Abstract
Objective
Individuals with schizophrenia exhibit disturbances in a number of cognitive, affective, sensory, and motor functions that depend on the circuitry of different cortical areas. The cognitive deficits associated with dysfunction of the dorsolateral prefrontal cortex result, at least in part, from abnormalities in GABA neurotransmission, as reflected in a specific pattern of altered expression of GABA-related genes. Consequently, the authors sought to determine whether this pattern of altered gene expression is restricted to the dorsolateral prefrontal cortex or could also contribute to the dysfunction of other cortical areas in subjects with schizophrenia.
Method
Real-time quantitative polymerase chain reaction was used to assess the levels of eight GABA-related transcripts in four cortical areas (dorsolateral prefrontal cortex, anterior cingulate cortex, and primary motor and primary visual cortices) of subjects (N=12) with schizophrenia and matched normal comparison subjects.
Results
Expression levels of seven transcripts were lower in subjects with schizophrenia, with the magnitude of reduction for each transcript comparable across the four areas. The largest reductions were detected for mRNA encoding somatostatin and parvalbumin, followed by moderate decreases in mRNA expression for the 67-kilodalton isoform of glutamic acid decarboxylase, the GABA membrane transporter GAT-1, and the α1 and δ subunits of GABAA receptors. In contrast, the expression of calretinin mRNA did not differ between the subject groups in any of the four areas.
Conclusions
Because the areas examined represent the major functional domains (e.g., association, limbic, motor, and sensory) of the cerebral cortex, our findings suggest that a conserved set of molecular alterations affecting GABA neurotransmission contribute to the pathophysiology of different clinical features of schizophrenia.
The core features of schizophrenia include disturbances in critical cognitive functions, such as working memory, that are mediated by the neural circuitry of the dorsolateral prefrontal cortex (1, 2). In the dorsolateral prefrontal cortex of subjects with schizophrenia, markers of inhibitory neurotransmission appear to be impaired (3). For example, reduced levels of mRNA encoding the 67-kilodalton isoform of glutamic acid decarboxylase (GAD67), the enzyme principally responsible for GABA synthesis (4), and the GABA membrane transporter GAT-1, which regulates the reuptake of synaptically released GABA, have been replicated in multiple postmortem studies of schizophrenia (5–12). These alterations in markers of GABA neurotransmission appear to involve specific subsets of GABA neurons. For example, mRNA encoding parvalbumin and somatostatin, each of which is expressed in a separate subset of GABA neurons, was decreased, whereas mRNA encoding calretinin, which is expressed in a third subset of GABA neurons, was unchanged in subjects with schizophrenia (11, 13). Furthermore, reduced GABA synthesis might be selectively mediated by a deficit in GAD67, because neither mRNA nor protein levels of GAD65, which contributes to GABA synthesis only under conditions of high synaptic demand (4), were altered in the dorsolateral prefrontal cortex of subjects with schizophrenia (8). In addition to these presynaptic markers of GABA neurotransmission, we recently reported decreased mRNA expression for several GABAA receptor subunits in the dorsolateral prefrontal cortex of subjects with schizophrenia (11), including the α1 and δ subunits, the major subunits in synaptic and extrasynaptic cortical GABAA receptors, respectively. Because normal working memory function depends upon GABA-mediated neurotransmission in the dorsolateral prefrontal cortex (14), these molecular alterations are thought to contribute to working memory disturbances in subjects with schizophrenia (3).
Individuals with schizophrenia also exhibit abnormalities in other cognitive, affective, sensory, and motor functions that depend on the circuitry of other cortical regions (15–17). Therefore, we sought to determine whether the specific pattern of changes in GABA-related transcript expression observed in the dorsolateral prefrontal cortex (i.e., decreased mRNA expression for GAD67, GAT-1, parvalbumin, somatostatin, GABAA α1, and GABAA δ and unaltered mRNA expression for calretinin and GAD65) is specific to this area only or could also contribute to the dysfunction of other cortical areas in subjects with the illness. Consequently, we assessed the levels of these eight transcripts in four cortical areas (the dorsolateral prefrontal cortex, the anterior cingulate cortex, and the primary motor and primary visual cortices) of subjects with schizophrenia and matched normal comparison subjects. Because these areas represent the major functional domains of the cerebral cortex (e.g., association, limbic, motor, and sensory functions, respectively), they provide a robust test of the hypothesis that a conserved set of molecular alterations underlie the pathophysiology of different clinical features of schizophrenia.
