Abstract
Objective: Neurocognitive impairments have been documented in adolescents with early-onset schizophrenia (EOS). There is still inconsistency regarding an average profile, which could be due to the fact that each study uses different tests. The purpose of this study was to examine whether the “Measurement and Treatment Research to Improve Cognition in Schizophrenia” (MATRICS) battery is useful in detecting differences between the patient group and the healthy controls, and to describe the neuropsychological pattern in the EOS group. Method: Neuropsychological functioning was examined in 31 adolescents with schizophrenia spectrum disorders and 67 healthy controls, using the MATRICS battery. Results: There were significant differences between the patients and the controls on every domain except for social cognition. Patients showed a generalized neurocognitive deficit of 0.8–1.8 SDs compared with controls, with verbal learning, working memory, and visual learning being the most affected areas. Conclusions: The MATRICS battery is sensitive in detecting differences between patients and controls in the adolescent population. However, we question the use of Mayer-Salovey-Caruso Emotional Intelligence Test in this age group. Results document a significant generalized deficit in adolescents with EOS.
Keywords: neurocognition, psychosis, adolesecents
Introduction
Cognitive impairments are regularly found in patients with adult-onset schizophrenia (AOS) and are a core feature of the disorder.1 The deficits span from early sensory information processing to attention, verbal/ visual learning and memory, and executive functions.2,3 New findings suggest that neuropsychological (NP) status is a better predictor of functional outcome than symptom status in AOS and a possible target for interventions.4 The lack of a consensus core battery to evaluate cognitive functioning has hampered both possibilities to compare findings across the now numerous studies on cognition in AOS and the development of treatments. This is one of the main reasons behind the National Institute of Mental Health “Measurement and Treatment Research to Improve Cognition in Schizophrenia” (MATRICS) initiative, which aimed at developing a consensus cognitive battery for use in clinical trials in schizophrenia.5 The MATRICS group has identified 7 cognitive domains that are dysfunctional in schizophrenia and thought to be a core feature of the disorder. From these 7 domains, 6 were included on the basis of multiple factor-analytic studies of cognitive performance in schizophrenia. The seventh domain, social cognition, was included because of its promising nature as a mediator of neurocognitive effects on functional outcome.6 The MATRICS test battery covers these domains and seeks to be a gold standard for measuring cognition in schizophrenia. The tests within the battery have been chosen with a thorough emphasis on reliability and validity.5–7
Cognitive deficits are also prevalent in patients with early-onset schizophrenia (EOS),8 and they seem to have more severe premorbid neurodevelopmental abnormalities and worse long-term outcome than AOS patients.9 To date, studies on cognitive functioning in EOS are rare. Overall, they support the presence of a global profile of cognitive deficits similar to findings in AOS, but the results on specific deficits are partly inconsistent8,10–23 (see table 1). While all studies assessing verbal memory, speed of processing, and working memory showed a significant deficit, the results varied considerably for all other domains.
Table 1.
Deficits Found in Specific Neurocognitive Domains in Early-Onset Schizophrenia Patients Compared With Healthy Adolescent Controls
| Verbal Memory | Visual Memory | Working Memory | Attention | Speed of Processing | Planning and Problem Solving | Mental Flexibility/Executive Functions | |
| Kenny et al10 | Yes (verbal list learning) | Not assessed | Yes (Trigram Recall With Interference Test) | Yes (PASAT/digit span distraction) | Yes (Category Instance Retrieval Test) | Not assessed | Yes (WCST) |
| Oie et al11 | Not assessed | Not assessed | Not assessed | No (covert visual attention task) | Not assessed | Not assessed | Not assessed |
