Abstract
Olfactory perception was examined in deficit syndrome (DS) and nondeficit syndrome (ND) schizophrenia patients. Participants included 22 controls (CN) and 41 patients with schizophrenia who were divided into DS (n = 15) and ND (n = 26) subtypes using the Schedule for the Deficit Syndrome (SDS). Olfactory perception for pleasant and unpleasant odors was assessed using the Brief Smell Identification Test. Participants were instructed to identifying each smell as well as provide hedonic judgment ratings of each smell on a 7-point scale (1 = extremely pleasant, 4 = neutral, and 7 = extremely unpleasant). Results indicated that when compared with the ND patients, the DS patients rated pleasant smells as being significantly less pleasant, although no difference between the groups was present for unpleasant smells, and both ND and DS groups significantly differed from CN on rating and identifying pleasant and unpleasant items. Additionally, lower smell identification accuracy was negatively correlated with SDS symptom severity, and valence ratings for pleasant odors were positively correlated with SDS diminished emotional range. Findings suggest that the DS is characterized by a unique pattern of olfactory valence judgment that is characterized by abnormalities in processing positively valenced stimuli.
Keywords: negative symptoms, olfaction, emotion, valence
Introduction
The deficit syndrome (DS) is thought to reflect a distinct subtype of patients within the broader diagnosis of schizophrenia.1 Validity for the DS subtype comes from evidence, suggesting that DS patients differ from nondeficit (ND) schizophrenia patients on a number of domains, including course of illness, risk factors, and etiology.2 Core features of the DS include deficits in affective and volitional domains, which are primary and stable for a period of at least 1 year. DS symptoms have proven highly treatment resistant,3–5 which may in part be due to a distinct underlying neuropathology associated with the DS condition.
Given the significance of identifying the biological correlates associated with these symptoms, there has been increased interest in delineating neuropathology associated with the DS. Initial studies found evidence consistent with frontal and parietal involvement using neuropsychological measures6–8 which was subsequently supported by neuroimaging studies indicating reduced regional cerebral blood flow (rCBF) within these regions.9–10 Kirkpatrick et al2 proposed that these early findings were consistent with dysfunction within dorsolateral prefrontal basal ganglia-thalamocortical circuitry. Several recent studies have also found evidence consistent with frontal and parietal dysfunction11–18; however, there has also been some support for impairment on tests reflecting temporal lobe dysfunction.14,17,19 In an attempt to clarify these discrepant findings, Cohen et al20 conducted a meta-analysis of 13 separate studies investigating neuropsychological impairment in DS and ND patients. Results of that meta-analysis as well as new data they presented failed to support a unified pattern of neurocognitive impairment when neuropsychological measures were grouped into frontal, parietal, and temporal domains. However, an analysis of domain-specific neurocognitive abilities provided a striking finding that olfactory processes were the most impaired of all neurocognitive domains.
Given the extent to which olfactory performance differentiates DS and ND patients, further investigation of olfactory dysfunction may provide insight into neural processes underlying the DS. Neuropathology inherent to smell identification deficits is likely to involve dysfunction within a distributed network of brain regions core to DS symptoms, namely the temporal lobe, prefrontal cortex, limbic system structures, and the thalamus. The prefrontal cortex may be central to this dysfunction considering that direct connections between the olfactory bulb and prefrontal cortex enable olfactory information to bypass sensory relay within the thalamus. However, as noted by Malaspina et al,21 it is also possible that this prefrontal dysfunction may be secondary to aberrant limbic system activation. Considering that both affective and olfactory impairments are core to DS symptoms, and are functions subsumed by limbic and frontal systems, it would be valuable to determine whether DS patients display impairment on tests designed to assess neuropathology inherent to these systems.
Several recent studies have examined valence-related olfactory abnormalities in schizophrenia and set the groundwork for investigating such dysfunction in the DS. In a comprehensive assessment of olfactory perception, Hudry et al22 found that patients were more deficient than controls at several olfactory processes, including the assignment of pleasantness ratings. Similarly, Moberg et al23 found patients to rate the pleasantness of stimuli abnormally, despite being nearly identical to controls with regard to intensity judgment. Crespo-Facorro et al24 furthered these results using positron emission tomography (PET) by demonstrating that schizophrenia patients rated pleasant smells as being significantly more neutral than controls and that these deficits in valence judgment were associated with aberrant prefrontal cortex and limbic system activation. Doop and Park25 also found schizophrenia patients to display abnormal pleasantness judgments and that these abnormalities were correlated with negative symptoms, such that more severe flat affect was associated with more aberrant hedonic judgment. Thus, although relatively few studies have investigated the nature of olfactory affect perception in schizophrenia, results from initial studies suggest that (1) schizophrenia patients judge odor valence abnormally, (2) these abnormalities are associated with negative symptoms, and (3) frontal/limbic circuitry may underlie olfactory hedonic dysfunction.
The current study examined whether DS and ND patients differed in relation to accurately naming and judging the valence of pleasant and unpleasant odors. It was hypothesized that DS patients would display lower overall olfactory identification accuracy and valence judgment abnormalities when judging the hedonic tone of pleasant and unpleasant odors, such that both pleasant and unpleasant odors would be rated as significantly less pleasant by DS than ND or control (CN) subjects. Additionally, consistent with previous research,26 smell identification performance was predicted to be correlated with greater severity of DS symptomatology, particularly diminished social drive and diminished emotional range.
