Abstract
Background
Although the number of infected people receiving highly active anti-retroviral therapy (HAART) in low- and middle- income countries increased dramatically, optimal disease management is not well defined.
Methods
We developed a model to compare the costs and benefits of three types of HIV monitoring strategies: symptom-based strategies, CD4-based strategies, and CD4 plus viral load strategies for starting, switching, and stopping HAART. We used clinical and cost data from southern Africa and performed a cost-effectiveness analysis. All assumptions were tested in sensitivity analyses.
Results
Compared to the symptom-based approaches, monitoring CD4 every 6 months and starting treatment at a threshold of 200 cells/μl was associated with a life expectancy gain of 6.5 months (61.9 vs. 68.4) and a discounted lifetime cost savings of $464 per person (4,069 vs. 3,605 discounted 2007 USD). CD4-based strategies where treatment was started at the higher threshold of 350 cells/μl provided an additional life expectancy gain of 5.3 months at a cost effectiveness of $107 per life-year gained compared to a threshold of 200 cells/μl. Monitoring viral load with CD4 was more expensive than monitoring CD4 alone, added 2.0 months of life, and had an incremental cost-effectiveness ratio of $5,414/life-year gained relative to monitoring CD4 counts. In sensitivity analyses, the cost-savings from CD4 monitoring compared to symptom-based approaches was sensitive to cost of inpatient care, and the cost-effectiveness of viral load monitoring was influenced by the per-test costs and rates of virologic failure.
Conclusions
Use of CD4 monitoring and early HAART initiation in southern Africa provides large health benefits relative to symptom-based approaches for HAART management. In southern African countries with relatively high costs of hospitalization, CD4 monitoring would likely reduce total health care expenditures. The cost-effectiveness of viral load monitoring depends on test prices and rates of virologic failure.
Two-thirds of the world's HIV-infected population resides in Africa, and the majority of the world's new infections occur in low- and middle- income countries. In the southern cone of Africa alone, which includes heavily affected countries such as South Africa, Botswana, Zimbabwe, Namibia, Angola, Malawi, Zambia, and Mozambique, approximately 11 million people are living with HIV.1, 2 Despite substantial progress in access to treatment, only 20% of adults who need highly active antiretroviral therapy (HAART) currently receive it.1 In addition, in many resource-limited regions, people who do receive HAART are managed without access to monitoring of CD4+ T-cell counts or HIV viral load, which may substantially reduce the effectiveness of HAART.3 Therefore, key questions in the management of HIV in resource-constrained settings are whether and how to monitor people with HIV, and when to initiate HAART. At present, little is known about the effectiveness and cost-effectiveness of evaluation and treatment initiation criteria in an African setting, and monitoring of infected individuals remains a major challenge for clinicians and health care systems.4, 5
In high-income countries, clinical monitoring, CD4 counts, and viral load are the most common tools used to determine treatment eligibility and to monitor HIV-infected patients.6 Multiple clinical trials examined strategies for choosing initial and sequential antiretroviral regimens in which CD4 and viral load are used for treatment decisions and serve as the primary measurements of efficacy.7, 8 In resource-limited regions, however, where laboratory monitoring is often not available, many HIV infected individuals are started on HAART when they develop a severe opportunistic disease.9
Previous studies in sub-Saharan Africa estimate the cost-effectiveness of HAART and timing of treatment initiation.10-12 However, no study looked at the use of monitoring strategies in these settings. In this study, we used an HIV treatment and monitoring model to estimate the effectiveness and cost-effectiveness of several strategies for treatment initiation, change, and discontinuation of infected individuals in southern Africa.
Methods
Overview & Model Structure
We developed a simulation model (Treeage Pro, Williamstown, MA) of the lifetime history of HIV+ patients from time of presentation for care until death. The purpose of the model was to evaluate the relative cost-effectiveness of three types of currently practiced management strategies of caring for patients with HIV in southern Africa: two symptom-based strategies where patients are managed using clinical criteria without CD4 or viral load monitoring; four CD4-based strategies, which includes CD4 monitoring in addition to clinical monitoring for treatment initiation and regimen change; and four strategies that includes both CD4 and viral load measurements, comparable to routine management of patients in resource-rich countries (see Supplementary Appendix for more details).
Each patient's health was characterized by CD4 counts, viral load, medication toxicity, and severe opportunistic diseases. The model followed each patient's health status monthly, but clinical and laboratory data was only available to decision-makers during follow-up visits, or sooner for acute clinical events.
Data for the model was taken from two established HIV cohorts in the Cape Town area: the Cape Town AIDS Cohort (CTAC), a group of HIV+ patients cared for in local hospital clinics; and the Médecins Sans Frontières community clinics in Khayelitsha (Table 1).13-16 For the base-case analyses we simulated a population of 100,000 patients, and in sensitivity analyses we simulated independent cohorts of 50,000 patients.
