Abstract
Background
Individuals with exaggerated exercise blood pressure (BP) tend to develop future hypertension. It is controversial if they have higher risk of death from cardiovascular disease (CVD).
Methods and Results
6,578 asymptomatic Lipid Research Clinic Prevalence Study participants (45% women, mean age 46 years, 74% untreated baseline BP <140/90mmHg [non-hypertensive]) performing submaximal Bruce treadmill tests were followed for 20 years (385 CVD deaths occurred). Systolic and diastolic BP at rest, Bruce stage 2, and maximal BP during exercise were significantly associated with CVD death. Comparing multivariate hazard ratios (HRs) and 95% confidence intervals per 10/5mmHg BP increments, the association was strongest for rest BP (systolic 1.21 [1.14–1.27]; diastolic 1.20 [1.14–1.26]), then Bruce stage 2 BP (systolic 1.09 [1.04–1.14]; diastolic 1.09 [1.05–1.13]), then maximal exercise BP (systolic 1.06 [1.01–1.10]; diastolic 1.04 [1.01–1.08]). Overall, exercise BP was not significant after adjustment for rest BP. However, hypertension status modified the risk associated with exercise BP (p, interaction=0.03). Among non-hypertensives, whether they had normal BP (<120/80mmHg) or prehypertension, Bruce stage 2 BP >180/90 vs. ≤180/90mmHg carried increased risk independent of rest BP and risk factors (adjusted HR for systolic 1.96 [1.40–2.74], p<0.001; diastolic 1.48 [1.06–2.06], p=0.02), and added predictive value (net reclassification improvement systolic 12.0% [−0.1–24.2%], diastolic 9.9% [−0.3–20.0%]; relative integrated discrimination improvement 14.3% and 12.0%, respectively).
Conclusions
In asymptomatic individuals, elevated exercise BP carried higher risk of CVD death, but became non-significant after accounting for rest BP. However, Bruce stage 2 BP >180/90mmHg identified non-hypertensive individuals at higher risk of CVD death.
Keywords: Exercise, blood pressure, cardiovascular disease, mortality
Blood pressure (BP) response to exercise has been studied in relation to incident hypertension. Several studies found that non-hypertensive individuals with exaggerated BP responses during Bruce and ergonomic stress protocols are more likely to develop clinical hypertension compared to those with a normal BP response.1–3 Subsequently, several studies examined whether exercise BP in healthy individuals predicts future morbidity and death from cardiovascular disease (CVD), the results of which have varied, depending in part on the stage of exercise when BP was measured.
Fagard and colleagues first noted that neither submaximal nor maximal exercise BP yielded additive prognostic information over baseline BP for CVD or all-cause mortality in their study of 143 hypertensive men.4 Contrarily, others found that exaggerated submaximal exercise BP was associated with future CVD death independent of rest BP,5–7 although in many of these studies, the ability of exercise BP to predict death was attenuated once BP was measured at maximum capacity.5, 7 A recent analysis from the Framingham Offspring study found exercise diastolic BP, but not systolic BP, measured at low level exercise (stage 2 Bruce protocol) was associated with CVD events independent of other risk factors and baseline BP.8 Given this conflicting data on the association with CVD endpoints, the American College of Cardiology/American Heart Association guidelines on exercise testing included exercise-induced hypertension as a marker for future clinical hypertension, but did not link it with CVD or mortality outcomes.9
Therefore, the present study aimed to evaluate whether exaggerated systolic or diastolic BP attained at low and submaximal exercise was associated with future CVD death in a large population of healthy men and women. We also aimed to determine whether exercise BP response provided incremental risk information to baseline BP and other risk factors.
Methods
Study Participants
Participants were derived from the Lipid Research Clinics Prevalence Study, a prospective cohort of North American individuals across 10 geographic locations and diverse socioeconomic and occupational groups, as previously described.10–11 Briefly, participants were recruited from selected target populations in North America meant to provide a cross-sectional view by virtue of size, economic, and geographical diversity. The populations fell into three broad categories: occupational groups, household or residential groups, and parents of school children. Recruited individuals were screened at visit 1 for triglycerides and cholesterol. Participants with elevated lipids, as well as an additional random sample of all-comers and subjects with borderline hyperlipidemia were asked to return for visit 2. In total, the random sample constituted 58.4% of the total study participants. A comprehensive medical history, physical examination, fasting blood samples, resting electrocardiogram, and exercise treadmill test were obtained at baseline visit 2 (1972–1976). Participants were ineligible if pregnant, had evidence of current or past myocardial ischemia or coronary bypass surgery, baseline systolic BP <90 or >200mmHg or diastolic BP >120mmHg, or were deemed ineligible for exercise testing by the study physicians.