Materials and Methods
Participants included 41 individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for schizophrenia and 22 healthy controls. Patients and controls did not significantly differ on age (schizophrenia patients: mean [M] = 41.85, SD = 11.8; CN: M = 40.05, SD = 12.13; F1,62 = 0.33, P = .57) or education (schizophrenia patients: M = 12.2, SD = 1.8; CN: M = 12.84, SD = 1.04; F1,62 = 2.22, P = .14). Approximately 69% of patients and 18.2% of CN were males (= 13.01, P < .001). Caucasians comprised 43.9% of patients and 68.2% of CN ( = 8.90, P = .063). Patient mean age of onset was 20.95 (SD = 4.0) with a length of illness duration of 21.7 (SD = 10.25) years. Brief Psychiatric Rating Scale (BPRS) total scores indicated that patients were experiencing a mild level of symptoms at the time of evaluation (M = 40.95, SD = 7.62). Individuals with schizophrenia were recruited from a local community outpatient mental health center in Nevada and were identified by their treating psychiatrists for inclusion in the study if they were English speaking (as determined by English being their primary language) and carried a primary diagnosis of DSM-IV-TR schizophrenia.27 This clinical diagnosis was subsequently confirmed using the Structured Clinical Interview for DSM-IV,28 which was informed by consultation with the treating psychiatrist and other program staff, and thorough review of medical records. Individuals with schizophrenia were excluded from participation if English was not their first language, they had history of traumatic brain injury or other medical or neurological condition that would affect central nervous system function, had a history of substance-use disorder within the past 6 months or another current DSM-IV Axis I disorder other than schizophrenia, a diagnosis of mental retardation, they were using prescribed or over-the-counter medications that could produce significant cognitive effects (other than those prescribed to treat schizophrenia), and had corrected vision worse than 20/50 as determined by evaluation using a standard wall chart.
Schizophrenia patients were divided into DS and ND groups using the Schedule for the Deficit Syndrome (SDS) rating scale, which has adequate reliability and validity.29 To ensure the accuracy of DS classification, consensus ratings were conducted by two of the authors (G.P.S. and D.N.A.), treatment providers (psychiatrists, counselors, case managers) were consulted, and medical records were reviewed to verify stability of negative symptom presentation and accuracy of primary vs secondary negative symptom distinction. This classification resulted in identification of 15 DS and 26 ND patients.
Healthy controls (CN) were recruited from the community and a local university. In addition to the aforementioned exclusionary criteria for the schizophrenia groups, healthy controls had no lifetime diagnosis of schizophrenia or bipolar disorder, history of neurological conditions, or current Axis I psychiatric disorder, as determined by the Structured Clinical Interview for DSM-IV. Individuals were also excluded if they were currently using any psychotropic medication or had a first- or second-degree relative diagnosed with or suspected to have a psychotic disorder as determined using a standardized interview.30 The research was approved by the local institutional review board for protection of human subjects, and all participants provided informed consent.
Measures
Chlorpromazine Equivalent Dosage, Clinical Symptomatology, and Estimated IQ
Chlorpromazine equivalent dosage was estimated using Woods.31 Measures completed to assess clinical symptoms included the SDS,29 BPRS,32 and Abnormal Involuntary Movement Scale (AIMS).33 IQ was estimated using the Vocabulary, Information, and Block Design subtests from the Wechsler Adult Intelligence Scale—III.34
Brief Smell Identification Test
Olfactory discrimination was assessed using the University of Pennsylvania Brief Smell Identification Test (B-SIT).35 The B-SIT is a standardized measure that requires identification of 12 common microencapsulated odors by selecting 1 of 4 multiple-choice answers consisting of various odor names. Smell identification scores are calculated by totaling the number of correct responses. Previous findings have suggested that as a proxy, the B-SIT is as effective as the original 40-item variant of the test, the University of Pennsylvania Smell Identification Test in measuring olfactory dysfunction with correlations ranging from 0.83 to 0.8936 and has a test-retest reliability of 0.71 over a period of 1 week.35,37 The B-SIT is also capable of detecting olfactory processing deficits in patients with schizophrenia and, previously, found to differentiate DS and ND subtypes.36
In addition to identifying the odor for each of the B-SIT items, participants also provided valence ratings for each item. In a procedure similar to that employed by Doop and Park,25 odor valence was rated on a 7-point scale (1 = extremely pleasant, 4 = neutral, and 7 = extremely unpleasant). After identifying each odor, participants were instructed to indicate how pleasant or unpleasant they found each odor. Individual items were divided into pleasant and unpleasant categories using normative valence ratings developed by Doty, Shaman, and Dann.38 For the present study, items which fell on the pleasant end of the neutral reference point using the Doty et al. norms were considered pleasant and items falling toward the unpleasant end of the neutral point were considered unpleasant. This division resulted in 7 pleasant and 5 unpleasant items. Pleasant and unpleasant items do not differ in intensity based upon normative data collected by Doty et al.38
Procedure
The current tests were administered as part of a large battery of symptom and neuropsychological measures. For each subject, demographic, diagnostic, and symptom ratings were completed prior to administration of the neurocognitive evaluations. The neurocognitive evaluation lasted for 3–4 h, and breaks were allowed as needed to diminish fatigue and maintain motivation. Patient and control participants recruited from the community received monetary compensation for participation. All evaluations were conducted by doctoral level researchers who were extensively trained to complete the procedures in a reliable and valid manner. Evaluations occurred in a quiet and private setting.