| Rund et al12 | Not assessed | Not assessed | Not assessed | No (DS-CPT) | Not assessed | Not assessed | Not assessed |
| Oie and Rund22 | Yes (CVLT) | Yes (Kimura Recurring Figures Test) | Not assessed | No (DS-CPT/dichotic listening) | Yes (Trail Making A and B/WISC-R digit symbol) | Not assessed | Yes (WCST/WISC-R similarities) |
| Kravariti et al13 | Yes (WMS-R) | Yes (WMS-R) | Yes (computerised executive golf task) | Yes (WMS-R) | Not assessed | No (3-D CTL Test/3-D CTL control) | Yes (computerised Trail Making Task) |
| Brickman et al14 | Yes (Serial Verbal Learning Test) | Not assessed | Not assessed | Yes (Trail Making A/Digit Span Test) | Not assessed | Not assessed | Yes (WSCT/Stroop/Trail Making B) |
| McClellan et al15 | Yes (CVLT-C) | Yes (WRAML) | Not assessed | Not assessed | Yes (VMI) | Yes (Controlled Oral Word Association) | Yes (WCST) |
| Ueland et al16 | Not assessed | Yes (Kimura Recurring Figures Test) | Yes (Digit Span Backward Task, WISC-R) | No (DS-CPT) | Yes (digit symbol, WISC-R) | Not assessed | Yes (WCST) |
| Rhinewine et al23 | Yes (CVLT) | Not assessed | Not assessed | Yes (Trail Making A/ CPT-IP) | Not assessed | Not assessed | Yes (WCST/ Trail Making B) |
| Fagerlund et al8 | Yes (CANTAB) | Not assessed | Not assessed | Yes (CANTAB) | Yes (Trail Making B) | Not assessed | Yes (WCST) |
| Kester et al17 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Yes (Iowa Gambling Task) | Not assessed |
| Roofeh et al18 | Yes (CVLT) | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Kravariti et al19 | Yes (WMS-R) | No(WMS-R) | Yes (Computerized analogue of the CANTAB) | No (Computerized Trail Making Task) | Not assessed | Yes (3-D CTL Test/ 3-D CTL Control) | No (Computerised Trail Making Task) |
| Vance et al20 | Not assessed | Yes (DMTS, CANTAB) | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed |
| Groom et al21 | Yes (RAVLT) | Not assessed | Not assessed | Yes (CPT-IP) | Not assessed | Not assessed | Yes (HSCT/FAS) |
Note: CVLT, California Verbal Learning Test; WMS-R, Wechsler Memory Scale–Revised; CANTAB, Cambridge Neuropsychological Test Automated Battery; RAVLT, Rey Auditory Verbal Learning Test; WRAML, Wide Range Assessment of Memory and Learning; DMTS, The Delayed Matching to Sample Trials; PASAT, Paced Auditory Serial Addition Test; DS-CPT, The Degraded Stimulus Continuous Performance Task; CPT-IP, Continuous Performance Test, Identical Pairs; WISC-R, Wechsler Intelligence Scale for Children–Revised; CTL, Computerised Tower of London Test; WCST, Wisconsin Card Sorting Test; HSCT, Hayling Sentence Completion Test; FAS, FAS Test of Orthographic Verbal Fluency; VMI, Test of Visual-Motor Integration.
Earlier works on identifying a specific NP profile in AOS provide support for the conclusion that these patients do show a distinct pattern of cognitive deficits, which are different from other diagnostic categories, such as depression.2,24–27 Few studies have to our knowledge investigated the cognitive profile of the EOS population. Two of them16,22 found that the patient group performed worse on every cognitive domain except for selective and sustained attention. Other findings8,13,23 suggests a general cognitive deficit across all domains investigated. Due to lack of studies on EOS, and the diversity in results, it is hard to establish a typical, distinct profile. A major problem in establishing a profile of cognitive deficits in EOS is the use of different NP test batteries across different studies making direct comparisons difficult.8
Because EOS is a rare disorder, the use of a standard test battery such as the MATRICS would be particularly useful because this would allow for comparing or merging small samples. The studies going into the scientific foundation of the MATRICS battery are however based on AOS patients, and the battery has to the best of our knowledge not yet been used in an adolescent sample. The aims of the current study are thus to examine whether the MATRICS battery will differentiate between patients with EOS and an adolescent control group. We will investigate if any areas are more affected than others and evaluate the practical use of the battery in this age group.
