degree of Evans blue dye uptake. Because Evans blue dye is a direct reflection of membrane permeability in muscle, this finding indicates that LTBP4 regulates membrane integrity in myofibers. The mechanism by which LTBP4 may do this is unknown, but its position in the matrix argues for a role external to myofibers and potentially a direct interaction with the extracellular face of the myofibers.
Muscle from DMD patients is known to express increased levels of TGF-β, and protein can be localized within the extracellular matrix (Bernasconi et al., 1995). TGF-β drives the synthesis of collagen and promotes the growth and differentiation of fibroblasts. It was proposed that in muscular dystrophy, myocyte necrosis or injury leads to TGF-β signaling, which promotes fibrosis at the expense of regeneration by satellite cells. TGF-β1 is capable of converting myoblast cells into myofibroblasts, raising the possibility that satellite cells are activated but differentiate into noncontractile tissue (Li et al., 2004). Phosphorylated SMAD2/3, an indication of TGF-β signaling, is present in mdx muscle fibers (Cohn et al., 2007). Antibodies that inactivate TGF-β or the angiotensin II receptor blocker losartan, which indirectly decreases TGF-β secretion, reduced SMAD signaling and decreased the severity of the mdx phenotype, including improved grip strength (Cohn et al., 2007).
Dystrophic muscle has a substantial mononuclear cell infiltrate that includes macrophages, lymphocytes, neutrophils, and eosinophils. The role of these immune cells is increasingly being considered in the pathogenesis of muscular dystrophy. For example, these cells are a likely source of proteases responsible for LTBP4 cleavage and subsequent TGF-β activation. Recently, Vetrone et al. (2009) identified a subset of T cells specifically associated with dystrophic skeletal muscle. These cells display T cell receptors with the Vβ8.1/8.2 rearrangement and express the extracellular matrix phosphoprotein, osteopontin. Deletion of osteopontin had several positive effects in the mdx mouse. Neutrophil invasion into the muscle was significantly reduced, and the number of immune-suppressing T lymphocytes, T regulatory cells, was increased. Overall, deleting osteopontin decreased TGF-β and fibrosis, and it increased grip strength in the mdx mouse. A more balanced view of the immune system, including T regulatory cells, is required in muscular dystrophy and is the subject of ongoing investigation.
Perspective
It is clear that multiple interacting molecular processes occur in dystrophic muscle as it transitions from its normal appearance and function at birth to the completely dysfunctional state of end-stage muscular dystrophy. Several questions remain. The first is whether fiber dysfunction contributes significantly to muscle weakness, or whether only loss of fibers contributes. There is extensive evidence of altered ion handling, and the RYR-stabilizing drug S-107 improves membrane function (Bellinger et al., 2009). Conversely, decreasing Ca2+ leak with dominant-negative TRPC6 reduces fibrosis and serum creatine kinase levels in muscular dystrophy (Millay et al., 2009). The increased water mass, or edema, in Sgca-null muscle implies some cell physiological defect, but the degree to which this contributes must be further explored (Consolino et al., 2005). It is also unclear whether extracellular matrix changes decrease force per se, or whether fibroblasts and extracellular matrix proteins simply prevent the formation of replacement muscle by occupying former muscle niches or forcing muscle stem cells to transdifferentiate to myofibroblasts (Li et al., 2004).
Future efforts will focus on interactions between the molecular processes that underlie the disease and place them in the order of their activation. It will also be important to determine which experimental findings are central to the disease process and thus are reasonable to target for therapeutic intervention. In the medium term, RYR stabilization or TGF-β inhibition, through S-107, losartan, or other means, is useful for treating larger animal models or humans with muscular dystrophy.
Acknowledgments
This work is supported by National Institutes of Health (grant NIH61322), Doris Duke Charitable Foundation (to E.M. McNally), and the American Heart Association (to J.A. Goldstein). The authors have no conflicts of interest related to this manuscript or its content.