Results
Prior to conducting the main analyses, the groups were compared on demographic and clinical variables. The DS, ND, and CN groups did not differ on age (DS: M = 45.8, SD = 12.5, range = 22–64; ND: M = 39.6, SD = 10.9, range = 20–62; CN: M = 40.0, SD = 12.1, range = 20–59; F2,62 = 1.49, P = .23), education, F2,62 = 1.31, P = .28, or proportion of right handedness, = 1.81, P = .40. No significant differences were found for estimates of smoking behavior, as measured by the percentage of individuals who smoke (DS M = 73.3%; ND M = 46.2%; CN M = 40.90%; = 4.13, P = .13) and the number of cigarettes smoked per day (DS M = 16.8, SD = 11.4; ND M = 16.6, SD = 5.1; CN M = 10.11, SD = 4.8; F2,62 = 2.22, P = .13). Groups significantly differed on IQ estimated from the Wechsler Adult Intelligence Scale Vocabulary, Information, and Block Design subtests, such that DS had lower IQ than ND who were lower than CN (Vocabulary F2,62 = 12.76, P < .001; Information F2,62 = 19.32, P < .001; Block Design F2,62 = 20.67, P < .001). Also, significant differences were present among DS, ND, and CN groups for race ( = 17.09, P < .05), with African Americans making up 60.0%, 26.9%, and 4.5% of the groups, respectively. Furthermore, DS and ND patients did not differ with regard to length of illness (F1,40 = 1.91, P = .19), age of onset (F1,40 = 1.45, P = .24), daily antipsychotic medication dosage (chlorpromazine equivalent dosage32) (F1,40 = 0.60; P = .43), or severity of extrapyramidal symptoms (AIMS total: F1,40 = .20, P = .66). Also, patients were prescribed a similar regiment of antipsychotic medication: conventional—DS = 13.3%; ND = 11.50%; atypical—DS = 93.3%; ND = 96.2%. DS displayed more severe negative symptoms (F1,40 = 15.20, P < .001), equal levels of disorganization (F1,40 = .01, P = .96), and a trend toward less severe positive symptoms (F1,40 = 3.10, P = .09). Both DS and ND patients had a higher proportion of male than female subjects (DS: % male = 60%; ND: % male = 69.2%) and a higher frequency of never having been married than having been married. Additional details regarding the demographic and clinical characteristics of the samples can be found in Strauss et al.39 These clinical and demographic characteristics are consistent with the original DS conceptualization.1
Smell Identification Accuracy Analyses
Analysis of covariance (ANCOVA), with number of cigarettes smoked per day and gender included as covariates, was used to determine if there were significant differences in smell identification accuracy among the three groups. The B-SIT total recognition scores were used as the dependent variable in this analysis. Statistically significant differences in smell identification accuracy were present among the groups, F2,60 = 11.77, P < .001. Means and SDs for total B-SIT scores were DS: M = 7.2 (SD = 1.78); ND: M = 9.6 (SD = 1.93); CN: M = 10.2 (SD = 1.04). Post hoc Tukey tests indicated that DS performed significantly worse than ND and CN (P < .001); however, differences between ND patients and CN were nonsignificant (P = .56).
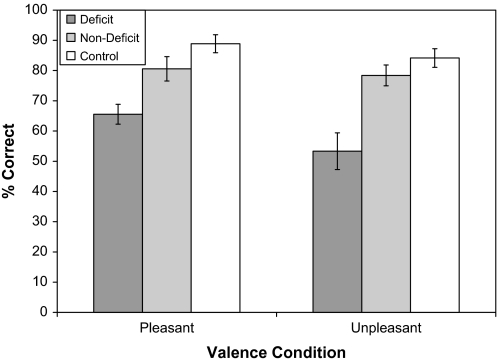
Repeated-measures ANCOVA was then conducted with sex and number of cigarettes smoked per day included as covariates to determine whether there were group differences in the identification of pleasant and unpleasant odors. Contrary to hypotheses, the valence × group interaction was not significant, F2,60 = 0.99, P = .38. The main effect for valence was significant, F1,60 = 3.63, P = .04 (η2 = 0.09), which can be attributed to all groups displaying greater accuracy for pleasant than unpleasant odors. There was also a significant between-subjects effect for group, F2,60 = 12.83, P < .001 (η2 = 0.33), which reflects that CN displayed the highest performance, followed by ND, and then DS (see figure 1). As can be seen in figure 1, results indicated that (1) patients do worse than controls on the identification of B-SIT items for both pleasant and unpleasant odors and (2) all three groups show a similar pattern of performance on the B-SIT with regard to identification of pleasant and unpleasant odors.
Fig. 1.
Means and SEs for Olfactory Identification Performance on Pleasant and Unpleasant Smell Items Among Deficit, Nondeficit, and Control Groups.