Method
Subjects
Brain specimens were obtained during autopsies conducted at the Allegheny County Medical Examiner's Office, Pittsburgh, after consent was obtained from next of kin. We analyzed 12 pairs of subjects, each pair consisting of a subject with schizophrenia and a normal comparison subject matched for gender, age, and postmortem interval. Schizophrenia and comparison subjects did not differ in terms of age (mean=47.6 years [SD=13.2] versus 46.0 years [SD=16.2], respectively; t=1.1, df=11, p=0.28) or postmortem interval (mean=18.0 hours [SD=11.0] versus 16.3 hours [SD=5.8]; t=0.90, df=11, p=0.39) (Table 1). Because this study was designed to determine if the pattern of GABA-related transcript expression in the dorsolateral prefrontal cortex of subjects with schizophrenia is also present in other cortical areas, we selected 12 pairs in which the subjects with schizophrenia exhibited the largest differences in GAD67 mRNA expression, as determined by in situ hybridization in a previous study (10).
TABLE 1. Demographic Characteristics of Subjects
| Normal Comparison Subjects |
Subjects With Schizophrenia |
Cortical Area |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair | Sex | Race | Age (years) | Postmortem Interval (hours) |
RNA Integritya |
Storage Time (months)b |
Cause of Death |
Sex | Race | Age (years) | Postmortem Interval (hours) |
RNA Integritya |
Storage Time (months)b |
Cause of Death |
DSM-IV Diagnosis |
Area 9 | Area 24 | Area 4 | Area 17 |
| 1 | Male | Black | 41 | 22.1 | 9.0 | 116 | Atherosclerotic coronary vascular disease | Male | White | 40 | 29.1 | 8.4 | 126 | Accidental asphyxiation | Chronic undifferentiated schizophrenia | √ | √ | √ | √ |
| 2 | Male | Black | 20 | 14.0 | 8.4 | 127 | Gun shot wound to chest | Male | Black | 27 | 16.5 | 7.4 | 123 | Heat stroke | Schizoaffective disorderc, d | √ | √ | √ | √ |
| 3 | Male | White | 65 | 21.2 | 9.0 | 110 | Atherosclerotic coronary vascular disease | Male | White | 63 | 18.3 | 8.0 | 120 | Atherosclerotic coronary vascular disease | Chronic undifferentiated schizophreniac, e | √ | √ | √ | √ |
| 4 | Male | White | 39 | 19.3 | 8.6 | 113 | Hypoplastic coronary artery disease | Male | White | 46 | 28.1 | 7.9 | 118 | Accidental combined drug overdose | Chronic paranoid schizophreniac, d, f, g | √ | √ | √ | √ |
| 5 | Male | White | 56 | 14.5 | 8.1 | 103 | Hypoplastic coronary artery disease | Male | White | 58 | 18.9 | 7.4 | 111 | Right middle cerebral artery infarction | Chronic undifferentiated schizophreniah | √ | √ | √ | √ |
| 6 | Male | Black | 19 | 7.0 | 9.2 | 96 | Trauma | Male | White | 25 | 5.0 | 9.3 | 77 | Suicide by drug overdose | Schizoaffective disorderc, d, f, h, i | √ | √ | √ | √ |
| 7 | Female | Black | 55 | 11.3 | 9.6 | 118 | Atherosclerotic coronary vascular disease | Female | White | 48 | 3.7 | 9.3 | 127 | Intracerebral hemmorrhage | Chronic disorganized schizophreniaf | √ | √ | ||
| 8 | Male | White | 42 | 26.1 | 8.7 | 100 | Atherosclerotic coronary vascular disease | Male | White | 50 | 40.5 | 8.1 | 125 | Suicide by combined drug overdose | Schizoaffective disorderd, j | √ | √ | √ | √ |
| 9 | Male | White | 54 | 8.0 | 9.1 | 72 | Cardiac tamponade | Male | Black | 52 | 8.0 | 7.7 | 87 | Peritonitis | Schizoaffective disorderj | √ | √ | ||
| 10 | Female | White | 73 | 19.7 | 7.7 | 10 | Anaphylactic reaction | Female | White | 71 | 23.8 | 7.0 | 60 | Atherosclerotic coronary vascular disease | Chronic undifferentiated schizophrenia | √ | √ | √ | |
| 11 | Male | White | 48 | 16.6 | 8.9 | 71 | Atherosclerotic coronary vascular disease | Male | White | 47 | 15.7 | 8.2 | 57 | Atherosclerotic coronary vascular disease | Continuous disorganized schizophreniad, i, j | √ | √ | √ | √ |
| 12 | Male | White | 40 | 15.8 | 8.4 | 95 | Atherosclerotic coronary vascular disease | Male | White | 44 | 8.3 | 8.1 | 56 | Myocarditis | Disorganized schizophreniad | √ | √ | √ | |
Assessed as RNA integrity number and measured on the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, Calif.).
Tissue stored at −80°C.
Taking benzodiazepines at time of death.
Taking valproic acid at time of death.
Alcohol abuse in remission at time of death.
Alcohol dependence current at time of death.
Other substance abuse current at time of death.
Not taking antipsychotic medication at time of death.
Other substance abuse in remission at time of death.
Alcohol dependence in remission at time of death.