Table 1. Variables and Sources.
| Variable | Base Case | Range | Source |
|---|---|---|---|
| Demographic variables | |||
| Age at presentation (mean ± SD) | 32.8±9.2 | 14-50 | Holmes16 |
| CD4 at presentation, cells/μl (mean ± SD) | 307±227 | 200-400 | Holmes16 |
| Viral load at presentation, log copies/ml (mean ± SD) | 5.0± 0.8 | 4-6 | Badri10 |
| Natural history variables | |||
| Mean monthly change in CD4 based on viral load (cells/μl) | Mellors, Rodriguez17, 18 | ||
| VL<500 | 1.67± 0.9 | ± 50% | |
| VL501-2000 | 3.33± 1.7 | ± 50% | |
| VL 2001-10,000 | 4.08± 2.1 | ± 50% | |
| VL 10,001-40,000 | 4.58± 2.4 | ± 50% | |
| VL >40,000 | 6.33± 3.2 | ± 50% | |
| Risk of developing severe opportunistic disease | Holmes, Badri16, 19 | ||
| CD4 <50 cells/μl | 10.5%/mo | ||
| CD4 51-200 cells/μl | 2.6%/mo | ||
| CD4 201-350 cells/μl | 1.1%/mo | ||
| CD4 >350 cells/μl | 0.26%/mo | ||
| Risk of death | Badri19 | ||
| CD4 <50 cells/μl | 2.1%/mo | ||
| CD4 51-200 cells/μl | 1.7%/mo | ||
| CD4 201-350 cells/μl | 1.1%/mo | ||
| CD4 >350 cells/μl | 0.8%/mo | ||
| Additional risk of death from severe opportunistic disease | Goldie12 | ||
| CD4 <50 cells/μl | 7.69%/mo | ||
| CD4 51-200 cells/μl | 4.48%/mo | ||
| CD4 201-350 cells/μl | 0.66%/mo | ||
| Treatment variables | |||
| Virologic suppression on HAART | |||
| 12 weeks | 84% | DART, Orrell, Coetzee15, 20, 21 | |
| 48 weeks | 72% | 50%-78% | |
| CD4 rise on first-line HAART | Battegay, Kaufmann, Lawn22-24 | ||
| First 6 months | 146 cells/μl | ||
| Months 7-12 | 46 cells/μl | ||
| Months 13-18 | 28 cells/μl | ||
| Months 19-24 | 21 cells/μl | ||
| Risk of HAART discontinuation due to toxicity | Robbins25, Orrell26, Calmy27, Amoroso28 | ||
| First line regimen 0-12 mo | 0.87%/mo | ± 50% | |
| First line regimen 12-36 mo | 0.25%/mo | ± 50% | |
| Second line regimen 0-12 mo | 1.8%/mo | ± 50% | |
| Second line regimen 12-36 mo | 0.42%/mo | ± 50% | |
| Utilization and cost variables | |||
| Annual number of inpatient days without a severe opportunistic disease | Badri et al10, 11 | ||
| CD4>350 (on/off HAART) | 0.14 / 1.9 | ± 50% | |
| CD4 201-350 (on/off HAART) | 0.39 / 3 | ± 50% | |
| CD4<=200 (on/off HAART) | 0.26 / 7.7 | ± 50% | |
| Annual number of inpatient days with a severe opportunistic disease | Badri et al10, 11 | ||
| CD4>350 (on/off HAART) | 0.37 / 5.7 | ± 50% | |
| CD4 201-350 (on/off HAART) | 0.52 / 10.8 | ± 50% | |
| CD4<=200 (on/off HAART) | 1.8 / 17.7 | ± 50% | |
| Annual number of outpatient visits | Badri et al10, 11 | ||
| CD4>350 (on/off HAART) | 4.3 / 4.1 | ± 50% | |
| CD4 201-350 (on/off HAART) | 3.9 / 5.0 | ± 50% | |
| CD4<=200 (on/off HAART) | 4.7 / 6.6 | ± 50% | |
| Costs (2007 USD) | |||
| Inpatient Day | $198 | $20-$200 | Badri; Cleary10, 29 |
| Outpatient visit | $30 | $3-$30 | Badri; Cleary10, 29 |
| Cost per viral load test* | $80 | $20-$140 | Badri, Elbeik10, 30 |
| Cost per CD4 test* | $25 | $5-$50 | Badri, Zijeneh10, 31 |
| First line HAART regimen (annual) | $322 | ± 50% | Badri, MSF10, 11, 32 |
| Second line HAART regimen (annual) | $640 | ± 50% | Badri, MSF10, 11, 32 |
Per test cost include an estimated cost of reagents, labor, parts, data management, and the rental or acquisition of the CD4 or viral load enumeration equipment.
