Abstract
Invasion of the human by a pathogen necessitates an immune response to control and eradicate it. When this response is inadequately regulated, systemic manifestations can result commonly manifested in physiologic changes described as “sepsis”. Recognition, diagnosis, and management of sepsis remain among the greatest challenges shared by the fields of neonatology and pediatric critical care medicine. Sepsis remains among the leading causes of death in both developed and under-developed countries with an incidence that is predicted to increase each year. Despite these sobering statistics, promising therapies derived from pre-clinical models have universally failed to obviate the substantial mortality and morbidity associated with sepsis. Thus, there remains a need for well-designed epidemiologic and mechanistic studies of neonatal and pediatric sepsis to improve our understanding of the causes—both early and late—of deaths attributed to the syndrome. In reviewing the definitions and epidemiology, developmental influences and regulation of the host response to sepsis, it is anticipated that an improved understanding of this host response will assist clinician-investigators in identifying improved therapeutic strategies.
Keywords: sepsis, septic shock, developmental influence, hemodynamics, coagulation cascade, immune function
Definitions characterizing the host responses in sepsis
“Sepsis” referring to the “decomposition of animal or vegetable organic matter in the presence of bacteria” 1 first appeared over 2700 years ago in the poems of Homer. Hippocrates also used the term “sepsis” and believed the decomposition could release “dangerous principles” that could cause “auto-intoxication” 2. Lewis Thomas furthered this concept when he proposed that the clinical responses seen in sepsis were the result of the host’s response to the infectious agent 3. In 1991, an American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference was convened to create a framework in which to define the systemic response to sepsis which resulted in defining criteria for the systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis and septic shock 4, 5. These criteria were refined a decade later (2001) by the participants of the International Sepsis Definitions Conference 6 and were based exclusively on adult criteria. The International Consensus Conference on Pediatric Sepsis and Organ Dysfunction was convened in 2002 to develop pediatric-specific definitions for SIRS, sepsis, severe sepsis, septic shock and multiple organ dysfunction syndrome (MODS) 7.
Through clinical observations, pediatricians and neonatologists had recognized that the systemic inflammatory response of tachycardia, tachypnea, hyperthermia and leukocytosis (Table 1) most commonly triggered by infection, could also be present following trauma, burn injury, pancreatitis and various other insults. As a result, this physiologic response was defined as the systemic inflammatory response syndrome (SIRS) with no reference to the presence of infection. Sepsis was defined as a SIRS response associated with infection based on either microbiologic cultures or strong clinical evidence of the presence of an infection. Severe sepsis was defined as sepsis plus evidence of organ dysfunction define around pediatric parameters (Table 2) while septic shock was defined as sepsis criteria plus the presence of “cardiovascular dysfunction” present after the administration of at least 40 ml/kg in 1 hour of fluid. Cardiovascular dysfunction included: age-specific hypotension (Table 3 shows age-related normal values); requirement of a vasoactive agent to maintain normal blood pressure; or evidence of poor end-organ perfusion (Table 2).
Table 1.
Definitions of systemic inflammatory response syndrome (SIRS), infection, sepsis, severe sepsis, and septic shock
Systemic Inflammatory Response Syndrome: The presence of at least two of the following four criteria, one of which must be abnormal temperature or leukocyte count:
|
Infection
|
Sepsis
|
| Severe sepsis |
Septic shock
|
Modified from 7.
Table 2.
Organ dysfunction criteria
Cardiovascular dysfunction: Despite administration of isotonic intravenous fluid bolus ≥40 mL/kg in 1 hr
|
Respiratory
|
Neurologic
|
Hematologic
|
Renal
|
Hepatic
|
Modified from 7.
Table 3.
Age-specific vital signs and laboratory variables
| Age Group | Heart Rate (Beats/Min) |
Respiratory Rate (Breaths/Min) | Leukocyte Count (Leukocytes × 103/mm) | Systolic Blood Pressure (mm Hg) | |
|---|---|---|---|---|---|
| Tachycardia | Bradycardia | ||||
| 0 days to 1 wk | >180 | <100 | >50 | >34 | <65 |
| 1 wk to 1 mo | >180 | <100 | >40 | >19.5 or <5 | <75 |
| 1 mo to 1 yr | >180 | <90 | >34 | >17.5 or <6 | <100 |
| 2–5 yrs | >140 | NA | >22 | >15.5 or <6 | <94 |
| 6–12 yrs | >130 | NA | >18 | >13.5 or<4.5 | <105 |
| 13 to <18 yrs | >110 | NA | >14 | >11 or <4.5 | <117 |
Modified from 7.
Though this report listed criteria for both “newborns” (aged 0 to 7 days) and “neonates” (aged 1 wk to month), preterm newborns were specifically excluded from the definitions 7. This omission related to a number of factors including: the charge of the Consensus Conference, insufficient representation by practitioners in neonatology, and a general agreement that premature infants fell outside the scope of clinical practice of the majority of Conference attendees. Furthermore, the diagnosis of sepsis can be challenging in the very preterm infant undergoing the physiologic transitional period immediately after birth. Other features complicating the delineation of this diagnosis include the subtlety of non-specific presenting signs and the contribution of transitional physiology making it imperative that clinicians maintain a high index of suspicion. This reliance on clinical “gestalt” has been substantiated by evidence showing that a physician’s clinical suspicion of culture positive sepsis has significant positive predictive value (>70%) in excess of most laboratory studies 8, 9. In some neonatal cases, the clinical presentation may be overtly obvious with significant apnea or respiratory distress, cyanosis, hypotension, bradycardia, poor perfusion, acidosis, and lethargy. Alternatively, the presence of one or more subtle and non-specific signs such as feeding intolerance, self-resolving apnea or bradycardia, mild tachypnea or tachycardia, abnormal serum glucose, or decreased activity may be the only warning before fulminant clinical deterioration 10, 11. Though some useful ancillary laboratory tests for the diagnosis of neonatal sepsis exist, the diagnosis remains a significant clinical challenge 12.