An independent committee of experienced research clinicians made consensus DSM-IV diagnoses for each subject on the basis of medical records and structured diagnostic interviews conducted with the decedent's family members, as described previously (18). All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
Tissue Preparation
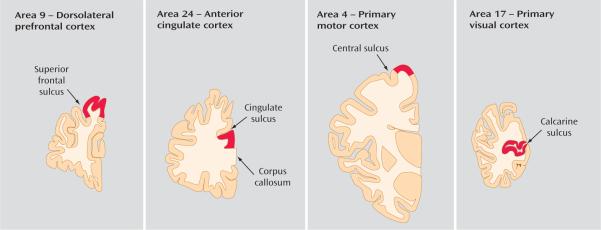
The right hemisphere of each brain was blocked coronally, immediately frozen, and stored at −80°C, as described previously (7). The tissue storage time did not differ between subjects with schizophrenia (mean=98.9 months, SD=29.4) and comparison subjects (mean=94.3 months, SD=31.6; t=0.72, df=11, p=0.50) (Table 1). The cortical gray matter of each cortical area (Figure 1) was dissected from cryostat sections (40 μm) of freshly frozen brain blocks in a manner that insured limited white matter contamination and excellent RNA preservation. Brodmann's area 9 of the dorsolateral prefrontal cortex was identified cytoarchitectonically (7) using coronal sections cut along the rostrocaudal axis of the superior frontal sulcus. The anterior cingulate cortex (area 24) was defined as the gray matter located between the dorsal surface of the corpus callosum and the fundus of the cingulate sulcus in coronal sections cut at the level of the anterior genu of the corpus callosum. The primary motor cortex (area 4) was identified cytoarchitectonically by the presence of Betz cells at the knee representation of the precentral gyrus. The primary visual cortex (area 17) was identified by the presence of the stria of Gennari within the calcarine sulcus in coronal sections cut 1 centimeter rostral to the occipital pole. Brain blocks were available from 12, 11, 10, and 9 subject pairs for the analyses of areas 9, 24, 4, and 17, respectively (Table 1). Total RNA was isolated from tissue homogenates and further purified using RNeasy Tissue Kit (Qiagen, Valencia, Calif.), and RNA integrity was assessed by measuring the RNA integrity number, using the Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, Calif.) (11). Although the mean value of RNA integrity in the normal comparison group (8.73, SD=0.52) was significantly higher than that of the schizophrenia group (8.07, SD=0.70; t=5.9, p<0.01), RNA samples from all subjects had RNA integrity values ≥7.0 (Table 1), indicating the excellent quality of total RNA for all subjects (19).
FIGURE 1. Locations of Cortical Gray Matter Excision for the Purpose of RNA Extractiona.
aAs shown in coronal sections through the right hemisphere of the human brain. Approximate locations where cortical gray matter was excised are in red.
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was converted into complementary DNA with random primers and quantitative real-time reverse transcriptase polymerase chain reaction (qPCR). The cycle threshold for each transcript of interest was determined using SYBR Green 1 dye and ABI PRISM 7000 (Applied Biosystems, Foster City, Calif.). The difference in cycle threshold between two transcripts equals the log2-transformed value of their expression ratio. High amplification efficiency (≥97%) was confirmed for each of the primer sets by performing individual standard curve analyses (data supplement Table 1, available online at http://ajp.psychiatryonline.org). In addition, all primer sets amplified specific single products in dissociation curve analyses. Each qPCR run for a given region included both subjects in a pair and amplified all 11 transcripts of interest in quadruplicate using a plate with 96 wells (2 subjects×11 transcripts×4 replications). Two qPCR runs (two plates) were performed for each subject pair for each region, with each run analyzing samples from a different reverse transcription. Reported expression levels represent the mean of the two qPCR runs.
Regional Survey of GABA-Related Transcript Expression
Eight GABA-related transcripts (GAD67, GAD65, GAT-1, parvalbumin, somatostatin, calretinin, and GABAA α1 and δ subunits) were amplified with three internal control transcripts encoding for beta-actin, cyclophilin A, and glyceraldehyde-3-phosphate dehydrogenase for each of the four cortical areas of each subject pair. These three internal control transcripts were selected based on their stable expression across subjects regardless of diagnosis (see data supplement Table 1). The difference in cycle threshold for each GABA-related transcript was calculated by subtracting the mean cycle threshold for beta-actin, glyceraldehyde-3-phosphate dehydrogenase, and cyclophilin A from the cycle threshold of each GABA-related transcript. Because this difference in cycle threshold (dCt) represents the log2-transformed expression ratio of each GABA-related transcript to the geometric mean of the three internal control transcripts (20), the expression level of each GABA-related transcript was determined as 2−dCt. For each GABA-related transcript in each cortical area, differences within each subject pair were determined as −d(dCt) (difference in cycle threshold for the comparison subject minus the difference in cycle threshold for the schizophrenia subject in each pair), which equals the log2-transformed expression ratio of the schizophrenia subject to the normal comparison subject (11).
