Abstract
Approximately 500,000 American premenopausal women have the Postural Orthostatic Tachycardia Syndrome (POTS). We tested the hypothesis that in POTS women during orthostasis, activation of the renin-angiotensin-aldosterone system is greater leading to better compensated hemodynamics in the mid-luteal phase (MLP) than early-follicular phase (EFP) of the menstrual cycle. Ten POTS women and 11 healthy women (controls) consumed a constant diet 3 d prior to testing. Hemodynamics and renal-adrenal hormones were measured supine and during 2-h standing. We found that blood pressure was similar, heart rate and total peripheral resistance were greater, cardiac output and stroke volume were lower in POTS than controls during 2-h standing. In controls, hemodynamic parameters were indistinguishable between menstrual phases. In POTS, cardiac output and stroke volume were lower, total peripheral resistance was greater in the EFP than MLP after 30 min of standing; but blood pressure and heart rate were similar between phases. Plasma renin activity [9±6 (SD) vs 13±9 ng/ml/h, P=0.04] and aldosterone (43±22 vs 55±25 ng/dl, P=0.02) were lower in the EFP than MLP in POTS after 2 h of standing. Catecholamine responses were similar between phases. The percentage rate of subjects having presyncope was greater in the EFP than MLP for both groups (Chi-square, P<0.01). These results suggest that the menstrual cycle modulates the renin-angiotensin-aldosteron system and affects hemodynamics during orthostasis in POTS. The high estrogen and progesterone in the MLP are associated with greater increases in renal-adrenal hormones and presumably more volume retention, which improve late standing tolerance in these patients.
Keywords: orthostatic intolerance, renin-angiotensin-aldosterone system, hemodynamics, sex hormones
INTRODUCTION
Patients with the Postural Orthostatic Tachycardia Syndrome (POTS, also called Chronic Orthostatic Intolerance) are unable to stand or remain upright for prolonged periods of time due to intolerable palpitations, light-headedness, weakness, or near-syncope. This disorder affects approximately 500,000 Americans,1 the vast majority of whom are young women. Severely affected patients are unable to work, attend school, or participate in recreational activities resulting in substantial morbidity. However, the underlying pathophysiology remains unclear. It has been proposed that the mechanisms for POTS are heterogeneous.2 We recently found that as a group, patients with POTS have a small heart coupled with reduced blood and plasma volume, which contributes to a small stroke volume, ultimately resulting in reflex tachycardia during orthostasis.3
The renin-angiotensin-aldosterone system (RAAS) plays an important role in the neurohumoral regulation of plasma volume and hemodynamic homeostasis in humans, especially during long-term orthostasis.4 Despite its importance in arterial pressure maintenance, results regarding the responses of the RAAS during upright posture in POTS patients are few and controversial; increased,5 decreased,6, 7 or unchanged8 plasma levels of renin and/or aldosterone have been reported.
Even though the vast majority of POTS patients are premenopausal women, there is no information available concerning the menstrual cycle effects on the RAAS in POTS. Hirshoren et al9 observed in young healthy women that fluid-regulatory hormones, plasma renin activity and aldosterone increased, and plasma norepinephrine decreased along the luteal phase; however, blood pressure, heart rate, and their responses to orthostasis remained unchanged. Chidambaram et al10 demonstrated that the renal-adrenal response to orthostatic stress was significantly augmented in the luteal phase compared with the follicular phase. These observations were made in healthy euvolemic women; whether similar results are observed in POTS women, who have a small heart coupled with reduced plasma volume, is uncertain.
The primary objective of this study was to test the hypothesis that in POTS women during orthostasis, activation of the RAAS is greater leading to better compensated hemodynamics in the mid-luteal phase (MLP) than early-follicular phase (EFP) of the menstrual cycle. To accomplish this objective, we evaluated comprehensively renal-adrenal and hemodynamic responses during prolonged standing in normally menstruating POTS women during the EFP (1 to 4 days after the onset of menstruation when both estrogen and progesterone are low), and during the MLP (19 to 22 days, when both hormones are high).
METHODS
Participants
The patient population consisted of 54 consecutive patients referred to our tertiary Autonomic Function Clinic between December 2004 and April 2008. Forty-six of these patients were screened, 28 ultimately were enrolled, and 10 normally menstruating POTS women agreed to participate in all phases of this study. All patients met the inclusion criteria for POTS,11 and had a heart rate rise ≥ 30 bpm or a rate that exceeded 120 bpm that occurred after 10 min of standing without any evidence of orthostatic hypotension.8 Eleven age-matched healthy women (controls) were also enrolled. All had self-reported regular menstrual cycles of ≈28 days, and had never taken or had not taken oral contraceptives for ≥ 6 mo.12 All were non-smokers. None was an endurance-trained athlete.13 All were screened with a careful medical history, physical examination, 12-lead electrocardiogram, and a 10-min stand test. Patients had stopped taking medications that could affect the autonomic nervous system ≥ 2 wk before screening and testing. All were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas. A summary of the descriptive data for POTS women and controls is presented in Table 1.
Table 1.
