Abstract
We recently showed that, in healthy individuals, emotional expression influences memory for faces both in terms of accuracy and, critically, in memory response bias (tendency to classify stimuli as previously seen or not, regardless of whether this was the case). Although schizophrenia has been shown to be associated with deficit in episodic memory and emotional processing, the relation between these processes in this population remains unclear. Here, we used our previously validated paradigm to directly investigate the modulation of emotion on memory recognition. Twenty patients with schizophrenia and matched healthy controls completed functional magnetic resonance imaging (fMRI) study of recognition memory of happy, sad, and neutral faces. Brain activity associated with the response bias was obtained by correlating this measure with the contrast subjective old (ie, hits and false alarms) minus subjective new (misses and correct rejections) for sad and happy expressions. Although patients exhibited an overall lower memory performance than controls, they showed the same effects of emotion on memory, both in terms of accuracy and bias. For sad faces, the similar behavioral pattern between groups was mirrored by a largely overlapping neural network, mostly involved in familiarity-based judgments (eg, parahippocampal gyrus). In contrast, controls activated a much larger set of regions for happy faces, including areas thought to underlie recollection-based memory retrieval (eg, superior frontal gyrus and hippocampus) and in novelty detection (eg, amygdala). This study demonstrates that, despite an overall lower memory accuracy, emotional memory is intact in schizophrenia, although emotion-specific differences in brain activation exist, possibly reflecting different strategies.
Keywords: amygdala, faces, facial expression, functional neuroimaging, sad, happy, symptomatology, recollection, familiarity
Introduction
Emotional dysfunction has long been considered a hallmark of schizophrenia.1 This deficit can have significant consequences for many aspects of an individual’s daily life. In particular, they may interfere with the successful formation of associations between relevant environmental cues and their emotional significance or salience.2 Indeed, it is now generally accepted that emotion can help cognition by prioritizing biologically relevant information.3 Specifically, when the information is appropriately associated with its emotional context, it can guide future behavioral responses by referring to these learned associations in memory. Incorrect storage of associations between emotion and environmental information could, however, lead an individual to assign inappropriate emotional significance to a situation, by referring to these aberrant memories, thus resulting in severe impairments in his/her social interactions.
A few studies have directly investigated the influence of emotion on memory performance in schizophrenia but have so far yielded conflicting findings. Namely, whereas some have reported group differences in the modulation of memory accuracy by emotion,4,5 others found that, despite an overall lower performance in patients, the effect of emotion on accuracy was similar for both groups.6,7 Given the differences in the task (eg, recognition vs recall), response type (old/new vs remember/know), time of testing (eg, immediate vs delayed), and material (eg, words vs pictures), it is difficult to draw any definite conclusions from these studies in terms of memory deficits associated with emotional stimuli.
Importantly, emotion can modulate recognition memory not only in terms of accuracy but also in relation to response bias; ie, the overall tendency to judge stimuli as previously seen or not, regardless of whether that was the case or not.8,9 Specifically, whereas accuracy is measured by the rate of hits minus false alarms, false memories are assessed by the response bias, which measures the tendency to incorrectly classify a new item as previously seen (corrected for overall accuracy). Notably, these 2 aspects of memory are largely independent29. For example, for a recognition test with an equal proportion of old and new stimuli, completely random responses would correspond to chance accuracy and no response bias, whereas a classification of all stimuli as previously seen (saying always “old”) would also result in a chance level for accuracy but would correspond to an extreme value of familiarity bias.
