Abstract
Dyneins are microtubule motors, the core of which consists of a ring of AAA+ domains. ATP-driven conformational changes of the AAA+ ring are used to drive the movement of a mechanical element (termed the linker domain) that provides the motor’s powerstroke and to change the affinity of the motor for microtubules (strong binding during the power stroke and weak binding to allow stepping and recocking of the linker domain). Dynein’s microtubule-binding domain (MTBD) is located at the end of a 10 nm long anti-parallel coiled coil (the stalk) and conformational changes that alter the affinity for microtubules must propagate through this coiled coil. A recent crystal structure of dynein’s MTBD sheds new light on how this long-range communication along a coiled coil might occur.
Keywords: dynein, coiled coil, stalk, microtuble binding domain
Introduction
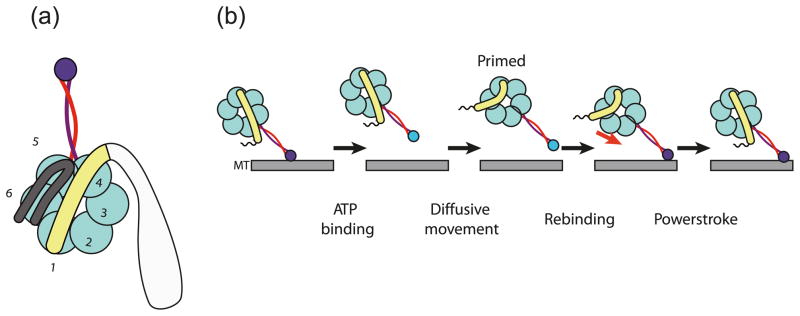
Dyneins are a family of motor proteins that couple ATP hydrolysis to movement along microtubules. Axonemal dyneins power the beating of cilia and flagella, while cytoplasmic dynein and intraflagella transport (IFT) dynein carry a large variety of cargos. The structure of the motor domain of dynein (Fig. 1a) consists of an N terminal linker domain, 6 AAA+ domains that form a ring, and a microtubule-binding domain (MTBD) at the end of a 10 nm long anti-parallel coiled-coil stalk that extends out from the AAA+ ring (between the 4th and 5th AAA+ domain) (Roberts et al. 2009). The events underlying dynein-driven movement are shown in Fig. 1b. At the heart of the mechanism, as with many other AAA+ proteins (White and Lauring 2007), are conformational changes in the AAA+ ring driven by ATP hydrolysis. In the case of dynein, the main nucleotide-binding site is the 1st AAA+ domain (AAA1) (Imamula et al. 2007). The 3rd AAA+ site (AAA3) also appears to play an important role in the mechanism, as ATP binding and hydrolysis mutations in AAA3 produce severe impairment in dynein motility (Kon et al. 2004; Cho et al. 2008). Comparable mutations in AAA2 and AAA4 have more subtle effects on motility. Thus, the roles that AAA2–AAA4 play in the dynein motility cycle remain unclear. The conformational changes in the AAA+ ring are amplified to produce a shift in the position of the linker domain (Burgess et al. 2003; Kon et al. 2005). This movement appears to provide the main, although not sole, conformational change responsible for motility (Shima et al. 2006).
Fig. 1.
Cartoon model of the dynein structure and ATP hydrolysis cycle. (a) Cartoon of the structure showing a ring of 6 AAA+ domains and the coiled-coil stalk emerging from between AAA4 and AAA5 with the microtubule-binding domain (MTBD) at its tip. The mechanical element (linker domain) spans across the face of the ring before connecting to AAA1. A C-terminal region coming out of AAA6 also stretches across the ring. (b) The ATP hydrolysis cycle. ATP binding causes release from the microtubule, followed by a recocking of the linker domain. Rebinding to the microtubule is followed by a displacement of the linker domain (powerstroke), which generates movement relative to the microtubule. The full-colour version of this Figure is available from http://bcb.nrc.ca.