Methods
Subjects
The inclusion criteria were patients with treatment referral to one of the psychiatric departments in the southern part of Norway for a disorder meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for a broad schizophrenia-spectrum disorder (schizophrenia, schizophreniform disorder, schizoaffective disorder, brief psychotic disorder, and psychosis not otherwise specified [NOS]) and age between 12 and 18 years. Patients with a history of central nervous system pathology or trauma (loss of consciousness for greater than 30 min and/or any neurological sequelae) or with an estimated IQ less than 70 were excluded. A total of 31 psychotic adolescents (table 2) were included; 16 (52%) with a schizophrenic disorder, 10 (32%) with psychotic disorder NOS, 3 (10%) with schizoaffective disorder, 1 (3%) with schizophreniform disorder, and 1 (3%) with brief psychotic disorder. Twenty-six (84%) were born and educated in Norway. Three patients did not have Norwegian as their mother tongue but could be tested and interviewed in Norwegian. One of these had for practical and cultural reasons a very short formal education. Twenty-three (75%) had started antipsychotic treatment before testing (median 24 wk, range 3–80). Of these, 3 patients (13%) used a combination of first- and second-generation antipsychotics and 20 (87%) used second-generation antipsychotics alone. None used first-generation antipsychotics separately.
Table 2.
Characteristics of Patients With Schizophrenia Compared With Normal Control Subjects
| Patients (n = 31) | Healthy Controls (n = 67) | Test Statistics |
|||
| Sex (female) | 15 (48%) | 34 (51%) | χ2 = .00 | df = 1 | ns |
| Hand dominance (R) | 27 (87%) | 60 (90%) | χ2 = .00 | df = 1 | ns |
| Age (y) | 15.8 (2.0) | 16.0 (1.9) | t = −0.49 | df = 96 | ns |
| Mother’s education (y) | 13.3 (2.9) | 14.3 (2.3) | t = −1.96 | df = 95 | ns |
| FSIQ (WASI)a | 97.1 (14.6) | 107.9 (14.1) | t = −3.49 | df = 96 | <.001 |
| GAFb | |||||
| Symptom | 47.5 (13.4) | ||||
| Function | 46.7 (15.5) | ||||
| PANSSc | |||||
| Positive | 14.9 (4.1) | ||||
| Negative | 12.1 (5.2) | ||||
| Total | 57.5 (12.5) | ||||
| Duration of untreated psychosis (wk) | 35.0 (51.5) | ||||
Full-Scale IQ from the Wechsler Abbreviated Scale of Intelligence.
Global Assessment of Functioning Scale—Split Version.
Positive and Negative Syndrome Scale, n = 28.
Healthy controls were recruited through personal letters to a randomly selected group from the Norwegian population register and from schools in the patient catchment area. They were matched to patients on gender, age, and length of mother's education. They were screened for mental problems using the Mini-International Neuropsychiatric Interview screening module28 and were excluded in the case of any positive answer to the screening questions. Due to the fact of overrepresentation of adolescents with mothers of high education in the control group, we systematically excluded the last 15 of the eligible, normal controls where the mother had more than 17 years of education. The final number of controls was then 67.
After complete description of the study to the subjects, written informed consent was obtained from patients and controls, as well as their parents, if the adolescents were below 16 years of age. The study is approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate.
Clinical Instruments
The patients were interviewed by clinical psychologists working as research fellows. They also had access to medical records and information from family members and treating clinicians. Diagnoses were established using the Structural Clinical Instrument of Diagnosis for DSM-IV Axis I disorders (SCID-I), modules A–D. All interviewers were trained in use of the SCID, participated in regular diagnostic consensus meetings led by a well-experienced clinical researcher in the field of diagnostics in psychotic disorders, and finished a training course in SCID assessment based on the training program at UCLA. Mean overall kappa for SCID diagnoses as assessed in the UCLA training course was 0.77.
Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS)29 and the Global Assessment of Functioning (GAF) Scale (split DSM-IV version).30
Neurocognition
Neurocognitive assessment was carried out by clinical psychologists with training in standardized NP testing. Assessments were done using the MATRICS battery with the addition of IQ tests. The battery covers the following 7 domains:
Speed of Processing
Category fluency31—verbal fluency for animals was tested for 60 seconds. Scores were obtained for the number of words produced. Symbol coding (Brief Assessment of Cognition in Schizophrenia)32—writing numbers corresponding to nonsense symbols as quickly as possible during a period of 90 seconds. Scores were obtained for the number of symbols coded right. Trail making A33—involves connecting consecutive numbers arranged in random order on a sheet of paper. The scores used were the total time for the completion.