Facial expressions constitute one class of emotional stimulus that is critical for conveying socially relevant information such as others’ social intentions and motivations,10,11 which have been shown to be an important component of the social dysfunction associated with schizophrenia (for reviews, see12,13). We recently conducted a series of studies to investigate the influence of emotional expression on recognition memory for faces in healthy individuals.14,15 In these previous studies, we observed that, as expected with negatively valenced stimuli,16,17 fearful faces were associated with a better accuracy compared with neutral and happy expressions. In contrast, and despite their negative valence, sad faces were associated with decreased memory accuracy. Critically, this effect was due to a significant familiarity bias. On the other hand, a tendency to classify faces as never seen before (novelty bias), in the absence of any changes in accuracy, was observed for happy expressions. Interestingly, behavioral novelty bias responses for both happy and sad expressions significantly correlated with amygdala activity, consistent with the proposed role of this region in emotional processing and novelty detection. Importantly, we ensured that these differences in memory accuracy and bias between expressions were unlikely to be due to differences in the physical features of the stimuli by directly comparing the stimuli through an objective measure of physical similarity (ie, eigenface analysis).14
Taken together, these findings show that the influence of emotion on memory depends not only on stimulus valence or arousal but also on a combination of these 2 dimensions consistent with the circumplex model which posits that individual emotions can be represented as points in a 2-dimensional emotion space, with valence and arousal (or intensity) as the main orthogonal axes.18,19 Furthermore, emotional expressions were shown to independently modulate memory accuracy and response bias. Thus, these results highlight the need to separate individual emotions when exploring their influence on memory, as well as the importance of measuring their possible effects on both accuracy and response bias. The fact that many of the previous studies of emotional memory in schizophrenia combined different negative emotions, often including fear and sadness, may have provided yet another confounding factor, in addition to those mentioned earlier, which could have contributed to the inconsistency in the results obtained. For example, sad faces are associated with similar negative valence as fearful ones but with different levels of arousal.19
Therefore, the goal of this fMRI study was to explore the influence of emotion on recognition memory in individuals with schizophrenia using a previously validated paradigm.14,15 In particular, we investigated the effects of specific emotions, namely sadness and happiness, on accuracy and response bias, 2 complementary indices of memory performance. Our specific objective was to determine whether the previously observed effects of these expressions on response bias in healthy individuals were also present in people suffering from schizophrenia. Importantly, the opposing effects of these 2 emotions on response bias allowed us to specifically identify any differences between groups in their influence on memory above and beyond the expected overall deficits in memory in the patient group. Furthermore, direct statistical comparisons between groups allowed us to assess to what extent the relation between behavioral and neural indices of this memory bias was similar between patients and controls as a function of emotional expression. We predicted that people with schizophrenia would present an overall reduced memory accuracy. On the other hand, we hypothesized that, in agreement with previous studies,4,20 emotional expressions would have a similar influence on memory response bias in patients as in healthy individuals.15 In terms of neural correlates, we anticipated group differences in regions within the prefrontal cortex and medial temporal lobes (amygdala, hippocampus, and parahippocampal gyrus) because several studies have already highlighted structural differences21–23 as well as abnormal activity of these regions in memory tasks, particularly when distinguishing between conscious recollection and familiarity-based recognition.24
Methods
Subjects
Twenty-five healthy individuals and 30 outpatients with Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV)–defined schizophrenia participated in the study. Five control subjects and 10 patients were excluded from the final analysis due to excessive movement during the scan (>3 mm, patients = 3, controls = 4), poor behavioral performance (<50%, patients = 5, controls = 1), or inability to complete the session (patients = 2). Demographic and clinical characteristics of the patients included in the final analysis are shown in Table 1. Diagnoses were made by the treating psychiatrist and confirmed by a psychologist from the outpatient clinic from the Douglas Mental Health University Institute using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, and by medical records. All patients were administered the Scale for the Assessment of Positive Symptoms25 and the Scale for the Assessment of Negative Symptoms.26 In addition, patients completed the Wechsler Abbreviated Scale of Intelligence. At the time of assessment, all patients had been clinically stable for at least 4 weeks and had been on a fixed medication regimen for at least 6 weeks. None of the patients had a concurrent mood disorder at the time of testing.
Table 1.
Demographic and Clinical Characteristics of the Groups
| Schizophrenia Group |
Controls Group |
P Value (2 Tailed) | |||||
| Mean | SD | Range | Mean | SD | Range | ||
| Age | 31.8 | 7.4 | 19–45 | 28.5 | 6.0 | 18–42 | 0.14 |
| WASI full-scale IQ | 100.6 | 15.3 | 67–123 | 106.1 | 11.3 | 82–126 | 0.21 |
| n | % | n | % | ||||
| Sex | 0.72 | ||||||
| Female | 9 | 45 | 10 | 50 | |||
| Male | 11 | 55 | 10 | 50 | |||
| Parental socioeconomic status | 0.26 | ||||||
| Upper | 2 | 10 | 3 | 15 | |||
| Upper-middle | 4 | 20 | 8 | 40 | |||
| Middle | 8 | 40 | 4 | 20 | |||
| Lower-middle | 2 | 10 | 3 | 15 | |||
| Lower | 4 | 20 | 2 | 10 | |||
| Handedness categories | 0.39 | ||||||
| Right handed | 15 | 75 | 18 | 90 | |||
| Moderately right handed | 1 | 5 | 0 | 0 | |||
| Ambidextrous handed | 2 | 10 | 1 | 5 | |||
| Moderately left handed | 1 | 5 | 0 | 0 | |||
| Left handed | 1 | 5 | 1 | 5 | |||
| Medication | — | ||||||
| Second generation antipsychotics (atypical) | 13 | 65 | — | — | — | ||
| Combination of atypical and conventional antipsychotics | 5 | 25 | — | — | — | ||
| Neuroleptic free | 2 | 10 | — | — | — | ||
| Antidepressant medication | 7 | 35 | — | — | — | ||
| Anticonvulsive medication | 2 | 10 | — | — | — | ||
| Anticholinergic medication | None | 0 | |||||
| Mean | SD | Range | Mean | SD | Range | ||
| Chlorpromazine equivalent (mg/d) | 377 | 402 | 0–1589 | — | — | — | — |
| Mean duration of illness (y) | 8.6 | 7 | 2–23 | — | — | — | |
| SAPS | 8.7 | 8.7 | 0–33 | — | — | — | — |
| SANS | 16 | 7.2 | 2–26 | — | — | — | — |
Note: WASI, Wechsler Abbreviated Scale of Intelligence; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms.