Altogether, 8,652 of 13,852 individuals screened underwent baseline exercise tests. Of these, we additionally excluded 1,877 individuals to reduce potential confounding by pre-existing disease or comorbidity for the following reasons: exercise duration <1 minute, known or suspected CVD (myocardial infarction, angina, stroke, claudication, heart surgery, congestive heart failure, or digitalis use), baseline age <30 or >70 years, treadmill test other than Bruce protocol, and those lost to follow-up (n=6). Another 197 individuals were excluded due to missing baseline or exercise BP measurements, resulting in 6,578 individuals for this analysis.
Follow-up was until death or December 31, 1995. The primary outcome of this study was CVD death, ascertained with death certificates, interviews with next of kin, medical records, the National Death Index, and the Epidemiology Research Index. All participants gave written informed consent at study enrollment. The Brigham and Women’s Institutional Review Board approved the present study. Drs. Weiss and Mora had full access to the data and take full responsibility for its integrity. All authors agree to the manuscript as written.
Exercise Test Protocol
Participants underwent a standard submaximal Bruce protocol treadmill exercise test,12 which was terminated when a predetermined target heart rate of ≥90% of maximal predicted heart rate for age and physical activity was achieved and maintained for one minute or until the end of the stage, or when target heart rate was exceeded by 8 beats per minute.10 An electrocardiogram was monitored prior to test initiation, continuously during exercise, and for 6-minutes post-exercise. Heart rate was monitored continuously. BP measurements were taken at the end of each 3-minute exercise stage by the cuff technique. Participants were allowed to touch the treadmill for balance, but not to pull or lean on it. The test could be terminated early at the discretion of the supervising physicians for significant symptoms, arrhythmias, hemodynamic instability, ST-segment changes on electrocardiogram, or if the participant was unwilling or unable to continue.
Baseline and Exercise Test Variables
The mean of two BP measurements taken at rest (supine) prior to exercise was used to define hypertension (systolic BP≥140mmHg, diastolic BP≥90mmHg, or use of anti-hypertensive medications). Medication use was assessed by questionnaires or examination of medications. Resting BP was categorized into 4 groups according to the 7th Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7) recommendations (<120/80 [normal], 120–139/80–89 [prehypertension], 140–159/90–99 [stage I hypertension], and ≥160/100mmHg [stage II hypertension]).13 Resting BP <140/90mmHg was further subcategorized into 2 levels of prehypertension defined by JNC 7, 120–129/80–84 and 130–139/85–89mmHg.
Maximum exercise systolic BP (ExSBPmax, in mmHg) was defined as maximum systolic BP recorded during exercise, and divided into 4 groups corresponding to approximate quartiles (≤160, 161–180, 181–200, and >200mmHg). Maximum exercise diastolic BP (ExDBPmax, in mmHg) was defined and divided similarly (≤80, 81–85, 86–95, >95mmHg). Since BP at low level exercise has been suggested to be more predictive of incident CVD events compared with maximal BP,6–8 we also examined Bruce stage 2 systolic (ExSBPlow) and diastolic (ExDBPlow ) BP divided into population quartiles (ExSBPlow≤146, 147–160, 161–180, and >180mmHg; ExDBPlow≤73, 74–80, 81–90, and >90mmHg). Peak exercise capacity, expressed in metabolic equivalents (METs), was calculated from total treadmill time.14
Statistical Analyses
STATA software (version 10.1) was used for statistical analyses. Mean ± standard deviation (SD) values were calculated for continuous variables, and statistical comparisons were made with ANOVA. Categorical variables were compared by χ2 statistics. Significance tests were two-tailed, with probability values <0.05 considered significant.