Valence Judgment Analyses
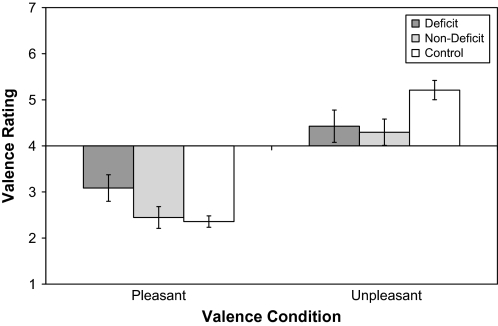
Repeated-measures ANCOVA was conducted with sex and the number of cigarettes smoked per day included as covariates to determine whether there were differences in valence judgment among groups. Results indicated a significant valence × group interaction, F2,60 = 3.98, P = .04 (η2 = 0.12), which is presented in figure 2. Tukey post hoc analyses further indicated that these differences were attributed to DS patients rating pleasant items as being less pleasant than CN and ND patients (P < .05), while ND patients and CN did not differ in pleasantness ratings. DS (P = .19) and ND (P = .07) also showed a trend toward rating unpleasant items as being less unpleasant than CN, but DS and ND did not differ in rating unpleasant items (P = .94). These results indicate that both DS and ND patients judge unpleasant items as being less unpleasant than CN. However, DS patients display a unique impairment in that they judge pleasant items as being less pleasant than either the ND or CN subjects.
Fig. 2.
Means and SEs for Olfactory Valence Judgment on Pleasant and Unpleasant Smell Items Among Deficit, Nondeficit, and Control Groups.
Because valence scores do not reflect a linear construct given that they simultaneously measure pleasantness, unpleasantness, and neutrality, it is impossible to determine whether the finding that DS patients judge pleasant items to be less pleasant reflects increasing unpleasant scores, decreasing pleasant scores, or increasing/decreasing neutrality. To address this issue, valence ratings were trichotomized into pleasant (score = 1–2), neutral (score = 3–5), and unpleasant ranges (score = 6–7), and group differences were examined in a 2 (item valence: pleasant, unpleasant) × 3 (valence interval: pleasant, neutral, unpleasant) × 3 (group: DS, ND, CN) repeated-measures ANCOVA. Sex and number of cigarettes smoked per day were included as covariates. ANCOVA indicated a significant item valence × valence interval × group interaction, F4,110 = 3.18, P = .01. Tukey post hoc analyses were used to further examine the interaction effects. For pleasant smells, these analyses indicated that (1) the DS patients rated significantly fewer pleasant items in the pleasant range than did the ND and CN groups (P < .05), (2) the DS, ND, and CN groups did not significantly differ in the frequency with which they rated pleasant items in the neutral range (P = .19), and (3) the DS patients were more likely to rate pleasant items as falling in the unpleasant range than the ND and CN groups (P < .05). With regard to unpleasant items, (1) DS and ND patients both rated unpleasant items as being in the pleasant range more often than CN (P < .05) but did not differ from each other (P = .62), (2) both DS and ND patients were less likely to rate unpleasant items in the unpleasant range than CN (P < .05) but did not differ from each other (P = .92), and (3) DS, ND, and CN did not differ in the frequency with which they judged unpleasant items as falling in the neutral range (P > .60). Thus, the DS group differed from the ND group in their ratings of pleasant smells but not unpleasant smells.
One-way ANOVAs were also conducted to examine group differences in valence rating across all B-SIT items (i.e., collapsing across both pleasant and unpleasant items). Results indicated significant differences among the groups with regard to the percentage of responses in the pleasant range, F2,60 = 2.19, P < .05; however, no differences were found for the neutral and unpleasant ranges. Post hoc Tukey analyses indicate that group differences in the total percentage of B-SIT responses falling in the pleasant range can be attributed to DS patients rating significantly fewer items in the pleasant range than ND patients (P < .05) (CN = not significant). Means and SDs are presented for the trichotimized and bipolar valence ratings in table 1.
Table 1.
Means and SDs for Olfactory Valence Conditions Among Deficit, Nondeficit, and Control Groups
| DS (n = 15) |
ND (n = 26) |
CN (n = 22) |
||||
| Mean | SD | Mean | SD | Mean | SD | |
| Bipolar valence ratingsa | ||||||
| Pleasant | 3.1 | 1.1 | 2.5 | 1.2 | 2.4 | 0.5 |
| Unpleasant | 4.4 | 1.4 | 4.3 | 1.4 | 5.2 | 0.9 |
| Total | 3.6 | 0.9 | 3.2 | 1.1 | 3.5 | 0.4 |
| Trichotimized valence ratingsb | ||||||
| Pleasant items | ||||||
| % Rated as Pleasant | 45.7 | 25.0 | 66.3 | 21.2 | 66.9 | 12.8 |
| % Rated as Neutral | 39.0 | 28.2 | 27.4 | 18.7 | 32.1 | 11.5 |
| % Rated as Unpleasant | 15.3 | 9.1 | 6.3 | 8.4 | 1.0 | 03.3 |
| Unpleasant items | ||||||
| % Rated as Pleasant | 18.7 | 18.2 | 23.2 | 18.3 | 1.0 | 6.1 |
| % Rated as Neutral | 48.0 | 30.1 | 44.8 | 31.9 | 49.0 | 28.2 |
| % Rated as Unpleasant | 33.3 | 25.8 | 32.0 | 24.7 | 50.0 | 18.8 |
| Across all items | ||||||
| % Rated as Pleasant | 36.7 | 19.3 | 53.0 | 18.2 | 43.6 | 16.4 |
| % Rated as Neutral | 42.8 | 21.8 | 30.0 | 18.9 | 35.2 | 19.9 |
| % Rated as Unpleasant | 20.5 | 18.8 | 17.0 | 15.3 | 21.2 | 14.5 |
Note: DS, deficit syndrome; ND, nondeficit syndrome; CN, control.