In addition to linker movement, the rearrangements in the AAA+ ring are linked to changes in the affinity of the MTBD for microtubules. ATP binding results in release of the MTBD, while its rebinding provides the trigger for the powerstroke (Imamula et al. 2007). When the stalk was first identified as an anti-parallel coiled coil, it was recognized that there must be some communication mechanism along its length (Gee et al. 1997). However, it was unclear whether this mechanism involved conformational changes along its length or a rigid body movement, such as a rotation about its axis or a swivel about the MTBD (Gee et al. 1997; Xie et al. 2006). As will be discussed in this review, recent data, including a crystal structure of the MTBD and proximal part of the stalk, provide evidence that communication involves conformational changes propagated along the coiled coil.
Structure of the dynein stalk and its interaction with microtubules
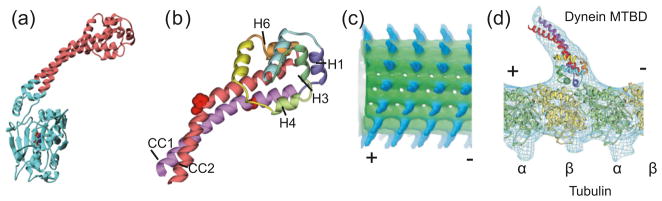
To produce a stable construct for crystallization, the dynein MTBD and the top part of the stalk were fused into the coiled coil of a known protein called seryl-tRNA synthetase (SRS) (Gibbons et al. 2005). A number of different constructs with different fusion sites were prepared (discussed in the next section). Crystals were obtained with the most thermally stable construct and resulted in a 2.3 Å resolution structure (Carter et al. 2008). The structure confirms, as expected, that the dynein stalk is a coiled coil (Fig. 2). The two helices in the stalk (CC1 coming out of AAA4 and CC2 returning back to AAA5) extend for 3 heptad repeats before being kinked by the presence of two highly conserved proline residues. Beyond the kink, the two helices come into contact with the rest of the MTBD, which consists of a novel fold of α helices. The microtubule binding interface, which is on the top surface of the MTBD furthest away from the point of entry of the stalk, is made up of helices H1, H3, and H6. This assignment was made both by the location of mutations that interfere with microtubule binding (Koonce and Tikhonenko 2000) and by docking the crystal structure into an electron-density map of a dynein stalk bound to microtubules that was obtained by cryoelectron microscopy (Fig. 2d).
Fig. 2.
Structure of the dynein microtubule-binding domain (MTBD). (a) Overview of the seryl-tRNA synthetase (SRS) fusion construct. The SRS is in cyan, while the MTBD is in red. (b) Close up view of the MTBD, with secondary structure elements labeled. The prolines that stabilize the kink in the stalk are shown as red spacefill. (c) Cryoelectron microscope image of dynein MTBDs (blue) bound to a microtubule (green). (d) MTBD and tubulin structures docked into an electron-density map (blue mesh) of a single microtubule protofilament decorated with dynein MTBDs. The full-colour version of this Figure is available from http://bcb.nrc.ca.
It is possible that the kink in the stalk plays an important role in dynein’s mechanism. It has been directly observed in the negative stain EM images of the whole cytoplasmic dynein motor domain (Roberts et al. 2009) and is also observed in the cryoelectron microscopy map of the stalk bound to microtubules (Fig. 2d). This would suggest that the microtubule-binding interface orients the kink so that the downstream part of the stalk is angled back toward the plus end (as in Fig. 1b). There is some evidence that the direction in which the dynein stalk points is important for determining the directionality of dynein (Carter et al. 2008; Ueno et al. 2008).