Disease Progression
Disease progression was determined by each patient's CD4 count, viral load, history of opportunistic diseases, and treatment history. We modeled CD4 as a continuous variable that determined the patient's risk of death and of developing opportunistic diseases. Viral load, also modeled as a continuous variable, guided the rate of change of CD4 counts in the absence of suppression of viral replication.17, 18 After successful initiation of HAART, a patient's viral load decreased to less than 400 copies/ml, and CD4 counts rose based on empirical data.22-25 When a patient developed a severe opportunistic disease, their risk of death increased by an amount inversely related to their CD4 at the time of the infection (Table 1).10, 19
Treatment Options
In resource-limited regions, the World Health Organization (WHO) formulary advises a first-line regimen consisting of a dual nucleoside reverse transcriptase inhibitor backbone with a non-nucleoside reverse transcriptase inhibitor, and a second-line protease inhibitor-based regimen.3 We used efficacy and safety data for first- and second-line regimens similar to the WHO formulary; however, consistent with current practice in resource-limited countries, we assumed no additional HAART regimens.24, 25, 27 At the start of the model, patients were antiretroviral-naïve, and after starting a first-line regimen, were switched to a second-line regimen for two reasons: medication toxicity, and failure of therapy (based on measured viral load, CD4 decrease, or opportunistic diseases in the various strategies, as shown in Table 2).15, 25, 33
Table 2. Monitoring Strategies.
| Strategy | Monitoring | HAART initiation | HAART regimen switch | Frequency of monitoring |
|---|---|---|---|---|
| 1 | Symptom-based | First severe OD | Second severe OD | NA |
| 2 | Symptom-based | First severe OD | Third severe OD | NA |
| 3 | CD4 only | First severe OD or CD4<200 cells/μl | CD4 drop to 50% highest measured level | 6 months |
| 4 | CD4 only | First severe OD or CD4<200 cells/μl | CD4 drop to 50% highest measured level | 3 months |
| 5 | CD4 only | First severe OD or CD4<350 cells/μl | CD4 drop to 50% highest measured level | 6 months |
| 6 | CD4 only | First severe OD or CD4<350 cells/μl | CD4 drop to 50% highest measured level | 3 months |
| 7 | CD4 and viral load | First severe OD or CD4<200 cells/μl | Elevated viral load | 6 months |
| 8 | CD4 and viral load | First severe OD or CD4<200 cells/μl | Elevated viral load | 3 months |
| 9 | CD4 and viral load | First severe OD or CD4<350 cells/μl | Elevated viral load | 6 months |
| 10 | CD4 and viral load | First severe OD or CD4<350 cells/μl | Elevated viral load | 3 months |
OD – Opportunistic disease
Management Strategies
We examined ten strategies: two symptom-based strategies and four versions of the CD4 and CD4-viral load strategies, where HAART was started at 200 cells/μl or 350 cells/μl, and monitoring frequency was 3 or 6 months (Table 2). In the symptom-based strategies, HAART was started when patients developed their first severe opportunistic disease, and treatment was changed when patients experienced toxicity related to the first-line regimen, or after developing their second or third opportunistic disease, suggesting failure of therapy. In the CD4-based strategies, CD4 was checked regularly, and HAART was started when the measured CD4 dropped below an initiation threshold, unless a patient first developed an opportunistic disease. The treatment regimen was changed with toxicity, or if the measured CD4 dropped to half of the highest measured CD4, suggesting failure of therapy. Finally, in the CD4-viral load strategies, patients were switched to a second-line regimen with measured virologic failure (greater than 1000 copies/ml).34 In all strategies, treatment was stopped in patients who experienced severe toxicity with second-line HAART, but was continued in patients with treatment failure on second-line treatment due to the survival advantages of a non-suppressive regimen compared with HAART cessation.35
Costs
We considered all direct HIV costs obtained from costing reports of the study cohorts.10, 29, 36 Cost of care included inpatient costs, outpatient costs, HAART costs, and testing costs. Per-test cost included cost of reagents, labor, parts, data management, maintenance, and the rental or acquisition of CD4 or viral load enumeration equipment.30, 31 Viral load was only measured once a patient was started on HAART, as no therapeutic decisions were made using viral load prior to onset of HAART. We measured the incremental cost-effectiveness of each strategy compared with the next least effective strategy in 2007 USD per life-year gained. All costs were converted to 2007 USD with a currency converter and a GDP deflator.37 We adopted a societal perspective, although some indirect costs such as the cost of lost wages and travel costs were excluded. We discounted all costs and benefits at 3% annually.
Sensitivity Analysis
We evaluated all parameters in one-way and multi-way sensitivity analyses. In particular, we examined clinical parameter estimates, including rates of virologic failure, rates of treatment discontinuation due to toxicity, rates of opportunistic diseases, and the increased mortality risk of opportunistic diseases. We also examined the sensitivity of the results to inpatient, outpatient, HAART, and testing costs independently and jointly (assuming that costs co-vary among regions and a reduction in one cost is related to other reduced costs).
Results
Model Validation
We calibrated our model against established models of HIV in resource-limited settings and cost studies from Cape Town, and validated the outcomes of the model by comparing model predictions to cost of care, life expectancy, and observed rates of development of severe opportunistic diseases (Table 3).11, 12, 16 Our model correlated well with expected values.
