Abstract
Quercetin is a potential chemopreventive and chemotherapeutic agent for pancreatic and other cancers. This study was to examine the distribution of quercetin in plasma, lung, liver, pancreas and pancreatic cancer xenografts in a murine in vivo model and the uptake of quercetin in pancreatic cancer MiaPaCa-2 cells in cellular in vitro model. Mice were randomly allocated to control diet, 0.2 and 1% quercetin diet groups utilizing the AIN93G-based diet (n=12 per group) for 6 weeks. In addition, 6 mice from each group were injected weekly with chemotherapeutic drug gemcitabine (120 mg/kg mouse, i.p.). MiaPaCa cells were collected from culture medium after cells were exposed to 30 µM of quercetin for 0.5, 1, 2, 4, 8, and 24 hrs. Levels of quercetin and 3-O’-methyl-quercetin in mice tissues and MiaPaCa-2 cells were measured by high-pressure liquid chromatography following enzymatic hydrolysis and then extraction. Our study showed that quercetin is accumulated in pancreatic cancer cells, and is absorbed in the circulating system, tumors and tissues of pancreas, liver and lung in vivo. A higher proportion of total quercetin found in tumors and pancreas are aglycones. Gemcitabine co-treatment with quercetin reduced absorption of quercetin in mice circulatory system and liver. Results from the study provide important information on the interpretation of chemo-therapeutic efficacy of quercetin.
Keywords: Quercetin, bioavailability, pancreas, pancreatic cancer, in vivo, HPLC
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer-related death for both men and women in the United States (1). The 5-year relative survival rate for all stages is approximately 5 percent. These statistics underlined the urgent need to identify new approaches and new agents for the treatment and prevention of pancreatic cancer. Recent epidemiological studies suggested that dietary flavonoids, such as kaempferol, quercetin and myricetin reduce pancreatic cancer risks (2–4). Extended research by our research team and the others supported epidemiological findings and provided some evidence that quercetin has many biological activities, such as antitumor and antiproliferative effects on a wide range of human cancer (see review(5)) including pancreas (6, 7). However, most studies investigated biological activities in cultured cells by using free form of quercetin or aglycone and did not take the absorption and metabolism into consideration for the interpretation of results. Dietary flavonoids were present in human circulating system predominantly as conjugates of glucuronides and sulphates that are likely to have differential biological activities and distribution pattern in tissues and cells compared with flavonoid aglycones (8–10). More importantly, bioactive food components must be sufficiently absorbed in the gastrointestinal tract and reach pharmacological levels in target tissues to have the potential to exert biological activity.
Quercetin (3,3’,4’,5,7-pentahydroxyflavone) is a naturally occurring flavone found in many fruits and vegetables in the form of quercetin glucosides. The mechanism of quercetin glucosides absorption involves the luminal hydrolysis of the glucosides by lactase phlorizin hydrolase followed by diffusion of released aglycone and/or the transport by sodium-dependant glucose transporter with subsequent deglycosylation within the enterocyte by cytosolic β-glucosidase (11). Quercetin aglycone is metabolized by the phase II drug-metabolizing enzymes, the uridine-5’diphosphate – glucuronosyl-transferase, sulfotransferase, and catechol-O-methyl transferase, which leads to the formation of quercetin glucuronide and sulphate conjugates with or without methylation on the catechol functional group of quercetin. Hydroxyl groups of quercetin may be multiply conjugated (12, 13). In blood the predominant forms of quercetin are conjugates (sulfate and glucuronidates with optional methylation), whereas organs like lung, liver, kidney etc. contain higher proportion of free quercetin and 3’O-methylated quercetin isorhamnetin. Quercetin has a relatively long plasma half-life of 12–28 hours (14).
Although bioavailability of quercetin intake by rats (15, 16), pigs, (16, 17) mice(18) and humans (19, 20) are reported, information on quercetin distribution in pancreatic cancer cells, pancreatic tissue and pancreatic tumor in xenograft are not available at present. In this study we have investigated the uptake of quercetin in pancreatic cancer MiaPaCa-2 cells in cellular in vitro. Further, we determined the distribution of quercetin in plasma, lung, liver, pancreas and tumor xenograft in a murine in vivo model fed long term with diets containing either 0.2 or 1% quercetin.
MATERIALS AND METHODS
Materials
All solvents used were HPLC grade (Fisher Scientific, Fairlawn, NJ). Quercetin dihydrate, isorhamnetin and β-Glucuronidase/sulfatase (type H-5 from Helix Pomatia) were purchased from Sigma-Aldrich (St. Louis, MO). Quercetin and tamarixetin were purchased from Chromadex (Irvine, CA). Internal standard 3, 3’,4’-trihydroxyflavone was purchased from Indofine (Hillsborough, NJ).
Cell culture
The human pancreatic cancer cell line MiaPaCa-2 (American Type Culture Collection, Manassas, VA) was cultured in DMEM supplemented with 10% heat inactivated fetal bovine serum, 100 units/ml penicillin, 100µg/ml streptomycin and 292 µg/ml glutamine and incubated at 37°C in a humidified atmosphere with 5% CO2. For the experimental procedures cells were plated in full media. After 24 hours they were starved over night before the addition of 30 µM quercetin (diluted from 100 mM stock solution in DMSO with cell culture medium) for the indicated amount of time. Final DMSO concentration was less than 0.1%. One ml aliquots of the media were frozen after the incubation time. Cells were washed 3 times with ice cold PBS, harvested by scraping and spun at 14,000 rpm for one minute. The supernatant was aspirated and the cell pellets frozen in liquid nitrogen and kept at −80 °C until further analysis. Protein concentration was measured with Pierce Protein Assay kit (Pierce, Rockford, IL).