Evidence for conformational changes in the dynein stalk
The first evidence that conformational changes could be transmitted through the stalk came from the seryl-tRNA synthetase (SRS) fusion experiments (Gibbons et al. 2005) mentioned earlier. Coiled coils are made up of repeats of 7 amino acids (heptads) in which residues in the 1st and 4th position are predominantly hydrophobic. The two αhelices in the coiled coil come together so that the residues in these positions pack together. Although identifying the heptad repeat pattern is relatively straightforward, determining the registry of a coiled coil (i.e., which residue in one helix packs against which residue in the other) is often harder. To determine the registry of the dynein stalk, Gibbons et al. (2005) fused it to the SRS coiled coil in such a way that the fusion site on CC2 was fixed, while the length of CC1 included in the construct was varied. The pattern that emerged was that the affinity of the MTBD for microtubules varied in a largely predictable pattern according to the registry in which the stalk coiled coil was fused to that of the SRS. The construct that was crystallized was in this so-called +β registry (in this construct, the number of amino acids between the fusion site and conserved prolines near to the MTBD was 26 in CC1 and 19 in CC2, and thus referred to as SRS-26:19) and was the one that had a low affinity for microtubules (>20 μmol·L−1). In contrast, reducing the length of CC1 by four amino acids (α registry, 22 amino acids in CC1, 19 in CC2 – SRS-22:19) resulted in a construct with a higher affinity (<2 μmol·L−1). This pattern of high and low affinity is repeated when longer lengths of the dynein stalk are fused to SRS but with different registers as above (Gibbons et al. 2005; Carter et al. 2008). This suggests that changes in the registry of the stalk coiled coil at the AAA+ proximal end can be propagated along its length to determine the properties of the MTBD.
More conclusive support for this proposal has been obtained recently using a recombinantly expressed dynein motor domain (Kon et al. 2009). Pairs of cysteines were introduced into the dynein stalk so that when dynein is treated with an oxidizing agent, disulphide crosslinks are formed that trap the stalk in either the +β or α registries. In direct confirmation of the SRS-fusion experiments, the authors showed that crosslinking in the +β registry gave rise to a low-affinity state for microtubules, whereas crosslinking in the α registry produced a high-affinity binding state. They also showed that the different registries correlated with different ATPase activities of the AAA+ ring, consistent with stalk communication being important for controlling both affinity and the nucleotide hydrolysis cycle.
The authors also provided direct evidence that microtubule binding and nucleotide hydrolysis can drive conformational changes in the stalk. Dynein bound to microtubules showed somewhat faster crosslink formation between cysteines in the α registry, where incubation of the dynein with ATP and vanadate (which drives it into a weak binding state) favored crosslink formation in the +β registry. Interestingly, however, even in the presence of microtubules, the +β registry crosslinks eventually formed; likewise, in the presence of ATP, vanadate α registry crosslinks were also eventually formed. This either implies that the stalk is a very dynamic structure or that the cysteines are close enough to form crosslinks, albeit less efficiently, even when the stalk is in the alternate conformation.
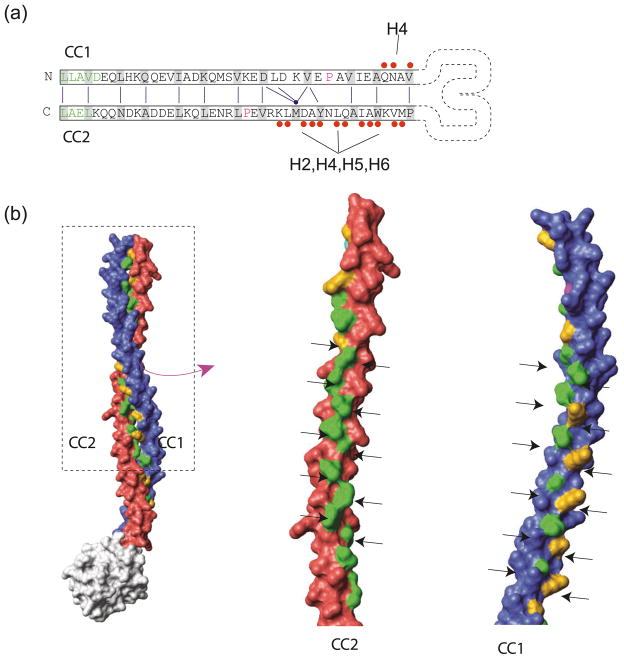
The structure of the stalk itself contains features that support the idea of conformational changes propagating along the stalk. One example is the asymmetry with which the two helices in the stalk coiled coil interact with the rest of the MTBD. Whereas CC2 makes extensive contacts (Fig. 3a) with the helices of the MTBD, CC1 makes hardly any and only with the two flexible helices thought to be directly at the microtubule-binding interface (packing against H3 and leading directly into H1). This arrangement is consistent with CC2 acting as a static rod against which CC1 can slide. Based upon our crystal structure, any movement of CC1 will directly impinge on microtubule binding, particularly by moving H1, which is part of the microtubule-binding interface.