Physical characteristics in POTS women and controls
| Variables | POTS Women (n = 10) | Controls (n = 11) | ||
|---|---|---|---|---|
| EFP | MLP | EFP | MLP | |
| Age (yr) | 27 ± 9 | 33 ± 10 | ||
| Height (cm) | 167 ± 6 | 166 ± 6 | ||
| Weight (kg) | 67 ± 12 | 66 ± 12 | 63 ± 7 | 64 ± 8 |
| BMI (kg/m2) | 24 ± 4 | 24 ± 4 | 23 ± 2 | 23 ± 2 |
| Menstrual Cycle Day (d) | 4.0 ± 0.4 | 20.9 ± 0.9* | 3.9 ± 0.5 | 21.5 ± 0.7* |
| Estradiol (pg/mL) | 33.9 ± 8.3 | 94.6 ± 54.6* | 32.4 ± 8.9 | 91.8 ± 46.7* |
| Progesterone (ng/mL) | 0.8 ± 0.5 | 7.2 ± 6.1*† | 0.9 ± 0.5 | 11.1 ± 5.7* |
| Plasma Volume (mL/kg) | 41 ± 5† | 41 ± 7† | 46 ± 5 | 46 ± 5 |
| Blood Volume (mL/kg) | 63 ± 8 | 62 ± 9 | 68 ± 7 | 68 ± 7 |
| Blood pH | 7.39 ± 0.01 | 7.38 ± 0.01 | 7.42 ± 0.02 | 7.41 ± 0.01 |
| Na+ (mmol/L) | 139 ± 1 | 138 ± 1 | 138 ± 1 | 138 ± 0.4 |
| K+ (mmol/L) | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.2 ± 0.1 | 4.3 ± 0.1 |
| Ca++ (mmol/L) | 1.17 ± 0.02 | 1.15 ± 0.02 | 1.11 ± 0.02 | 1.15 ± 0.02 |
| 24-h Urine Output (mL) | 2390 ± 900 | 2693 ± 1174 | 2059 ± 860 | 2338 ± 799 |
| Osmolality (mOs/kg) | 296 ± 121 | 344 ± 231 | 366 ± 93 | 360 ± 105 |
| pH | 6.7 ± 0.2 | 6.6 ± 0.2 | 6.5 ± 0.2 | 6.4 ± 0.2 |
| Na+ (mmol/24 h) | 185.9 ± 48.6 | 196.0 ± 72.2 | 148.2 ± 53.7 | 169.4 ± 48.8 |
| K+ (mmol/24 h) | 65.6 ± 32.2 | 64.2 ± 26.4 | 52.4 ± 20.6 | 56.7 ± 17.5 |
| Ca++ (mmol/24 h) | 2.0 ± 1.2 | 2.1 ± 1.5 | 1.3 ± 0.7 | 1.7 ± 0.9 |
Values are mean ± standard deviation. EFP, early-follicular phase; MLP, mid-luteal phase; BMI, body mass index.
P < 0.05 compared to MLP within the same group.
P < 0.05 compared to controls during the same menstrual phase.
Hemodynamic Measurements
Heart Rate and Blood Pressure
Heart rate was monitored from the electrocardiogram (Hewlett-Packard), and beat-to-beat arterial pressure was derived by finger photoplethysmography (Portapres). Arm cuff blood pressure was measured by electrosphygmomanometry (SunTech), with a microphone placed over the brachial artery to detect Korotkoff sounds. Respiratory excursions were detected by a nasal cannula.
Cardiac Output
Cardiac output was measured with the acetylene rebreathing technique,14 from the disappearance rate of acetylene in expired air, measured with a mass spectrometer (Marquette), after adequate mixing in the lung has been confirmed by a stable helium concentration. This method has been validated against standard invasive techniques, including thermodilution and direct Fick.15, 16
Stroke volume was calculated from cardiac output and the heart rate measured during rebreathing. Total peripheral resistance was calculated as the quotient of mean arterial pressure and cardiac output, multiplied by 80 (expressed as dyn·s·cm−5). Mean arterial pressure was calculated as [(systolic pressure − diastolic pressure)/3] + diastolic pressure.
Experimental Protocol
All subjects were studied twice, once during the EFP and once during the MLP, with the order counterbalanced. Cycle phase was determined by the onset of menstruation and by the detection of the luteinizing hormone surge by an ovulation prediction kit (OvuQuick) and was verified by circulating estradiol and progesterone concentrations on each study day. Luteal phase progesterone > 2 ng/ml was confirmed in all but 3 POTS and 1 control women. Subjects were on an isocaloric diet consisting of 200 mEq sodium, 100 mEq potassium, and 1000 mg calcium. Fluid intake was ad libitum 3 days prior to testing and assessed by 24-h urine output the day prior to testing to verify dietary compliance. Subjects were required not to exercise at least 24 h prior to testing. They took a pregnancy test and showed negative results on each study day.