Table 3. Model Validation and Calibrations.
| Outcome | Previous literature | Model | Comments |
|---|---|---|---|
| Life years gained12 | 5.8 | 5.8 | Using CD4 of 200 or a single opportunistic disease to start treatment. |
| Discounted lifetime costs11 | $5,088 | $4,552 | Using a strategy where CD4 and viral load were checked every 6 months. |
| Rate of severe opportunistic diseases (per 100 person-years) 16 | Most opportunistic diseases listed in WHO Stage III and Stage IV were considered severe.3 | ||
| <50 | 133.1 | 125.5 | |
| 51-200 | 31.7 | 31.6 | |
| 201-350 | 13.6 | 12.9 | |
| >350 | 3.1 | 3.4 |
Base Case Analyses
CD4 Monitoring
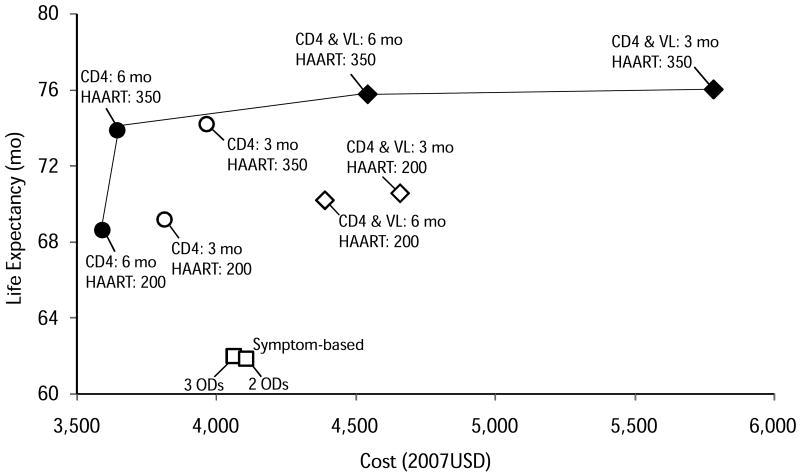
All CD4-based strategies resulted in higher life expectancy and were less costly than the symptom-based approaches (Table 4 and Figure 1). The most effective symptom-based strategy yielded a discounted estimated life expectancy of 61.9 months at a lifetime cost of $4,069, while the least effective CD4-based strategy (where HAART was started at 200 cells/μl) cost $3,605 and had an estimated life expectancy of 68.4 months (Figure 1). That is, a lifetime cost saving of $464 and a life expectancy increase of 6.5 months. Table 3 shows that the increase in life expectancy is associated with a large decrease in the number of severe opportunistic diseases (314 fewer severe opportunistic diseases per 1,000 people over their lifetime), and that the increased treatment and testing costs when CD4 was monitored were more than offset by the decrease in inpatient costs compared to the symptom-based approach.
Table 4. Clinical Benefits and Cost-Effectiveness of Alternative Monitoring Strategies.
| Strategy | Life expectancy (mo) | Discounted lifetime costs (2007USD) | ODs, per 1000* | ICER ($/life-years gained) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Testing | HAART | Total | |||||
| 1 | CD4 and VL every 3 months, HAART at 350 | 75.87 | 730 | 805 | 2313 | 1941 | 5,789 | 525 | 101,251 |
| 2 | CD4 and VL every 6 months, HAART at 350 | 75.72 | 743 | 804 | 1126 | 1879 | 4,552 | 526 | 5,414 |
| 3 | CD4 every 3 months, HAART at 350 | 74.04 | 762 | 785 | 606 | 1820 | 3,973 | 589 | Extended dominance** |
| 4 | CD4 every 6 months, HAART at 350 | 73.73 | 778 | 784 | 307 | 1784 | 3,653 | 589 | 107 |
| 5 | CD4 and VL every 6 months, HAART at 200 | 70.10 | 1269 | 762 | 928 | 1437 | 4,397 | 628 | Dominated by strategy 4† |
| 6 | CD4 every 3 months, HAART at 200 | 69.05 | 1058 | 748 | 575 | 1444 | 3,825 | 677 | Dominated by strategy 4† |
| 7 | CD4 every 6 months, HAART at 200 | 68.41 | 1113 | 744 | 283 | 1465 | 3,605 | 719 | |
| 8 | Symptom-based – Three ODs‡ | 61.94 | 2374 | 724 | 0 | 972 | 4,069 | 1,033 | Dominated by strategy 7† |
Average number of severe opportunistic diseases per 1,000 infected people over their lifetime.
Extended dominance refers to a mix of strategies, in this case strategies 2 and 4, that, in some combination, are more effective and less costly than the dominated strategy (3 above). No single strategy by itself is more effective and less costly than strategy 3.
There is at least one strategy that is more effective and less costly than any dominated strategy.
OD – Opportunistic disease. The number of opportunistic diseases refers to the decision when to switch from first- to second-line treatment in the symptom-based strategy.
Figure 1. Health and Cost Outcomes of Monitoring Strategies.