Cell quercetin extraction
Frozen cell pellets (3×106 cells) were broken by a pellet pestle and then extracted 3 times with 80µl of MeOH. In each extraction the mixture was vortexed for 1 min. and then centrifuged at 13,000g for 5 min. Combined MeOH was dried with a SpeedVac at RT and the residue reconstituted in 200̣ µl of H2O: MeOH (1:3). Aliquots of culture medium were acidified with equal volume of aqueous methanol (methanol: water: acetic acid 45:50:5) and then centrifuged. For both cell pellet and culture medium, aliquots of 50̣ µl were injected into the HPLC.
Animals and diets
Animal studies were approved by the Chancellor’s Animal Research Committee of the University of California, Los Angeles, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Nude mice (Charles River Laboratories, San Diego, CA) were housed 4/cage in a room with controlled temperature (20–22 °C). The mice diet was AIN-93G purified rodent diet (Dyets, Bethlehem, PA) supplement with or without quercetin (5% w/w) for 8 weeks. Diet was replaced every week and the stability of quercetin was analyzed in day 0 (as control), 1, 2, 4 and 7 by HPLC following extraction. Mice blood was drawn by retro-orbital bleeding and plasma was collected in week 2, 4 and 6 weeks and pooled from 4 mice for each sample. After eight weeks the animals were anesthetized and whole blood was taken by cardiac puncture. The lung and liver were preserved as freshly frozen and in 10 % formalin before processing. The pancreas was preserved as freshly frozen. Tissues were processed at the Tissue Procurement & Histology Core Laboratory for hematoxylin and eosin staining. They were evaluated by a pathologist.
The orthotopic xenograft model was performed as described earlier (21). Briefly, 3×106 MiaPaCa-2 cells were injected subcutaneously into the flank of nude mice. After 4 weeks the donor tumor was harvested and minced to fragments of approximately 1 mm3. One tumor fragment was placed into the tail of the pancreas of recipient nude mice. Starting 2 days prior to the surgery the mice were fed the AIN-93G diet. In the 2nd test, the animals were divided in 2 groups of 8 animals each: control and quercetin 5% for 8 weeks. In the 3rd test, animals were divided in 6 groups of 6 animals each: control, gemcitabine, quercetin 0.2%, quercetin 0.2% with gemcitabine, quercetin 1% and quercetin 1% with gemcitabine. Quercetin was administered orally mixed with the powder diet in the respective percentage starting day 1 after surgery. Gemcitabine (120 mg/kg) was given ip day 10, 17, 24, 31 and 38. After six weeks the animals were anesthetized and blood and tissues were obtained similar as described.
Quercetin extraction in mice plasma and tissues
Plasma sample is acidified with 0.1 volume of 0.58M acetic acid to limit flavonoid losses (22). To the acidified mice plasma samples (110 µL, equivalent to 100 µL plasma) in a 2 ml microcentrifuge tube was added 29 µL of β-glucuronidase in 0.2 M sodium acetate buffer (pH 5.0) containing 500 U β-glucuronidase and 12.5 U sulfatase. An aliquot of 71 µL of 0.2 M sodium acetate buffer (pH 5.0) containing 1% ascorbic acid was added. Mixture was vortexed and then incubated at 37°C for 2 hours. After the incubation period, 600 µL of ethyl acetate was added followed by addition of 20 µL of internal standard 3, 3’,4’-trihydroxyflavone in H2O (25 µM). Quercetin and its metabolites were extracted with 600 µL ethyl acetate three times. In each extraction mixture was vortexed for 1 min and then centrifuged at 600 rpm for 5 min. Supernatant was transferred to a glass test tube and combined. Solvent was removed in a SpeedVac at RT until complete dryness. Residue was reconstituted in 150 µL of acetone, vortexed and then sonicated for 1 min., followed by adding 50 µL of H2O. Supernatant was then transferred to a glass insert and an aliquot of 25 µL mixture was injected to HPLC. For mice tissues analysis, frozen tissue was weighed and homogenized in buffer with a tissue grinder and internal standard was added. The mixture was then hydrolyzed and extracted similarly as plasma samples.
High-Pressure Liquid Chromatography
(HPLC) HPLC analysis was performed with a RP-18 Luna column (150 × 4.6 mm, 3 µm, Phenomenex, Torrance, CA) on an Agilent 1100 HPLC system (Santa Clara, CA) comprising of an autosampler and quaternary pump coupled to a photodiode array detector. The mobile phase consisted of a binary gradient of 0.1% (v/v) ortho-phosphoric acid in water (eluent A) and acetonitrile (eluent B), used with flow rate 0.6 mL/min in the following conditions: 20–35% B (0–12 min); 35–70% B (12–18 min); and 70–20% B (18–23 min). Column temperature was held on 30 °C. The chromatograms were recorded at 370 nm and 258 nm for quercetin and its conjugates and metabolites. Data were analyzed with the Hewlett Packard Chemstation® software. Concentrations of quercetin in cell pellets and culture media were determined by HPLC using external calibration. Mice plasma and tissue concentrations were determined by internal calibration. Concentration of the stock solutions was determined spectrophotometrically using Beer’s Law. Calibration standards were prepared from the stock solutions by series dilution. The calibration curves generated from standard solutions of quercetin showed a linear relationship between peak area and concentration in the range of 12.5 ng/mL to10 µg/mL. The detection limit for quercetin is 6 ng/mL with 25 µl injection.