Statistical Analysis
To determine the schizophrenia-related expression pattern of each GABA-related transcript across cortical areas, we employed a multivariate analysis of variance (MANOVA) model. The observations from the four cortical regions were treated as repeated measurements with an unconstrained covariance matrix. The fixed effects of diagnosis, cortical area, and interaction between diagnosis and cortical area were included and tested. Diagnosis was considered as a between-subjects effect and cortical area was considered as a within-subject effect. Because each region of each pair was analyzed separately in a different qPCR run, a systematic pair effect reflecting differences in qPCR runs was possible. Thus, pair and the interaction between cortical area and pair were considered as blocking effects. The expression levels of GABA-related transcripts were log2-transformed in order to stabilize the variability across different cortical areas. The analysis was implemented using SAS Proc Mixed (SAS Institute, Cary, N.C.).
In each cortical area, differences in GABA-related transcript expression were rank-ordered according to the mean magnitude of the log2-transformed values. Spearman's rank-order correlation was calculated across any two cortical areas, and a permutation test was performed to assess the null hypothesis that the rank orders were not related between areas. The null hypothesis was rejected if p values were smaller than the adjusted significance levels for multiple comparisons and rank orders were considered to be concordant between cortical areas.
To control for multiple comparisons, significance levels were adjusted for simultaneous inference using the Bonferroni-Holm method (7), in which p values are ordered from smallest (i=1) to largest (i=N) among multiple comparisons, and the significance level for each comparison is defined as α=0.05/([N+1]−i).
Results
Changes in GABA-Related Transcript Expression Across Cortical Areas
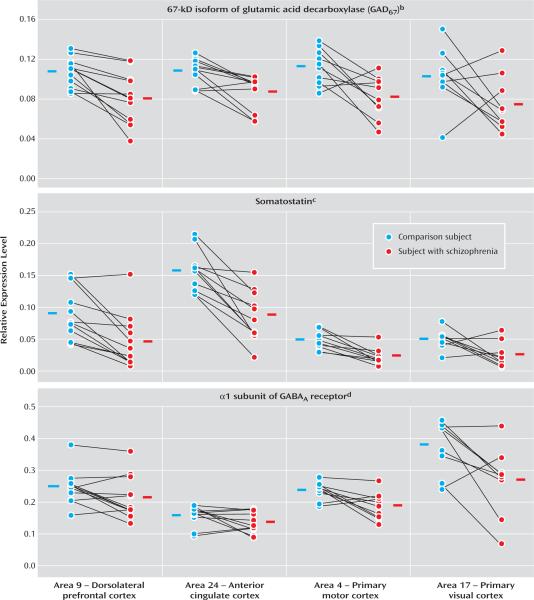
Regional patterns of expression for three transcripts are shown in Figure 2. Within both subject groups, the levels of GAD67 mRNA were comparable across all cortical areas (Figure 2). As a consequence, the mean reduction in levels of GAD67 mRNA was similar across regions (28%, 21%, 28%, and 32% in Brodmann's areas 9, 24, 4, and 17, respectively). MANOVA revealed a significant effect of diagnosis (F=18, df=1, 6.5, p=0.005), but no effect of cortical area (F=1.6, df=3, 7.1, p=0.28). The interaction between diagnosis and area was not significant (F=0.49, df=3, 7, p=0.70), indicating that the magnitude of between-group differences in GAD67 mRNA expression did not differ across the four cortical areas. In addition, within-subject pair differences in GAD67 mRNA expression in area 9 detected by qPCR were highly correlated (r=0.78, p=0.003) with those determined by in situ hybridization for the same subject pairs (10), substantiating our decision to measure mRNA using reverse transcriptase qPCR.
FIGURE 2. GABA-Related Transcript Expression Levels Across the Four Cortical Areas of Subjects With Schizophrenia and Matched Normal Comparison Subjectsa.
aExpression levels of each GABA-related transcript were determined as ratios to the geometric mean of the three most stable control transcript (beta-actin, cyclophilin A, and glyceraldehyde-3-phosphate dehydrogenase).
bSignificant effect of diagnosis only (F=18, df=1, 6.5, p=0.005).
cSignificant effect of diagnosis (F=36, df=1, 9.1, p<0.001) and area (F=130, df=3, 6.5, p<0.001).
dSignificant effect of diagnosis (F=8.3, df=1, 9.2, p=0.018) and area (F=220, df=3, 5.5, p<0.001).
Expression levels of somatostatin and GABAA α1 mRNA were also reduced in schizophrenia subjects across the four areas (Figure 2). However, in contrast to the findings for GAD67 mRNA, expression levels of somatostatin and GABAA α1 mRNA differed across cortical areas in both subject groups. Somatostatin mRNA levels were highest in area 24, intermediate in area 9, and lowest in areas 4 and 17 (Figure 2), whereas GABAA α1 mRNA levels appeared highest in area 17, intermediate in areas 9 and 4, and lowest in area 24 (Figure 2). Indeed, MANOVA revealed significant effects of both diagnosis (somatostatin: F=36, df=1, 9.1, p<0.001; GABAA α1: F=8.3, df=1, 9.2, p=0.018) and area (somatostatin: F=130, df=3, 6.5, p<0.001; GABAA α1: F=220, df=3, 5.5, p<0.001) for these two transcripts. However, interactions between diagnosis and area for somatostatin (F=1.3, df=3, 6.9, p=0.36) and GABAA α1 (F=2.9, df=3, 5.4, p=0.14) were not significant, indicating that the magnitude of reduction in these types of mRNA did not differ across the four cortical areas in subjects with schizophrenia.