Open symbols represent strategies that were dominated by other strategies either through strict dominance (less effective and more costly than another strategy) or extended dominance (less effective and more costly than a mix of other strategies). All CD4-based strategies were more effective and less costly than the symptom-based strategies. Starting HAART at CD4 350/μl was always more effective than starting at 200/μl, regardless of viral load monitoring. More frequent monitoring was generally more effective than less frequent, but was dominated in most cases. The squares represent the symptom-based strategies, circles represent the CD4-based strategies, and diamonds represent the CD4-viral load strategies.
CD4 Threshold
Starting HAART at CD4 350/μl was always more effective than starting at 200/μl, regardless of the monitoring strategy. When CD4 count alone was monitored every 6 months, starting HAART at 350 cells/μl rather than 200 cells/μl was associated with a gain of 5.3 months of life expectancy at an incremental lifetime cost of $48, or $107 per life-year gained. Compared to a symptom-based approach, the gain in life expectancy was 11.8 months. Starting HAART at 350 cells/μl led to higher HAART costs, outpatient costs, and testing costs, but to decreased inpatient costs. Compared to the lower threshold for initiation of therapy, individuals also had 18% fewer severe opportunistic diseases (589 vs. 719 per 1000 people).
Viral Load Monitoring
We estimated the benefits and costs of viral load monitoring for determining treatment failure and the timing of a regimen change. Adding viral load to CD4 monitoring was associated with further increase in life expectancy. When testing every 6 months and starting HAART at 350 cells/μl, adding viral load to CD4 testing was associated with a life expectancy gain of 2 months. However, viral load testing was associated with increased lifetime cost of $899 per person, mostly due to increased testing costs. Compared to monitoring CD4 every six months and starting HAART at 350 cells/μl, the incremental cost effectiveness ratio of adding viral load was $5,414 per life-year gained.
Monitoring Frequency
Finally, testing every three months instead of every six months was associated with modest increases in life expectancy and significant increases in lifetime costs. For equivalent strategies, testing every three months was associated with a gain of 2-19 days. The incremental cost effectiveness ratio of testing CD4 and viral load every three months and starting HAART at 350 cells/μl was about $100,000 per life-year gained compared to a similar strategy with monitoring every six months.
Sensitivity Analysis
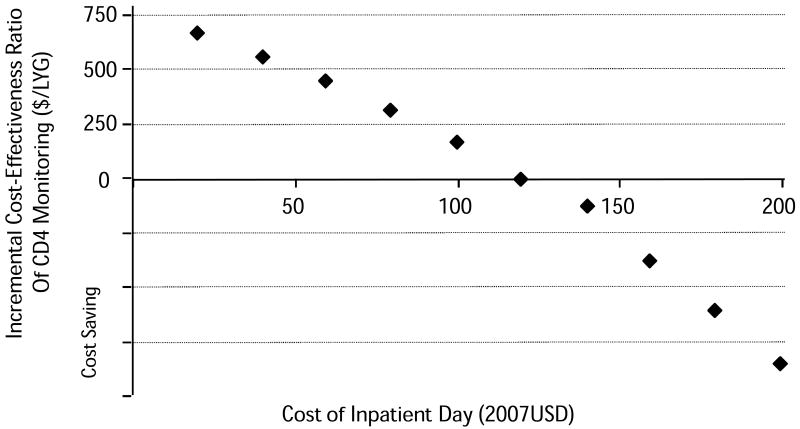
CD4 monitoring reduced net costs compared with symptom-based management because it reduced expensive hospitalizations for opportunistic infections. In parts of southern Africa, hospitals are not available, and medical care may be very elementary. To assess the importance of inpatient care, we varied the cost of an inpatient day over the range reported for southern Africa.38 As the cost of an inpatient day fell from $198 per day (based on data from South Africa) to $120 per day, CD4 monitoring remained cost saving (Figure 2). However, when the cost of an inpatient was further reduced, CD4 monitoring increased total costs compared to symptom-based management, but the incremental cost-effectiveness ratio remained less than $700 per life-year gained even when inpatient costs were reduced to $20 per day, the lowest value reported for southern Africa.38
Figure 2. Effect of Inpatient Costs on Cost-Effectiveness of CD4 Monitoring.
The incremental cost-effectiveness ratio of monitoring CD4 and starting HAART at 200 cells/μl compared to a symptom-based strategy is represented on the Y axis. A negative ratio suggests that monitoring CD4 was cost saving. The symptom-based approach is cheaper than monitoring CD4 when inpatient stay costs less than $120 per day. At $20 per day, the incremental cost-effectiveness ratio is a little under $700 per life-year gained.
The cost-effectiveness of viral load monitoring was sensitive to cost of testing and virologic failure (Figure 3). In a comparison with strategies where CD4 was monitored every six months and HAART was started at 350 cells/μl, halving the cost of viral load testing brought the incremental cost-effectiveness ratio down to $2,869 per life-year gained, and at a per-test cost of $20, the ratio was $1,635. Additionally, high rates of virologic failure, which might occur where adherence is low or rates of resistance are high, increased the importance of viral load monitoring. Where rates of failure were twice as high as our base case estimate, the incremental cost effectiveness ratio of viral load testing came down to $3,257 per life-year gained, while halving the rates of failure increased the ratio to $8,776 per life-year gained.