Data represent means and SDs for raw scores on a 7-point scale (1 = extremely pleasant and 7 = extremely unpleasant).
Data represent the percentage of pleasant, unpleasant, and total BSIT items rated as falling in pleasant (score 1–2), neutral (score 3–5), and unpleasant (score 6–7) ranges when the valence scale was trichotimized.
Associations Between DS Symptoms and Olfactory Performance
Spearman correlations were also calculated to determine associations between B-SIT performance and SDS symptom variables. On account of the relatively small sample of DS patients, analyses were not conducted separately for DS and ND patients but rather for the whole sample. Results indicated that B-SIT total scores were significantly correlated with 5 of the 6 individual symptom domains (not diminished emotional range). Pleasant item accuracy was most significantly correlated with poverty of speech and curbed interest. Unpleasant item accuracy was significantly correlated with all symptom domains except diminished emotional range. Thus, more severe symptoms were associated with poorer accuracy on both pleasant and unpleasant items, but diminished emotional range, sense of purpose, and social drive were less associated with identifying pleasant odors.
With regard to valence judgment using raw scores on the bipolar 1–7 scale, results indicated significant correlations among valence judgment for pleasant odors and emotional range, curbed interest, sense of purpose, and social drive, indicating that the tendency to rate items as being less pleasant is associated with more severe negative symptoms. Judgment of unpleasant items was not significantly correlated with SDS symptoms. Spearman correlations were also calculated between the 6 SDS scales and trichotimized valence ratings. Results indicated that the percentage of pleasant items rated as pleasant was negatively correlated with SDS scales of emotional range, sense of purpose, and social drive, suggesting that more severe negative symptoms are associated with decreased likelihood of rating pleasant items as highly pleasant. The percentage of pleasant items rated as unpleasant was positively correlated with SDS symptoms of emotional range, curbed interests, and sense of purpose, suggesting that a tendency to judge pleasant items as unpleasant is associated with more severe negative symptoms. The percentage of pleasant items rated as neutral was not significantly correlated with SDS items. Similarly, unpleasant items were not significantly correlated with SDS symptom ratings (see table 2). It is important to note that these correlational analyses were exploratory and that many of these associations reported would not survive Bonferroni correction for multiple comparisons.
Table 2.
Correlations Among Olfactory Performance Measures and Symptom Ratings
| Restricted Affect | Emotional Range | Poverty of Speech | Curbed Interests | Sense of Purpose | Social Drive | |
| Accuracy | ||||||
| B-SIT total | −0.29* | −0.20 | −0.32* | −0.37* | −0.31* | −0.38** |
| Pleasant item accuracy | −0.18 | 0.03 | −0.24* | −0.24* | −0.12 | −0.16 |
| Unpleasant item accuracy | −0.37** | −0.15 | −0.36** | −0.42** | −0.29* | −0.27* |
| Bipolar valence ratingsa | ||||||
| Pleasant item valence | 0.19 | 0.50** | 0.11 | 0.25* | 0.28* | 0.36** |
| Unpleasant item valence | −0.01 | 0.09 | −0.02 | −0.03 | 0.01 | −0.08 |
| Trichotimized valence ratingsb | ||||||
| Pleasant items | ||||||
| % Rated as Pleasant | −0.18 | −0.35** | −0.17 | −0.22 | −0.25* | −0.26* |
| % Rated as Neutral | 0.16 | 0.16 | 0.11 | −0.02 | 0.10 | 0.09 |
| % Rated as Unpleasant | 0.13 | 0.31* | 0.23 | 0.44** | 0.32* | 0.24 |
| Unpleasant items | ||||||
| % Rated as Pleasant | −0.06 | −0.17 | −0.05 | 0.08 | −0.02 | 0.01 |
| % Rated as Neutral | 0.13 | 0.15 | −0.01 | −0.06 | 0.13 | 0.03 |
| % Rated as Unpleasant | −0.06 | −0.01 | −0.04 | −0.01 | −0.07 | −0.08 |
| Across all items | ||||||
| % Rated as Pleasant | −0.15 | −0.35** | −0.10 | −0.16 | −0.22 | −0.19 |
| % Rated as Neutral | 0.13 | 0.14 | 0.03 | −0.08 | 0.10 | 0.03 |
| % Rated as Unpleasant | −0.02 | 0.12 | 0.01 | 0.14 | 0.06 | 0.04 |
Note: B-SIT, Brief Smell Identification Test.
Data represent means and SDs for raw scores on a 7-point scale (1 = extremely pleasant and 7 = extremely unpleasant).
Data represent the percentage of pleasant, unpleasant, and total BSIT items rated as falling in pleasant (score 1–2), neutral (score 3–5), and unpleasant (score 6–7) ranges when the valence scale was trichotimized.
*P < .05; **P < .01.