Cox proportional hazard models were used to examine the association of baseline and exercise BP with time to CVD death. We considered 3 levels of adjustment: 1) age and sex, 2) plus non-BP variables (age, sex, diabetes, low- and high-density lipoprotein [LDL and HDL] cholesterol, triglycerides, smoking, body mass index, and family history), 3) plus rest BP (for models examining exercise BP). We further adjusted the model with non-BP variables for exercise capacity to account for differences in achieved exercise workload. The proportional hazard assumption was satisfied using Schoenfeld residuals and the natural logarithm of follow-up time.
To examine the additional value of exercise BP according to baseline hypertension, individuals were stratified according to baseline presence or absence of hypertension and evaluated for exercise BP. Similar analyses were performed for participants with baseline prehypertension and normal BP. Statistical tests for interaction between baseline and exercise BP in relation to CVD death were obtained using likelihood ratio tests.
The likelihood ratio χ2 statistic was used to evaluate the goodness-of-fit of predictive models. Model discrimination was examined using the c-index,15 a generalization of the area under the receiver operator characteristic curve. Risk reclassification was assessed by categorizing the predicted 10-year CVD death risk for each model into 3 categories (≤2.5%, 2.5% to 10%, and >10%) or 4 categories (≤2.5%, 2.5% to 5%, 5% to 10%, and >10%). We computed the Net Reclassification Improvement (NRI),16 which compares the shifts in reclassified categories by observed outcome, and the Integrated Discrimination Improvement (IDI),16 which compares the integrals of sensitivity and specificity under two models. Due to the fewer number of events among those with normal BP or prehypertension during this 10-year interval, we assessed the NRI and IDI for the combined group (non-hypertensives).
RESULTS
Baseline Characteristics
The cohort of 6,578 asymptomatic study participants was middle-aged (mean age 46 years), with a large proportion (45%) of women (Table 1). Of the total, 4,844 (74%) had untreated resting BP <140/90mmHg (non-hypertensive). Table 2 shows baseline demographic, clinical, and exercise test characteristics according to categories of Bruce stage 2 BP. Except for regular exercise which occurred more frequently in increasing quartiles of ExSBPmax and ExDBPlow, the remaining characteristics were similar in Bruce 2 and maximum BP categories regardless of stage of exercise and are thus shown by ExSBPlow and ExDBPlow in Table 2. Individuals in increasing categories of ExSBP and ExDBP were older with more prevalent diabetes, hypertension, and dyslipidemia. Just over half of individuals with baseline hypertension also had ExSBPmax>200mmHg (56.5%) and ExSBPlow>180mmHg (50.3%). Smoking and a premature family history of coronary disease were somewhat less prevalent in those with highest ExSBP, and smoking was less prevalent in those with the highest ExDBP.
Table 1.
Baseline characteristics
| Men n=3650 | Women N=2928 | |
|---|---|---|
| Demographic and clinical * | ||
| Age, years | 44.8 (10.3) | 46.6 (11.4) |
| Hypertension | 28.3 | 24.0 |
| Current smoker | 37.7 | 33.7 |
| Diabetes | 3.6 | 2.7 |
| Family history CHD <60years┼ | 16.4 | 18.7 |
| Total cholesterol ≥ 240 mg/dL | 33.2 | 33.4 |
| LDL cholesterol ≥160 mg/dL | 36.0 | 33.6 |
| HDL cholesterol <40 mg/dL | 38.2 | 10.6 |
| Triglycerides ≥200 mg/dL | 27.6 | 11.9 |
| Body mass index, kg/m2 | 26.8 (3.5) | 24.9 (4.7) |
| Regular exerciseǂ | 30.7 | 12.9 |
| Exercise test | ||
| Rest BP, mmHg | 125 (15) | 121 (18) |
| Systolic | ||
| Diastolic | 81 (10) | 77 (10) |
| Bruce stage 2 BP, mmHg | ||
| Systolic | 169 (25) | 158 (25) |
| Diastolic | 83 (14) | 81 (14) |
| Maximum BP during exercise, mmHg | ||
| Systolic | 187 (24) | 172 (26) |
| Diastolic | 88 (13) | 86 (14) |
| Proportion achieving target HR | 76.0 | 63.5 |
| Exercise capacity, METs | 10.4 (2.3) | 7.3 (2.3) |
| STD ≥1mm | 4.1 | 4.6 |
Values shown are mean (SD) or percentage
Abbreviations: HR, heart rate; CHD, coronary heart disease; LDL, low density lipoprotein; HDL, high density lipoprotein; METs, metabolic equivalents; STD, ST-segment depressions.