Statistics
Descriptive statistics, such as mean and standard deviation, were used to summarize the results. Statistical comparisons were made using a paired two-tailed Student’s t test with a confidence level of 95%. Statistical significance was defined by P-value of 0.05.
RESULTS
Quercetin degradation in aqueous cell culture media and penetration into cells in culture
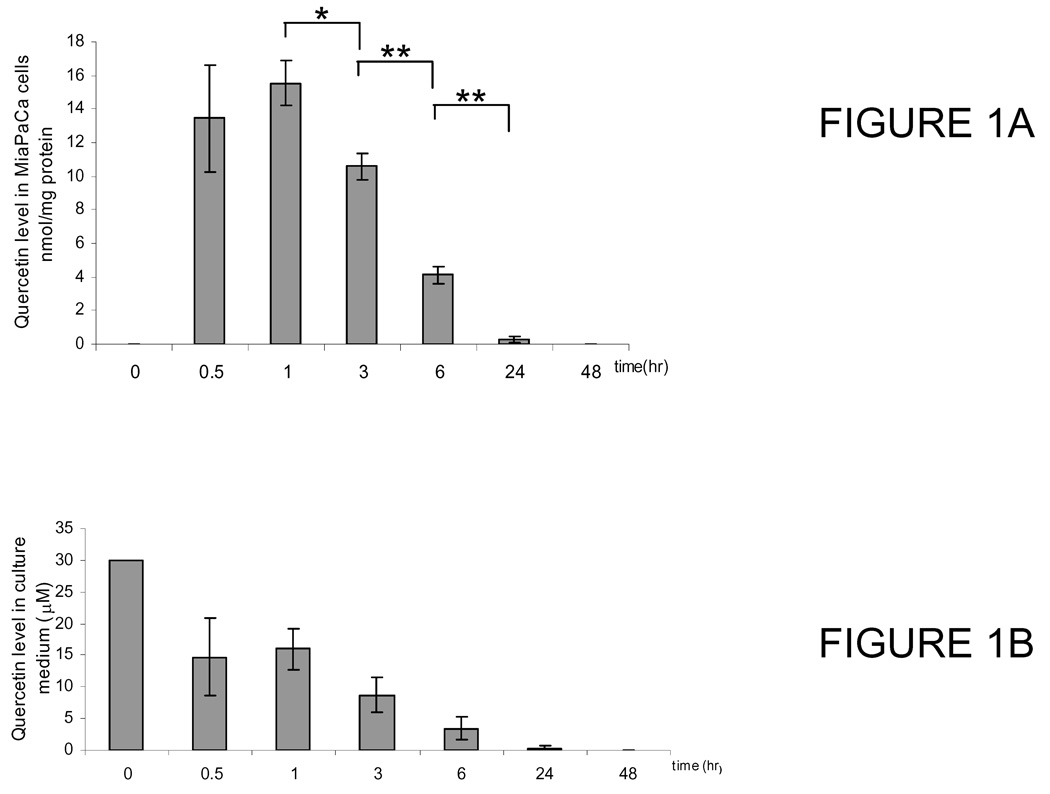
Quercetin is highly labile in cell culture conditions and undergoes degradation and oxidation along with metabolism (23, 24). To examine quercetin taken up into MiaPaCa-2 cells and its changes following exposure to cell culture condition, we collected samples of cells and medium after cells were treated with 30 µM of quercetin and incubated for 0.5, 1, 2, 4, 8, and 24 hrs. Control samples were collected without quercetin treatment. HPLC profile of quercetin uptake by cells showed that quercetin is eluted at 16.7 min. Quercetin concentration plateaus at 1 hr and then decreases steadily. At 24 hrs, its level fell from 6.92 (± 1.20) nmol of peak concentration to 0.15 (± 0.12) nmol (Fig. 1A). Meanwhile, small but detectable amount of quercetin metabolites isorhamnetin (3’-methyl-quercetin, Rt= 19.2 min) and tamarixetin (4’-O-methyl quercetin, Rt=18.9 min) were observed starting 0.5 hr, both of which increase slightly with time and were present until 24 hrs. A peak at Rt 14.4 min was also observed in cells exposed to quercetin. The area of the peak appears increasing with exposure time up to 6 hr then disappearing at 24 hr. The on-line UV-Vis absorption spectrum of the peak is identical to that of quinone as published by Spencer (25). Quercetin, with an intrinsic catechol moiety in its structure, is known to form quinone and quinone methides in cellular in vitro model that is the subject of research interests due to its pro-oxidant chemistry (23, 25). Subsequently, we examined the stability of quercetin in cell culture medium following incubation. Quercetin level declined to half of its initial concentration in 1 hr and fell to 0.34 (± 0.59) µM in 24 hr (Fig. 1B). Also trace but detectable amount of quercetin metabolites isorhamnetin and tamarixetin were observed starting from 0.5 hr but disappeared at 24 hr exposure. To examine if MiaPaCa-2 cells were responsible for the rapid disappearance of quercetin and the production of two O-methylated quercetins, we have performed similar experiments in absence of MiaPaCa-2 cells. We found that the HPLC profile and the change of quercetin level in cell culture medium were parallel to those of experiments in presence of cells, with 30.18 ± 0.91, 14.44 ± 5.60, 15.53 ± 3.41, 5.01 ± 2.05, 0.63 ± 1.09 and 0.16 ± 0.16 at 0, 0.5, 1, 3, 6 and 24 hrs, respectively. Quinone species is also formed in culture medium in presence or absence of cells. These experiments demonstrated approximately 15-fold of quercetin ratio between medium and cells (3 × 106). Thus, the MiaPaCa-2 cells are capable of accumulating quercetin.