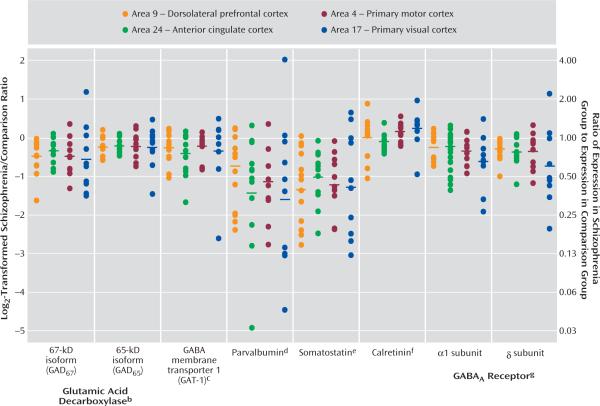
For each GABA-related transcript, the differences in expression within each subject pair across the four cortical areas are shown in Figure 3. For scale linearity, transcript expression differences in each pair are plotted as log2-transformed ratios of schizophrenia subject to matched comparison subject. The log2-transformed ratios for GAD67, GAD65, GAT-1, parvalbumin, somatostatin, GABAA α1, and GABAA δ mRNA were <0 for the majority of subject pairs, indicating reduced expression in subjects with schizophrenia. For each transcript, the magnitude of decrease appeared to be similar across the four cortical areas. MANOVA revealed a significant effect of diagnosis for each of these seven transcripts, without significant interaction between diagnosis and area, and the effect of diagnosis remained significant after correction for multiple comparisons (Figure 3). In addition, area had a significant effect on all transcript levels except for GAD67, indicating different transcript expression levels across cortical areas. Finally, in agreement with previous studies using in situ hybridization (13) or microarrays (11), we did not detect a significant difference in calretinin mRNA expression between subjects with schizophrenia and normal comparison subjects in any cortical area.
FIGURE 3. Summary of Differences in GABA-Related Transcript Expression in Subjects With Schizophrenia Across the Four Cortical Areasa.
aDifferences in expression within each subject pair for each transcript are plotted as log2-transformed ratios of schizophrenia subjects to normal comparison subjects. Corresponding nontransformed schizophrenia/normal comparison expression ratios are shown on the right axis. Horizontal bars indicate the mean for all subject pairs in each cortical area.
bSignificant effect of diagnosis only for GAD67 (F=18, df=1, 6.5, p=0.005) and both diagnosis (F=11, df=1, 11, p=0.008) and area (F=20, df=3, 9.4, p<0.001) for GAD65.
cSignificant effect of both diagnosis (F=4.9, df=1, 11, p=0.048) and area (F=15, df=3, 11, p<0.001).
dSignificant effect of both diagnosis (F=21, df=1, 7.6, p=0.002) and area (F=870, df=3, 6.5, p<0.001).
eSignificant effect of both diagnosis (F=36, df=1, 9.1, p<0.001) and area (F=130, df=3, 6.5, p<0.001).
fSignificant effect of area only (F=150, df=3, 9, p<0.001).
gSignificant effect of both diagnosis and area for GABAA receptor α1 subunit (F=8.3, df=1, 9.2, p=0.018; F=220, df=3, 5.5, p<0.001, respectively) and δ subunit (F=14, df=1, 6.5, p=0.008; F=113, df=3, 7.6, p<0.001, respectively).
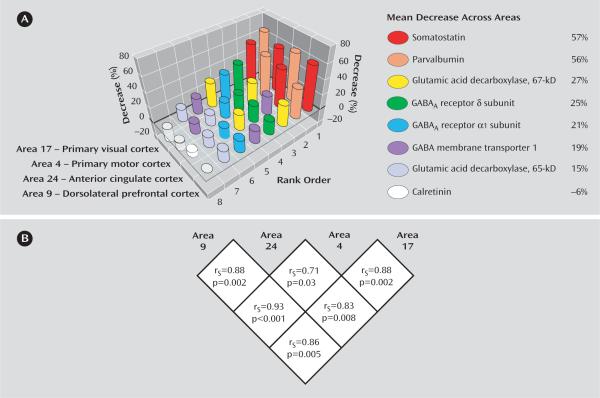
The pattern of change in transcript expression across cortical areas is summarized in Figure 4. Rank-orders based on the magnitude of decrease in transcript expression in subjects with schizophrenia were significantly preserved across the four cortical areas, rejecting the null hypothesis even after correction for multiple comparisons (Figure 4). In addition, the relative magnitudes of difference between subjects with schizophrenia and normal comparison subjects for each transcript appeared to be conserved across the four areas. Somatostatin and parvalbumin mRNA exhibited the largest decrease (>50% in every area), followed by GAD67, GABAA α1, GABAA δ, and GAT-1 mRNA, which showed average decreases of ~20%–30%. GAD65 mRNA showed the smallest decrease in subjects with schizophrenia with an average 15% decrease across cortical areas.