Healthy controls were screened with the Nonpatient Edition of the Structured Clinical Interview for DSM-IV Axis I Disorders to rule out current or past Axis I psychiatric disorders. They were group matched to the patients in terms of age, sex, handedness,27 primary language, and parental socioeconomical status as assessed with the Hollingshead 2-factor index of social position.28 Exclusion criteria for all participants were illicit substance or alcohol misuse within the past 2 years, a history of neurological illness, head injury, or other significant medical illness.
Stimuli
We used a previously validated set of 168 photographs of different individuals depicting sad, happy, or neutral facial expressions.15 Two equivalent subsets of stimuli were made from this set, each including 28 sad, happy, and neutral faces (half male).
Task Procedure
The experiment consisted of 2 runs, encoding and recognition. During the encoding phase, one stimulus subset was presented twice, in pseudorandom order (counterbalanced across subjects). Subjects were asked to perform a gender decision on the faces by clicking on a mouse with their right hand and also to try to remember them.
During the recognition phase, subjects were presented with the original, previously seen stimuli together with those from the other set, never seen before. Subjects were instructed to determine if each face had been previously seen or not (old/new judgment). Following the scanning session, subjects rated all the faces in terms of valence using a visual analog scale (data from 6 controls and 5 patients were not available due to technical problems).
The stimulus duration was 2.5 s. In order to achieve a good estimate of baseline activity, several so-called null events (black screen) were also interspersed pseudorandomly. Specifically, we used 2 different stimulus anset asynchronies (2.5 and 5 s) with a frequency of occurrence of 0.71 and 0.29, respectively. This resulted in a desynchronized (jittered) presentation of the stimuli with respect to the onset of volume acquisitions.
fMRI Acquisition
Scanning took place at the Montreal Neurological Institute, using a 1.5-T Siemens Sonata. Functional T2*-weighted echoplanar images were acquired (repetition time [TR] = 2540 ms, echo time [TE] = 50 ms, flip angle = 90°, field of view [FOV] = 256 mm, matrix = 64 × 64) covering the entire brain (30 interleaved slices parallel to the anterior-posterior commissural plane, voxel size 4 × 4 × 4 mm). Two functional runs of 350 volumes each were acquired (encoding and recognition). An anatomical volume was also acquired (voxel size 1 × 1 × 1 mm3). Stimulus presentation and subjects’ response recording were done with a PC laptop running E-PRIME.
Data Analysis
Behavioral Data
Memory performance was calculated according to the 2-high threshold model29 by means of the discrimination index Pr:
and the response bias Br
where H and FA represent hit and false alarm rates, respectively (the subtraction of 0.5 is not part of the original formula of the 2-high threshold model, but it was added so that an absence of bias would correspond to a value of 0). Notably, Pr and Br measures are independent29: whereas the former provides an unbiased estimate of memory accuracy, the latter is an index of the overall tendency to respond “old” or “new” regardless of accuracy. In this case, positive values indicate a familiarity bias (ie, a tendency to say old) and negative ones correspond to a novelty bias (ie, a propensity to say new).
Neuroimaging
Functional data were analyzed using SPM2. Before the analysis, images were time corrected to account for differences in sampling times for different slices, motion corrected, spatially normalized (final voxel size 2 × 2 × 2 mm), and smoothed (8 mm full-width at half maximum). We defined 12 event types based on facial expression (sad, happy, and neutral), presentation (old and new), and accuracy (correct and incorrect, based on each subjects’ performance). As in our previous study,15 we were specifically interested in assessing the relation between behavioral and neural responses associated with response bias. To do so, a linear contrast of the parameter estimates corresponding to subjective old (ie, hits and false alarms) minus subjective new (ie, misses and correct rejections) events for each expression was computed for each subject. These contrasts were then taken to a second-level random effects correlation analysis with the corresponding behavioral response bias.
Areas commonly activated by patients and controls were identified using a conjunction procedure according to the minimum statistic compared with the conjunction null hypothesis,30 by setting a threshold of 0.005 for each contrast separately, thus yielding an overall threshold of P < .0052. Group differences (eg, controls minus patients) were computed with a 2-sample t test on the corresponding contrasts. Importantly, only those voxels showing a significant correlation for one group (eg, controls) were reported (ie, inclusive masking at P = .001).
In addition, we examined the relation between contrast maps between patients and controls by computing the Tanimoto coefficient31 associated with the contrast subjective old minus new responses correlated with the behavioral response bias:
This coefficient, ranging from 0 to 1, provides a measure of the overall degree of spatial overlap (in terms of location and magnitude) in the activations of both groups. That is, T = 1 would reflect identical activation maps between groups, whereas T = 0 would represent orthogonal activation patterns. Statistical inferences (ie, P values) relative to the null distribution were obtained by using a permutation test (n = 10 000).