Fig. 3.
Structural evidence for conformational changes occurring in the dynein stalk coiled coil. (a) Contacts of stalk coiled-coil helices (CC1 and CC2) with other elements in the MTBD. CC1 makes a small number of contacts with H4, whereas CC2 makes extensive contacts with the rest of the domain. (b) Pattern of hydrophobic residues in the core of the coiled coil. Left panel shows the model of the mouse cytoplasmic dynein stalk produced by extending the coiled coil from the known structure. Core residues are colored green (hydrophobic) and orange (hydrophilic). The right panel shows the separation of CC1 and CC2 with the two lines of residues in the core marked by arrows. In CC2, both sides are predominantly hydrophobic; in CC1, the left side is hydrophobic, whereas the right is hydrophilic. The full-colour version of this Figure is available from http://bcb.nrc.ca.
The distinctive and conserved pattern of hydrophobic residues in CC1 is also suggestive that it might be designed to move relative to CC2. The regular heptad repeat pattern of most coiled coils results in a stripe of hydrophobic residues, two amino acids wide, that runs up the α helix. These residues undergo a knobs-in-holes packing (each hydrophobic knob fits in a hole formed by 3 hydrophobic residues from the opposing α helix) in the core of the coiled coil, making it a very stable structure. The free energy cost of disrupting such packing and exposing hydrophobic residues would generally prohibit all but the smallest conformational changes. A model of the dynein stalk, made by extending the structure observed in the MTBD crystal structure, clearly shows that CC2 has a regular heptad repeat pattern (Fig. 3b) although as with many coiled coils there are some hydrophilic residues (gold) in the core. In the case of CC1, however, the hydrophobic residues are present mainly on one side of the core stripe, whereas the other side is predominately hydrophilic. This feature is conserved and shows up in sequence alignments as a conserved hydrophobic residue every 7 amino acids (Gibbons et al. 2005). A half-heptad sliding of the coiled coil, such as that proposed on the basis of SRS fusion and stalk crosslinking experiments, would shift the line of hydrophobic residues from one side of the coiled coil core to the other. As a minimal number of hydrophobic residues are exposed, the energy barrier of this transformation may be within the range provided by ATP hydrolysis.
Comparison of various models for conformational changes in the dynein coiled coil
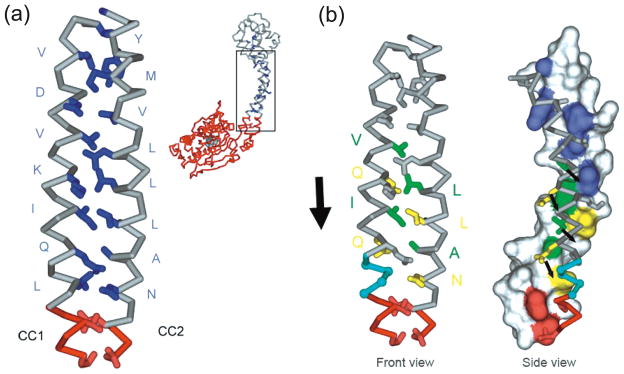
Our current model for communication along the stalk is a sliding of the helices relative to each other (Fig. 4) by a half heptad. The proposal is that the line of hydrophobic residues in CC1 would slide in the grooves formed between the core residues in CC2. The movement would correspond to a transition between the weak binding +β registry observed in the crystal structure and the strong binding α registry trapped by SRS fusion experiments. While capable of explaining the experimental observations, there is as yet no direct proof that such a sliding occurs, nor any calculations showing that it is energetically feasible. It is perhaps useful therefore to discuss other possible mechanisms.