Attention/Vigilance
Continuous Performance Test, Identical Pairs (CPT-IP)34—a test of monitoring numbers on a data screen and press the button whenever 2 digits in a row are presented identically. Mean d′ value across 2-, 3-, and 4-digit conditions was used as the score. d’ is an index of signal/noise discrimination computed by the CPT-IP program.
Working Memory
University of Maryland—Letter-Number Span35: requires mental reordering of orally presented list with letters and numbers and repeating them back. Total number correct was used as score. Spatial Span (Wechsler Memory Scale III)36—involves remembering the locations of a series of blocks pointed to by the administrator, forwards and backwards, respectively. Sum of raw scores on both conditions are used as measurement.
Verbal Learning
Hopkins Verbal Learning Test-Revised37—a list of 12 words were presented 3 times. The sum of words repeated after these learning trials was used as a measure of verbal learning.
Visual Learning
Brief Visuospatial Memory Test-Revised38—6 geometrical figures were displayed for 10 seconds, 3 times altogether. The sum of number of points awarded after recalled drawings on 3 learning trials is used as the score of visual learning.
Reasoning and Problem Solving
Mazes (Neuropsychological Assessment Battery [NAB])39—7 mazes, with gradually increasing difficulty, were distributed on single sheets of paper, to be resolved with a pencil. Points are awarded based on the time used to solve the maze. The score used was the sum of points awarded after 7 consecutive trials.
Social Cognition
Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT)40—the part Managing Emotions (D & H) were distributed. The score of this branch of the test was calculated using general consensus scoring. These scores were computed with the MATRICS scoring program.
Intelligence
The 4 Wechsler Abbreviated Scale of Intelligence (WASI) subscales (vocabulary, similarities, block design, and matrix reasoning) were used to calculate full-scale IQ, using Norwegian norms.41
Statistical Analyses
Data analysis was done using the statistical package SPSS for Windows (version 16.0). All tests were 2 tailed. If not indicated otherwise, the applied methods were Student t test for group comparisons of continuous data, chi-square for group comparisons of categorical data, and Pearson r for correlations. The level of significance was set at P = .05. For figure 1, the raw test scores were transformed to standard equivalents (z scores) on the basis of the means and SDs of the normal comparison group. By definition, the normal comparison group mean is represented by the zero line, with SD = 1 for all functions. Where high scores indicated impairment, scores were transformed (direction reversed) so that high scores always indicated better cognitive functioning. We then renormed the sum scores for the 2 domains working memory and speed of processing. To assess the percentage of patients considered neuropsychologically impaired on each neurocognitive domain, a cutoff score of 1.5 SD below the mean of the normal control group was used as a threshold for impairment and 1.0 SD below the mean of the control group as a threshold for slight impairment. Using the impairment cutoff (1.5 SD), we classified the subjects further into the categories “moderately impaired” (performing deviantly on 2–4 domains) and ”severely impaired” (performing deviantly in 5–7 domains).2
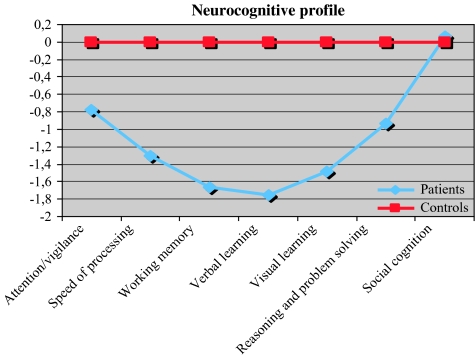
Fig. 1.
Scores on 7 Neurocognitive Composite Scores for Patients With Schizophrenia Compared With Normal Control Subjects.
To investigate the possible differences between the patients with psychosis NOS and the group with narrow schizophrenia-spectrum disorders, we performed a 1-way analysis of variance (ANOVA) followed up by Tukey post hoc tests for group comparison.
To investigate the possible differences between the patients that were nonmedicated by the time of testing (n = 8) and the ones who were on medication, we performed a 1-way ANOVA followed up by Tukey post hoc tests for group comparison.
To further investigate the issue of a generalized vs. specific impairment, we performed a multivariate analysis of covariance (MANCOVA) to adjust for IQ on the cognitive functions of the MATRICS battery.
Results
Demographic characteristics of patients with schizophrenia-spectrum disorders and healthy control subjects, as well as clinical characteristics of the patient group, are listed in table 2.