Results
Behavior
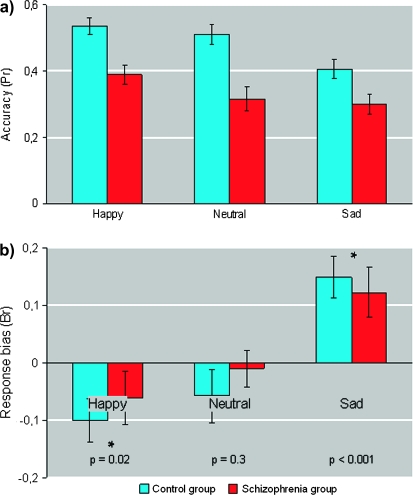
Memory accuracy (Pr) and response bias (Br) for both groups, as a function of expression are shown in figures 1a and 1b, respectively. A mixed-effect ANOVA for memory accuracy with expression (happy, sad, and neutral) as within-subject factor and group (patients and controls) as the between factor revealed a significant main effect of expression (F(2,76) = 8.1, P = .001) and group (F(1,38) = 17.6, P < .001) with no interaction between these factors (F(2,76) = 0.8, P = .4). Post hoc tests confirmed that the emotion effect was mainly due to a difference between happy and sad expressions (t[39] = 4.3, P < .001), with also a significantly better accuracy for happy compared with neutral faces (t[39] = 2.5, P = .02). The group difference was, as expected, due to an overall lower memory accuracy in the schizophrenia group.
Fig. 1.
(a) Accuracy (Pr) and (b) Memory Response Bias (Br) for Each Facial Expression (Happy, Neutral, and Sad) for Healthy Subjects (Blue) and Individuals With Schizophrenia (Red). Error bars represent one standard error of the mean. For the memory response bias, P values correspond to the comparison with 0 (ie, no response bias), as described in the “Results” section.
A similar analysis for response bias also showed a significant main effect of expression (F(2,76) = 17.5, P < .001) but no significant main effect of group (F(1,38) = 0.3, P = .6) or interaction between expression and group (F(2,76) = 0.04, P = .9). Post hoc tests confirmed a significant familiarity bias for sad faces (t[39] = 3.4, P < .001), a novelty bias for happy expressions (t[39] = 2.5, P = .02), and no significant bias for neutral ones (t[39] = 1.07, P = .3). As further support to the absence of group difference for both Pr and Br, we also performed the planned comparisons for each emotion separately and found there was no significant difference between groups for any of them (all P > .5).
A repeated-measure ANOVA of the valence ratings yielded a significant main effect of expression (F(2,54) = 335.8, P < .0001) but no main effect of group (F(1,38) = 0.1, P = .8) or interaction between expression and group (F(2,54) = 1.1, P > .3). In addition, ratings by patients and controls for each stimulus were highly correlated (r = .98, P < .0001), confirming that both groups rated all the faces similarly in terms of their emotional value.
The inclusion of IQ measures and antipsychotic medication (equivalent chlorpromazine dose) in the analyses did not have a significant effect on either Pr (IQ: F(2,54) = 1.2, P = .3; antipsychotic medication: F(2,54) = 2.5, P = .09) or Br (IQ: F(2,54) = 2.4, P = .1; antipsychotic medication: F(2,54) = 2.7, P = .08).
fMRI
Comparison Between Patients and Controls
In order to identify the brain regions associated with memory response bias, we conducted a correlation analysis between the contrasts subjective old (ie, hits and false alarms) minus subjective new (correct rejections and misses) and the behavioral memory bias response for each subject, for each expression (see “Methods” section and15). We then directly identified the similarities and differences in the activation patterns between schizophrenia patients and healthy individuals by conducting 2 independent analyses using the contrast mentioned above.
Group Similarities (Conjunction)
Table 2 shows areas commonly activated by both groups for the correlation between behavioral bias and the subjective old minus subjective new contrast for happy and sad expressions. Notably, the conjunction for sad faces revealed significant right amygdala, middle temporal gyrus, and bilateral parahippocampal gyrus activations. In the case of happy expressions, significant activations were observed in the caudate and parahippocampal gyrus.
Table 2.
Significant Activations for the Conjunction of the Control and Schizophrenia Groups for the Correlation Between the Behavioral Response Bias and the Contrast Subjective Old Minus New
| Coordinates |
|||||||
| Left |
Right |
Minimum z score | |||||
| Region | x | y | z | x | y | z | |
| Happy | |||||||
| Caudate | −12 | −26 | 24 | 3.35 | |||
| 24 | −30 | 20 | 3.45 | ||||
| Parahippocampal gyrus | −34 | −26 | −18 | 3.67 | |||
| 28 | −58 | 20 | 3.98 | ||||
| Sad | |||||||
| Middle temporal gyrus | 46 | −66 | 20 | 4.22 | |||
| Parahippocampal gyrus | 22 | −36 | −6 | 2.69 | |||
| −32 | −36 | −12 | 3.01 | ||||
| Amygdala | 24 | −6 | −26 | 2.19 | |||
Note: Coordinates of local maxima (P < .0052 uncorrected) are in Montreal Neurological Institute (MNI) space.