Epidemiology of pediatric sepsis
Worldwide, sepsis is one of the most common causes of death in children. One of the first pediatric-specific studies analyzing data from 5 centers reported over 21 to 25% of the 726 patients met criteria for “sepsis syndrome” with a mortality of 11% 13. Proulx et al. 14 analyzed over 1000 admissions over a one-year period and observed that SIRS was present in 82% of PICU patients while sepsis was present in 23% with severe sepsis and septic shock was detected in 4% and 2% of patients. Though sepsis-specific mortality rates were not reported, the overall mortality rate of the entire cohort (n=1058) was 6% and did differ among those patients with multiple organ dysfunction, many of whom were triggered by sepsis 14. Watson et al. examined discharge data to show that severe sepsis accounted for 9675 of the 1.6 million discharges of patients ≤19 years old resulting in a national estimate of 42,371 cases of pediatric sepsis per year (0.6 cases/1,000 population) 15. Not all centers reported data for premature low birth weight or very low birth weight neonates which were by convention included as “infants” in the analysis 15. The grouping of “infants” (any child < 1 year) comprised nearly half the children with severe sepsis and nearly half the sepsis cohort had at least one co-morbid condition. The highest incidence of severe sepsis was found in neonates (5.2 cases/1,000 population) compared to 5 to 14 year olds (0.2 cases/1,000 population) and the mortality rate reported across the entire cohort (including some neonates within the infant category) with severe sepsis was 10.3% estimating 4,364 deaths per year nationally. These data positioned sepsis as the third leading cause of death in children nationwide. In a follow up study from 1995 and 1999, Watson et al found the rate of severe sepsis had increased 11% mostly due to an increase in the number of very low birth weight neonates succumbing to sepsis 16. Despite this increase in cases of severe sepsis, the mortality due to severe sepsis in previously healthy children was found to be 9% 16.
Odetola et al. conducted a similar retrospective study of hospitalized children (age 0 to 19 years) with severe sepsis (sepsis with at least one organ dysfunction as estimated from ICD-9 coding) within the 2003 Kids’ Inpatient Database (KID) which included almost 3 million pediatric discharges from 3438 hospitals in 36 states 17. The authors identified nearly 13,000 hospitalizations for severe sepsis in the database providing a national estimate of 21,448 severe sepsis admissions with an overall mortality rate of 4.2%. There was similar increase in the prevalence of and mortality from severe sepsis in the younger aged cohort (less than 4 years), with a notable increase in adolescents as compared to 4–10 year olds. Perhaps most importantly, these investigators noted the significant association of both co-morbid conditions and existing organ dysfunction to worsening outcomes and higher resource utilization 17.
In the most recent epidemiology study of pediatric sepsis, Czaja and colleagues investigated readmission rates and late mortality for children (1 month to 18 years old) following severe sepsis 18. 7183 children were diagnosed with severe sepsis from 1990 through 2004, 6.8% of whom died during with the authors termed the “sentinel admission” or within 28 days of discharge. Importantly, death certificates confirmed that an additional 434 (6.5%) of those who had survived an initial 28 days after admission, subsequently died during the follow-up period with the highest late death rate occurring within 2 years of the initial hospitalization 18. Although most of the early, as well as the late deaths, occurred in children with co-morbidities (8% early death, 10.4% late death), 8% of children with no co-morbidities died during their initial hospitalization.
Neonatal sepsis is also a significant global killer responsible for over 1 million deaths annually and is among the top ten causes of neonatal death in the United States 19. Stoll et al. reported that the incidence of neonatal sepsis is dependent upon gestational age and time of onset (early-onset sepsis-EOS [<72 hours after birth] versus late-onset sepsis-LOS [>72 hours after birth]) 20–23. Deficiencies in the immune system function of neonates further delineated below increase the risk of morbidity for survivors (only 28% alive and considered normal at 18 months) and mortality that exceeds 70% for the most immature neonates 24. Risk factors for developing sepsis in the premature neonate have been described and are reviewed 10, 20, 21, 25–30. Notable findings from the 1996 Neonatal Network report included the observation that culture-proven EOS was uncommon, occurring in only 1.9% of nearly 8000 VLBW neonates. Group B streptococcus (31%), Escherichia coli (16%) and Haemophilus influenzae (12%) were the most common pathogens associated with EOS in this era. The fact that antibiotic therapy for suspected sepsis was often started at birth in VLBW neonates and continued for 5 or more days, despite a negative blood culture result in 98% of cases 27 underscores the challenge of excluding sepsis in the symptomatic VLBW neonate. In this report, 26% of VLBW neonates with EOS died but it was not clear that all deaths were attributable to infection as only 4% of the 950 deaths that occurred in the first 72 hours of life were attributed to infection 27. Key maternal factors such as Group B Streptococcus (GBS) positive vaginal culture, prolonged rupture of membranes, intrapartum fever, and chorioamnionitis are strongly associated with EOS 28. Gender (male), birth weight (<1000 g), and gestational age (<30 weeks) as well as common clinical interventions associated with prematurity (e.g. intubation, mechanical ventilation, and central venous access) are also associated with an increased risk of sepsis 20, 21, 24. These data illustrate that sepsis is a major health problem in pediatrics.
Key developmental differences impacting neonatal and pediatric sepsis
Severe sepsis manifests with concurrent derangements in almost every single organ system. The degree to which any of these derangements are manifest is variable and influenced by multiple host and pathogen factors, including the patient’s age 15, 31, gender 32, 33, race 34, 35, and genetic background 36–39, as well as the presence of co-morbid conditions 15, 40–42, the patient’s underlying immune status 15, 40, and the specific pathogen involved 43, 44 (see accompanying article). There are several key developmental differences in the host response to infection and therapy that clearly delineate pediatric sepsis as a separate, albeit related, entity from adult sepsis. A complete picture of pediatric sepsis therefore requires a thorough understanding of those developmental differences and how they contribute to organ dysfunction in the pediatric patient with septic shock.