Figure 1.
(A) Uptake of quercetin in MiaPaCa-2 cells at different time points as indicated. Values are means ± SEM of 3 separate measurements; (B) Change of quercetin level in cell culture medium with incubation. Preparation of cells and quantitative measurement of quercetin in culture medium at different time points as indicated. Values are means ± SEM of 3 separate measurements (*, P < 0.05, **, P < 0.01).
Plasma absorption of quercetin in mice after oral administration of quercetin diets
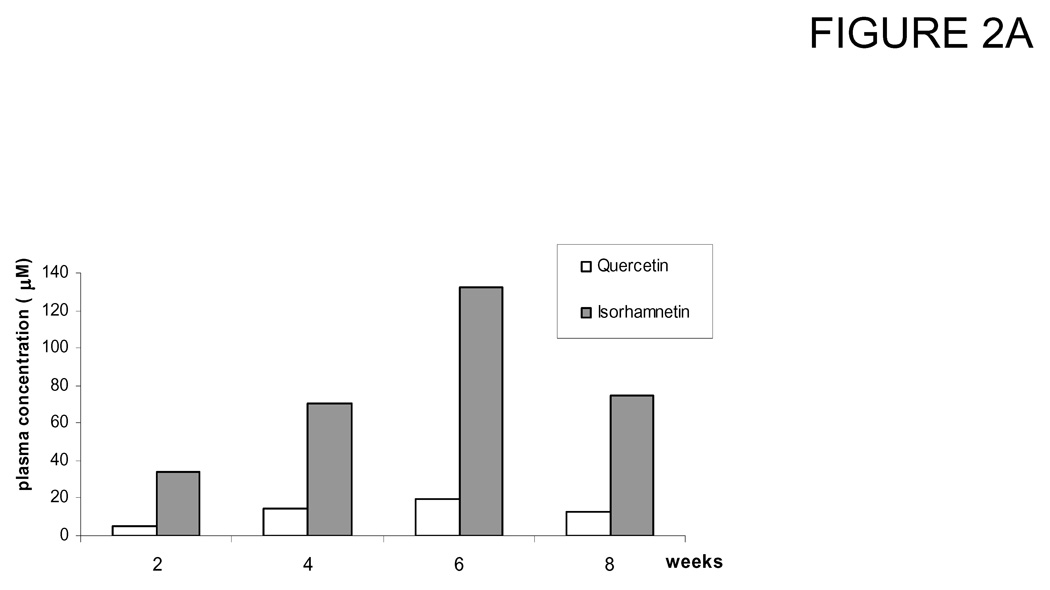
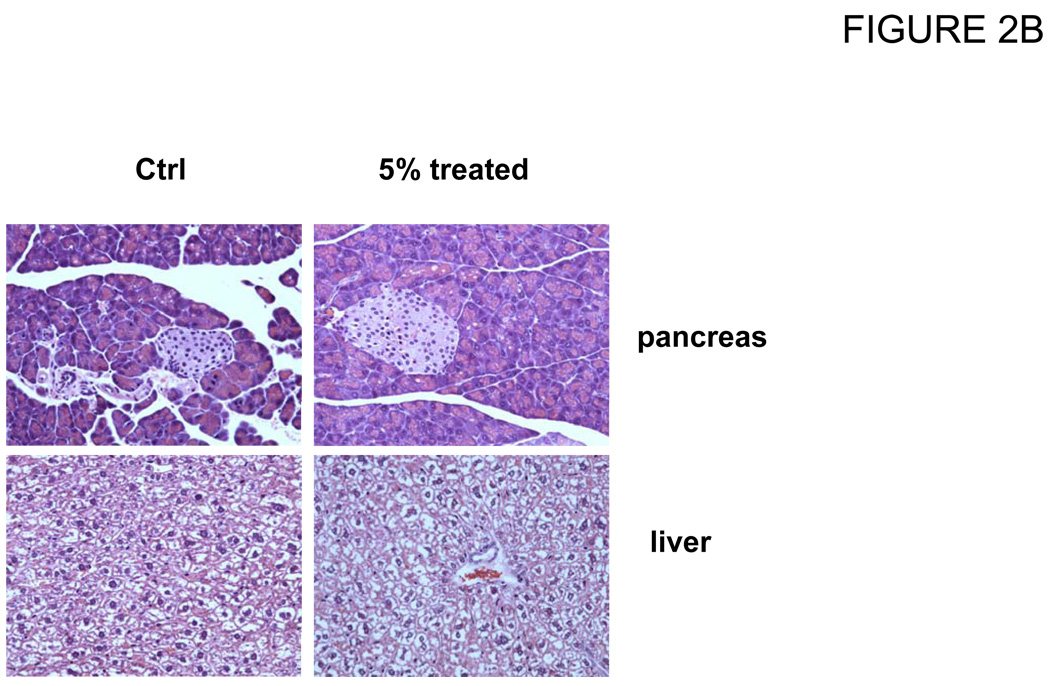
In the first experiment, a group of 4 mice was treated with a 5% of quercetin diet for 8 weeks (calculated as 5.3 g/(kg body wt · d) quercetin intake). The dosage and length of feeding were selected in order to determine the quercetin bioavailability and toxicity in mice. During the course of study stability of quercetin in the diet was measured at day 1, 2, 3, 4 and 7. Mice plasma were collected in week 2, 4 and 6 weeks and pooled from 4 mice for each sample. Data showed that quercetin was absorbed into the mice blood time-dependently and gave total concentration (quercetin plus isorhamnetin) of 39.4, 84.5 and 152.0 µM at 2, 4 and 6 weeks, respectively. However, at the time mice were sacrificed after 8 weeks feeding, plasma concentration decreased to 87.8 µM ± 17.9 (Fig. 2A). Mice also exhibited slightly increased body weight but did not have signs of other adverse effects as revealed by measurements of blood chemistry panel (Table 1) and histology (Figure 2B). We observed large variation in blood alanine aminotransferase (ALT) and alanine transaminase (AST) levels for control mice. It is likely due to the small sample size. In spite of it, all tests were normal for all the animals and organs except one animal in the control group having an AST of 423 U /L (normal: 37 – 329 U / L). Clinically this mouse had a normal liver function and the other liver chemistries (ALT and Bilirubin) were normal.