Fig. 4.
The half-heptad sliding model of dynein stalk communication. (a) Close up view of the amino acids in the core of the stalk coiled coil (in dark blue). (b) Removal of 4 residues marked in light blue produces a construct with a 10-fold higher affinity for microtubules. This suggests the left hand helix will slide so that its residues colored in yellow and green will pair up with the equivalently colored residues in the right hand helix. The large arrow shows the proposed direction of sliding of CC1 and small arrows, in the side view, show the potential movement of individiual residues. The full-colour version of this Figure is available from http://bcb.nrc.ca.
A more extreme possibility than sliding is that the coiled coil undergoes a general melting (Gee and Vallee 1998). Such a large conformational change is reminiscent of the coiled-coil transitions occurring during the pH-dependant conformational changes observed in influenza hemagglutinin (Skehel and Wiley 2000). The best evidence against such a model comes from electron microscopy of dynein molecules in various nucleotide states (Sale et al. 1985; Burgess 1995; Burgess et al. 2003; Ueno et al. 2008; Roberts et al. 2009), which suggest there are no large changes in the structure of the stalk. Although the flexibility of the stalk has been observed to increase going from the apo (high affinity) to the ATP vanadate-bound (low-affinity) state (Burgess et al. 2003), its overall dimensions remain fairly constant and consistent with that of a coiled coil (Burgess et al. 2003; Roberts et al. 2009). The other issue that must be addressed with a melting of the stalk is the entropic cost of unburying the hydrophobic residues in the stalk core.
It is also worth considering whether larger movements between the stalk helices should be considered in analogy to the intermolecular sliding observed between dimers of the nuclear pore protein Nup58/45 (Melcék et al. 2007). In that case, sliding movements of ~11 Å were observed, corresponding to a whole heptad movement. However, the contacts between the α helices are entirely mediated by hydrophilic residues. The crystal structures suggested that contacts between these residues were made and broken as the helices slide past each other. These contacts appear very different from that in the dynein stalk, where the primary contribution is from hydrophobic residues. However, assuming that the half-heptad model is correct, it is porbable that the hydrophilic residues in CC1 would have to make and break contacts as CC1 and CC2 slide past each other.
Perhaps the most likely alternative to the half-heptad model is some more subtle rearrangement of the coiled coil, such as a small rotation of CC1 and CC2 with respect to each other. The best evidence for such a mechanism comes from the field of transmembrane receptors, many of which are thought to transmit signals by long-range conformational changes in the association of α helices across the plasma membrane (Matthews et al. 2006). Hulko et al. (2006) determined the structure of a 4-helix coiled coil called the HAMP domain that sits directly under the membrane portion of many bacterial transmembrane receptors and transmits the transmembrane signal to the cytoplasmic enzymatic domain (e.g., histidine kinase). This HAMP structure showed an unusual (knobs-to-knobs) packing that led the authors to suggest that the mechanism of signaling is the transition from this packing to the more normal knobs-in-holes variety. Such a conformational change would result in a ~28° rotation of each of the 4 helices with respect to each other. It is possible that a similar sort of rotation could occur along the length of the dynein stalk coiled coil, caused by relatively small shifts in the knobs-in-holes packing (Walshaw and Woolfson 2001).