Developmental differences in hemodynamics
Septic shock is due to a combination of decreased intravascular volume (either absolute or relative hypovolemia), myocardial dysfunction, and abnormalities in peripheral vasoregulation. Absolute hypovolemia (i.e. decreased intravascular volume secondary to poor oral intake, vomiting, diarrhea, or increased insensible losses, etc.) or relative hypovolemia (i.e. decreased intravascular volume secondary to capillary leak, increased venous capacitance, etc.) is the most common cause of shock in children 45, 46. However, abnormalities in peripheral vasoregulation and/or myocardial dysfunction likely play a greater role in the hemodynamic derangements associated with pediatric septic shock, especially in neonates and young infants 47–51. Ceneviva and colleagues 52 categorized 50 children with fluid-refractory shock based upon hemodynamic data obtained with a pulmonary artery catheter into three categories: (i) a hyperdynamic state characterized by a high cardiac output (> 5.5 L/min/m2 BSA) and low systemic vascular resistance (< 800 dynes sec/cm5) (classically referred to as warm shock); (ii) a hypodynamic state characterized by low cardiac output (< 3.3 L/min/m2 BSA) and normal to low systemic vascular resistance (SVR); or (iii) a hypodynamic state characterized by low cardiac output and high SVR (> 1200 dynes sec-cm5) (classically referred to as cold shock). Thus, in contrast to adults in which septic shock is characterized by a high cardiac output and low SVR, most children had low cardiac output and high systemic vascular resistance (cold shock) and required vasodilators to decrease SVR, increase CI, and improve peripheral perfusion 52. These findings were confirmed in multiple studies 53–58. For example, Feltes et al 58 reported decreased left ventricular systolic function and increased afterload in 5 out of 10 children with septic shock. Thus, myocardial depression is a common pathophysiological feature in pediatric septic shock raising the question as to differences unique to myocardial function.
Significant developmental differences in both myocardial structure and function compromise the compensatory response of children and neonates to sepsis 49, 59, 60. For example, changes in excitation-contraction coupling occur due to the immaturity of the calcium regulation system (T tubules, sarcoplasmic reticulum, L-type Ca2+ channels). These developmental differences lead to alterations in the normal mechanisms regulating the Ca2+-induced Ca2+ release (CICR) that triggers excitation-contraction coupling, such that the neonatal myocardium is more dependent upon extracellular calcium versus intracellular calcium for contractility as compared to the mature heart 61–64 explaining why neonates are more sensitive to calcium channel antagonists 62. Further differences in the neonatal myocardium include decreased expression of ATP-sensitive K+ channels (KATP) 65 and alterations in β-adrenergic receptor signal transduction 66. KATP channels are inhibited by intracellular ATP and activated by intracellular nucleoside diphosphates (e.g. ADP). These channels are activated in response to ischemia or hypoxia and are therefore important for the adaptations that must occur in sepsis 67–69.
The infant’s myocardium also has a relatively decreased left ventricular mass in comparison to the adult myocardium 70, 71, as well as an increased ratio of type I collagen (decreased elasticity) to type III collagen (increased elasticity) 72. In addition, the infantile myocardium functions at a relatively high contractile state, even at baseline 49, 73. Collectively, these developmental changes result in a relatively limited capacity to increase stroke volume during stress 49, 71, 74, and hence neonates and young infants are critically dependent upon an increase in heart rate to generate increased cardiac output. However, while adults can easily double their heart rate to compensate for decreased stroke volume, infants are unable to compensate in this manner due to the relatively higher baseline heart rate 75. In addition, myocardial perfusion occurs to the greatest degree during diastole and depends directly upon the difference between diastolic blood pressure and left atrial pressure, and inversely with heart rate. Thus, as the heart rate increases, diastolic filling will eventually reach a point at which further increases in cardiac output are limited. Cardiac output, however, is maintained for a time via peripheral vasoconstriction (increased systemic vascular resistance = increased afterload) in an attempt to maintain adequate preload 54–56, 76. Finally, systolic performance in neonates and young infants is critically dependent upon afterload 73, 77. An increase in systemic afterload in the setting of septic shock therefore results in markedly reduced left ventricular systolic performance and myocardial dysfunction. Collectively, these hemodynamic changes which are clinically manifested as cold shock (decreased CI, increased SVR) are more commonly observed in critically ill children with septic shock.
Developmental differences in the coagulation cascade
Sepsis is one of the most common causes of disseminated intravascular coagulation (DIC) which results from uncontrolled thrombin generation and subsequently leads to both microvascular thrombosis that contributes to end organ dysfunction and paradoxically, bleeding diathesis due to the consumption of coagulation factors 78–80. One of the seminal findings that has contributed to an improved molecular understanding of sepsis-induced organ dysfunction is this observed “switch” from a homeostatic balance of anti- versus pro-coagulant factors to a skewing towards pro-coagulation with impaired anti-coagulation favoring microvascular thromboses. A number of factors contribute to dysregulation of the coagulation cascade in sepsis: activation of procoagulant pathways, consumption of clotting factors, alterations in fibrinolysis, and reduced anti-coagulant activity resulting in what is commonly described as disseminated intravascular coagulation (DIC) (reviewed in 79, 81–83. Simultaneous to enhanced fibrin production, attenuated fibrinolysis caused by increased plasminogen activator inhibitor type 1 (PAI-1), as well as dysfunction and/or depletion of natural anti-coagulants [antithrombin III, protein C, protein S and tissue factor pathway inhibitor (TFPI)] occurs. A correlation between low AT III and mortality in sepsis provided the impetus for studying AT III replacement; however, no consistent benefit has been observed in either adults 84–87, children 88, 89, or neonates 90.