The experiment was performed in the morning or afternoon ≥ 2 h after a light breakfast or lunch, and ≥ 72 h after the last caffeinated or alcoholic beverage in a quiet, environmentally controlled laboratory with an ambient temperature of ≈25° C. The subject was placed in the supine position and an intravenous catheter was inserted into an antecubital vein for blood samples. Hemodynamic variables were measured after ≥ 30 min in the supine position and every 10 min after the subject began 2-h standing. Blood samples were collected after ≥ 1 h in the supine position, and after 30 min, 1 and 2 h of standing. Because Jacob et al17 previously found that neurohumoral responses did not reach a plateau after 1 h of standing, we implemented more prolonged (i.e., 2 h) standing in this study. Estradiol, progesterone, plasma renin activity, vasopressin, and aldosterone were measured by radioimmunoassay techniques,18 while plasma catecholamines were measured by high-performance liquid chromatography.19 Plasma volume was measured by a modified carbon monoxide rebreathing technique (please see Online Supplement at http://hyper.ahajournals.org for details).20, 21
Statistical Analysis
Data are expressed as mean ± standard deviation unless otherwise noted. Physical characteristics between groups were compared using Mann-Whitney rank sum tests, and between menstrual phases within groups were compared using Wilcoxon signed rank tests. Hemodynamic and renal-adrenal responses during 2-h standing between phases and between groups were analyzed using two way repeated-measures analysis of variance, and the Holm-Sidak method was used post hoc for multiple comparisons. The percentage rate of subjects having presyncope between menstrual phases and groups was compared using Chi-square tests. All statistical analyses were performed with a personal computer-based analysis program (SigmaStat, SPSS). A P value of < 0.05 was considered statistically significant.
RESULTS
POTS versus Controls
POTS women and controls were not different in age, height, weight, and body mass index (Table 1). Blood electrolytes, 24-h urine output, osmolality, and urine electrolytes did not differ between groups (Table 1). Plasma volume was lower in POTS women than in controls (Table 1, P = 0.04).
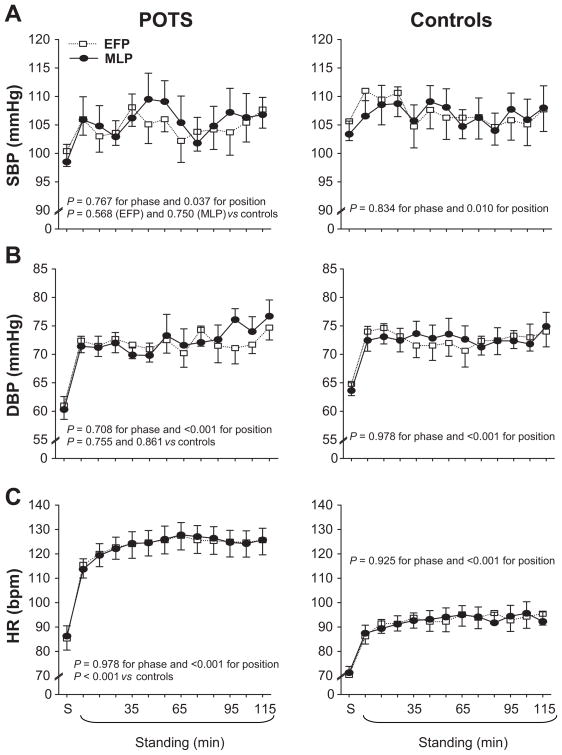
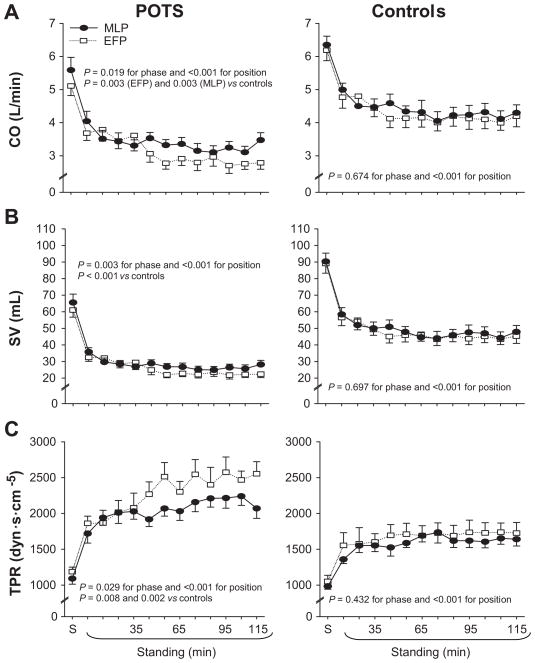
Systolic pressure remained stable, and diastolic pressure increased during 2-h standing; these responses were not different between groups (Figure 1A and 1B). Heart rate increased during 2-h standing, and was much greater in POTS women compared to controls (Figure 1C). Both cardiac output and stroke volume decreased during 2-h standing, and were much lower in POTS women (Figure 2A and 2B). Total peripheral resistance increased during 2-h standing, and was much greater in POTS women than in controls (Figure 2C). Both heart rate and total peripheral resistance were negatively correlated with stroke volume, indicating that tachycardia and strong vasoconstriction were function of a lower stroke volume in POTS women (Figure S1 in the Online Supplement).
Figure 1.
Systolic blood pressure (SBP, A), diastolic blood pressure (DBP, B), and heart rate (HR, C) responses during 2-h standing in the EFP (open symbols) and MLP (closed symbols) of the menstrual cycle in POTS women and controls. S, supine. Values are expressed as mean ± standard error.
Figure 2.