Family history: premature coronary heart disease in a parent or sibling.
Regular exercise: self-reported participation in regular strenuous activity or hard labor.
Table 2.
Baseline and exercise test characteristics according to Bruce stage 2 exercise BP groups
| Bruce stage 2 exercise systolic BP, mmHg | |||||
|---|---|---|---|---|---|
| ≤146 | 147–160 | 161–180 | >180 | P, trend | |
| Demographic and clinical * | |||||
| Age, years | 41.0 (8.5) | 42.6 (8.8) | 44.9 (9.8) | 49.8 (11.0) | <0.001 |
| Hypertension | 6.3 | 14.8 | 27.6 | 50.3 | <0.001 |
| Current smoker | 38.6 | 35.2 | 38.1 | 34.1 | 0.03 |
| Diabetes | 1.0 | 2.0 | 3.1 | 6.1 | <0.001 |
| Family history of CHD <60years┼ | 19.5 | 17.3 | 18.2 | 15.3 | 0.03 |
| Total cholesterol, mg/dL | 211 (49) | 218 (44) | 225 (44) | 228 (45) | <0.001 |
| LDL cholesterol, mg/dL | 137 (40) | 143 (41) | 149 (41) | 150 (41) | <0.001 |
| HDL cholesterol, mg/dL | 52 (15) | 50 (15) | 49 (15) | 49 (16) | <0.001 |
| Triglycerides, mg/dL | 130 (217) | 148 (122) | 167 (144) | 184 (178) | <0.001 |
| Body mass index, kg/m2 | 24.3 (3.7) | 25.7 (3.7) | 26.6 (4.1) | 27.2 (4.1) | <0.001 |
| Regular exerciseǂ | 24.4 | 25.9 | 25.0 | 21.7 | 0.07 |
| Exercise test | |||||
| Rest BP | |||||
| Systolic, mmHg | 111.3 (11.4) | 118.7 (12.0) | 125.2 (13.3) | 135.9 (16.9) | <0.001 |
| Diastolic, mmHg | 73.3 (9.1) | 77.9 (9.4) | 81.3 (9.5) | 85.2 (10.2) | <0.001 |
| Bruce stage 2 BP | |||||
| Systolic, mmHg | 134.4 (9.1) | 154.8 (4.5) | 171.9 (6.0) | 200.0 (15.7) | --- |
| Diastolic, mmHg | 74.8 (10.6) | 80.0 (11.0) | 83.7 (13.0) | 91.4 (16.0) | <0.001 |
| Maximum BP during exercise | |||||
| Systolic, mmHg | 155.1 (17.0) | 171.2 (14.2) | 187.0 (15.2) | 209.9 (18.5) | <0.001 |
| Diastolic, mmHg | 79.4 (9.6) | 84.5 (10.2) | 88.7 (12.0) | 96.6 (15.1) | <0.001 |
| Proportion achieving target HR | 75.7 | 77.0 | 76.2 | 67.1 | <0.001 |
| Exercise capacity, METs | 10.0 (2.3) | 9.9 (2.3) | 9.6 (2.2) | 8.8 (2.1) | <0.001 |
| Bruce stage 2 exercise diastolic BP, mmHg | |||||
|---|---|---|---|---|---|
| ≤73 | 74–80 | 81–90 | >90 | P, trend | |
| Demographic and clinical * | |||||
| Age, years | 42.8 (10.4) | 43.2 (9.3) | 45.0 (9.7) | 47.5 (10.4) | <0.001 |