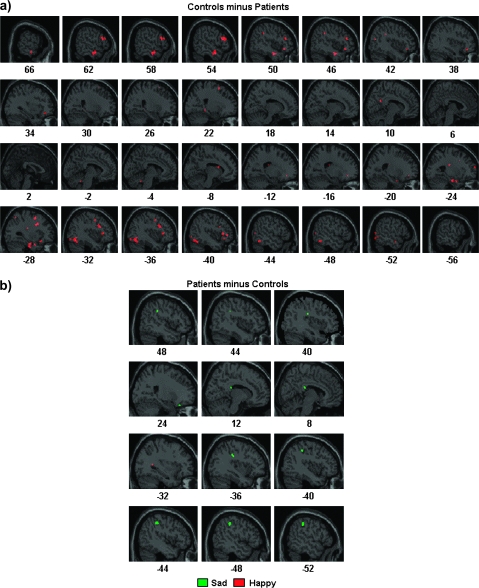
Group Differences
As shown in table 3 and figures 2 and 3, only a few regions exhibited significant differential activations between groups for sad faces confirming the similar patterns associated with memory bias for both groups. Critically, no significant amygdala activation was observed even when lowering the statistical threshold (P = .05 uncorrected). In contrast, in the case of happy faces, schizophrenia patients showed significantly less activations than healthy individuals over a widespread network, especially within the frontal lobes, as well as temporal regions, including the amygdala, hippocampus, and parahipocampal gyrus (figures 2a and 3a). Interestingly, several regions, including the amygdala, exhibited opposite patterns for controls and patients, namely significant positive and negative correlations with behavioral bias, respectively (figure 3b).
Table 3.
Significant Activations for the Group Differences in the Correlation Between the Behavioral Response Bias and the Contrast Subjective Old Minus New Responses
| Coordinates |
Correlation |
||||||||
| Left |
Right |
Group Difference | Controls | Patients | |||||
| Region | x | y | z | x | y | zz score | z score | z score | |
| Controls > patients | |||||||||
| Happy | |||||||||
| Superior frontal gyrus | −26 | 62 | 16 | 3.26 | 3.46* | 0.00 | |||
| −4 | 26 | 60 | 3.29 | 3.45* | −1.58 | ||||
| Middle frontal gyrus | 36 | 36 | −12 | 3.65 | |||||
| 54 | 32 | 20 | 4.34 | 3.59* | −3.61* | ||||
| −36 | 0 | 46 | 4.24 | 4.30* | −2.05 | ||||
| Inferior frontal gyrus | −20 | 36 | −8 | 3.29 | |||||
| 46 | 28 | −10 | 3.55 | ||||||
| −44 | 28 | −8 | 3.53 | ||||||
| 56 | 22 | 20 | 3.87 | 3.28* | −2.59 | ||||
| −32 | 22 | −16 | 4.00 | 3.41* | −3.29* | ||||
| Precentral gyrus | −32 | 4 | 32 | 4.58 | 7.07* | −1.36 | |||
| Insula | −28 | −22 | 24 | 3.41 | 3.83* | −0.43 | |||
| Superior parietal lobule | −30 | −60 | 54 | 3.25 | 4.17* | −1.17 | |||
| Inferior parietal lobule | 48 | −40 | 36 | 3.95 | 4.26* | −1.28 | |||
| Middle temporal gyrus | 52 | 0 | −20 | 4.09 | 4.59* | −1.96 | |||
| −54 | −4 | −18 | 3.38 | 3.58* | −0.89 | ||||
| −64 | −36 | −2 | 3.59 | 3.54* | −1.45 | ||||
| 42 | −70 | 18 | 3.54 | ||||||
| −54 | −70 | 8 | 3.46 | 3.44* | −1.14 | ||||
| Inferior temporal gyrus | 48 | −8 | −22 | 3.69 | 4.03* | −1.08 | |||
| 62 | −14 | −22 | 4.15 | 4.30* | −2.50 | ||||
| Fusiform gyrus | −48 | −54 | −16 | 3.74 | 3.39* | −2.17 | |||
| −40 | −60 | −18 | 3.58 | 3.31* | −1.66 | ||||
| Uncus | −30 | −16 | −34 | 3.99 | 3.59* | −2.63 | |||
| Precuneus | 10 | −54 | 26 | 3.31 | 4.08* | −0.10 | |||
| Middle occipital gyrus | −54 | −70 | −8 | 3.32 | 3.57* | −1.54 | |||
| Inferior occipital gyrus | −38 | −84 | −10 | 3.44 | 3.23* | −1.86 | |||
| Hippocampus | −28 | −14 | −24 | 4.06 | 4.43* | −1.49 | |||
| Parahippocampal gyrus | 22 | −36 | −6 | 3.38 | |||||
| Amygdala | −28 | 0 | −26 | 3.65 | 3.23* | −2.02 | |||
| 24 | −2 | −26 | 3.13 | ||||||
| 32 | −4 | −14 | 3.16 | ||||||
| Sad | |||||||||
| No significant voxels | |||||||||
| Patients > controls | |||||||||
| Happy | |||||||||
| Parahippocampal gyrus | −30 | −50 | 8 | 3.61 | −3.36* | 2.50 | |||
| Sad | |||||||||
| Inferior frontal gyrus | 26 | 26 | −22 | 3.62 | −1.57 | 4.26* | |||
| Insula | 40 | −22 | 26 | 3.52 | −1.03 | 3.71* | |||
| Postcentral gyrus | −36 | −26 | 34 | 4.09 | −3.41* | 3.29* | |||
| Inferior parietal lobule | −50 | −34 | 42 | 4.00 | −3.30* | 3.27* | |||
| Posterior cingulate | 10 | −30 | 22 | 3.81 | −1.02 | 5.25* | |||
Note: Coordinates of local maxima are in MNI space. *P < .001 (uncorrected).