Table 3 summarizes the raw scores of the 2 groups on individual NP tests. Patients with schizophrenia were significantly more impaired than healthy control subjects on every measure, except social cognition. The differences are largest on the domains verbal learning, visual learning, and working memory. As a group, the patients performed between 0.8 and 1.8 SD below the control group.
Table 3.
Neuropsychological Test Results for Patients With Schizophrenia Compared With Normal Control Subjects
| Patients |
Controls |
|||||
| Mean | SD | Mean | SD | t | P | |
| WASI | ||||||
| Vocabulary | 45.7 | 10.9 | 52.3 | 9.9 | −2.97 | .004 |
| Similarities | 31.1 | 6.2 | 36.0 | 7.0 | −3.34 | <.001 |
| Block design | 43.7 | 16.3 | 54.4 | 12.0 | −3.68 | <.001 |
| Matrix R | 25.3 | 4.8 | 27.0 | 4.1 | −1.82 | .071 |
| Category fluency | 18.4 | 5.6 | 23.2 | 4.8 | −4.09 | <.001 |
| Symbol coding | 46.3 | 10.8 | 61.9 | 11.8 | −6.24 | <.001 |
| Trail Making A | 35.4 | 14.1 | 27.7 | 10.0 | 2.93 | .004 |
| CPT-IP | 1.7 | 0.7 | 2.2 | 0.7 | −3.50 | <.001 |
| Letter-number span | 11.8 | 2.8 | 16.1 | 3.0 | −6.73 | <.001 |
| Spatial span total | 15.0 | 2.6 | 19.1 | 2.9 | −6.79 | <.001 |
| HVLT-R total | 22.8 | 5.4 | 28.9 | 3.5 | −6.75 | <.001 |
| BVMT-R total | 22.6 | 8.7 | 28.6 | 4.1 | −4.71 | <.001 |
| Mazes (NAB) total | 17.8 | 5.7 | 21.7 | 4.2 | −3.83 | <.001 |
| MSCEIT | 87.0 | 11.9 | 87.0 | 8.8 | .02 | .982 |
Note: WASI, Wechsler Abbreviated Scale of Intelligence; CPT-IP, Continuous Performance Test, Identical Pairs; HVLT-R, Hopkins Verbal Learning Test-Revised; BVMT-R, Brief Visuospatial Memory Test-Revised; NAB, Neuropsychological Assessment Battery; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test.
The z score profile on the MATRICS battery is presented in figure 1. As figure 1 shows, there was a clear difference in performance pattern across groups.
The percentages of patients and controls that performed 1.0 SD and 1.5 SD below the mean, respectively, are presented in table 4. In total, 52% of the patients showed impairments within 2 or more domains using a cutoff of 1.5 SD below the mean of the normal subjects, ie, 32% were classified as moderately impaired and 20% as severely impaired. The corresponding figures for the healthy controls were 12 % and 0 %, respectively.
Table 4.
Percentage of Patients and Healthy Controls With Neurocognitive Impairment Using Cutoffs of 1 SD and 1.5 SD Below the Mean of the Controls
| 1 SD |
1.5 SD |
|||
| Patients | Controls | Patients | Controls | |
| Attention/vigilance | 41 | 13 | 29 | 8 |
| Speed of processing | 48 | 10 | 26 | 3 |
| Working memory | 77 | 12 | 39 | 5 |
| Verbal learning | 61 | 9 | 58 | 6 |
| Visual learning | 48 | 13 | 42 | 6 |
| Reasoning and problem solving | 48 | 16 | 29 | 10 |
| Social cognition | 16 | 12 | 16 | 6 |
Because some test performances can be influenced by differences in general intelligence, we repeated the analysis correcting for differences in IQ, using a MANCOVA analysis to ascertain that there are differences over and above those directly related to IQ differences. This analysis reproduces the exact same patterns of differences for all neurocognitive domains (F′s ranging from 1.1 to 26.6, all dfs = 1, P′s ranging from .000 to .044), with the possible exception of the speed of processing domain (Category Fluency Test [F = 3.3, df = 1, P = .07] and Trail Making Test [F = 0.6, df = 1, P = .43]).
There were no significant differences between the psychosis NOS group and the narrow schizophrenia-spectrum group on any test (F′s ranging from 1.4 to 26.0, dfs ranging from 76 to 97, P′s ranging from .102 to .924) (1-way ANOVA with the 2 diagnostic groups and normal controls).