Patients with sepsis also have substantial depletion of protein C (Reviewed: 91–93). Given encouraging pre-clinical studies with the use of APC in sepsis, clinical trials in adults were commenced (e.g. Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial 94. In this study, APC was associated with a statistically significant reduction in 28-day mortality in sepsis in adults. In further analysis, APC appeared to confer benefit only on those with the highest severity of illness (APACHE score >25); subsequent studies have been executed in an attempt to identify the adult patient population most benefiting from this therapy 94–96. The pediatric Phase III study employing APC in severe sepsis was stopped after an interim analysis showed that APC was unlikely to improve the resolution of organ dysfunction and that its use was associated with an increased risk of serious adverse events in children less than 60 days 97, 98. To date, only case reports have examined the use of APC in neonatal sepsis 99–101. Taken together, the current state of the use of APC in sepsis remains controversial in adults and appears to be of no benefit in either children or neonates.
There are important developmental differences in the coagulation and fibrinolytic system that impact the pathophysiology and management of DIC in children versus adults 102. For example, neonates and infants are at an increased risk for bleeding complications, primarily due to lower circulating levels of vitamin K-dependent procoagulant factors (factor II, VII, IX, and X), a decreased capacity to generate thrombin, and decreased circulating levels of coagulation inhibitors. The neonatal coagulation system contains all of the essential factors necessary for an intact coagulation system, though the amounts of factors are decreased relative to adult levels 102. Similarly, while there are no distinct differences in platelet quantity between children and adults, platelets are relatively hyporesponsive to physiologic agonists 103. These unique differences may explain the increased risk of mortality in younger children with DIC, compared to older children 104 and adults and why the therapeutic response to modulators of coagulation differ.
Developmental differences in the inflammatory response to sepsis
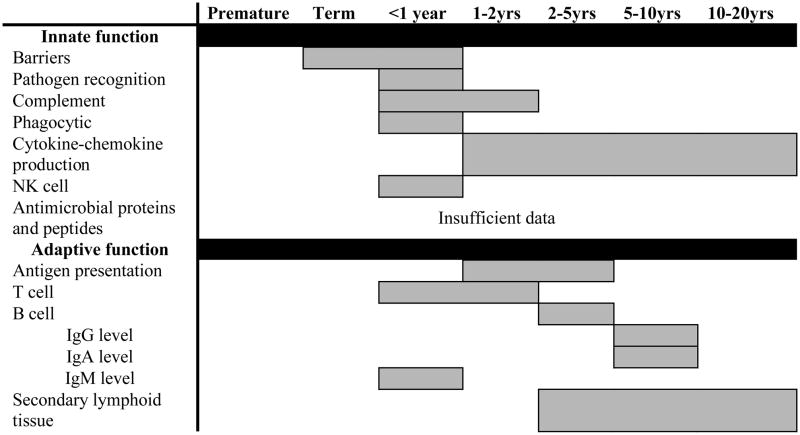
In order to contextualize the developmental differences in the host response to sepsis, specific contributions of some mediators and mechanisms must be briefly summarized. Key mediators of the inflammatory response to host invasion include numerous gene products (e.g. cytokines and chemokines, coagulation-related factors, and immune active proteins) all of which are critical to ensuring recruitment and activation of immune cells necessary for pathogen eradication. Literally hundreds of gene products play some role in the complex host response to sepsis and a comprehensive review of these beyond the scope of any single report on this subject. The reader is directed to recent reviews of the mediators of this pathophysiologic response in sepsis 105–108 and gene expression profiling (accompanying article). Here, we briefly highlight some key developmental differences in the host innate and adaptive immune responses (reviewed 109 and summarized in Table 4).
Table 4.
Timing of acquisition of mature, immune function where gray shading indicates the estimated age range when near-adult level function is attained.
 |
While a number of mediators responsible for the initiation and propagation of sepsis were identified early on, few studies attempted to identify those responsible for the eventual resolution of inflammation. This concept appeared to be important as failure to reduce proinflammatory mediators over the course of sepsis was associated with higher mortality rates 110–112. Also, patients who died from ARDS produced lower levels of IL-10 as compared to survivors 113. More recently, the hypothesis that an over-exuberant counter-regulatory process can lead to gene deactivation in sepsis and thus, increase mortality in both children 114 and adults 115 has identified a need to better understand this mechanism.
We now understand that immune activation triggered by pathogens also drives expression of endogenous counter-regulators of inflammation resulting in a state termed the “compensatory anti-inflammatory response syndrome (CARS)”. Such mediators include both cytokine antagonists (e.g. soluble TNF receptor, IL-1Ra) and “anti-inflammatory” cytokines (e.g. IL-10 and TGF-β) (reviewed 116–118). Clearly other regulatory cytokines (e.g. TGF-β, IL-13) possess anti-inflammatory properties and contribute to the endogenous regulation of the acute inflammatory response to sepsis. Based on additional observations, there is a growing recognition that this compensatory anti-inflammatory response may play a greater role in the pathobiology of septic shock and multiple organ failure in children as compared to adults 119.
For example, Wynn and colleagues compared the host inflammatory response and subsequent mortality in a fecal slurry model of generalized peritonitis between neonatal mice (age 5–7 days) and young adult mice (age 7–10 weeks). Compared with young adult mice, sepsis in the neonatal mice was associated with a markedly attenuated systemic inflammatory response, decreased bacterial clearance, and increased mortality 120. Similar studies in a hemorrhagic shock model demonstrated decreased lung inflammation and injury in immature mice versus adult mice 121.
Consistent with these results, Barsness and colleagues collected peritoneal macrophages during laparoscopic surgery in children (mean age 3.6 years) and adults (mean age 46.9 years) and treated them ex vivo with IL-1β and LPS 122. Both IL-1β- and LPS-induced pro-inflammatory cytokine (TNF-α and IL-6) production were markedly increased in the peritoneal macrophage cultures obtained from children versus adults. Importantly, the anti-inflammatory response, as determined by IL-10 production, was much greater in the cultures obtained from children such that the ratio of IL-10 to TNF-α was significantly higher macrophage cultures from children as compared to adults, suggesting a predominant anti-inflammatory phenotype 122. These data have provided the impetus to further understand the immune function differences that exist across the entire spectrum of ages all of which are subject to sepsis.