Footnotes
Abbreviations used in this paper:
- DMD
- Duchenne muscular dystrophy
- ROS
- reactive oxygen species
- TGF
- transforming growth factor
- TRPC
- transient receptor potential canonical
References
- Amann K.J., Renley B.A., Ervasti J.M. 1998. A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J. Biol. Chem. 273:28419–28423 10.1074/jbc.273.43.28419 [DOI] [PubMed] [Google Scholar]
- Bellinger A.M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A.R. 2009. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15:325–330 10.1038/nm.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi P., Torchiana E., Confalonieri P., Brugnoni R., Barresi R., Mora M., Cornelio F., Morandi L., Mantegazza R. 1995. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J. Clin. Invest. 96:1137–1144 10.1172/JCI118101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R.D., van Erp C., Habashi J.P., Soleimani A.A., Klein E.C., Lisi M.T., Gamradt M., ap Rhys C.M., Holm T.M., Loeys B.L., et al. 2007. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13:204–210 10.1038/nm1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolino C.M., Duclos F., Lee J., Williamson R.A., Campbell K.P., Brooks S.V. 2005. Muscles of mice deficient in alpha-sarcoglycan maintain large masses and near control force values throughout the life span. Physiol. Genomics. 22:244–256 10.1152/physiolgenomics.00311.2004 [DOI] [PubMed] [Google Scholar]
- Cox G.A., Cole N.M., Matsumura K., Phelps S.F., Hauschka S.D., Campbell K.P., Faulkner J.A., Chamberlain J.S. 1993. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 364:725–729 10.1038/364725a0 [DOI] [PubMed] [Google Scholar]
- Duclos F., Straub V., Moore S.A., Venzke D.P., Hrstka R.F., Crosbie R.H., Durbeej M., Lebakken C.S., Ettinger A.J., van der Meulen J., et al. 1998. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J. Cell Biol. 142:1461–1471 10.1083/jcb.142.6.1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong P.Y., Turner P.R., Denetclaw W.F., Steinhardt R.A. 1990. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 250:673–676 10.1126/science.2173137 [DOI] [PubMed] [Google Scholar]
- Hack A.A., Ly C.T., Jiang F., Clendenin C.J., Sigrist K.S., Wollmann R.L., McNally E.M. 1998. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J. Cell Biol. 142:1279–1287 10.1083/jcb.142.5.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack A.A., Cordier L., Shoturma D.I., Lam M.Y., Sweeney H.L., McNally E.M. 1999. Muscle degeneration without mechanical injury in sarcoglycan deficiency. Proc. Natl. Acad. Sci. USA. 96:10723–10728 10.1073/pnas.96.19.10723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack A.A., Lam M.Y., Cordier L., Shoturma D.I., Ly C.T., Hadhazy M.A., Hadhazy M.R., Sweeney H.L., McNally E.M. 2000. Differential requirement for individual sarcoglycans and dystrophin in the assembly and function of the dystrophin-glycoprotein complex. J. Cell Sci. 113:2535–2544 [DOI] [PubMed] [Google Scholar]
- Heydemann A., Ceco E., Lim J.E., Hadhazy M., Ryder P., Moran J.L., Beier D.R., Palmer A.A., McNally E.M. 2009. Latent TGF-beta-binding protein 4 modifies muscular dystrophy in mice. J. Clin. Invest. 119:448–450 10.1172/JCI38618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf F.W., Turner P.R., Denetclaw W.F., Jr., Reddy P., Steinhardt R.A. 1996. A critical evaluation of resting intracellular free calcium regulation in dystrophic mdx muscle. Am. J. Physiol. 271:C1325–C1339 [DOI] [PubMed] [Google Scholar]
- Iwata Y., Katanosaka Y., Shijun Z., Kobayashi Y., Hanada H., Shigekawa M., Wakabayashi S. 2005. Protective effects of Ca2+ handling drugs against abnormal Ca2+ homeostasis and cell damage in myopathic skeletal muscle cells. Biochem. Pharmacol. 70:740–751 10.1016/j.bcp.2005.05.034 [DOI] [PubMed] [Google Scholar]
- Li Y., Foster W., Deasy B.M., Chan Y., Prisk V., Tang Y., Cummins J., Huard J. 2004. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am. J. Pathol. 164:1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R., Nishikawa A., Tanaka H. 1995. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J. Biochem. 118:959–964 [DOI] [PubMed] [Google Scholar]
- Menke A., Jockusch H. 1991. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 349:69–71 10.1038/349069a0 [DOI] [PubMed] [Google Scholar]
- Millay D.P., Goonasekera S.A., Sargent M.A., Maillet M., Aronow B.J., Molkentin J.D. 2009. Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc. Natl. Acad. Sci. USA. 106:19023–19028 10.1073/pnas.0906591106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C., Wong S., Elson E.L. 1995. Mechanical function of dystrophin in muscle cells. J. Cell Biol. 128:355–361 10.1083/jcb.128.3.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H.L. 1993. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA. 90:3710–3714 10.1073/pnas.90.8.3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins K.W., Humston J.L., Mehta A., Tate V., Ralston E., Ervasti J.M. 2009. Dystrophin is a microtubule-associated protein. J. Cell Biol. 186:363–369 10.1083/jcb.200905048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova I.N., Patel J.R., Ervasti J.M. 2000. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J. Cell Biol. 150:1209–1214 10.1083/jcb.150.5.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman H.H., Sweeney H.L., Shrager J.B., Maguire H.C., Panettieri R.A., Petrof B., Narusawa M., Leferovich J.M., Sladky J.T., Kelly A.M. 1991. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 352:536–539 10.1038/352536a0 [DOI] [PubMed] [Google Scholar]
- Turner P.R., Westwood T., Regen C.M., Steinhardt R.A. 1988. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature. 335:735–738 10.1038/335735a0 [DOI] [PubMed] [Google Scholar]
- Turner P.R., Fong P.Y., Denetclaw W.F., Steinhardt R.A. 1991. Increased calcium influx in dystrophic muscle. J. Cell Biol. 115:1701–1712 10.1083/jcb.115.6.1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrone S.A., Montecino-Rodriguez E., Kudryashova E., Kramerova I., Hoffman E.P., Liu S.D., Miceli M.C., Spencer M.J. 2009. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J. Clin. Invest. 119:1583–1594 10.1172/JCI37662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Weisleder N., Collet C., Zhou J., Chu Y., Hirata Y., Zhao X., Pan Z., Brotto M., Cheng H., Ma J. 2005. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7:525–530 10.1038/ncb1254 [DOI] [PubMed] [Google Scholar]
- Whitehead N.P., Pham C., Gervasio O.L., Allen D.G. 2008. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. 586:2003–2014 10.1113/jphysiol.2007.148338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A.V., Niday A.K., Voelker K.A., Call J.A., Evans N.P., Granata K.P., Grange R.W. 2006. Passive mechanical properties of maturing extensor digitorum longus are not affected by lack of dystrophin. Muscle Nerve. 34:304–312 10.1002/mus.20588 [DOI] [PubMed] [Google Scholar]