Cardiac output (CO, A), stroke volume (SV, B), and total peripheral resistance (TPR, B) during 2-h standing in the EFP (open symbols) and MLP (closed symbols) of the menstrual cycle in POTS women and controls. S, supine. Values are expressed as mean ± standard error.
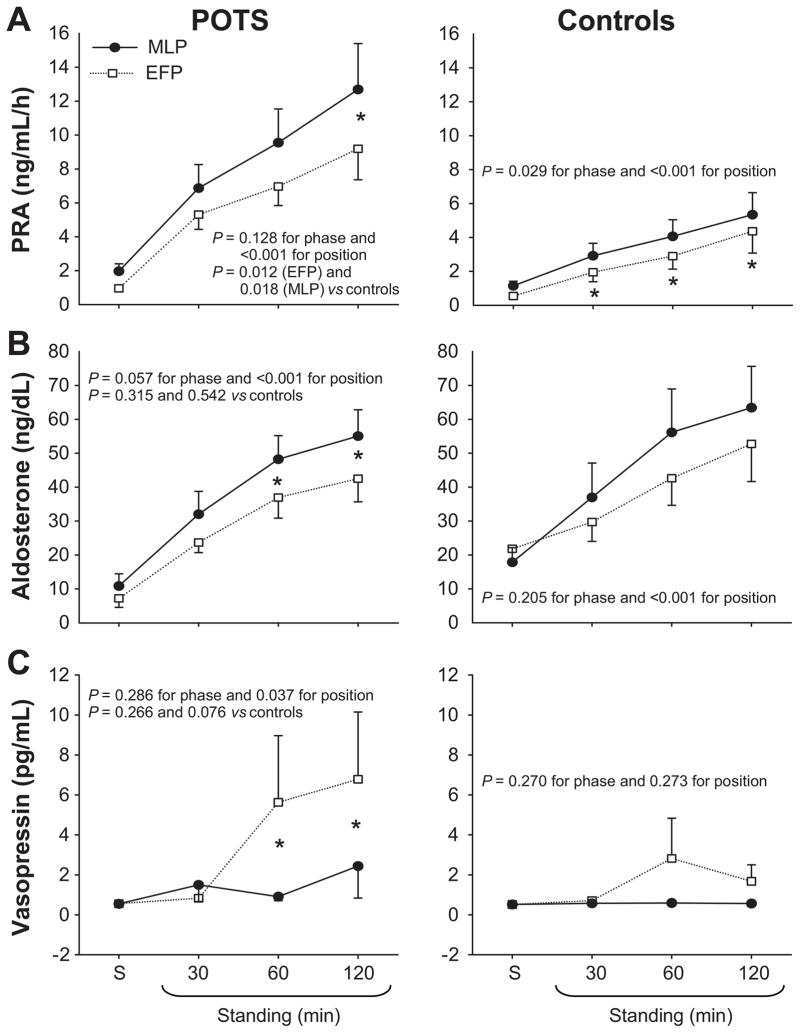
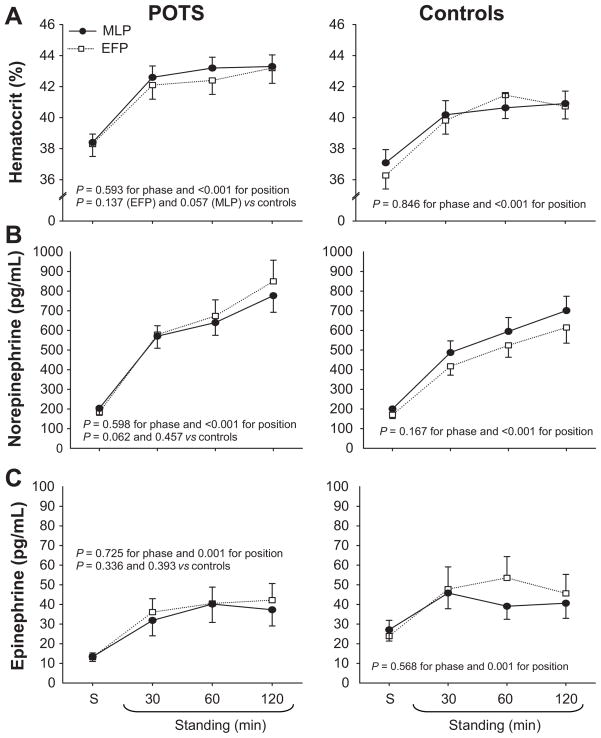
Plasma renin activity increased progressively during 2-h standing, and was greater in POTS women compared to controls (Figure 3A). This can not be explained by different sodium intakes as the urine sodium excretion was greater, although not significantly so, in POTS women than in controls (Table 1). Rather, a reduced plasma volume in POTS women may be responsible for the greater increases in plasma renin activity during standing. Aldosterone also increased gradually during 2-h standing, but did not differ between groups (Figure 3B). As a consequence, the aldosterone-to-renin ratio was lower in POTS women than in controls after 2 h of standing (5.5 ± 2.7 vs 14.0 ± 7.2 during the MLP; P < 0.01). Vasopressin increased after 1 and 2 h of standing in POTS women during the EFP, which was associated with presyncope (Figure 3C). Hematocrit increased during 2-h standing, and tended to be greater in POTS women during the MLP (Figure 4A). Plasma norepinephrine increased progressively during 2-h standing, and tended to be greater in POTS women during the EFP compared to controls (Figure 4B). Plasma epinephrine increased during 2-h standing, and did not differ between groups (Figure 4C).