Developmental differences in the immune system
There are other significant differences in the host immune response between the different ages. Clearly, compared to immunologically mature populations, neonates have an increased risk for the development and progression of a systemic infection. Broad deficits across both innate and adaptive immune function have been identified and have been reviewed in detail (summarized in Table 4) 123–125. In particular, the preterm neonate exhibits significant vulnerability due to exacerbated immunologic immaturity as well the need for life-sustaining clinical interventions that increase the likelihood for infection. Adaptive immune function is hindered by deficiencies in: 1) T cell function: TH2 skewed cytokine responses, increased requirement for CD4 T cell stimulation, decreased CD8 T cell cytolytic activity, abundant and potent T regulatory population; 2) B cell function: weak immunoglobulin (Ig) production [predominantly IgM], poor Ig class switching, decreased maternal-derived serum IgG due to premature delivery, poor antibody response to polysaccharide antigens, poor T cell-dependent B cell stimulation, and limited antecedent antigen exposure prior to birth; and 3) underdeveloped secondary lymphoid tissues.
While the contribution of the distinct adaptive immune system function in neonates in the setting of sepsis has not been fully defined in humans, experimental work in mice suggests substantial differences exist. In contrast to findings in adult mice, transgenic neonatal mice lacking an adaptive immune system showed no difference in sepsis survival when compared to wild-type neonatal mice 125. Altered adaptive immune system function leaves the neonate largely dependent upon the innate immune system for defense against pathogenic challenge. The neonatal innate immune system is also limited in function as compared to adults and children. Deficits exist in: barrier integrity, circulating complement components, expression of antimicrobial proteins and peptides (intercellular and circulating), production of type I interferons and TH1 polarizing cytokines. Furthermore, there are quantitative and qualitative impairments in neutrophil, monocyte, macrophage, and dendritic cell function and decreased response to most Toll-like receptor agonists. The net effect of these deficits is a functional immunocompromised state that leaves the premature neonate extremely susceptible to microbial invasion. Future studies aimed at characterizing the unique neonatal pathophysiologic response, including defining critically important elements and the capacity for positive immunomodulation, are necessary 126.
Conclusion
Sepsis, a syndrome characterized by variable, systemic physiologic changes triggered by infection, continues to provide an extraordinary challenge to clinicians managing critically ill neonates, children and adolescents. The incidence of sepsis continues to increase with a mortality rate—both early and late—that position it among the leading causes of death in children. Developmental differences that impact the hemodynamic, inflammatory, coagulation and immune responses make it difficult to extrapolate data from adult studies to these vulnerable pediatric populations. In light of emerging data that suggest developmental influences on epigenetic regulation of gene expression in sepsis, it is imperative that neonatal and pediatric clinician-investigators drive and execute sepsis studies in their respective populations. Only by mechanistic studies complemented with well-crafted, network-based interventional trials, will we impact on this most challenging pathologic syndrome.
Acknowledgments
Grant support: Supported by K12HD047349 to TTC; R01GM064619 and 1RC1HL100474-01 to HRW; RO1HL097361, RO1GM066839 and UL1RR024986 to TPS.
Abbreviations
- EOS
early-onset-sepsis
- LOS
late-onset-sepsis
- SIR
systemic inflammatory response syndrome
- MODS
multiple organ dysfunction syndrome
- DIC
disseminated intravascular coagulation
- APC
activated protein C
References
- 1.Geroulanos S, Douka ET. Historical perspective of the word “sepsis”. Intensive Care Med. 2006;32(12):2077. doi: 10.1007/s00134-006-0392-2. [DOI] [PubMed] [Google Scholar]
- 2.Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, et al. Sepsis and Major Abdominal Surgery Lead to Flaking of the Endothelial Glycocalix. J Surg Res. 2009 doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Thomas L. Germs. N Engl J Med. 1972;287:553–555. doi: 10.1056/NEJM197209142871109. [DOI] [PubMed] [Google Scholar]
- 4.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101(6):1481–1483. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20(6):724–726. doi: 10.1097/00003246-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 8.Fischer JE, Harbarth S, Agthe AG, Benn A, Ringer SA, Goldmann DA, et al. Quantifying uncertainty: physicians’ estimates of infection in critically ill neonates and children. Clin Infect Dis. 2004;38(10):1383–1390. doi: 10.1086/420741. [DOI] [PubMed] [Google Scholar]
- 9.Ng PC. Diagnostic markers of infection in neonates. Arch Dis Child Fetal Neonatal Ed. 2004;89(3):F229–235. doi: 10.1136/adc.2002.023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer CR, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J. 1998;17(7):593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Sankar MJ, Agarwal R, Deorari AK, Paul VK. Sepsis in the newborn. Indian J Pediatr. 2008;75(3):261–266. doi: 10.1007/s12098-008-0056-z. [DOI] [PubMed] [Google Scholar]
- 12.Lam HS, Ng PC. Biochemical markers of neonatal sepsis. Pathology. 2008;40(2):141–148. doi: 10.1080/00313020701813735. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111(3):324–328. doi: 10.1016/s0022-3476(87)80448-1. [DOI] [PubMed] [Google Scholar]
- 14.Proulx F, Fayon M, Farrell CA, Lacroix J, Gauthier M. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109(4):1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 15.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 16.Watson RS, Carcillo JA. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S3–5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 17.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 18.Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 19.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;57(2):1–32. [PubMed] [Google Scholar]