Fig. 2.
Between-Group Comparisons for (a) Control > Schizophrenia and (b) Schizophrenia > Control of the Correlation Between Brain Activity Associated With Subjective Old Minus Subjective New Response and the Behavioral Response Bias for Sad (Green) and Happy (Red) Expressions. The statistical parametric map was thresholded at P < .001 and overlaid on the canonical magnetic resonance image of SPM.
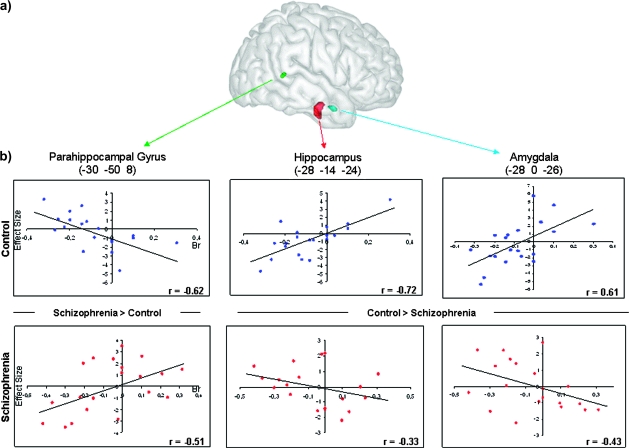
Fig. 3.
(a) Three Dimensional Rendering of the Clusters for the Amygdala (Blue), Hippocampal (Red), and Parahippocampal (Green) Activations From the Between-Group Comparisons for Happy Expressions. (b) Scatterplots of the correlations between brain activity associated with subjective old minus subjective new response and the behavioral response bias for the control (top) and schizophrenia (bottom) groups.
To further quantify the similarities and differences in the overall pattern of activations between patients and controls associated with the response bias, we computed the Tanimoto index for the activation maps corresponding to this contrast. Consistent with the conjunction and interaction analyses, the index for sad faces was TS = 0.16, which was significantly larger than the expected mean value from the null distribution (T0 = 0.10, P < .0001), indicating a substantial overlap between activations from both groups. In contrast, the Tanimoto index for happy faces, TH = 0.006 was significantly smaller than that expected by chance (T0 = 0.04, P < .0001). This value provides further evidence that the correlation between behavioral bias and the subjective old minus subjective new contrast for happy faces in controls and patients yielded activations in different, largely nonoverlapping clusters.
Discussion
Behavior
As expected, schizophrenia patients exhibited a significantly lower overall recognition memory performance than controls. The critical finding, however, was that emotional expression modulated memory performance in the same way in patients and controls, across 2 independent behavioral measures, namely accuracy and response bias.
All these indices pointed toward a tendency in all participants, both controls and patients, to classify sad faces as previously seen, regardless of whether this had been the case or not, together with a novelty bias for happy expressions. These results are in agreement with previous studies showing an intact modulation of accuracy6,7 and response bias4 by emotion in schizophrenia.
Neural Correlates
Brain activity associated with the response bias for sad faces was consistent with the behavioral findings. Indeed, patients and controls showed little difference in their activations, as determined by the conjunction and interaction analyses. The presence of a common network of activated regions, with similar effect sizes, was further confirmed by the Tanimoto index which quantifies the degree of overlap between groups in terms of location and magnitude.
In contrast, response bias for happy faces in patients was associated with a substantially different network from that observed in control subjects. This difference in activation patterns was reflected in the small number of significant voxels obtained in the conjunction analysis and the large areas showing a significantly larger activation in controls than patients. Furthermore, the Tanimoto index for happy faces was significantly smaller than the one expected by chance.