Figure 3.
Plasma renin activity (PRA, A), aldosterone (B), and vasopressin (C) responses during 2-h standing in the EFP (open symbols) and MLP (closed symbols) of the menstrual cycle in POTS women and controls. S, supine. Values are mean ± standard error. *P < 0.05 EFP vs MLP within the group.
Figure 4.
Hematocrit (A), plasma norepinephrine concentration (B), and plasma epinephrine concentration (C) responses during 2-h standing in the EFP (open symbols) and MLP (closed symbols) of the menstrual cycle in POTS women and controls. S, supine. Values are mean ± standard error.
Both plasma renin activity and hematocrit were negatively correlated with stroke volume, suggesting that a greater reduction in central blood volume can cause a lower strove volume, and thereafter, a greater activation of the renal system during prolonged standing in POTS women (Figure 2S in the Online Supplement).
EFP versus MLP
Circulating levels of estradiol and progesterone were greater in the MLP than EFP for both groups (Table 1, both P < 0.01). During the MLP, progesterone was lower in POTS women than in controls (P = 0.04), even though menstrual cycle days did not differ between groups. Plasma volume and blood volume were not affected significantly by the menstrual cycle in both groups (Table 1).
The menstrual cycle did not influence supine hemodynamics in POTS women and controls (Figures 1 and 2, Table S1 in the Online Supplement). Supine plasma renin activity was greater in the MLP than EFP for both groups, and supine aldosterone was greater in the MLP in POTS women only (Figure 3, Table S1). The menstrual cycle did not affect blood pressure and heart rate responses during 2-h standing in both groups (Figure 1). In controls, hemodynamic parameters were indistinguishable between phases. Interestingly, in POTS women during the initial 30 min of standing, cardiac output, stroke volume, and total peripheral resistance responses were not different between phases; however, cardiac output and stroke volume were lower, while total peripheral resistance was greater in the EFP than MLP after 30 min of standing (Figure 2, all P < 0.05).
Plasma renin activity was lower in the EFP than MLP in both groups after 2 h of standing (Figure 3A). Aldosterone was lower in the EFP than MLP in POTS women after 1 and 2 h of standing, and it trended similarly in controls (Figure 3B). Plasma catecholamine concentrations were not affected by the menstrual cycle in both groups (Figure 4B and 4C).
Three POTS women and 2 controls developed presyncope during the EFP, while 1 POTS woman and no controls had presyncope during the MLP. The percentage rate of subjects suffering from presyncope was greater in the EFP than MLP for POTS women (30% vs 10%, Chi-square, P < 0.01) and controls (9% vs 0%, Chi-square, P < 0.01), suggesting a role of both estrogen and progesterone in promoting orthostatic tolerance in women. The rate was not different between groups (Chi-square, P = 0.22).
DISCUSSION
Our major findings are that (1) plasma renin activity increases were greater in POTS women than in controls during 2-h standing, presumably due to a reduced plasma volume in POTS; however, aldosterone responses did not differ between groups; (2) both plasma renin activity and aldosterone were lower in the EFP than MLP in POTS women during 2-h standing; (3) in POTS women during the initial 30 min (i.e., early) of standing, cardiac output, stroke volume, and total peripheral resistance responses were not different between phases; however, cardiac output and stroke volume were lower, while total peripheral resistance was greater in the EFP than MLP after 30 min (i.e., late) of standing; and (4) the percentage rate of subjects having presyncope was greater in the EFP than MLP for both POTS and healthy women.
These results suggest that the menstrual cycle modulates the RAAS and affects hemodynamics during prolonged standing in POTS women. The high estrogen and progesterone in the MLP are associated with greater increases in renal-adrenal hormones and presumably more volume retention, which improve late standing tolerance in these patients.
RAAS Responses in POTS
We found that standing plasma renin activity was markedly greater in POTS women than in controls, while standing aldosterone did not differ between groups. These observations are consistent in part with some5, 6 but not all8 previous studies. The reasons for these conflicting results are unclear, though timing of hormonal measurements obviously contributes. In addition, differences in salt intake (i.e., 150 mEq sodium per day in previous studies versus 200 in our study) may be one potential explanation, since it has been demonstrated that dietary sodium can modulate the responses of the RAAS in humans.22, 23 We used the relatively high salt diet because many patients had already been requested by their physicians to increase dietary salt intake or to take salt tablets; moreover, the high salt diet would allow us to detect a greater RAAS response during prolonged standing. The second possible explanation may be the influences of the menstrual cycle. It has been shown that the fluctuations of estrogen and progesterone during the menstrual cycle affect plasma renin activity and aldosterone in healthy women.9, 10, 23 All previous studies did not control for, or standardize phase of the menstrual cycle in POTS women.5, 6, 8 In contrast, our study was well controlled not only for the diet but also for the menstrual cycle.