Figure 2.
Change of quercetin and isorhamnetin concentrations in plasma of mice fed a 5% quercetin diet for 8 weeks. Mice were fed with a diet containing 5% of quercetin for 8 weeks, blood were collected and pooled at different time as indicated. Concentrations of quercetin and isorhamnetin in plasma were measured by HPLC as described in Materials and Methods. (A) Plasma absorption of quercetin and isorhamnetin at different time point. (B) Histology of liver and pancreas of nude mice fed with 5% quercetin diet for 8 weeks.
Table 1.
Blood chemistry panel of mice fed a 5% quercetin diet for 8 weeks
| Groups | Control | 5% Quercetin Diet |
|---|---|---|
| ALT 7.0 – 227.0 U/L | 54.0 ± 25.5 | 35.0 ± 3.6 |
| AST 37.0 – 329.0 U/L | 262.5 ± 227.0 | 126.3 ± 28.0 |
| BUN 2.0 – 71.0 mg/dl | 23.5 ± 2.1 | 18.0 ± 1.7 |
| Creatinin 0.1 – 2.1 mg/dl | 0.3 ± 0.0 | 0.3 ± 0.1 |
| Total Bilirubin 0.1 – 1.1 mg/dl | 0.6 ± 0.1 | 0.4 ± 0.1 |
ALT, alanine aminotransferase; AST, alanine transaminase; BUN, blood urea nitrogen;
HPLC profile of mice plasma shows two peaks with retention times at 16.7 and 19.1 as standard quercetin and methylated 3’-O-methyl quercetin after samples were hydrolyzed with β--glucuronidase / sulfatase. Compared with the retention times and online UV/Vis maxima of the DAD detector responses of the authentic samples facilitated the confirmation of these peaks. Majority of the quercetin (approximately 83%) is metabolized to isorhamnetin, which is in accordance with a previous report in that about 79% of the metabolites in the free access rat plasma were methylated (26).
In the 2nd test, 16 tumor-bearing mice administered with 5% quercetin or control diets for 8 weeks. Average plasma concentration is 50.23 (±15.37) µM for quercetin and 58.02 (±11.07) µM for isorhamnetin as measured after enzymatic hydrolysis. Because 5% quercetin intake did not produce tumor growth inhibition, further data were not collected and lower doses were adopted.
In the 3rd study, analysis of blood of tumor-bearing mice administered lower doses with 1.0 and 0.2% quercetin diets for 6 weeks (calculated as 1.5 and 0.3 g/(kg body wt · d) quercetin intake, respectively) revealed a dose-dependant plasma total concentration after enzyme hydrolysis (Table 2). Plasma samples in 1 % quercetin intake group showed free quercetin and isorhamnetin, albeit in very lower level (about 4–15% of total levels), as measured without enzymatic hydrolysis. It also showed about 3 times higher concentrations of total quercetin compared to the 0.2% quercetin intake group. In both intake groups the level of methylated quercetin was consistently about one half of the total quercetin and was noticeably less than that from the plasma of the nude mice with 5% quercetin intake. Further, we observed the group of mice fed with quercetin diet and injected with gemcitabine at the same time showed lower blood levels of quercetin and methylated quercetin (Table 3) compared with the group fed with quercetin diet only. Generally, plasma levels of quercetin and isorhamnetin are lower in both quercetin plus gemcitabine groups. Statistic significance was observed in quercetin level in 0.2% quercetin intake group and isorhamnetin level in 1% intake group.
Table 2.
Quercetin and isorhamnetin levels in plasma of xenograft mice fed with 0.2, and 1% of quercetin diet for 6 weeksa
| 0.2% quercetin diet | 1% quercetin diet | |
|---|---|---|
| µM | ||
| Quercetin | 3.29 ± 0.40 | 8.68 ± 1.21 (0.19 ± 0.09b) |
| Isorhamnetin | 3.27 ± 0.91 | 13.97 ± 2.11 (0.07 ± 0.04b) |
Values are mean ± SEM;
aglycone.
Table 3.
Quercetin and isorhamnetin levels in plasma of xenograft mice fed with 0.2 and 1% quercetin diet with and without gemcitabinea
| 0.2% quercetin diet | 1% quercetin diet | |||
|---|---|---|---|---|
| Q | Q+ G | Q | Q+G | |
| µM | ||||
| Quercetin | 4.42 ± 0.51b | 2.35 ± 0.10 | 9.43 ± 1.49 | 7.94 ± 2.00 |
| Isorhamnetin | 4.46 ± 1.96 | 2.27 ± 0.19 | 18.19 ± 3.31b | 9.95 ± 1.24 |
Values are mean ± SEM;
different from Q+G co-treatment, P<0.05.