FIGURE 4. Rank-Order by Magnitude of Change in GABA-Related Transcript Expression Across Cortical Areasa.
aA) Eight GABA-related transcripts rank-ordered by the magnitude of difference in their expression for each cortical area. B) Spearman's rankorder correlation coefficients calculated between the rank-orders of any given two cortical areas.
In order to determine if these changes in GABA-related transcript expression were influenced by the use of substances that affect GABA neurotransmission, such as alcohol or benzodiazepines and mood stabilizers, we compared subject pairs with or without reported substance use in the pair's subject with schizophrenia at the time of death. Differences in expression within subject pairs did not differ between subject pairs with and without reported alcohol abuse/dependence or between pairs with and without reported use of benzodiazepines/mood stabilizers for any of the eight GABA-related transcripts in area 9 (data supplement Figure 2). In addition, no significant differences in expression between pairs with and without a diagnosis of schizoaffective disorder were observed in area 9 (data supplement Figure 2). Similar findings were present in the other three cortical areas (data not shown).
Discussion
Expression levels of seven GABA-related transcripts were reduced in subjects with schizophrenia in each of four cortical regions, with the magnitude of decrease for each transcript comparable across the four cortical areas. The largest decreases were detected for mRNA encoding somatostatin and parvalbumin, each of which is selectively expressed by a distinct subset of GABA neurons. General mediators of GABA neurotransmission, such as GAD67 and GAT-1 mRNA (which are expressed by all GABA neurons) and GABAA receptors α1 and δ subunits (which are expressed by both GABA and pyramidal neurons), showed moderate decreases in subjects with schizophrenia. mRNA for GAD65 exhibited the smallest, although still significant, decrease across the four areas (Figure 3). In contrast, the expression of calretinin mRNA, which is expressed in ~50% of GABA neurons that contain neither parvalbumin nor somatostatin (21), did not differ between subject groups in any of the four areas.
These findings suggest that intrinsic cortical GABA neurotransmission in schizophrenia, with selective involvement of the parvalbumin- and somatostatin-containing subsets of GABA neurons, is altered in a similar manner across cortical regions that are markedly different in cytoarchitecture, connectivity, and function. Furthermore, impaired GABA neurotransmission is unlikely to be restricted to the cortical areas or subjects evaluated in this study, since reductions in GAD67 and GAT-1 mRNA and/or protein have been previously reported in the auditory cortices of the superior temporal gyrus and in the anterior cingulate cortex in other subject cohorts (22–24). Therefore, impaired cortical GABA neurotransmission in schizophrenia is not likely due to factors more characteristic of altered dorsolateral prefrontal cortex (relative to at least some other cortical regions) circuitry in schizophrenia, such as reduced pyramidal cell dendritic spine density (18) or reduced excitatory input from the hippocampus (25) or mediodorsal thalamus (26). Instead, the conserved pattern of alteration in transcript levels is consistent with the presence of one or more common upstream mechanisms that are operative across cortical areas. For example, polymorphisms in genes encoding GAD67 (12) and GABAA receptor subunits (27) have been associated with both schizophrenia and alterations in their transcript levels. Furthermore, a recent study indicated that the signaling of neuregulin-1 through its receptor ErbB4, both of which are the products of putative susceptibility genes in schizophrenia (28), regulates inhibitory neurotransmission by a subset of cortical GABA neurons (29). Finally, parvalbumin- and somatostatin-containing cortical GABA neurons originate within the medial ganglionic eminence, whereas calretinin-containing GABA neurons are derived from the caudal ganglionic eminence (30). In addition, both time of birth (30) and transcription factors that regulate early differentiation (31) differ across these subpopulations of GABA neurons. Therefore, alterations in factors that are associated with the origin or maturation of both parvalbumin- and somatostatin-containing, but not calretinin-containing, GABA neurons could account for both cell type specificity and regional conservation of GABA neuron disturbances in schizophrenia.
The results of this study also address the question of whether cortical pathology is more pronounced in the dorsolateral prefrontal cortex than in other cortical regions in subjects with schizophrenia. For example, previous studies found that both the size of neurons (32) and the density of pyramidal neuron dendritic spines (18) were significantly decreased in the dorsolateral prefrontal cortex, but not the primary visual cortex, of subjects with schizophrenia. However, our current observations do not support the idea of preferential involvement of the dorsolateral prefrontal cortex, at least with regard to GABA-related transcript expression. Interestingly, we have previously found conserved reduction in the somal volume of layer 3 pyramidal neurons across the dorsolateral prefrontal cortex in Brodmann's area 9, primary auditory area 41, and auditory association area 42 in subjects with schizophrenia (33–35). Thus, at least several types of cortical pathology, affecting both inhibitory and excitatory neurons, might commonly be present across different cortical areas regardless of their functional properties, whereas other types of pathology might be relatively region-specific.