However, one observation is common in all previous and the current study; namely, that POTS patients had a reduced aldosterone-to-renin ratio. Raj et al8 termed this dysregulation in POTS the “renin-aldosterone paradox”. It might be possible that the levels of aldosterone are so high in the upright posture that the aldosterone response has reached a physiologic maximum in POTS women. Interestingly, a similar reduced aldosterone-to-renin ratio was also observed in healthy individuals after a period bed rest (i.e., simulated microgravity exposure), in which “deconditioning” (i.e., cardiac atrophy, hypovolemia, etc.) occurs.24–26 Numerous studies have shown that real or simulated microgravity exposure can elicit a “POTS-like” syndrome even in healthy fit people. Conversely, a blunted adrenal response to angiotensin II or renin has been found in ~40% of patients with essential hypertension, and the concept of non-modulating hypertension is based on this theory.27 It has also been found that non-modulating hypertensive patients have a greater decrease in plasma volume when shifted from a high salt diet to a low salt diet28 and a greater increase in plasma norepinephrine concentration during standing compared to modulating hypertensive patients.29 Whether abnormalities in the RAAS contribute to POTS or whether the reduced aldosterone-to-renin ratio is a result of POTS (i.e., “deconditioning”) needs to be determined.
Menstrual Cycle Effects in POTS Women
Consistent with previous findings in healthy women,10, 30, 31 we observed that plasma renin activity and aldosterone increases during 2-h standing were greater in the MLP than EFP in POTS women. Studies using oral estrogen and progesterone in postmenopausal women have demonstrated that both hormones can activate the RAAS.32, 33 However, other data suggest that only progesterone activates the RAAS, while estrogen might inhibit the activation of this system.34, 35 Szmuilowicz et al23 showed that progesterone may directly contribute to increased luteal phase aldosterone production independent of the RAAS.
Activation of the RAAS can lead not only to salt and water retention but also to vasoconstriction. Given the higher plasma renin activity and aldosterone, we would predict greater cardiac output, stroke volume, and total peripheral resistance during 2-h standing in the MLP than EFP in POTS women. However, standing total peripheral resistance was actually lower in the MLP, even though standing cardiac output and stroke volume were indeed greater. There are several possibilities for the lower standing total peripheral resistance during the MLP in POTS women. First, Chapman et al30 found that the hormonal changes in the luteal phase had a specific renal vasodilating effect, overriding secondary activation of other renal vasoconstricting systems such as the RAAS. Second, animal and human studies showed that estrogen could upregulate nitric oxide and stimulate an increase in endothelial nitric oxide synthesis,36, 37 producing a direct vasodilatory effect.38 In addition, nitric oxide can downregulate angiotensin II type 1 receptors in vascular tissue39 and adrenal glands,40 and mitigate the actions of angiotensin II. Third, standing cardiac output and stroke volume were greater in the MLP in POTS women, which in turn could attenuate the increase in total peripheral resistance during upright posture via the baroreflex mechanism.
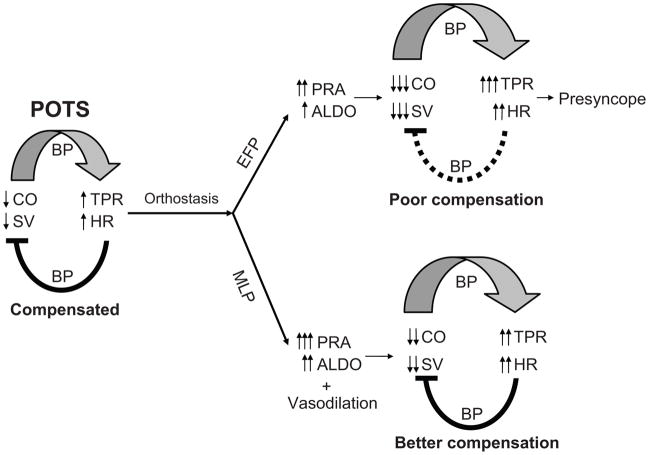
In POTS women during early standing, cardiac output, stroke volume, and total peripheral resistance responses were not different between phases; however, cardiac output and stroke volume were lower, while total peripheral resistance was greater in the EFP than MLP after 30 min of standing. These results suggest that a lower activation of the RAAS in the EFP, rather than any fundamental difference in the gravitationally mediated hemodynamics of the upright posture, or intrinsic impairment of baroreflex function may account for the different responses during different menstrual phases. The menstrual cycle modulated renal-adrenal increases during 2-h standing in both POTS and healthy women, but it affected hemodynamics in POTS women only. It is possible that the vasodilatory effects of sex hormones may depend on the degree of vasoconstriction. POTS women had greater vasoconstriction in response to upright posture compared to controls, and thus, the vasodilatory effects of estrogen and progesterone were greater in these patients. It is also possible that the sensitivity or density of estrogen and progesterone receptors on the blood vessels may be greater in POTS women than in controls. However, blood pressure and heart rate responses during prolonged standing in POTS women did not vary during the menstrual cycle, suggesting that POTS per se is not caused by the fluctuations of sex hormones but rather that these fluctuations influence the physiologic compensation to orthostasis. Figure 5 depicts possible mechanisms for orthostatic tolerance in POTS women during different menstrual phases.
Figure 5.