Figure 3. Sensitivity Analysis of Viral Load Monitoring.
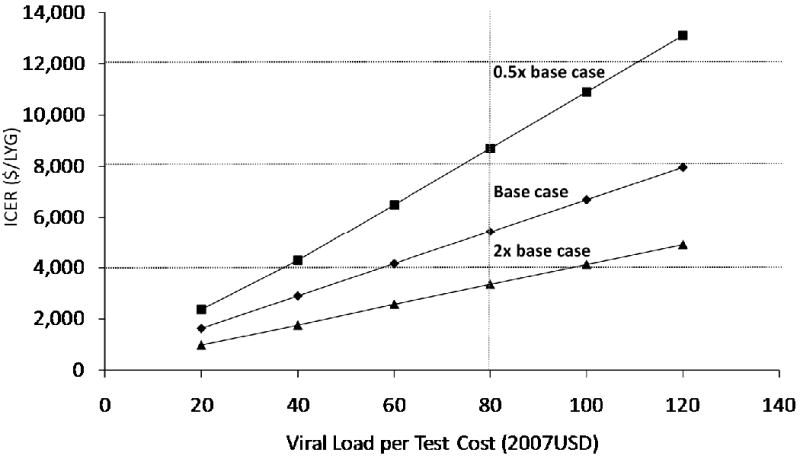
The incremental cost-effectiveness ratio (ICER) is sensitive to both the rate of virologic failure and the cost per test of viral load monitoring. The squares represent the relationship between per-test costs and the ICER when the rate of virologic failure was halved compared with the base case, while the triangles represent the relationship when the rate of virologic failure was doubled, highlighting the importance of viral load monitoring in settings with high rates of virologic failure.
Finally, we evaluated changes in the rates of treatment discontinuation due to toxicities, rates of virologic suppression, rates of CD4 change, and the cost of HAART in sensitivity analyses. Within the range of variability we examined (Table 1), none of these sensitivity analyses changed our results substantively.
Discussion
We evaluated the relative merits of different HIV monitoring strategies in resource-limited settings based on data from southern Africa. We found that CD4 monitoring could substantially increase length of life and reduce total costs relative to the symptom-based approaches currently practiced in many regions, especially outside of major urban areas. Monitoring CD4 increased length of life through earlier initiation of HAART and prevention of severe opportunistic diseases. Compared with the most effective symptom-based strategy, initiation of HAART at a CD4 count of 200 cells/μl or 350 cells/μl increased life expectancy by nearly 7 and 12 months, respectively.
These gains in life expectancy are substantial. Previous studies suggest that use of HAART compared to no HAART increases life expectancy by approximately 20 months.12 Thus, addition of CD4 monitoring and initiation of treatment when CD4 counts reach 350 cells/μl provides a 60% additional gain in longevity compared with introduction of antiretroviral therapy. For the population eligible for HAART in southern Africa, the achievable gains in length of life is large: initiating antiretroviral therapy for one million people at a CD4 count of 200 cells/μl would provide 542,000 life years over providing HAART without CD4 monitoring, and initiating HAART at 350 cells/μl would provide additional 440,000 life years.
In South Africa, and perhaps in several other countries, much of this gain in longevity could be obtained while reducing total expenditures for HIV care by avoiding expensive hospitalizations for opportunistic diseases, which outweighed the higher costs of CD4 testing and HAART. The reduction in total costs is large in South Africa in part because of the relatively high cost of inpatient care, and our analysis suggests that monitoring CD4 may reduce costs of HIV care also in Namibia, Botswana, and Swaziland, where the quality of the epidemic is similar and inpatient care costs are relatively high.38 However, even in Malawi, where the healthcare infrastructure is very basic, healthcare costs are low, and use of inpatient care is inconsistent, the incremental cost-effectiveness ratio of monitoring CD4 every 6 months and starting HAART at 200 cells/μl was $670 per life-year gained.38 A threshold of twice the per capita GDP is often cited as an acceptable incremental cost-effectiveness ratio for developing countries.39, 40 By that standard, monitoring CD4 is cost-effective in all parts of southern Africa. Our analysis also suggests that even in the most resource-limited settings, starting HAART at 350 cells/μl is an effective and cost-effective intervention.
Our analysis highlights that the sizeable worldwide investments to make HAART available could be strongly leveraged by using CD4 monitoring to initiate treatment prior to onset of serious opportunistic diseases and severe immunocompromise. Recent evidence shows that, in resource-limited settings, where HAART is commonly started at low CD4 counts or with opportunistic diseases, rates of death after treatment initiation are much higher than in Europe and North America, especially in the first few months of treatment.41, 42
Addition of viral load monitoring resulted in an additional increase in life expectancy of 2 months relative to use of only CD4 monitoring. Two months is an important additional benefit. However, this gain in effectiveness came at a less favorable incremental cost-effectiveness ratio than did CD4 monitoring because viral load testing is substantially more expensive and provides about one quarter of the benefit of CD4 testing. If the price of viral load testing were significantly reduced, the cost effectiveness would improve markedly. In developed countries, where cost-effectiveness acceptability thresholds are substantially higher, viral load monitoring is considered a cost-effective intervention. Viral load monitoring has other benefits, such as reduced transmission by limiting the number of people with non-suppressed HIV replication, and fewer accumulated resistance mutations. Because we did not include these potential benefits, we may have underestimated the overall benefits of viral load testing.