Also, there were no significant differences between the group that was not medicated at the time of testing and the medicated group on any test (F′s ranging from 0.7 to 20.9, dfs ranging from 71 to 89, P′s ranging from .31 to 1.0) (1-way ANOVA with the medicated and unmedicated patients and normal controls). The association between neurocognitive domain scores and medication dosage at time of testing was additionally explored. There were no significant correlations between defined daily dose (DDD) and for any of the domain scores (Pearson r ranging from −.15 to .24, P‘s ranging from .25 to .91), the only partial exception was Trail Making A Test (r = 0.35, P = .11).
Discussion
Our data shows that the MATRICS battery clearly is able to differentiate between the patients and the healthy controls in every cognitive domain except social cognition and must therefore be regarded as a sensitive neurocognitive battery.
Sensitivity is an important issue in this field of research. Palmer et al42 suggested the concept of a “continuum of neurocognitive functioning” to better understand NP test results. This model is validated here by our finding of 20% of the patients being severely impaired, 32% moderately impaired, and 48% unimpaired, suggesting that psychosis in adolescence may exist in the context of relatively preserved cognition. Because there are no existing instruments that can be used to measure premorbid cognition for patients with EOS, we cannot infer anything about a possible decline from their presumed premorbid level. There were no differences in the level of impairment between patients with a narrow schizophrenia diagnosis and patients with psychotic disorder NOS (mainly patients with monosymptomatic hallucinosis), indicating that they were not overrepresented in the unimpaired group.
Also, our data show a general and explicit cognitive deficit in the patient group relative to the controls, with the apparent exception of social cognition. However, other studies43 have found several areas of cognitive functioning relatively spared, indicating that the previously used NP tests were not sensitive enough. Our findings of impairments in all 4 higher order neurocognitive domains (executive function, working memory, visual learning, and verbal learning) support other studies implicating both frontal and temporal hippocampal involvement in the pathophysiology in schizophrenia.2
One partially unexpected finding is that the area of reasoning and problem solving appears as the second least impaired domain. Studies of adults with schizophrenia regularly show that reasoning and problem solving is the most affected area, besides learning and memory.44 Our results could be explained by random sample characteristics or it may be that concept formation deficits are milder than others in this phase. This notion is in part supported by the fact that the Matrix-R part of the WASI was the least impaired one. The finding could also be related to test characteristics. The Mazes Test was chosen because it—compared with tests like the Wisconsin Card Sorting Test—has a low potential for learning effects and thus can be used in repeated testing. However, it does not take into account mental flexibility and verbal abilities and might be an easier test for persons with compromised cognitive functioning.
There were no differences at all between the 2 groups in social cognition as measured by the MSCEIT. This might have 2 explanations, either that both groups performed well or that both groups performed poorly. Supporting the first interpretation are findings that patients with schizophrenia do not differ from healthy controls when it comes to having knowledge about how to act in social situations. The problems appear when they have to actually perform it.45 This is an argument for using role-play tests to assess deficits in social cognition in this group. Supporting the second interpretation is the finding that the controls in our sample perform far below expectation compared with the American norms for the age groups 20–22 years, the lowest age group with existing norms. Being made for the adult population, the situations described in the MSCEIT vignettes are far from every day life, also for healthy adolescents. The clinical impression when administrating the test was that both the instructions and the vignettes were hard to understand and answers were given in a fairly random order. The alternatives are on the other hand rare. The first 6 MATRICS cognitive domains were based on multiple factor-analytic studies of cognitive performance in schizophrenia.5 Because there were very few test data available for the domain of social cognition, the domain was included because it was viewed as a promising mediator of neurocognitive effects on functional outcomes.46 In lieu of better alternatives or age-dependent norms, it appears very important not to evaluate MSCEIT results in adolescent patients without an age- and gender-matched control group.