Discussion
Consistent with previous studies, results indicate that DS patients evidenced significantly poorer olfactory identification performance than ND patients and CN.11,21,26,35,36,40 These findings also extend the work of previous studies indicating that negative symptoms defined more broadly are associated with poor olfactory perception and acuity.41–44 Results extend previous research on olfaction in DS schizophrenia by separating out individual B-SIT items to examine potential differences in accurately identifying and judging the valence of pleasant and unpleasant odors. Relative to ND patients and CN, DS patients were found to identify both pleasant and unpleasant items with less accuracy. This finding of diminished accuracy for both odor categories was specific to DS patients because ND patients and CN did not differ in relation to identifying either pleasant or unpleasant items. Thus, DS patients were simply poorer on identifying both affective categories, and data did not indicate a differential deficit in identifying pleasant and unpleasant items.
However, a differential deficit was found when individual items were examined in relation to valence judgment. When asked to rate individual B-SIT items for pleasantness on a 7-point scale, DS patients were uniquely found to rate pleasant items as significantly less pleasant than CN and ND patients. No differences were found with regard to judging unpleasant smells because DS, ND, and CN subjects provided similar valence ratings for unpleasant smells. Furthermore, when valence ratings were trichotomized into pleasant, neutral, and unpleasant ranges, and group differences were examined in relation to the percentage of responses falling into these 3 ranges, several interesting findings emerged. First, when the percentage of items rated as falling into pleasant, neutral, and unpleasant ranges were analyzed across all B-SIT items (ie, collapsing across pleasant and unpleasant items), DS patients rated significantly fewer items as falling in the pleasant range than ND and CN subjects. However, no differences were found across groups with regard to the percentage of items rated in neutral and unpleasant ranges. Second, when only the pleasant items were analyzed, results indicated that DS patients were less likely to rate pleasant items as falling in the pleasant range than ND and CN subjects. While abnormal valence judgment is consistent with previous studies examining olfactory hedonics in patients with schizophrenia22–25 (however, see Rupp et al45), these findings provide the first evidence, suggesting that valence judgment may be more abnormal in, or even selective to, DS patients. Findings also point to a reduced responsiveness to positively valenced stimuli, which is consistent with our previous study on automatic attention bias in this sample of DS patients, which showed a tendency for DS patients to have less automatic responsiveness to positive words.39 Furthermore, the DS, ND, and CN did not significantly differ in the frequency with which pleasant items were rated as falling in the neutral range. However, the DS patients more often judged these pleasant items as being in the unpleasant range than did the ND or CN groups. This finding is somewhat consistent with a recent meta-analysis of emotional experience conducted by Cohen and Minor,46 which found that across 26 published studies, schizophrenia patients were significantly more likely than controls to judge pleasant and neutral stimuli as aversive. As suggested by Cohen and Minor,46 an aversive reaction to pleasant stimuli may take place when evaluating stimuli that have simultaneous pleasant and unpleasant properties. Upon closer inspection of our data, we did not find evidence of any individual pleasant stimuli that were more likely to be rated in the aversive range than others. However, when we examined the relationship between accuracy on pleasant items and the number of pleasant items rated as having valence that fell within the unpleasant range across all patients, we did observe a significant negative association (r = −.33, P = .024), suggesting that when patients are unable to correctly identify the name of a pleasant odor they are also more likely to experience the valence of that pleasant item as aversive. This may suggest that ambiguous stimuli may induce negative affect in schizophrenia patients. Because a bipolar valence scale was used in the current study, it is impossible to say whether these ambiguous stimuli are indeed responded to with ambivalence or the co-occurrence of positive and aversive emotional states. Alternatively, this finding may simply reflect that the DS patients are more prone to making valence judgment errors in general, which may result from generally poorer cognitive functioning. Future studies are needed that use both bipolar scales (such as the one used in the current study) and unipolar scales to obtain separate pleasant and unpleasant ratings on pleasant stimuli to directly address the issue of ambivalence and rule out the possibility that the tendency to rate pleasant stimuli as aversive is not simply reflective of systematic rating errors that occur because of rating scale parameters and cognitive deficits present in patients with high negative symptoms.
When trichotomized ratings for unpleasant items were analyzed, DS and ND patients did not differ in how frequently they judged items as falling within pleasant, neutral, and unpleasant ranges; however, both patient groups rated unpleasant items as falling in the pleasant range more frequently than CN and rated fewer unpleasant items as falling in the unpleasant range than CN. Again, these abnormal valence judgments are consistent with previous studies examining olfactory hedonics in patients with schizophrenia.22–25
Correlations calculated between deficit symptoms and olfactory measures provided additional insight into the nature of olfactory dysfunction in schizophrenia. Results indicated that poorer olfactory identification is associated with more severe deficit symptoms and that this pattern is generally consistent across both pleasant and unpleasant items. SDS symptoms of curbed interest, sense of purpose, restricted affect, poverty of speech, and social drive showed a higher correlation with unpleasant than pleasant item accuracy, suggesting that hard-to-identify unpleasant items are more easily identified as the severity of these symptoms decreases. However, because correlational analyses were exploratory and Bonferroni correction for multiple comparisons was not applied, significant relationships should be interpreted with this limitation.