| Hypertension | 12.1 | 15.8 | 26.5 | 47.1 | <0.001 |
| Current smoker | 38.1 | 39.4 | 33.8 | 33.9 | 0.001 |
| Diabetes | 1.7 | 1.9 | 3.7 | 4.6 | <0.001 |
| Family history of CHD <60years┼ | 17.4 | 18.2 | 17.2 | 17.9 | 0.89 |
| Total cholesterol, mg/dL | 214 (43) | 219 (43) | 224 (47) | 228 (50) | <0.001 |
| LDL cholesterol, mg/dL | 140 (39) | 144 (41) | 146 (43) | 150 (41) | <0.001 |
| HDL cholesterol, mg/dL | 52 (15) | 50 (16) | 50 (15) | 48 (16) | <0.001 |
| Triglycerides, mg/dL | 135 (106) | 146 (114) | 162 (148) | 191 (284) | <0.001 |
| Body mass index, kg/m2 | 24.8 (3.6) | 25.6 (4.0) | 26.4 (4.0) | 27.2 (4.3) | <0.001 |
| Regular exerciseǂ | 21.8 | 26.7 | 26.1 | 21.9 | 0.001 |
| Exercise test | |||||
| Rest BP | |||||
| Systolic, mmHg | 116.4 (14.2) | 118.8 (14.3) | 124.0 (14.7) | 132.7 (17.0) | <0.001 |
| Diastolic, mmHg | 75.0 (9.3) | 76.7 (9.4) | 80.8 (9.2) | 86.2 (10.7) | <0.001 |
| Bruce stage 2 BP | |||||
| Systolic, mmHg | 153.3 (22.8) | 156.7 (21.6) | 166.9 (22.1) | 184.9 (23.7) | <0.001 |
| Diastolic, mmHg | 65.0 (7.5) | 78.5 (2.2) | 87.4 (2.9) | 102.0 (8.2) | --- |
| Maximum BP during exercise | |||||
| Systolic, mmHg | 169.6 (23.8) | 173.5 (23.1) | 182.2 (22.6) | 198.6 (23.8) | <0.001 |
| Diastolic, mmHg | 73.6 (8.2) | 82.6 (5.6) | 91.2 (6.1) | 105.0 (9.2) | <0.001 |
| Proportion achieving target HR | 74.6 | 75.7 | 74.9 | 71.4 | 0.06 |
| Exercise capacity, METs | 9.6 (2.3) | 9.9 (2.2) | 9.6 (2.2) | 9.2 (2.2) | <0.001 |
Values shown are mean (SD) or percentage
See text for abbreviations.
Family history: premature coronary heart disease in a parent or sibling.
Regular exercise: self-reported participation in regular strenuous activity or hard labor.
Exercise Blood Pressure and CVD Death
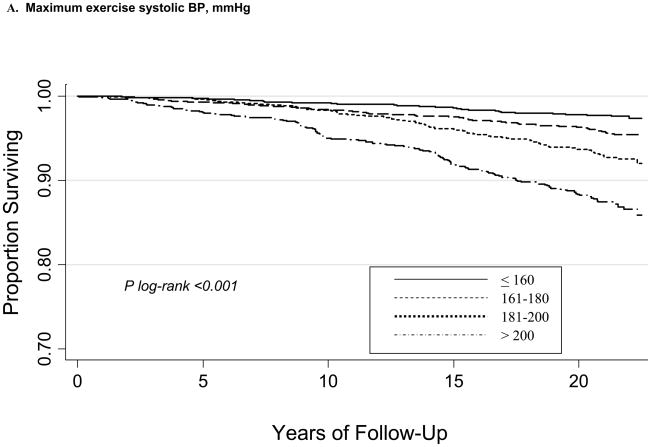
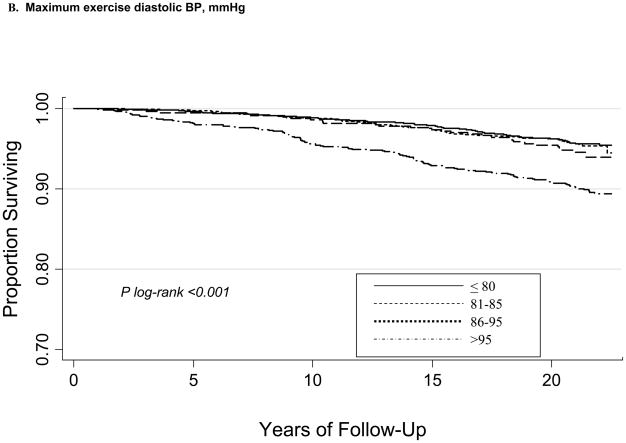
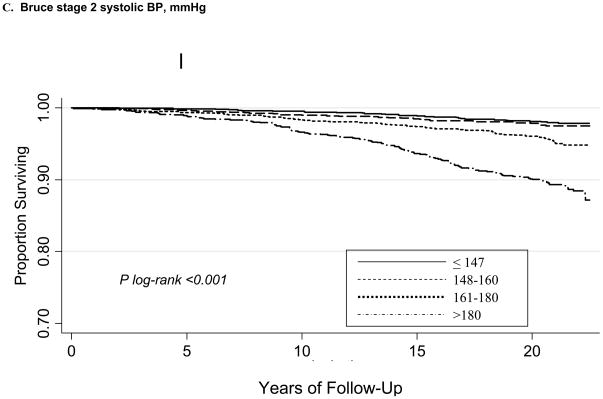
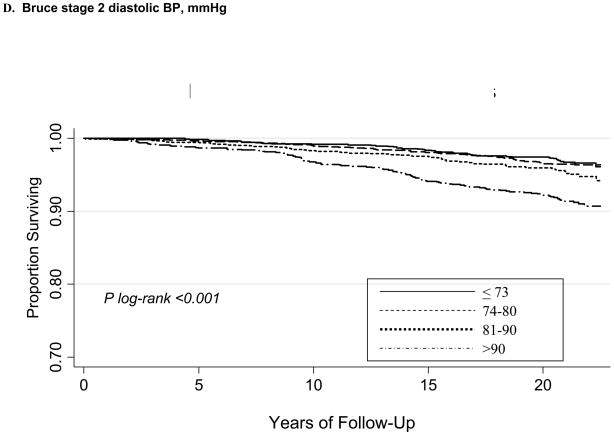
During a mean follow-up of 20.1±4.0 years, there were 385 CVD deaths. Kaplan-Meier curves according to maximum (ExSBPmax and ExDBPmax) and Bruce stage 2 BP (ExSBPlow and ExDBPlow) categories show significant associations with survival free of CVD death (Figure), with a wider curve separation noted for systolic compared with diastolic exerciseBP curves.