Q: quercetin diet; Q+G: quercetin diet with gemcitabine injection every week.
Tissue levels of quercetin in xenograft mice after oral administration of 0.2 and 1% quercetin diet
To investigate the levels of quercetin in pancreas and tumor as target tissues, we also incorporated tissues of lung and liver as metastases to the liver and lung are common findings especially with tumors of the pancreas. Generally, all tissues from mice fed with 1% of quercetin diet accumulated 4–10 times higher concentrations of quercetin and methylated quercetin compared to those from mice fed with 0.2% quercetin diet (Table 4). Level of total quercetin is the highest in liver, followed by pancreas, lung and tumor while in lower intake group, highest concentration was observed in pancreas, followed by liver and tumor. In addition, majority of the quercetin and methylated quercetin were found in their aglycone forms in these tissues including tumor. Among them, pancreas accumulated 84% quercetin aglycone and tumor 65%. Gemcitabine treatment resulted in significant reduction in quercetin only in the liver of mice fed with 0.2% quercetin diet (0.30 ± 0.07 vs. 1.07 ± 0.1, p<0.05). Qinone species, present in cell extract as well as in culture medium, was not found in any tissue or plasma of mice.
Table 4.
Quercetin and isorhamnetin levels in tissues of xenograft mice treated with 1% and 0.2% quercetin for 6 weeksa
| 0.2% quercetin diet | 1% quercetin diet | |||
|---|---|---|---|---|
| Q | I | Q (aglycone) | I (aglycone) | |
| nmol/g wet tissue | ||||
| Liver | 0.65 ± 0.13 | 0.37 ± 0.09 | 5.12 ± 1.24 (5.45 ± 1.28) | 3.69 ± 0.88 (3.56 ± 0.92) |
| Lung | 0.67 ± 0.18 | NDb | 3.04 ± 0.41 (2.17 ± 0.45) | 3.44 ± 0.51 (1.36 ± 0.38) |
| Pancreas | 1.16 ± 0.47 | 0.69 ± 0.21 | 4.72 ± 1.60 (3.98 ± 1.48) | 3.46 ± 0.32 (3.14 ± 1.27) |
| Tumor | 0.51 ± 0.12c | 0.30 ± 0.09c | 2.64 ± 1.15d (1.69e) | 2.16 ± 0.35d (1.61e) |
Values are mean ± SEM.
ND, not detected.
n=5.
n=6.
n=2.
Q, quercetin; I, isorhamnetin.
DISCUSSION
This study shows for the first time that quercetin and its metabolites are distributed in pancreas and xenograft tumors in mice after administered with 0.2 and 1% quercetin diets. Our data demonstrated that quercetin is distributed in the organs of liver, lung, pancreas and tumor tissue and its levels were within the same order of magnitude. Tissues including liver and lung in the 1% intake group accumulated about 4–10 times higher concentration of total quercetin than that in 0.2% intake group. In 1% quercetin intake group, level of total quercetin is the highest in liver, followed by pancreas, lung and tumor while in lower intake group, highest concentration was observed in pancreas, followed by liver, tumor and lung. These results are in agreement with a previous study in that quercetin is widely distributed in SC tumor, liver, lung, spleen, heart etc. in tumor-bearing mice (27). Furthermore, we found that all investigated organs contained a considerable proportion of deconjugated quercetin and isorhamnetin, ranging from 40–100% of the total quercetin concentration. In tumor 64% of quercetin and 75% of isorhamnetin are aglycone forms. A similar metabolism pattern has been found in rats and pigs after oral intake of quercetin diet. Rat tissues of lung, liver and kidney were shown to possess a high deconjugation activity due to the presence of enzymes with β-glucuronidase activity (16, 17). This high enzyme activity also resulted in an ex vivo conversion of 3-O-glucuronide to free aglycone during the extraction, which may also cause in vivo conversion in the tissues (23). Enzyme with β-glucuronidase activity can also be released under certain physiologic condition such as inflammation (28), carcinogenic tissue (29) and tumor, the tissues of the latter contain large amounts of β-glucuronidase (30, 31).
The glucuronidation of quercetin could take place at the 3-,7-, 3’-, and 4’-positions when quercetin was incubated with human liver cell-free extract (10). When incubation with mouse liver microsomes, 4 quercetin metabolites were observed (18). Many of the glucuronide metabolites retain or decrease the biological activity but mostly the antioxidant activities were examined (10, 32, 33). Quercetin aglycone is generally considered as the more active form. In our study all pancreas, liver, lung and tumor tissues accumulate significant amount of free quercetin. The high and selective accumulation of chemopreventive agents at the tumor site is essential for the success of disease treatment in vivo. Studies show that the efficiency of quercetin glucosides absorption is higher than that for the aglycone itself in human (14) as well as in animal (34). To this end, our animal fed with quercetin aglycone may provide a disadvantageous condition for its absorption into various tissues including the tumor. The high level of quercetin, along with the high ratio of aglycone/conjugates found in pancreas and tumor may contribute to the tumor inhibitory activity of quercetin.