- 20.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 21.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 Pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 23.Haque KN, Khan MA, Kerry S, Stephenson J, Woods G. Pattern of culture-proven neonatal sepsis in a district general hospital in the United Kingdom. Infect Control Hosp Epidemiol. 2004;25(9):759–764. doi: 10.1086/502473. [DOI] [PubMed] [Google Scholar]
- 24.Kermorvant-Duchemin E, Laborie S, Rabilloud M, Lapillonne A, Claris O. Outcome and prognostic factors in neonates with septic shock. Pediatr Crit Care Med. 2008;9(2):186–191. doi: 10.1097/PCC.0b013e31816689a8. [DOI] [PubMed] [Google Scholar]
- 25.Shah GS, Budhathoki S, Das BK, Mandal RN. Risk factors in early neonatal sepsis. Kathmandu Univ Med J (KUMJ) 2006;4(2):187–191. [PubMed] [Google Scholar]
- 26.Salem SY, Sheiner E, Zmora E, Vardi H, Shoham-Vardi I, Mazor M. Risk factors for early neonatal sepsis. Arch Gynecol Obstet. 2006;274(4):198–202. doi: 10.1007/s00404-006-0135-1. [DOI] [PubMed] [Google Scholar]
- 27.Stoll BJ, Gordon TG, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Early-onset sepsis in very low birth weight neonates: A report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):72–80. doi: 10.1016/s0022-3476(96)70192-0. [DOI] [PubMed] [Google Scholar]
- 28.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103(6):e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 29.Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O’Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000;105(1 Pt 1):21–26. doi: 10.1542/peds.105.1.21. [DOI] [PubMed] [Google Scholar]
- 30.Escobar GJ, Li DK, Armstrong MA, Gardner MN, Folck BF, Verdi JE, et al. Neonatal sepsis workups in infants >/=2000 grams at birth: A population-based study. Pediatrics. 2000;106(2 Pt 1):256–263. doi: 10.1542/peds.106.2.256. [DOI] [PubMed] [Google Scholar]
- 31.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 32.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26(2):167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 33.Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132(6):1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 34.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 35.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(3):279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest. 2003;124(3):1103–1115. doi: 10.1378/chest.124.3.1103. [DOI] [PubMed] [Google Scholar]
- 37.Villar J, Maca-Meyer N, Perez-Mendez L, Flores C. Bench-to-bedside review: Understanding genetic predisposition to sepsis. Critical Care. 2004;8:180–189. doi: 10.1186/cc2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahmer MK, Randolph A, Vitali S, Quasney MW. Genetic polymorphisms in sepsis. Pediatr Crit Care Med. 2005;6(3 Suppl):S61–73. doi: 10.1097/01.PCC.0000161970.44470.C7. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland AM, Walley KR. Bench-to-bedside review: Association of genetic variation with sepsis. Crit Care. 2009;13(2):210. doi: 10.1186/cc7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]
- 43.Brouwer MC, de Gans J, Heckenberg SG, Zwinderman AH, van der Poll T, van de Beek D. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9(1):31–44. doi: 10.1016/S1473-3099(08)70261-5. [DOI] [PubMed] [Google Scholar]
- 44.van der Poll T, Opal SM. Host-pathogen interactions in sepsis. Lancet Infect Dis. 2008;8(1):32–43. doi: 10.1016/S1473-3099(07)70265-7. [DOI] [PubMed] [Google Scholar]
- 45.Perkin RM, Levin DL. Shock in the pediatric patient. Part II. Therapy. J Pediatr. 1982;101(3):319–332. doi: 10.1016/s0022-3476(82)80053-x. [DOI] [PubMed] [Google Scholar]
- 46.Perkin RM, Levin DL. Shock in the pediatric patient. Part I. J Pediatr. 1982;101(2):163–169. doi: 10.1016/s0022-3476(82)80110-8. [DOI] [PubMed] [Google Scholar]
- 47.Dasgupta SJ, Gill AB. Hypotension in the very low birthweight infant: the old, the new, and the uncertain. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F450–454. doi: 10.1136/fn.88.6.F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill AB, Weindling AM. Echocardiographic assessment of cardiac function in shocked very low birthweight infants. Arch Dis Child. 1993;68(1 Spec No):17–21. doi: 10.1136/adc.68.1_spec_no.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luce WA, Hoffman TM, Bauer JA. Bench-to-bedside review: Developmental influences on the mechanisms, treatment and outcomes of cardiovascular dysfunction in neonatal versus adult sepsis. Crit Care. 2007;11(5):228. doi: 10.1186/cc6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz SM, Duffy JY, Pearl JM, Nelson DP. Cellular and molecular aspects of myocardial dysfunction. Crit Care Med. 2001;29(10 Suppl):S214–219. doi: 10.1097/00003246-200110001-00003. [DOI] [PubMed] [Google Scholar]
- 51.Walther FJ, Siassi B, Ramadan NA, Wu PY. Cardiac output in newborn infants with transient myocardial dysfunction. J Pediatr. 1985;107(5):781–785. doi: 10.1016/s0022-3476(85)80417-0. [DOI] [PubMed] [Google Scholar]
- 52.Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102(2):e19. doi: 10.1542/peds.102.2.e19. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds EM, Ryan DP, Sheridan RL, Doody DP. Left ventricular failure complicating severe pediatric burn injuries. J Pediatr Surg. 1995;30(2):264–269. doi: 10.1016/0022-3468(95)90572-3. discussion 269–270. [DOI] [PubMed] [Google Scholar]
- 54.Pollack MM, Fields AI, Ruttimann UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13(6):454–459. doi: 10.1097/00003246-198506000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975;51(5):867–874. doi: 10.1161/01.cir.51.5.867. [DOI] [PubMed] [Google Scholar]
- 56.Pollack MM, Fields AI, Ruttimann UE. Sequential cardiopulmonary variables of infants and children in septic shock. Crit Care Med. 1984;12(7):554–559. doi: 10.1097/00003246-198407000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Mercier JC, Beaufils F, Hartmann JF, Azema D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16(1):27–33. doi: 10.1097/00003246-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Feltes TF, Pignatelli R, Kleinert S, Mariscalco MM. Quantitated left ventricular systolic mechanics in children with septic shock utilizing noninvasive wall-stress analysis. Crit Care Med. 1994;22(10):1647–1658. [PubMed] [Google Scholar]