Why has CD4 monitoring not been universally adopted in resource-limited settings? The initial investment in CD4 technology and infrastructure is expensive. The cost of CD4 flow cytometers, which require highly trained personnel and laboratories with refrigeration, is high, and ministries of health and public health programs may not be able or willing to make the investment. In addition, the cost of an individual CD4 test, while modest in comparison to the cost of HAART or viral load monitoring, may limit access to testing and treatment. Finally, the WHO guidelines encourage using a CD4 threshold of 200 cells/μl for HAART initiation, but they acknowledge the limited capability to expand monitoring capacity.
These challenges are increasingly surmountable. Recent advances in CD4 enumeration technology allow for lower per-test cost, as well as smaller machines that require relatively little infrastructure, maintenance, and technical expertise.43 Alternative financing mechanisms may allow health care systems to minimize the initial investment in equipment through reagent rental agreements and amortization. Both the reductions in technical challenges, and our finding that CD4 monitoring is cost effective or cost saving, support expanding CD4 monitoring as a valuable tool in scaling-up treatment in southern Africa. Use of CD4 monitoring to determine treatment initiation, and initiating HAART early, will benefit a substantial proportion of those individuals for whom treatment would be otherwise delayed until life-threatening symptoms develop.
Our analysis has several limitations. Although the phase and prevalence of the epidemic in South Africa is similar to other countries in the region, most of the data for our model comes from a single region. Some opportunistic diseases, most notably tuberculosis, place a unique burden in that region and may limit the generalizability of our results. In addition, although our estimates of the health benefits of alternative management strategies are likely applicable more broadly in Africa, the study cohorts in Cape Town received care in a setting with potential access to clinics and hospitals. In settings in which individuals with opportunistic diseases have no access to hospitals, their mortality will be higher, and their cost of care will be lower than we projected. In those settings, more efforts to prevent severe opportunistic diseases may have additional mortality benefits.
We also used some data from clinical trials. While clinical trials may provide the best or only source of data, events such as treatment failure and response to HAART may differ from other settings. In addition, we used a societal perspective for this analysis, where all costs and benefits are included. However, additional perspectives may be relevant to parts of southern Africa where costs and benefits are accrued by different parts of the healthcare system. For example, the perspective of a donor organization which bears costs but sees no direct benefits may be important where donors play an important role in the healthcare system. Finally, our model is not intended to restrict the use of viral load monitoring in southern Africa. Rather, we highlight the importance of CD4 monitoring and early treatment initiation as the priority in improving the care of individuals in southern Africa.
The rapid increase in access to treatment in resource-limited regions represents a major progress towards reducing HIV morbidity and mortality. Our analysis shows that, where HAART is available, CD4 monitoring with earlier treatment initiation provides a substantial increase in length of life which, in some settings, may be achievable while reducing total expenditures for HIV. As the number of people receiving HAART increases, the potential health benefit and cost savings from use of CD4 monitoring will also increase.
Acknowledgments
This research is supported in part by the Agency for Healthcare Research and Quality (T32-HS000028), the Department of Veterans Affairs, and the National Institute on Drug Abuse (R01 DA15612-01). The supporting organizations had no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Bendavid had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author Contribution and Conflicts of Interests (Author Forms Submitted Separately): Eran Bendavid: I participated in originating the concept, in conducting the data collection, construction of the model, and data analysis. I did most of the writing of the paper, including the final revision. I had full access to all the data in the study and had final responsibility for the decision to submit for publication. I have no conflict of interests.
Sean D. Young: I participated in elucidating the original concept, in conducting the data collection, construction of the model, and revising the manuscript. I have seen and approved the final version and I have no conflict of interests.
Ahmed M. Bayoumi: I participated in reviewing and constructing the model, in advising on issues related to cost-effectiveness analysis, and in writing and revising the manuscript. I have seen and approved the final version, and I have no conflict of interests.
Gillian D. Sanders: I declare that I participated in reviewing and constructing the model, in advising on issues related to cost-effectiveness analysis, and in writing and revising the manuscript. I have seen and approved the final version. I consult for GSK Biologicals on work related to HPV vaccines, and I receive funding from Vertex Pharmaceuticals through a Duke University research grant for HCV treatment work.
David A. Katzenstein: I participated in defining the original concept, in clarifying issues relating to HIV treatment & monitoring in resource-limited countries, in advising about HIV monitoring technologies, and in writing and revising the manuscript. I have seen and approved the final version. I am an inventor on a patent assigned to Stanford University for PCR assays used in HIV viral load enumeration, but I have no present or potential conflict of interest with this work.