We demonstrated that quercetin is absorbed into mice blood in a dose-responsive manner. Plasma of tumor-bearing mice fed with 0.2 and 1 % of quercetin diets produced dose-dependant concentration increase in total quercetin and its metabolites. Plasma samples in 1% quercetin treated group showed quercetin and isorhamnetin aglycones in very low level which is in line with the reported studies in that aglycones are generally either absent in blood or present in low concentrations. During the course of absorption, quercetin is conjugated in the small intestine and later in the liver. This process includes methylation, sulfation and glucuronidation, which is a metabolic detoxication process common to many xenobiotics that restricts their potential toxic effects and facilitates its biliary and urinary elimination by increasing its hydrophilicity. Eleven quercetin metabolites have been reported and their structure identified in plasma and therefore the conjugation mechanisms are considered highly efficient (19). We found >80% of the absorbed quercetin is methylated to isorhamnetin. The high methylation of quercetin was observed starting week 2 and remained the same until week 8 in blood of nude mice.
We observed that the ratio of methylated/non- methylated quercetin was lower in plasma of both 0.2 and 1% quercetin intake groups comparing to that of nude mice with high quercetin intake. Although comparison needs to be made with cautious due to the difference in dosages, lower dose has been reported to favor the higher proportion of methylated quercetin in human plasma (35). To this end, our data may suggest tumor implantation and bearing may affect metabolism in mice. In addition, chemotherapeutic drug gemcitabine co-treatment with quercetin reduced absorption of quercetin in circulatory system and liver. Absorption of quercetin into blood and the subsequent metabolic profile were reported to be influenced by various factors including the route of administration (26), dosage (33), food matrix (36, 37), etc. Chronic quercetin exposure has been reported to affect fatty acid catabolism in rat lung (38). It is possible that gemcitabine alone alters liver metabolism or that frequent ip injection changes the eating habit and therefore the blood absorption.
In summary, this study showed that quercetin can be accumulated in pancreatic cancer MiaPaCa-2 cells in vitro, and absorbed effectively in the circulating system, tumors and tissues of pancreas, liver and lung in vivo. While higher proportion of quercetin and methylated quercetin found in organs of pancreas, lung and liver, as well as tumors are aglycones, conjugates were found almost exclusively in plasma (>98%). Lower ratio of methylated/non- methylated quercetin was found in plasma of both 0.2 and 1% quercetin intake groups comparing to that of nude mice. Chemotherapeutic drug gemcitabine co-treatment with quercetin reduced absorption of quercetin in circulatory system and liver but not in the other tissues investigated. As quercetin is eliminated mainly in bile, its levels in pancreas and liver generate high concentration variation. It is important to note that quercetin (1%) co-treatment with gemcitabine significantly inhibit the growth of tumor in orthotopic murine model(39). Our data provided important information on the therapeutic efficacy of quercetin.
Supplementary Material
ACKNOWLEDGEMENT
This research is supported by NIH grant AT003960.
ABBREVIATIONS USED
- ALT
alanine aminotransferase
- AST
alanine transaminase
- BUN
blood urea nitrogen
- DAD
diode array detector
- G
gemcitabine
- HPLC
high-pressure liquid chromatography
- I
isorhamnetin
- Q
quercetin
- Rt
retention time
Literature Cited
- 1.American Cancer Society. Cancer Statistics. 2009
- 2.Nothlings U, Murphy SP, Wilkens LR, Boeing H, Schulze MB, Bueno-de-Mesquita HB, Michaud DS, Roddam A, Rohrmann S, Tjonneland A, Clavel-Chapelon F, Trichopoulou A, Sieri S, Rodriguez L, Ye W, Jenab M, Kolonel LN. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am. J. Clin. Nutr. 2008;88(6):1653–1662. doi: 10.3945/ajcn.2008.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nothlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Flavonols and pancreatic cancer risk: the multiethnic cohort study. Am. J. Epidemiol. 2007;166(8):924–931. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 4.Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J, Stolzenberg-Solomon RZ. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland) Cancer Epidemiol. Biomarkers Prev. 2008;17(3):553–562. doi: 10.1158/1055-9965.EPI-07-2523. [DOI] [PubMed] [Google Scholar]
- 5.Jagtap S, Meganathan K, Wagh V, Winkler J, Hescheler J, Sachinidis A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009;16(12):1451–1462. doi: 10.2174/092986709787909578. [DOI] [PubMed] [Google Scholar]
- 6.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67(2):616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 7.Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, Pandol SJ. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int. J. Cancer. 2002;98(5):761–769. doi: 10.1002/ijc.10202. [DOI] [PubMed] [Google Scholar]
- 8.Manach C, Morand C, Crespy V, Demigne C, Texier O, Regerat F, Remesy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426(3):331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 9.Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. 1998;275(1 Pt 2):R212–R219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- 10.Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000;29(12):1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 11.Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G. Absorption of quercetin-3-glucoside and quercetin-4'-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003;65(7):1199–1206. doi: 10.1016/s0006-2952(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003;42(1):29–42. doi: 10.1007/s00394-003-0397-3. [DOI] [PubMed] [Google Scholar]