Possible mechanisms for plasma renin activity (PRA), aldosterone (ALDO), and hemodynamics including cardiac output (CO), stroke volume (SV), total peripheral resistance (TPR), heart rate (HR), and blood pressure (BP), during prolonged standing in POTS women during the early follicular phase (EFP) and mid-luteal phase (ML) of the menstrual cycle.
Limitations
First of all, even though many patients had POTS symptoms during 2-h standing, we did not document or quantify these symptoms during testing. However, patients’ overall well-being was assessed by the Short-Form 36 on each study day, and the results were not different between phases (Table S2). Secondly, we do not know whether some POTS women had the premenstrual syndrome. Rosenfeld et al41 found that women with premenstrual syndrome had increased fluid-regulatory hormones and disturbed fluid distribution only during the late-luteal phase, while our study was performed in the EFP and MLP. Thirdly, we did not measure luteinizing hormone and follicle-stimulating hormone, and thus, we were unable to determine the contributions of these hormones to renal-adrenal and hemodynamic responsiveness in POTS women.
Perspectives
Results from our study suggest that POTS per se is not caused by the fluctuations of sex hormones during the menstrual cycle, and therefore, we have ruled out a potential mechanism for this disorder. Conversely, these fluctuations can affect the physiologic compensation to orthostasis in POTS women. Given the fact that POTS women are more susceptible to orthostatic intolerance when both estrogen and progesterone levels are low, one simple approach would be to employ supplemental pharmacologic therapy, or perhaps behavioral modification during the EFP. Although POTS women demonstrated the anticipated changes in the RAAS during the menstrual cycle, namely, greater plasma renin activity and aldosterone increases during prolonged standing in the MLP than EFP, the aldosterone-to-renin ratio was lower in these patients compared to healthy women. This observation could suggest a rationale for the common use of fludrocortisone in such patients, though the optimal timing and dose of this medication is unclear. Whether the blunted adrenal response is a consequence or signature of POTS or whether abnormalities in the RAAS contribute to this syndrome needs to be determined in future studies.
Supplementary Material
Acknowledgments
The time and effort put forth by the subjects is greatly appreciated. The authors thank Robin P. Shook, Kazunobu Okazaki, Jeffrey L. Hastings, M. Dean Palmer, Daniel L. Creson, Colin L. Conner, Diane Bedenkop, and Peggy Fowler for their valuable laboratory assistance.
Sources of Funding
This study was supported by National Institutes of Health K23 grant (HL075283), National Space Biomedical Research Institute grant (CA00701), and the Clinical and Translational Research Center (formerly, the General Clinical Research Center) grant (RR00633).
Footnotes
Disclosures
None.
References
- 1.Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999;317:75–77. doi: 10.1097/00000441-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS) J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Q, VanGundy TB, Galbreath MM, Shibata S, Jain M, Hastings LJ, Bhella PS, Levine BD. Cardiac origins of the Postural Orthostatic Tachycardia Syndrome. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2010.02.043. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowell LB. Human Cardiovascular Control. New York Oxford: Oxford University Press; 1993. Neural-humoral adjustments to orthostasis and long-term control; pp. 81–117. [Google Scholar]
- 5.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 6.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–133. doi: 10.1016/s0002-9343(97)00133-2. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 2006;110:255–263. doi: 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 9.Hirshoren N, Tzoran I, Makrienko I, Edoute Y, Plawner MM, Itskovitz-Eldor J, Jacob G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J Clin Endocrinol Metab. 2002;87:1569–1575. doi: 10.1210/jcem.87.4.8406. [DOI] [PubMed] [Google Scholar]
- 10.Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol. 2002;13:446–452. doi: 10.1681/ASN.V132446. [DOI] [PubMed] [Google Scholar]
- 11.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS) Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 12.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 13.Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. doi: 10.1161/01.cir.84.3.1016. [DOI] [PubMed] [Google Scholar]
- 14.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–209. [PubMed] [Google Scholar]
- 15.Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis SS, Levine BD, Prisk GK, Shykoff BE, Elliott AR, Rosow E, Blomqvist CG, Pawelczyk JA. Simultaneous determination of the accuracy and precision of closed-circuit cardiac output rebreathing techniques. J Appl Physiol. 2007;103:867–874. doi: 10.1152/japplphysiol.01106.2006. [DOI] [PubMed] [Google Scholar]
- 17.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM, Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol. 1998;84:914–921. doi: 10.1152/jappl.1998.84.3.914. [DOI] [PubMed] [Google Scholar]