In contrast to accuracy ratings, valence judgment ratings for unpleasant items were unrelated to deficit symptomatology. However, pleasant item ratings were highly correlated with diminished emotional range, suggesting that as the percentage of pleasant items rated as pleasant increases, diminished emotional experience also decreases. Similarly, as the percentage of pleasant items rated as unpleasant increases, the severity of diminished emotional experience also increases. These findings are consistent with original conceptualizations of the DS, in as much as diminished emotional range interferes with the ability to assign appropriate valence ratings to positive emotions and, by extension, the ability to normally experience these pleasant stimuli. Because all the DS and ND groups were combined for these correlation analyses (small n precluded separate analyses for DS and ND groups) these findings should not be viewed as specifically pertaining to the DS. Whether similar patterns of correlations are present in DS and ND patients will need to be addressed in future studies with larger groups of patients.
Given that the neural mechanisms underlying olfactory identification and valence judgment are well delineated, these behavioral results also provide some insight into the neural mechanisms underlying DS schizophrenia. Using PET, Crespo-Facorro et al24 found that in patients with schizophrenia, regions central to evaluating pleasant smells in the prefrontal cortex were recruited for decoding unpleasant smells, which may have occurred as a compensatory mechanism due to reduced activation in limbic structures that are typically active in the evaluation of negative stimuli. Volumetric imaging data do not support subcortical structural abnormalities in the amygdala and hippocampus,43 suggesting that these abnormalities are functional in nature. Furthermore, functional abnormalities identified in previous research are consistent with studies examining general (ie, nonhedonic) olfactory perception, indicating reduced metabolism in the inferior frontal gyrus and orbitofrontal cortex.47–49 When the current behavioral findings are viewed in relation to results reported by previous imaging studies, valence-related abnormalities reported here may suggest that dysfunctional limbic and frontal circuitry, possibly within the amygdala and orbitofrontal cortex, are related to the neuropathology of the DS. Additionally, given that social affiliation is thought to be related to olfaction, and that social and olfactory impairments may share an underlying neuropathology,26 findings may shed light onto the biological underpinnings of social abnormalities in schizophrenia. Associations between olfactory performance and social drive reported by this and other studies support this notion and suggest that investigation of the neurobiological substrates of social drive impairment is warranted.21,26
The current findings should be viewed with certain limitations. First, the B-SIT, although sensitive to differentiating DS and ND patients in this and prior studies,36 provides only a limited range of olfactory perception. As such, future studies may benefit from using more sensitive olfactory measures to further examine the nature of the valence-related abnormalities reported here. Using disparate pleasant and unpleasant odors introduces other confounds when judging odor valence, such as basal intensity, familiarity, and chemical composition differences. It is therefore unclear as to whether use of a different chemical make-up in olfactory stimuli may result in different findings. It is also unknown whether odor familiarity influences valence judgment and whether these factors might differentially influence judgment among DS and ND patients. Finally, the moderately small sample size and subsequently reduced power further suggests that replication is needed, and neuroimaging studies are required to directly assess olfactory valence perception in DS patients before neuroanatomical conclusions can be substantiated. Nonetheless, findings indicate that the DS is associated with a unique pattern of olfactory performance, providing further evidence that the DS represents a distinct group of schizophrenia patients with prominent affective disturbance.
Acknowledgments
We would like to acknowledge the patients and staff at Mojave Adult, Child, and Family Services, as well as members of the Neuropsychology Research Program at the University of Nevada Las Vegas who assisted in completion of this study: Tara Hoyt, Shaida Jetha, Sally Barney, Christ Timko, Lucy Kamalani, Lisa Barnes, Amanda Villamar, and Steven Pace. Research was supported by an internal grant to D.N. Allen through the University of Nevada Las Vegas.
References
- 1.Carpenter WT, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: The concept. Am J Psychiatry. 1998;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- 2.Kirkpatrick B, Buchanan RW, Ross DE, et al. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 3.Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan RW, Breier A, Kirkpatrick B, et al. Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry. 1998;155:751–760. doi: 10.1176/ajp.155.6.751. [DOI] [PubMed] [Google Scholar]
- 5.Liberman RP, Mintz J, Zarate R. Comparison of efficacy of social skills training for deficit and nondeficit negative symptoms in schizophrenia. Am J Psychiatry. 1997;154:424–425. doi: 10.1176/ajp.154.3.424. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan RW, Strauss ME, Kirkpatrick B, et al. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry. 1994;51:804–811. doi: 10.1001/archpsyc.1994.03950100052005. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan RW, Kirkpatrick B, Heinrichs DW. Clinical correlates of deficit syndrome of schizophrenia. Am J Psychiatry. 1990;147:290–294. doi: 10.1176/ajp.147.3.290. [DOI] [PubMed] [Google Scholar]
- 8.Tamminga CA, Thaker GK, Buchanan R, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 9.Heckers S, Goff D, Schacter DL, et al. Functional imaging of memory retrieval in deficit vs. nondeficit schizophrenia. Arch Gen Psychiatry. 1999;56:1117–1123. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- 10.Lahti AC, Holcomb HH, Medeoff DR, et al. Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry. 2001;158:1797. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- 11.Seckinger RA, Goudsmit N, Coleman E, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res. 2004;69:55. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 12.Bryson G, Whelahan HA, Bell M. Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res. 2001;102:29–37. doi: 10.1016/s0165-1781(01)00245-1. [DOI] [PubMed] [Google Scholar]