- 13.Day AJ, DuPont MS, Ridley S, Rhodes M, Rhodes MJ, Morgan MR, Williamson G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998;436(1):71–75. doi: 10.1016/s0014-5793(98)01101-6. [DOI] [PubMed] [Google Scholar]
- 14.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995;62(6):1276–1282. doi: 10.1093/ajcn/62.6.1276. [DOI] [PubMed] [Google Scholar]
- 15.Carbonaro M, Grant G. Absorption of quercetin and rutin in rat small intestine. Ann. Nutr. Metab. 2005;49(3):178–182. doi: 10.1159/000086882. [DOI] [PubMed] [Google Scholar]
- 16.de Boer VC, Dihal AA, van der Woude H, Arts IC, Wolffram S, Alink GM, Rietjens IM, Keijer J, Hollman PC. Tissue distribution of quercetin in rats and pigs. J. Nutr. 2005;135(7):1718–1725. doi: 10.1093/jn/135.7.1718. [DOI] [PubMed] [Google Scholar]
- 17.Bieger J, Cermak R, Blank R, de Boer VC, Hollman PC, Kamphues J, Wolffram S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008;138(8):1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- 18.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004;52(4):935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 19.Mullen W, Edwards CA, Crozier A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006;96(1):107–116. doi: 10.1079/bjn20061809. [DOI] [PubMed] [Google Scholar]
- 20.Graefe EU, Derendorf H, Veit M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int. J. Clin. Pharmacol. Ther. 1999;37(5):219–233. [PubMed] [Google Scholar]
- 21.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31(3):258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 22.Justino GC, Santos MR, Canario S, Borges C, Florencio MH, Mira L. Plasma quercetin metabolites: structure-antioxidant activity relationships. Arch. Biochem. Biophys. 2004;432(1):109–121. doi: 10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Awad HM, Boersma MG, Boeren S, van der Woude H, van ZJ, Van Bladeren PJ, Vervoort J, Rietjens IM. Identification of o-quinone/quinine methide metabolites of quercetin in a cellular in vitro system. FEBS Lett. 2002;520(1–3):30–34. doi: 10.1016/s0014-5793(02)02754-0. [DOI] [PubMed] [Google Scholar]
- 24.Boulton DW, Walle UK, Walle T. Fate of the flavonoid quercetin in human cell lines: chemical instability and metabolism. J. Pharm. Pharmacol. 1999;51(3):353–359. doi: 10.1211/0022357991772367. [DOI] [PubMed] [Google Scholar]
- 25.Spencer JP, Kuhnle GG, Williams RJ, Rice-Evans C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. Biochem. J. 2003;372(Pt 1):173–181. doi: 10.1042/BJ20021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai Y, Saito S, Nishikawa T, Ishisaka A, Murota K, Terao J. Different profiles of quercetin metabolites in rat plasma: comparison of two administration methods. Biosci. Biotechnol. Biochem. 2009;73(3):517–523. doi: 10.1271/bbb.80516. [DOI] [PubMed] [Google Scholar]
- 27.Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006;12(10):3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 28.Shimoi K, Nakayama T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005;400:263–272. doi: 10.1016/S0076-6879(05)00015-7. [DOI] [PubMed] [Google Scholar]
- 29.Oi N, Hashimoto T, Kanazawa K. Metabolic conversion of dietary quercetin from its conjugate to active aglycone following the induction of hepatocarcinogenesis in fisher 344 rats. J. Agric. Food Chem. 2008;56(2):577–583. doi: 10.1021/jf072556c. [DOI] [PubMed] [Google Scholar]
- 30.Yuan L, Wagatsuma C, Yoshida M, Miura T, Mukoda T, Fujii H, Sun B, Kim JH, Surh YJ. Inhibition of human breast cancer growth by GCP (genistein combined polysaccharide) in xenogeneic athymic mice: involvement of genistein biotransformation by beta-glucuronidase from tumor tissues. Mutat. Res. 2003;523–524:55–62. doi: 10.1016/s0027-5107(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 31.Yue H, Yang B, Zhang H, Zhu SD, Du XJ, Feng XL, Yu Z, Xia YT, Yu JP. Clinical significance of TGF- beta1 and beta-glucuronidase synchronous detection in human pancreatic cancer. Hepatobiliary. Pancreat. Dis. Int. 2002;1(2):309–311. [PubMed] [Google Scholar]
- 32.Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nutr. 2004;80(1):15–21. doi: 10.1093/ajcn/80.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Santos MR, Rodriguez-Gomez MJ, Justino GC, Charro N, Florencio MH, Mira L. Influence of the metabolic profile on the in vivo antioxidant activity of quercetin under a low dosage oral regimen in rats. Br. J. Pharmacol. 2008;153(8):1750–1761. doi: 10.1038/bjp.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morand C, Manach C, Crespy V, Remesy C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000;33(5):667–676. doi: 10.1080/10715760000301181. [DOI] [PubMed] [Google Scholar]
- 35.DuPont MS, Bennett RN, Mellon FA, Williamson G. Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. J. Nutr. 2002;132(2):172–175. doi: 10.1093/jn/132.2.172. [DOI] [PubMed] [Google Scholar]
- 36.Nishijima T, Iwai K, Saito Y, Takida Y, Matsue H. Chronic ingestion of apple pectin can enhance the absorption of quercetin. J. Agric. Food Chem. 2009;57(6):2583–2587. doi: 10.1021/jf803547h. [DOI] [PubMed] [Google Scholar]
- 37.Tamura M, Nakagawa H, Tsushida T, Hirayama K, Itoh K. Effect of pectin enhancement on plasma quercetin and fecal flora in rutin-supplemented mice. J. Food Sci. 2007;72(9):S648–S651. doi: 10.1111/j.1750-3841.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 38.de Boer VC, van Schothorst EM, Dihal AA, van der Woude H, Arts IC, Rietjens IM, Hollman PC, Keijer J. Chronic quercetin exposure affects fatty acid catabolism in rat lung. Cell Mol. Life Sci. 2006;63(23):2847–2858. doi: 10.1007/s00018-006-6316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angst E, Park J, Moro A, Lu QY, King JC, Go VLW, Eibl G, Hines OJ. The AACR 101st Annual Meeting 2010; April 17–21; Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.