Evidence indicates that the reduced expression of GABA-related transcripts in schizophrenia is not attributable to the pharmacological treatment of the illness. First, transcript levels for GAD67, somatostatin, and GABAA α1 and δ subunits were not altered in the dorsolateral prefrontal cortex of monkeys chronically exposed to typical (haloperidol) or atypical (olanzapine) antipsychotic medications that produce trough plasma levels in the therapeutic range for humans (11). Second, transcript levels for GAD67, GAT-1, parvalbumin, and somatostatin were not altered in the dorsolateral prefrontal cortex of a different set of monkeys exposed to haloperidol at higher plasma levels, which produced marked extrapyramidal symptoms and required treatment with benztropine mesylate (7, 9, 13). Third, in our previous studies, transcript levels for GAD67, GAT-1, parvalbumin, somatostatin, and GABAA α1 and δ subunits were reduced in the dorsolateral prefrontal cortex of subjects with schizophrenia who were not taking antipsychotic medication at the time of death (11). Finally, in the present study, each GABA-related transcript showed comparable magnitudes of decrease across the four cortical areas, all of which differ both in the density of dopamine afferents that they receive (36) and in their expression of dopamine receptors (37) and are thus likely to be differentially influenced by antipsychotics.
The available data also indicate that the observed transcript changes are not driven by other confounding factors commonly associated with the illness. For example, in the present study (data supplement Figure 2) and in our previous microarray studies (11), we did not observe a significant effect of alcohol abuse/dependence or the use of benzodiazepines/mood stabilizers on decreases in GAD67, GAD65, GAT-1, parvalbumin, somatostatin, GABAA α1, and GABAA δ mRNA levels in any cortical area. Thus, it is unlikely that exposure to substances whose psychotropic effects are mediated through GABA neurotransmission produced the observed transcript changes. In addition, our previous studies clearly demonstrated that decreases in GAD67, GAT-1, parvalbumin, somatostatin, GABAA α1, and GABAA δ mRNA levels were not influenced by death by suicide in subjects with schizophrenia (11).
The impact of impaired GABA neurotransmission, especially via parvalbumin- and somatostatin-containing GABA neurons, on information processing in the dorsolateral prefrontal cortex may reveal how the conserved regional pattern of GABA-related transcript alteration observed in the present study could contribute to cortical dysfunction more broadly in schizophrenia. The network of parvalbumin-containing neurons, formed via both chemical and electrical synapses, plays a central role in generating oscillatory activities at the gamma band range (30–80 Hz) (38), whereas the network of somatostatin-containing neurons appears to be important for producing oscillations at the theta range (4–7 Hz) (39). In the dorsolateral prefrontal cortex, gamma band oscillations are induced and sustained during the delay period in working memory tasks (40), and their power increases in proportion with memory load (41), indicating that gamma band oscillations are associated with local neuronal processing critical for the maintenance and manipulation of information. On the other hand, theta band oscillations are recorded across widely distributed areas during working memory tasks (42), indicating their importance in orchestrating global interactions among distributed neuron networks. Together, these findings suggest that altered GABA neurotransmission by parvalbumin- and somatostatin-containing GABA neurons would have marked effects on the oscillatory activity of cortical neuronal networks required for dorsolateral prefrontal cortex functions. Consistent with this interpretation, recent studies have demonstrated impaired performance and reduced frontal gamma (43) and theta (44) activity in subjects with schizophrenia during cognitive tasks. Interestingly, cortical oscillations, especially at the gamma band range, have also been associated with primary motor (45) and visual (46) processing, and abnormal visual perception accompanied by reduced gamma band activity has been reported in occipital cortical areas of individuals with schizophrenia (47). Furthermore, abnormal gamma and theta oscillations observed in schizophrenia subjects during auditory processing tasks (48) might reflect altered GABA neurotransmission in auditory cortices (23, 24). Therefore, altered GABA neurotransmission mediated by parvalbumin- and somatostatin-containing GABA neurons may contribute to the dysfunction of multiple cortical areas in subjects with schizophrenia by disturbing cortical oscillations.
In addition to altered transcript expression in GABA neurons, this study revealed comparable reductions across cortical areas in mRNA levels for GABAA receptor α1 and δ subunits, both of which are expressed in cortical pyramidal and GABA neurons (49). Because α1 and δ subunits are frequently present in synaptic and extrasynaptic receptors, respectively (50), these findings suggest that reduced phasic (synaptic) as well as tonic (extrasynaptic) inhibition in both pyramidal and GABA neurons might be a feature shared by multiple cortical areas. Although it remains to be determined if these changes represent a primary pathology affecting postsynaptic GABA signaling or a process secondary to the alteration of presynaptic GABA neurons, reduced phasic and tonic inhibition appear to influence the selection and integration of excitatory inputs to cortical neurons (50) and could contribute to impaired information processing in each cortical area.