One possible limitation of the study is that the patient group's IQ scores were significantly lower than that of the control group. This is a complex issue because IQ is known to be impaired premorbidly47 and also covary with current NP performance.48 It is thus problematic to use IQ as a matching variable because it can be argued to be an inherent part of the disorder. We did, however, find that the cognitive functions in the MATRICS are impaired over and above IQ. Another limitation is that we did not have adequate control over medication effects. However, it would be unethical to withdraw medical treatment from the patients, and if we had a selection of medically naive patients, it would probably be skewed in the sense that patients with no medical treatment often have less symptoms and better functioning. Also, we found no relationship between DDD and cognitive performance, indicating that antipsychotic medication is unlikely to explain the poor performance of the patient group. An additional limitation is that there are currently no existing norms for our age group for the MATRICS battery. Our healthy control sample is matched for age, gender, and mothers’ education but is relatively small and not selected as a normative sample. It is assumed that people volunteering to control samples tend to perform better than the average and also belong to a higher socioeconomical level than patient groups.
A strength of the study is that the MATRICS battery is used on an EOS population for the first time.
In summary, the results in this study suggest that the MATRICS battery is sensitive in detecting differences between EOS patients and controls. The different test composing the battery also functions well, with the possible exception of the MSCEIT that might be insensitive to impairments in social cognition in this group.
Funding
The Department of Psychology, University of Oslo, Norway; Regional Health Authority, Region South-East, Norway.
Acknowledgments
We would like to thank Professor Kjetil Sundet for invaluable help with the statistical analyses and Ph.D Hallvard Føllesdal for constructive discussions regarding test results. We would also like to thank Ph.D Kjell Ivar Øvergård for valuable comments on the analyses. The authors of this article do not have any commercial associations that might pose a conflict of interest in connection with the manuscript.
References
- 1.Dickinson D, Iannone VN, Wilk CM, Gold JM. General and specific cognitive deficits in schizophrenia. Biol Psychiatry. 2004;55:826–833. doi: 10.1016/j.biopsych.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Rund BR, Sundet K, Asbjørnsen A, et al. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr Scand. 2006;113:350–359. doi: 10.1111/j.1600-0447.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 3.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Leung WW, Bowie CR, Harvey PD. Functional implications of neuropsychological normality and symptom remission in older outpatients diagnosed with schizophrenia: a cross-sectional study. J Int Neuropsychol Soc. 2008;14:479–488. doi: 10.1017/S1355617708080600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, Part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 6.Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, Part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Nuechterlein KH, Kern RS, et al. Functional co-primary measures for clinical trials in schizophrenia: results from the MATRICS psychometric and standardization study. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- 8.Fagerlund B, Pagsberg AK, Hemmingsen RP. Cognitive deficits and levels of IQ in adolescent onset schizophrenia and other psychotic disorders. Schizophr Res. 2006;85:30–39. doi: 10.1016/j.schres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Kumra S, Charles Schulz S. Editorial: research progress in early-onset schizophrenia. Schizophr Bull. 2008;34:15–17. doi: 10.1093/schbul/sbm123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenny JT, Friedman L, Findling RL, et al. Cognitive impairment in adolescents with schizophrenia. Am J Psychiatry. 1997;154:1613–1615. doi: 10.1176/ajp.154.11.1613. [DOI] [PubMed] [Google Scholar]
- 11.Oie M, Rund BR, Sundet K. Covert visual attention in patients with early-onset schizophrenia. Schizophr Res. 1998;34:195–205. doi: 10.1016/s0920-9964(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 12.Rund BR, Zeiner P, Sundet K, Oie M, Bryhn G. No vigilance deficit found in either young schizophrenic or ADHD subjects. Scand J Psychol. 1998;39:101–107. doi: 10.1111/1467-9450.00062. [DOI] [PubMed] [Google Scholar]
- 13.Kravariti E, Morris RG, Rabe-Hesketh S, Murray RM, Frangou S. The Maudsley early-onset schizophrenia study: cognitive function in adolescent-onset schizophrenia. Schizophr Res. 2003;65:95–103. doi: 10.1016/s0920-9964(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 14.Brickman AM, Buchsbaum MS, Bloom R, et al. Neuropsychological functioning in first-break, never-medicated adolescents with psychosis. J Nerv Ment Dis. 2004;192:615–622. doi: 10.1097/01.nmd.0000138229.29157.3e. [DOI] [PubMed] [Google Scholar]