- 18.Boer P, Sleumer JH, Spriensma M. Confirmation of the optimal pH for measuring renin activity in plasma. Clin Chem. 1985;31:149–150. [PubMed] [Google Scholar]
- 19.van der Hoorn FA, Boomsma F, Man in ‘t Veld AJ, Schalekamp MA. Determination of catecholamines in human plasma by high-performance liquid chromatography: comparison between a new method with fluorescence detection and an established method with electrochemical detection. J Chromatogr. 1989;487:17–28. doi: 10.1016/s0378-4347(00)83003-0. [DOI] [PubMed] [Google Scholar]
- 20.Burge CM, Skinner SL. Determination of hemoglobin mass and blood volume with CO: evaluation and application of a method. J Appl Physiol. 1995;79:623–631. doi: 10.1152/jappl.1995.79.2.623. [DOI] [PubMed] [Google Scholar]
- 21.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m) J Appl Physiol. 2006;101:1386–1393. doi: 10.1152/japplphysiol.00342.2006. [DOI] [PubMed] [Google Scholar]
- 22.Adler GK, Moore TJ, Hollenberg NK, Williams GH. Changes in adrenal responsiveness and potassium balance with shifts in sodium intake. Endocr Res. 1987;13:419–445. doi: 10.3109/07435808709035467. [DOI] [PubMed] [Google Scholar]
- 23.Szmuilowicz ED, Adler GK, Williams JS, Green DE, Yao TM, Hopkins PN, Seely EW. Relationship between aldosterone and progesterone in the human menstrual cycle. J Clin Endocrinol Metab. 2006;91:3981–3987. doi: 10.1210/jc.2006-1154. [DOI] [PubMed] [Google Scholar]
- 24.Vernikos J, Dallman MF, Keil LC, O’Hara D, Convertino VA. Gender differences in endocrine responses to posture and 7 days of -6 degrees head-down bed rest. Am J Physiol. 1993;265:E153–161. doi: 10.1152/ajpendo.1993.265.1.E153. [DOI] [PubMed] [Google Scholar]
- 25.Waters WW, Platts SH, Mitchell BM, Whitson PA, Meck JV. Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. Am J Physiol Heart Circ Physiol. 2005;288:H839–847. doi: 10.1152/ajpheart.00220.2004. [DOI] [PubMed] [Google Scholar]
- 26.Millet C, Custaud MA, Maillet A, Allevard AM, Duvareille M, Gauquelin-Koch G, Gharib C, Fortrat JO. Endocrine responses to 7 days of head-down bed rest and orthostatic tests in men and women. Clin Physiol. 2001;21:172–183. doi: 10.1046/j.1365-2281.2001.00315.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollenberg NK, Williams GH. Nonmodulation and essential hypertension. Curr Hypertens Rep. 2006;8:127–131. doi: 10.1007/s11906-006-0008-9. [DOI] [PubMed] [Google Scholar]
- 28.Williams GH, Tuck ML, Sullivan JM, Dluhy RG, Hollenberg NK. Parallel adrenal and renal abnormalities in young patients with essential hypertension. Am J Med. 1982;72:907–914. doi: 10.1016/0002-9343(82)90851-8. [DOI] [PubMed] [Google Scholar]
- 29.Conlin PR, Braley LM, Menachery AI, Hollenberg NK, Williams GH. Abnormal norepinephrine and aldosterone responses to upright posture in nonmodulating hypertension. J Clin Endocrinol Metab. 1992;75:1017–1021. doi: 10.1210/jcem.75.4.1400865. [DOI] [PubMed] [Google Scholar]
- 30.Chapman AB, Zamudio S, Woodmansee W, Merouani A, Osorio F, Johnson A, Moore LG, Dahms T, Coffin C, Abraham WT, Schrier RW. Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. Am J Physiol. 1997;273:F777–782. doi: 10.1152/ajprenal.1997.273.5.F777. [DOI] [PubMed] [Google Scholar]
- 31.Sealey JE, Itskovitz-Eldor J, Rubattu S, James GD, August P, Thaler I, Levron J, Laragh JH. Estradiol- and progesterone-related increases in the renin-aldosterone system: studies during ovarian stimulation and early pregnancy. J Clin Endocrinol Metab. 1994;79:258–264. doi: 10.1210/jcem.79.1.8027239. [DOI] [PubMed] [Google Scholar]
- 32.Hassager C, Riis BJ, Strom V, Guyene TT, Christiansen C. The long-term effect of oral and percutaneous estradiol on plasma renin substrate and blood pressure. Circulation. 1987;76:753–758. doi: 10.1161/01.cir.76.4.753. [DOI] [PubMed] [Google Scholar]
- 33.Jespersen CM, Arnung K, Hagen C, Hilden T, Nielsen F, Nielsen MD, Giese J. Effects of natural oestrogen therapy on blood pressure and renin-angiotensin system in normotensive and hypertensive menopausal women. J Hypertens. 1983;1:361–364. doi: 10.1097/00004872-198312000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension. 1999;33:1190–1194. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- 35.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 36.Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol. 1997;272:H748–752. doi: 10.1152/ajpheart.1997.272.2.H748. [DOI] [PubMed] [Google Scholar]
- 37.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 39.Ichiki T, Usui M, Kato M, Funakoshi Y, Ito K, Egashira K, Takeshita A. Downregulation of angiotensin II type 1 receptor gene transcription by nitric oxide. Hypertension. 1998;31:342–348. doi: 10.1161/01.hyp.31.1.342. [DOI] [PubMed] [Google Scholar]
- 40.Usui M, Ichiki T, Katoh M, Egashira K, Takeshita A. Regulation of angiotensin II receptor expression by nitric oxide in rat adrenal gland. Hypertension. 1998;32:527–533. doi: 10.1161/01.hyp.32.3.527. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfeld R, Livne D, Nevo O, Dayan L, Milloul V, Lavi S, Jacob G. Hormonal and volume dysregulation in women with premenstrual syndrome. Hypertension. 2008;51:1225–1230. doi: 10.1161/HYPERTENSIONAHA.107.107136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.