One important question is whether such relatively small changes are consistent with the SRS-fusion and crosslinking data. In the case of the SRS experiments, a large number of different registries were tested and almost all of them gave a low affinity for microtubules (with the exception of the α registry). We interpreted this to mean that if the registry was not exactly that required to produce a high affinity (α) form, then there would be a break in the registry around the fusion site and the rest of the stalk and MTBD would default to the more stable +β registry observed in the crystal structure. Such a break in a coiled coil is actually observed in an artificially shortened form of Ndc80 whose structure was recently determined (Ciferri et al. 2008). This same explanation could clearly also be applied to smaller conformational changes, where the default conformation is favored in most constructs, whereas the high-affinity conformation is only favored in the fusions predicted to be in the α registry. In the case of the cross-linking studies (Kon et al. 2009), it may be possible to explain the effects in terms of small conformational changes, if one assumes that the cysteines can form disulphide bonds even when they are not directly across the coiled coil from one another. Formation of diagonal cysteine crosslinks could favor one conformation of the stalk, whereas a crosslink between directly opposing cysteines would favor an alternate conformation.
In summary, in the absence of direct structural information, it is not possible to totally rule out one model of communication over another. However, one issue that is of interest is whether small conformational changes would provide a suitable mechanism for communication in dynein whose stalk, unlike the helices in transmembrane proteins, may have to bend in response to force. It may be that a half-heptad sliding mechanism provides enough of an energy barrier to prevent dynein spontaneously changing its affinity in response to force. On the other hand, given that dynein’s affinity for microtubules depends on the direction in which force is being applied (Gennerich et al. 2007), it may be that distortion of the stalk plays a direct role in controlling dynein’s affinity for microtubules.
In all of the models for communication discussed so far, the conformational changes have occurred as a concerted movement along the whole length of the stalk. However, it is also possible that they could propagate sequentially (Carter et al. 2008). Such a wave of rearrangements (either small rotations or larger sliding movements) may lower the overall energy barrier for the conformational change. An interesting analogy to this suggestion comes in the form of voltage sensitive ion channels, where the relative movement of the S4 helix is responsible for gating. Crystal structures of these channels show that part of S4 is in the more extended 310 helix form. This led the authors to speculate that this zone of 310 helix propagates along the S4 helix (Long et al. 2007).
Conclusions and perspectives
The outward helix in the dynein stalk (CC1) appears to move with respect to the return helix (CC2), directly connecting conformational changes in the AAA+ ring with those in the microtubule-binding interface. The pattern of conservation of hydrophobic residues in the coiled-oil core of CC1 hints that this conformational change may involve a half-heptad shift, although more subtle movements are also possible. Coiled coils are a very common motif in proteins and it is interesting to speculate how many others are involved in long distance communication. One example is the Rad50 protein. AFM studies suggest that binding DNA to the ATPase domain at one end of a coiled coil can change the interaction properties of the hook domain at the other end (Moreno-Herrero et al. 2005).
Footnotes
This paper is one of a selection of papers published in this special issue entitled 8th International Conference on AAA Proteins and has undergone the Journal’s usual peer review process.