The absence of group differences on accuracy and bias for either happy or sad faces, together with the large differences in brain activations only for happy faces, is particularly intriguing. One possible interpretation of this effect relies on differences in terms of the mechanisms or strategies employed by the 2 groups, specifically with regard to familiarity (retrieval of memories based on the feeling that an item is old in the absence of confirmatory contextual information) vs recollection (retrieval of memories accompanied by the recovery of specific contextual details).32,33
In general, old responses can be based on familiarity or recollection.34,35 We have previously argued15 that the tendency to classify sad faces as previously seen is mostly based on a sense of familiarity elicited by an empathic response to these stimuli, which would bias subjects to focus more on the emotional expression and the feeling generated by them, rather than on their physical attributes. That is, participants should rely mostly on familiarity-based judgments to decide whether a sad face was new or old. Thus, given that it has been shown that individuals with schizophrenia are not impaired in familiarity-based memory,36–38 it would be expected that both groups should exhibit similar behavioral and neural patterns associated with the response bias for sad faces, as observed in our study. Consistent with this hypothesis, the between-group conjunction analysis for sad faces yielded a significant activation in the parahippocampal gyrus, a region typically considered being involved in familiarity-based memory.39
In contrast, even though happy faces resulted in similar behavioral responses in both groups, they elicited different brain activation patterns, which could reflect differences in the use of recollection- vs familiarity-based judgments between groups. Specifically, control subjects engaged a larger neural network than patients, including several regions thought to be important for processes associated with recollection-based retrieval. For example, the anterior frontal cortex and inferior parietal cortex have been proposed to be associated with recollection,40,41 as well as with the postretrieval monitoring and the evaluation of the retrieved information.40,42 In addition, activation in the superior parietal lobule has been posited to depend on the amount of information being retrieved,43 which is typically larger during recollection than familiarity-based memory retrieval. Furthermore, consistent with the hypothesized increased reliance of patients in familiarity-based retrieval, the group comparison revealed that the parahippocampal gyrus was significantly more activated in patients than controls for happy faces.
Finally, the reduced hippocampal activation observed in patients as compared with controls subjects for the response bias for happy faces is in agreement with a recent meta-analysis of neuroimaging studies of episodic memory in schizophrenia24 in which a deactivation of this region, shown to be involved in conscious recollection,32,42 was observed when comparing high vs low retrieval conditions. Importantly, although these findings have been interpreted as representing a functional deficit of this structure, the reduced activation could also reflect structural differences between patients and controls as several studies have reported a smaller hippocampal volume21,22 and/or an alteration in shape44,45 in individuals with schizophrenia.
Alternatively, the reduced activation in patients in the hippocampus and other previously mentioned regions involved in recollection, as well as the increased response in the parahippocampal gyrus, could be a consequence, rather than a cause, of their behavior. For instance, it has been suggested that schizophrenia patients are more confident in their responses than controls, even if this does not always lead to increased errors.46 Thus, it is possible that patients made their decisions based simply on a feeling of familiarity and therefore did not need to resort to more elaborate processing (ie, recollection) to obtain the necessary level of confidence to make their response.37
Importantly, although we hypothesize that control subjects relied mostly on recollection to assess whether a happy face was previously presented, they likely used familiarity judgments as well. Interestingly, the conjunction analyses for both happy and sad faces revealed a common activation in patients and controls of the parahippocampal gyrus, in agreement with this hypothesis.
Finally, the group comparison for the response bias associated to the 2 emotional expressions revealed significant difference in the amygdala activation as a function of emotion. This provides additional support for the differential effect of happy and sad expressions on response bias in schizophrenia patients compared with controls. Indeed, patients exhibited a significant amygdala activation associated with novelty bias for sad faces (see table 2), similar to that previously reported in healthy controls and consistent with a role of this structure in the detection of novel stimuli, particularly those with biological or emotional relevance.47 In contrast, no significant amygdala activation for that contrast was found for happy faces in patients, whereas a significant one was present in controls, leading to a significant interaction (see table 3 and figures 2 and 3). Importantly, this emotion-specific abnormal amygdala activation pattern argues against a generalized structural or functional deficit of this structure in schizophrenia.
In conclusion, our results have several implications for the understanding of emotional processing in schizophrenia. In particular, we have shown that differences in neural processing of emotional information between schizophrenia patients and healthy individuals can occur in the absence of behavioral differences. Therefore, reduced activations in patients in brain areas linked to emotion and/or memory should not be automatically interpreted as representing a deficit or inability to recruit these regions but may indicate use of different strategies or approaches (eg, familiarity vs recollection) to solve the task. That is, different strategies can be used depending on the particular characteristics of the stimuli being processed, in this case, their emotional expression.
Funding
Canadian Institutes of Health Research 53280.
Acknowledgments
We thank Drs Joober, Lal, Malla, and Debruille for help with patient recruitment.