- 59.Seri I. Circulatory support of the sick preterm infant. Semin Neonatol. 2001;6(1):85–95. doi: 10.1053/siny.2000.0034. [DOI] [PubMed] [Google Scholar]
- 60.Noori S, Seri I. Pathophysiology of newborn hypotension outside the transitional period. Early Hum Dev. 2005;81(5):399–404. doi: 10.1016/j.earlhumdev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Wibo M, Bravo G, Godfraind T. Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res. 1991;68(3):662–673. doi: 10.1161/01.res.68.3.662. [DOI] [PubMed] [Google Scholar]
- 62.Brillantes AM, Bezprozvannaya S, Marks AR. Developmental and tissue-specific regulation of rabbit skeletal and cardiac muscle calcium channels involved in excitation-contraction coupling. Circ Res. 1994;75(3):503–510. doi: 10.1161/01.res.75.3.503. [DOI] [PubMed] [Google Scholar]
- 63.Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Mejia-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol. 2004;286(3):H971–978. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- 64.Huang Y, Zhou Y, Castiblanco A, Yang W, Brown EM, Yang JJ. Multiple Ca(2+)-binding sites in the extracellular domain of the Ca(2+)-sensing receptor corresponding to cooperative Ca(2+) response. Biochemistry. 2009;48(2):388–398. doi: 10.1021/bi8014604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, et al. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5(1):1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. Beta 2-adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res. 1995;76(1):40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- 67.Findlay I. The ATP sensitive potassium channel of cardiac muscle and action potential shortening during metabolic stress. Cardiovasc Res. 1994;28(6):760–761. doi: 10.1093/cvr/28.6.760. [DOI] [PubMed] [Google Scholar]
- 68.Buckley JF, Singer M, Clapp LH. Role of KATP channels in sepsis. Cardiovasc Res. 2006;72(2):220–230. doi: 10.1016/j.cardiores.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103(5):1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 70.Ichihashi K, Ewert P, Welmitz G, Lange P. Changes in ventricular and muscle volumes of neonates. Pediatr Int. 1999;41(1):8–12. doi: 10.1046/j.1442-200x.1999.01008.x. [DOI] [PubMed] [Google Scholar]
- 71.Joyce JJ, Dickson PI, Qi N, Noble JE, Raj JU, Baylen BG. Normal right and left ventricular mass development during early infancy. Am J Cardiol. 2004;93(6):797–801. doi: 10.1016/j.amjcard.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 72.Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. 1994;23(5):1204–1208. doi: 10.1016/0735-1097(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 73.Crepaz R, Gentili L, Taddei F, Romeo C, Pitscheider W. Developmental changes of cardiac mechanics during fetal and postnatal life. Diagnostic role of Doppler echocardiography. G Ital Cardiol. 1998;28(2):187–192. [PubMed] [Google Scholar]
- 74.Rowland DG, Gutgesell HP. Noninvasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am J Cardiol. 1995;75(12):818–821. doi: 10.1016/s0002-9149(99)80419-6. [DOI] [PubMed] [Google Scholar]
- 75.Carcillo JA, Kuch BA, Han YY, Day S, Greenwald BM, McCloskey KA, et al. Mortality and functional morbidity after use of PALS/APLS by community physicians. Pediatrics. 2009;124(2):500–508. doi: 10.1542/peds.2008-1967. [DOI] [PubMed] [Google Scholar]
- 76.Carcillo JA, Pollack MM, Ruttimann UE, Fields AI. Sequential physiologic interactions in pediatric cardiogenic and septic shock. Crit Care Med. 1989;17(1):12–16. doi: 10.1097/00003246-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Crepaz R, Cemin R, Pedron C, Gentili L, Trevisan D, Pitscheider W. Age-related variations of left ventricular endocardial and midwall function in healthy infants, children, and adolescents. Ital Heart J. 2005;6(8):634–639. [PubMed] [Google Scholar]
- 78.Kenet G, Strauss T, Kaplinsky C, Paret G. Hemostasis and thrombosis in critically ill children. Semin Thromb Hemost. 2008;34(5):451–458. doi: 10.1055/s-0028-1092875. [DOI] [PubMed] [Google Scholar]
- 79.Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008;34(5):459–468. doi: 10.1055/s-0028-1092876. [DOI] [PubMed] [Google Scholar]
- 80.Levi M. The coagulant response in sepsis. Clin Chest Med. 2008;29(4):627–642. viii. doi: 10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 81.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med. 2001;29(7 Suppl):S28–34. doi: 10.1097/00003246-200107001-00013. discussion S34–25. [DOI] [PubMed] [Google Scholar]
- 82.Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost. 1998;24(1):33–44. doi: 10.1055/s-2007-995821. [DOI] [PubMed] [Google Scholar]
- 83.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 84.Baudo F, Caimi TM, de Cataldo F, Ravizza A, Arlati S, Casella G, et al. Antithrombin III (ATIII) replacement therapy in patients with sepsis and/or postsurgical complications: a controlled double-blind, randomized, multicenter study. Intensive Care Med. 1998;24(4):336–342. doi: 10.1007/s001340050576. [DOI] [PubMed] [Google Scholar]
- 85.Warren BL, Eid A, Singer P, Pillay SS, Carl P, Novak I, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: A randomized controlled trial. JAMA. 2001;286:1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 86.Hoffmann JN, Wiedermann CJ, Juers M, Ostermann H, Kienast J, Briegel J, et al. Benefit/risk profile of high-dose antithrombin in patients with severe sepsis treated with and without concomitant heparin. Thromb Haemost. 2006;95(5):850–856. [PubMed] [Google Scholar]
- 87.Wiedermann CJ, Hoffmann JN, Juers M, Ostermann H, Kienast J, Briegel J, et al. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med. 2006;34(2):285–292. doi: 10.1097/01.ccm.0000194731.08896.99. [DOI] [PubMed] [Google Scholar]
- 88.Munteanu C, Bloodworth LL, Korn TH. Antithrombin concentrate with plasma exchange in purpura fulminans. Pediatr Crit Care Med. 2000;1(1):84–87. doi: 10.1097/00130478-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 89.Kreuz WD, Schneider W, Nowak-Gottl U. Treatment of consumption coagulopathy with antithrombin concentrate in children with acquired antithrombin deficiency--a feasibility pilot study. Eur J Pediatr. 1999;158 (Suppl 3):S187–191. doi: 10.1007/pl00014353. [DOI] [PubMed] [Google Scholar]