- 15.McClellan J, Prezbindowski A, Breiger D, McCurry C. Neuropsychological functioning in early onset psychotic disorders. Schizophr Res. 2004;68:21–26. doi: 10.1016/S0920-9964(03)00058-6. [DOI] [PubMed] [Google Scholar]
- 16.Ueland T, Oie M, Inge Landro N, Rund BR. Cognitive functioning in adolescents with schizophrenia spectrum disorders. Psychiatry Res. 2004;126:229–239. doi: 10.1016/j.psychres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr Res. 2006;85:113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Roofeh D, Cottone J, Burdick KE, et al. Deficits in memory strategy use are related to verbal memory impairments in adolescents with schizophrenia-spectrum disorders. Schizophr Res. 2006;85:201–212. doi: 10.1016/j.schres.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Kravariti E, Morris RG, Rabe-Hesketh S, Murray RM, Frangou S. Comparative profile analysis of cognitive function in recent-onset and chronic patients with adolescent-onset schizophrenia. Schizophr Res. 2007;94:240–244. doi: 10.1016/j.schres.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Vance A, Hall N, Casey M, Karsz F, Bellgrove MA. Visuospatial memory deficits in adolescent onset schizophrenia. Schizophr Res. 2007;93:345–349. doi: 10.1016/j.schres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Groom MJ, Jackson GM, Calton TG, et al. Cognitive deficits in early-onset schizophrenia spectrum patients and their non-psychotic siblings: a comparison with ADHD. Schizophr Res. 2008;99:85–95. doi: 10.1016/j.schres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Oie M, Rund BR. Neuropsychological deficits in adolescent-onset schizophrenia compared with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1216–1222. doi: 10.1176/ajp.156.8.1216. [DOI] [PubMed] [Google Scholar]
- 23.Rhinewine JP, Lencz T, Thaden EP, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. 2005;58:705–712. doi: 10.1016/j.biopsych.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ, Shear PK. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry. 2000;157:1095–1100. doi: 10.1176/appi.ajp.157.7.1095. [DOI] [PubMed] [Google Scholar]
- 25.Basso MR, Bornstein RA. Relative memory deficits in recurrent versus first-episode major depression on a word-list learning task. Neuropsychology. 1999;13:557–563. doi: 10.1037//0894-4105.13.4.557. [DOI] [PubMed] [Google Scholar]
- 26.Albus M, Hubmann W, Walheim C, Sobizak N, Franz U, Mohr F. Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand. 1996;94:87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 27.Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry. 1996;153:490–496. doi: 10.1176/ajp.153.4.490. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini—International Neuropsychiatric Interview (M.I.N.I): the development and validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 29.Kay RS, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen G, Hagtvet KA, Karterud S. Generalizability studies of the Global Assessment of Functioning-Split version. Compr Psychiatry. 2007;48:88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 32.Keefe RSE. Brief Assessment of Cognition in Schizophrenia. Durham, NC: Duke University Medical Center; 1999. [Google Scholar]
- 33.Army Individual Test Battery: Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 34.Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 35.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio, Tx: The Psychological Corporation; 1997. [Google Scholar]
- 37.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test-Revised. Odessa, Fla: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 38.Benedict RHB. Brief Visuospatial Memory Test—Revised. Odessa, Fla: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 39.White T, Stern RA. Neuropsychological Assessment Battery. Lutz, Fla: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 40.Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test. Toronto, ON: MHS Publishers; 2002. [Google Scholar]
- 41.Wechsler Abbreviated Scale of Intelligence (WASI), Norwegian Manual Supplement. Stockholm, Sweden: Harcourt Assessment, Inc; 2007. [Google Scholar]
- 42.Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff DL, Harris MJ. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 43.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 44.Seidman LJ, Kremen WS, Koren D, Faraone SV, Goldstein JM, Tsuang MT. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr Res. 2002;53:31–44. doi: 10.1016/s0920-9964(01)00162-1. [DOI] [PubMed] [Google Scholar]
- 45.Vaskinn A, Sundet K, Friis S, et al. Emotion perception and learning potential: Mediators between neurocognition and social problem-solving in schizophrenia? J Int Neuropsychol Soc. 2008;14:279–88. doi: 10.1017/S1355617708080314. [DOI] [PubMed] [Google Scholar]
- 46.Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- 47.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 48.Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Res. 2008;158:181–194. doi: 10.1016/j.psychres.2006.01.022. [DOI] [PubMed] [Google Scholar]