References
- 1.Bleuler E. Dementia Praecox or the Group of Schizophrenia. New York, NY: International Universities Press; 1950. [Google Scholar]
- 2.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Damasio AR. Descartes’ Error. New York, NY: Avon Books; 1994. [Google Scholar]
- 4.Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol. 2007;116:43–55. doi: 10.1037/0021-843X.116.1.43. [DOI] [PubMed] [Google Scholar]
- 5.Neumann A, Blairy S, Lecompte D, Philippot P. Specificity deficit in the recollection of emotional memories in schizophrenia. Conscious Cogn. 2007;16:469–484. doi: 10.1016/j.concog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Danion JM, Kazes M, Huron C, Karchouni N. Do patients with schizophrenia consciously recollect emotional events better than neutral events? Am J Psychiatry. 2003;160:1879–1881. doi: 10.1176/appi.ajp.160.10.1879. [DOI] [PubMed] [Google Scholar]
- 7.Mathews JR. Barch: episodic memory for emotional and nonemotional words in schizophrenia. Ann N Y Acad Sci. 2004;18:721–740. [Google Scholar]
- 8.Windmann S, Urbach TP, Kutas M. Cognitive and neural mechanisms of decision biases in recognition memory. Cereb Cortex. 2002;12:808–817. doi: 10.1093/cercor/12.8.808. [DOI] [PubMed] [Google Scholar]
- 9.Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychon Bull Rev. 2007;14:423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- 10.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 11.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 12.Edwards J, Jackson HJ, Pattison PE. Emotion recognition via facial expression and affective prosody in schizophrenia: a methodological review. Clin Psychol Rev. 2002;22:789–832. doi: 10.1016/s0272-7358(02)00130-7. [DOI] [PubMed] [Google Scholar]
- 13.Mandal M, Pandey R, Prasad A. Facial expressions of emotions and schizophrenia: a review. Schizophr Bull. 1998;24:399–412. doi: 10.1093/oxfordjournals.schbul.a033335. [DOI] [PubMed] [Google Scholar]
- 14.Sergerie K, Lepage M, Armony JL. A process-specific functional dissociation of the amygdala in emotional memory. J Cogn Neurosci. 2006;18:1359–1367. doi: 10.1162/jocn.2006.18.8.1359. [DOI] [PubMed] [Google Scholar]
- 15.Sergerie K, Lepage M, Armony JL. Influence of emotional expression on memory recognition bias: a functional magnetic resonance imaging study. Biol Psychiatry. 2007;62:1126–1133. doi: 10.1016/j.biopsych.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Fischer H, Sandblom J, Nyberg L, Herlitz A, Backman L. Brain activation while forming memories of fearful and neutral faces in women and men. Emotion. 2007;7:767–773. doi: 10.1037/1528-3542.7.4.767. [DOI] [PubMed] [Google Scholar]
- 17.Cahill L, Haier RJ, Fallon J, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell J. A circumplex model of affect. J Pers Soc Psychol. 1980;39:1161–1178. [Google Scholar]
- 19.Posner J, Russell JA, Peterson BS. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 2005;17:715–734. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall J, Harris JM, McKirdy JW, Johnstone EC, Lawrie SM. Emotional memory in schizophrenia. Neuropsychologia. 2007;45:1152–1159. doi: 10.1016/j.neuropsychologia.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 22.Gur RE, Turetsky BI, Cowell PE, et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- 23.Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 26.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;155:49–58. [PubMed] [Google Scholar]
- 27.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 28.Miller DC. Hollingshead two-factor index of social position. In: Miller DC, editor. Handbook of Research Design and Social Measurement. Newbury Park, CA: Sage Publications; 1991. pp. 351–359. [Google Scholar]
- 29.Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Tanimoto TT. IBM Internal Report. IBM Technical Reports Series; 1957. [Google Scholar]
- 32.Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 33.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandler G. Recognizing: the judgment of previous occurrence. Psychol Rev. 1980;87:252–271. [Google Scholar]
- 35.Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huron C, Danion JM. Impairment of constructive memory in schizophrenia. Int Clin Psychopharmacol. 2002;17:127–133. doi: 10.1097/00004850-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Thoma P, Zoppelt D, Wiebel B, Daum I. Recollection and familiarity in negative schizophrenia. Neuropsychologia. 2006;44:430–435. doi: 10.1016/j.neuropsychologia.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 38.van Erp TG, Lesh TA, Knowlton BJ, et al. Remember and know judgments during recognition in chronic schizophrenia. Schizophr Res. 2008;100:181–190. doi: 10.1016/j.schres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 40.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 41.Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J Cogn Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- 42.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenton ME, Gerig G, McCarley RW, Szekely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry Res. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 46.Moritz S, Woodward TS, Rodriguez-Raecke R. Patients with schizophrenia do not produce more false memories than controls but are more confident in them. Psychol Med. 2006;36:659–667. doi: 10.1017/S0033291706007252. [DOI] [PubMed] [Google Scholar]
- 47.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]