- 13.Tiryaki A, Yazici KM, Anil EA. Reexamination of the characteristics of the deficit schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2003;253:221. doi: 10.1007/s00406-003-0434-5. [DOI] [PubMed] [Google Scholar]
- 14.Galderisi S, Maj M, Mucci A, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. 2002;159:983. doi: 10.1176/appi.ajp.159.6.983. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan RW, Strauss ME, Breier A, et al. Attentional impairments in deficit and nondeficit forms of schizophrenia. Am J Psychiatry. 1997;154:363–370. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- 16.Horan WP, Blanchard JJ. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr Res. 2003;65:125–137. doi: 10.1016/s0920-9964(02)00410-3. [DOI] [PubMed] [Google Scholar]
- 17.Brazo P, Marié RM, Halbecq I. Cognitive patterns in subtypes of schizophrenia. Eur Psychiatry. 2002;17:155. doi: 10.1016/s0924-9338(02)00648-x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen AS, Docherty NM. Negative vs. deficit syndrome: prediction of attentional impairment. Schizophr Bull. 2004;30:827–835. doi: 10.1093/oxfordjournals.schbul.a007135. [DOI] [PubMed] [Google Scholar]
- 19.Putnam KM, Harvey PD. Cognitive impairment and enduring negative symptoms: a comparative study of geriatric and nongeriatric schizophrenia patients. Schizophr Bull. 2000;26:867. doi: 10.1093/oxfordjournals.schbul.a033501. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AS, Saperstein AM, Gold JM, et al. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33:1201–1212. doi: 10.1093/schbul/sbl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malaspina D, Coleman E, Goetz RR. Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry. 2002;51:809–815. doi: 10.1016/s0006-3223(01)01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudry J, Saoud M, D'Amato T, et al. Ratings of different olfactory judgements in schizophrenia. Chem Sens. 2002;27:407–416. doi: 10.1093/chemse/27.5.407. [DOI] [PubMed] [Google Scholar]
- 23.Moberg PJ, Arnold SE, Doty RL. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160:1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- 24.Crespo-Facorro B, Paradiso S, Andreasen NC. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 25.Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophr Res. 2006;81:317–319. doi: 10.1016/j.schres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV–TR Axis I Disorders—Patient Edition (SCID-I/P 2/2001 Revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 29.Kirkpatrick B, Buchanan RW, McKenney PD, et al. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:19–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 30.Frantom LV, Allen DN, Cross C. Neurocognitive endophenotypes for bipolar disorder. Bipolar Disord. 2008;10:387–399. doi: 10.1111/j.1399-5618.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 31.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 32.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 33.Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976. pp. 218–222. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scales—Third Edition. Australia: Harcourt Assessment; 1997. [Google Scholar]
- 35.Doty RL, Marcus A, Lew WWL. Development of the 12-item cross-cultural smell identification test (CC-SIT) Laryngoscope. 1996;106:353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 36.Goudsmit N, Coleman E, Seckinger RA, et al. A Brief Smell Identification Test discriminates between deficit and non-deficit schizophrenia. Psychiatry Res. 2003;120:155–164. doi: 10.1016/s0165-1781(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 37.Doty RL. The Brief Smell Identification Test Administration Manual. Philadelphia, PA: Sensonics, Inc.; 2001. [Google Scholar]
- 38.Doty RL, Shamam P, Dann W. Development of the University of the Pennsylvania Smell Test standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–495. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 39.Strauss GP, Allen DN, Duke LA, et al. Automatic affective processing impairments in patients with deficit syndrome schizophrenia. Schizophr Res. 2008;102:76–87. doi: 10.1016/j.schres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Moberg PJ, Arnold SE, Doty RL. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 41.Brewer WJ, Edwards J, Anderson V, et al. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021–1031. doi: 10.1016/0006-3223(95)00594-3. [DOI] [PubMed] [Google Scholar]
- 42.Geddes J, Huws R, Pratt P. Olfactory acuity in the positive and negative syndromes of schizophrenia. Biol Psychiatry. 1991;29:774–778. doi: 10.1016/0006-3223(91)90196-s. [DOI] [PubMed] [Google Scholar]
- 43.Corcoran C, Whitaker A, Coleman E, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283–93. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brewer WJ, Wood SJ, Pantelis C, et al. Olfactory sensitivity through the course of psychosis: relationships to olfactory identification, symptomatology and the schizophrenia odour. Psychiatry Res. 2007;149:97–104. doi: 10.1016/j.psychres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Rupp CI, Fleischhacker WW, Kemmler G. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74:149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Cohen AS, Minor KS. Emotional experience in schizophrenia patients revisited: meta-analysis of laboratory studies. Schizophr Bull. doi: 10.1093/schbul/sbn061. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark C, Kopala L, Hurwitz T, Li D. Regional metabolism in microsmic patients with schizophrenia. Can J Psychiatry. 1991;36:645–650. doi: 10.1177/070674379103600904. [DOI] [PubMed] [Google Scholar]
- 48.Wu J, Buchsbaum MS, Moy K, et al. Olfactory memory in unmedicated schizophrenics. Schizophr Res. 1993;9:41–47. doi: 10.1016/0920-9964(93)90008-7. [DOI] [PubMed] [Google Scholar]
- 49.Bertollo DN, Cowen MA, Levy AV. Hypometabolism in olfactory cortical projection areas of male patients with schizophrenia: an initial positron emission tomography study. Psychiatry Res. 1996;60:113–116. doi: 10.1016/0165-1781(96)02619-4. [DOI] [PubMed] [Google Scholar]