- 90.Bassler D, Schmidt B. Antithrombin replacement in neonates: is there any indication? Thromb Res. 2006;118(1):107–111. doi: 10.1016/j.thromres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 91.Esmon CT. Protein C anticoagulant pathway and its role in controlling microvascular thrombosis and inflammation. Crit Care Med. 2001;29(7 Suppl):S48–51. doi: 10.1097/00003246-200107001-00018. discussion 51–42. [DOI] [PubMed] [Google Scholar]
- 92.Esmon CT. The normal role of Activated Protein C in maintaining homeostasis and its relevance to critical illness. Crit Care. 2001;5(2):S7–12. doi: 10.1186/cc1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faust SN, Heyderman RS, Levin M. Coagulation in severe sepsis: a central role for thrombomodulin and activated protein C. Crit Care Med. 2001;29(7 Suppl):S62–67. doi: 10.1097/00003246-200107001-00022. discussion S67–68. [DOI] [PubMed] [Google Scholar]
- 94.Vincent JL, Bernard GR, Beale R, Doig C, Putensen C, Dhainaut JF, et al. Drotrecogin alfa (activated) treatment in severe sepsis from the global open-label trial ENHANCE: further evidence for survival and safety and implications for early treatment. Crit Care Med. 2005;33(10):2266–2277. doi: 10.1097/01.ccm.0000181729.46010.83. [DOI] [PubMed] [Google Scholar]
- 95.Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, et al. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13(3):R103. doi: 10.1186/cc7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut J-F, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 97.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, et al. Drotrecogin alfa (activated) in children with severe sepsis: A multicentre phase III randomised controlled trial. Lancet. 2007;369:836–843. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 98.Goldstein B, Nadel S, Peters M, Barton R, Machado F, Levy H, et al. ENHANCE: results of a global open-label trial of drotrecogin alfa (activated) in children with severe sepsis. Pediatr Crit Care Med. 2006;7(3):200–211. doi: 10.1097/01.PCC.0000217470.68764.36. [DOI] [PubMed] [Google Scholar]
- 99.Albuali WH, Singh RN, Fraser DD, Scott LA, Kornecki A. Drotrecogin alfa (activated) treatment in a neonate with sepsis and multi organ failure. Saudi Med J. 2005;26(8):1289–1292. [PubMed] [Google Scholar]
- 100.Frommhold D, Birle A, Linderkamp O, Zilow E, Poschl J. Drotrecogin alpha (activated) in neonatal septic shock. Scand J Infect Dis. 2005;37(4):306–308. doi: 10.1080/00365540510031412. [DOI] [PubMed] [Google Scholar]
- 101.Sajan I, Da-Silva SS, Dellinger RP. Drotrecogin alfa (activated) in an infant with gram-negative septic shock. J Intensive Care Med. 2004;19(1):51–55. doi: 10.1177/0885066603258652. [DOI] [PubMed] [Google Scholar]
- 102.Kuhle S, Male C, Mitchell L. Developmental hemostasis: pro- and anticoagulant systems during childhood. Semin Thromb Hemost. 2003;29(4):329–338. doi: 10.1055/s-2003-42584. [DOI] [PubMed] [Google Scholar]
- 103.Israels SJ, Rand ML, Michelson AD. Neonatal platelet function. Semin Thromb Hemost. 2003;29(4):363–372. doi: 10.1055/s-2003-42587. [DOI] [PubMed] [Google Scholar]
- 104.Hazelzet JA, Risseeuw-Appel IM, Kornelisse RF, Hop WC, Dekker I, Joosten KF, et al. Age-related differences in outcome and severity of DIC in children with septic shock and purpura. Thromb Haemost. 1996;76(6):932–938. [PubMed] [Google Scholar]
- 105.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37(1):291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 106.Krawczyk-Michalak K, Glapinski A, Brzezinska-Blaszczyk E. Toll-like receptors and their role in regulation of the inflammatory response in sepsis. Anestezjol Intens Ter. 2008;40(4):253–259. [PubMed] [Google Scholar]
- 107.Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of Toll-like receptors in the development of sepsis. Shock. 2008;29(3):315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 108.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wynn JL, Neu J, Moldawer LL, Levy O. Potential of immunomodulatory agents for prevention and treatment of neonatal sepsis. J Perinatol. 2009;29(2):79–88. doi: 10.1038/jp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103(2):565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 111.Calandra T, Glauser MP. Cytokines and septic shock. Diagn Microbiol Infect Dis. 1990;13(5):377–381. doi: 10.1016/0732-8893(90)90006-h. [DOI] [PubMed] [Google Scholar]
- 112.Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J, et al. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 113.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, et al. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125(3):191–196. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 114.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pachot A, Lepape A, Vey S, Bienvenu J, Mougin B, Monneret G. Systemic transcriptional analysis in survivor and non-survivor septic shock patients: a preliminary study. Immunol Lett. 2006;106(1):63–71. doi: 10.1016/j.imlet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 116.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 117.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101(1):36–47. [PubMed] [Google Scholar]
- 118.Ward NS, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29(4):617–625. viii. doi: 10.1016/j.ccm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113(6):1625–1631. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 120.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28(6):675–683. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 121.Zingarelli B, Hake PW, O’Connor M, Burroughs TJ, Wong HR, Solomkin JS, et al. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor gamma. Crit Care Med. 2009;37(6):1978–1987. doi: 10.1097/CCM.0b013e31819feb4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC., Jr Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg. 2004;39(6):912–915. doi: 10.1016/j.jpedsurg.2004.02.009. discussion 912–915. [DOI] [PubMed] [Google Scholar]
- 123.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 124.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 125.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750–1758. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19(3):290–297. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]