In summary, the findings of this study suggest that disturbances in the expression of a set of GABA-related mRNA commonly found in the dorsolateral prefrontal cortex in subjects with schizophrenia are conserved across at least three other regions of the neocortical mantle. Thus, disturbances in GABA neurotransmission by specific cell types through certain types of GABAA receptors could represent a common pathophysiology for different domains of cortical dysfunction in schizophrenia, raising the possibility that pharmacological agents with the appropriate specificity for certain GABA-related targets might be effective for a range of clinical features of the illness.
Supplementary Material
Acknowledgments
Dr. Lewis has received research support from the Bristol-Myers Squibb Foundation, Merck, and Pfizer and has served as a consultant to Bristol-Myers Squibb, Pfizer, Roche, Sepracor, and Wyeth. Dr. Sampson is a statistical consultant for Johnson and Johnson. All other authors report no competing interests.
Supported by a NARSAD Young Investigator Award (Dr. Hashimoto) and by NIH grants MH-043784 (Dr. Lewis), MH-045156 (Dr. Lewis), and MH-067234 (Dr. Mirnics). The authors thank Mary L. Brady and Keriann Hansen for their assistance in preparing the manuscript.
Footnotes
Presented in part at the 36th annual meeting of the Society for Neuroscience, Atlanta, Oct. 14–18, 2006, and at the International Congress on Schizophrenia Research, Colorado Springs, Colo., March 28–April 1, 2007.
References
- 1.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia, I: regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 2.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 3.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 4.Battaglioli G, Liu H, Martin DL. Kinetic differences between the isoforms of glutamate decarboxylase: implications for the regulation of GABA synthesis. J Neurochem. 2003;86:879–887. doi: 10.1046/j.1471-4159.2003.01910.x. [DOI] [PubMed] [Google Scholar]
- 5.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 6.Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABAA receptor α-1 subunit messenger RNA and human GABA transporter-1 (hGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 7.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 9.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto T, Arion D, Unger T, Maldonado-Alvidés JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABAA blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan EV, Shear PK, Lim KO, Zipursky RB, Pfefferbaum A. Cognitive and motor impairments are related to gray matter volume deficits in schizophrenia. Biol Psychiatry. 1996;39:234–240. doi: 10.1016/0006-3223(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 16.Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 17.Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 18.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM, Kleinman JE. Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biol Psychiatry. 2006;60:650–658. doi: 10.1016/j.biopsych.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34.1–34.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 22.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 23.Konopaske GT, Sweet RA, Wu Q, Sampson A, Lewis DA. Regional specificity of chandelier neuron axon terminal alterations in schizophrenia. Neuroscience. 2006;138:189–196. doi: 10.1016/j.neuroscience.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 24.Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 26.Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petryshen TL, Middleton FA, Tahl AR, Rockwell GN, Purcell S, Aldinger KA, Kirby A, Morley CP, McGann L, Gentile KL, Waggoner SG, Medeiros HM, Carvalho C, Macedo A, Albus M, Maier W, Trixler M, Eichhammer P, Schwab SG, Wildenauer DB, Azevedo MH, Pato MT, Pato CN, Daly MJ, Sklar P. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10:1074–1088. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- 28.Straub RE, Weinberger DR. Schizophrenia genes—famine to feast. Biol Psychiatry. 2006;60:81–83. doi: 10.1016/j.biopsych.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 32.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 33.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 34.Sweet RA, Pierri JN, Auh S, Sampson AR, Lewis DA. Reduced pyramidal cell somal volume in auditory association cortex of subjects with schizophrenia. Neuropsychopharmacology. 2003;28:599–609. doi: 10.1038/sj.npp.1300120. [DOI] [PubMed] [Google Scholar]
- 35.Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432:119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 37.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 38.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 39.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 40.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 42.Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE. Gating of human theta oscillations by a working memory task. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Res Cogn Brain Res. 2005;25:936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Brown P, Salenius S, Rothwell JC, Hari R. Cortical correlate of the Piper rhythm in humans. J Neurophysiol. 1998;80:2911–2917. doi: 10.1152/jn.1998.80.6.2911. [DOI] [PubMed] [Google Scholar]
- 46.Friedman-Hill S, Maldonado PE, Gray CM. Dynamics of striate cortical activity in the alert macaque, I: incidence and stimulus-dependence of gamma-band neuronal oscillations. Cereb Cortex. 2000;10:1105–1116. doi: 10.1093/cercor/10.11.1105. [DOI] [PubMed] [Google Scholar]
- 47.Spencer KM, Nestor PG, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritschy JM, Mohler H. GabaA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 50.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.