References
- Burgess SA. Rigor and relaxed outer dynein arms in replicas of cryofixed motile flagella. J Mol Biol. 1995;250(1):52–63. doi: 10.1006/jmbi.1995.0357. [DOI] [PubMed] [Google Scholar]
- Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421(6924):715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- Carter AP, Garbarino JE, Wilson-Kubalek EM, Shipley WE, Cho C, Milligan RA, et al. Structure and functional role of dynein’s microtubule-binding domain. Science. 2008;322(5908):1691–1695. doi: 10.1126/science.1164424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C, Reck-Peterson SL, Vale RD. Regulatory AT-Pase sites of cytoplasmic dynein affect processivity and force generation. J Biol Chem. 2008;283(38):25839–25845. doi: 10.1074/jbc.M802951200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133(3):427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee M, Vallee R. The role of the dynein stalk in cytoplasmic and flagellar motility. Eur Biophys J. 1998;27(5):466–473. doi: 10.1007/s002490050157. [DOI] [PubMed] [Google Scholar]
- Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390(6660):636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- Gennerich A, Carter AP, Reck-Peterson SL, Vale RD. Force-induced bidirectional stepping of cytoplasmic dynein. Cell. 2007;131(5):952–965. doi: 10.1016/j.cell.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons IR, Garbarino JE, Tan CE, Reck-Peterson SL, Vale RD, Carter AP. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J Biol Chem. 2005;280(25):23960–23965. doi: 10.1074/jbc.M501636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, et al. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell. 2006;126(5):929–940. doi: 10.1016/j.cell.2006.06.058. [DOI] [PubMed] [Google Scholar]
- Imamula K, Kon T, Ohkura R, Sutoh K. The coordination of cyclic microtubule association/dissociation and tail swing of cytoplasmic dynein. Proc Natl Acad Sci USA. 2007;104(41):16134–16139. doi: 10.1073/pnas.0702370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43(35):11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- Kon T, Mogami T, Ohkura R, Nishiura M, Sutoh K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nat Struct Mol Biol. 2005;12(6):513–519. doi: 10.1038/nsmb930. [DOI] [PubMed] [Google Scholar]
- Kon T, Imamula K, Roberts AJ, Ohkura R, Knight PJ, Gibbons IR, et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nat Struct Mol Biol. 2009;16(3):325–333. doi: 10.1038/nsmb.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce MP, Tikhonenko I. Functional elements within the dynein microtubule-binding domain. Mol Biol Cell. 2000;11(2):523–529. doi: 10.1091/mbc.11.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Matthews EE, Zoonens M, Engelman DM. Dynamic helix interactions in transmembrane signaling. Cell. 2006;127(3):447–450. doi: 10.1016/j.cell.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Melcák I, Hoelz A, Blobel G. Structure of Nup58/45 suggests flexible nuclear pore diameter by intermolecular sliding. Science. 2007;315(5819):1729–1732. doi: 10.1126/science. 1135730. [DOI] [PubMed] [Google Scholar]
- Moreno-Herrero F, de Jager M, Dekker NH, Kanaar R, Wyman C, Dekker C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature. 2005;437(7057):440–443. doi: 10.1038/nature03927. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Numata N, Walker ML, Kato YS, Malkova B, Kon T, et al. AAA+ Ring and linker swing mechanism in the dynein motor. Cell. 2009;136(3):485–495. doi: 10.1016/j.cell. 2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale WS, Goodenough UW, Heuser JE. The substructure of isolated and in situ outer dynein arms of sea urchin sperm flagella. J Cell Biol. 1985;101(4):1400–1412. doi: 10.1083/jcb. 101.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima T, Kon T, Imamula K, Ohkura R, Sutoh K. Two modes of microtubule sliding driven by cytoplasmic dynein. Proc Natl Acad Sci USA. 2006;103(47):17736–17740. doi: 10.1073/pnas.0606794103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69(1):531–569. doi: 10.1146/annurev.biochem. 69.1.531. [DOI] [PubMed] [Google Scholar]
- Ueno H, Yasunaga T, Shingyoji C, Hirose K. Dynein pulls microtubules without rotating its stalk. Proc Natl Acad Sci USA. 2008;105(50):19702–19707. doi: 10.1073/pnas. 0808194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshaw J, Woolfson DN. Socket: a program for identifying and analysing coiled-coil motifs within protein structures. J Mol Biol. 2001;307(5):1427–1450. doi: 10.1006/jmbi.2001. 4545. [DOI] [PubMed] [Google Scholar]
- White SR, Lauring B. AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic. 2007;8(12):1657–1667. doi: 10.1111/j.1600-0854.2007.00642.x. [DOI] [PubMed] [Google Scholar]
- Xie P, Dou SX, Wang PY. Model for unidirectional movement of axonemal and cytoplasmic dynein molecules. Acta Biochim Biophys Sin (Shanghai) 2006;38(10):711–724. doi: 10. 1111/j.1745-7270.2006.00223.x. [DOI] [PubMed] [Google Scholar]