Douglas K. Owens: I participated in defining the original concept, in constructing the model, in advising on issues related to cost-effectiveness analysis, and in writing and revising the manuscript. I have seen and approved the final version, and I have no conflict of interests.
References
- 1.UNAIDS. 2006 Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2006. [Google Scholar]
- 2.UNAIDS. AIDS Epidemic Update. Geneva: 2007. [Google Scholar]
- 3.Hammer S, Havlir D, Klement E, et al. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public health Approach. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Bishai D, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21(10):1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 5.Stover J, Walker N, Garnett GP, et al. Can we reverse the HIV/AIDS pandemic with an expanded response? The lancet. 2002;360(9326):73–77. doi: 10.1016/S0140-6736(02)09339-X. [DOI] [PubMed] [Google Scholar]
- 6.Health and Human Services: Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents: Panel on Antiretroviral Guidelines for Adults and Adolescents. 2006 [Google Scholar]
- 7.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA. 2006;296(7):769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 8.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003 Dec 11;349(24):2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. Aids. 2004 May 21;18(8):1159–1168. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]
- 10.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 11.Badri M, Maartens G, Mandalia S, et al. Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med Jan. 2006;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Cote d'Ivoire. N Engl J Med. 2006 Sep 14;355(11):1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 13.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002 Jun 15;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 14.Coetzee D, Boulle A, Hildebrand K, Asselman V, Van Cutsem G, Goemaere E. Promoting adherence to antiretroviral therapy: the experience from a primary care setting in Khayelitsha, South Africa. AIDS. 2004;18 3:S27–S31. doi: 10.1097/00002030-200406003-00006. [DOI] [PubMed] [Google Scholar]
- 15.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004 Apr 9;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 16.Holmes CB, Wood R, Badri M, et al. CD4 Decline and Incidence of Opportunistic Infections in Cape Town, South Africa: Implications for Prophylaxis and Treatment. J Acquir Immune Defic Syndr. 2006 Aug 1;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 17.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12):1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 19.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006 Oct 7;368(9543):1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 20.DART Virology Group and Trial Team. Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20(10):1391–1399. doi: 10.1097/01.aids.0000233572.59522.45. [DOI] [PubMed] [Google Scholar]
- 21.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17(9):1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 22.Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. The Lancet infectious diseases. 2006;6(5):280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Archives of internal medicine. 2003;163(18):2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003 Dec 11;349(24):2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antiviral therapy. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 27.Calmy A. CROI. Los Angeles Conference Center; 2007. Outcomes of Adults Receiving Second-line ART in Médecins Sans Frontières-supported Projects in Resources-Limited Countries. [Google Scholar]
- 28.Amoroso A. CROI. Los Angeles: 2007. ART-Associated Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia. [Google Scholar]
- 29.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-Effectiveness Of Antiretroviral Treatment For HIV-Positive Adults In A South African Township. Cape Town: Health Systems Trust; 2004. [Google Scholar]
- 30.Elbeik T, Charlebois E, Nassos P, et al. Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000 Mar;38(3):1113–1120. doi: 10.1128/jcm.38.3.1113-1120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zijenah LS, Kadzirange G, Madzime S, et al. Affordable flow cytometry for enumeration of absolute CD4+ T-lymphocytes to identify subtype C HIV-1 infected adults requiring antiretroviral therapy (ART) and monitoring response to ART in a resource-limited setting. Journal of translational medicine. 2006;4:33–33. doi: 10.1186/1479-5876-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Untangling the web of price reductions: a pricing guide for the purchase of ARVs for developing countries. 9th. Médecins Sans Frontières; Jul, 2006. http://www.accessmed-msf.org/ [Google Scholar]
- 33.Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of Antiretroviral Treatment in Khayelitsha, South Africa - a primary data analysis. Cost effectiveness and resource allocation. 2006;4:1–14. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293(7):817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 35.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 36.Govender V, McIntyre D, Grimwood A, Maartens G. The Costs and Perceived Quality of Care for People Living with HIV/AIDS in the Western Cape Province in South Africa. Bethesda, MD: Partnerships for Health Reform; 2000. [Google Scholar]
- 37.Implicit Price Deflators for Gross Domestic Product. [May 11, 2007];Bureau of Economic Analysis: National Economic Accounts. www.bea.gov.
- 38.CHOosing Interventions that are Cost Effective (WHO-CHOICE) [September 27, 2007]; http://www.who.int/choice/en/
- 39.Macroeconomics and health: investing in health for economic development Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 40.World Health Report: Reducing Risk, Promoting Healthy Life. Geneva: World Health Organization; 2002. [Google Scholar]
- 41.ART-LINC, ART-CC. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. The Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 42.Egger M. CROI. LA Conference Center; 2007. Outcome of ART in Resource-Limited and Industrialized Countries. [Google Scholar]
- 43.Rodriguez WR, Christodoulides N, Floriano PN, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS medicine. 2005;2(7):e182–e182. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]