Figure 1.
Kaplan-Meier survival curves for CVD death according to maximum exercise systolic BP categories (A), maximum exercise diastolic BP categories (B), Bruce stage 2 systolic BP categories (C), and Bruce stage 2 diastolic BP categories (D).
Exercise Systolic Blood Pressure
In age-and sex-adjusted analyses (Table 3), higher levels of systolic BP measured at rest, Bruce stage 2, and during exercise were associated with increased risk of CVD death. Based on hazard ratios (HRs) per 10 mmHg-increments in systolic BP, the magnitude of association was stronger for rest systolic BP, followed by ExSBPlow, then ExSBPmax. We also repeated these analyses per 1-standard deviation increment in BP (data not shown) and found consistent results, i.e. that the strength of association was strongest for rest BP followed by Bruce stage 2 BP and then maximal exercise BP. With further adjustment for diabetes, lipids, smoking, body mass index, and family history, HRs were somewhat attenuated but remained significant at the highest quartiles (adjusted HR 1.88, 95% CI 1.23–2.87, for ExSBPlow>180mmHg; and 1.66, 95% CI 1.14–2.40, for ExSBPmax>200mmHg).
Table 3.
Association of baseline and exercise BP with CVD death
| Age- and sex-adjusted | |||||||
|---|---|---|---|---|---|---|---|
| Rest BP, range, mmHg | ≤120/80 | 120–139/80–89 | 140–159/90–99 | ≥160/100 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 1.73 (1.30–2.31) | 2.73 (1.99–3.74) | 2.89 (1.93–4.32) | <0.001 | 1.21 (1.15–1.28) | <0.001 |
| Diastolic, HR (95%CI) | Ref. | 1.17 (0.91–1.51) | 2.35 (1.80–3.06) | 2.40 (1.64–3.53) | <0.001 | 1.20 (1.15–1.26) | <0.001 |
| Bruce stage 2 BP, range, mmHg | ≤146/73 | 147–160/74–80 | 161–180/81–90 | >180/90 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 0.97 (0.60–1.57) | 1.46 (0.96–2.24) | 2.22 (1.47–3.34) | <0.001 | 1.10 (1.06–1.15) | <0.001 |
| Diastolic, HR (95%CI) | Ref. | 1.13 (0.77–1.66) | 1.37 (0.95–1.99) | 1.80 (1.27–2.56) | <0.001 | 1.10 (1.06–1.15) | <0.001 |
| Maximum BP during exercise, range, mmHg | ≤160/80 | 161–180/81–85 | 181–200/86–95 | >200/95 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 1.20 (0.82–1.75) | 1.48 (1.00–2.17) | 1.94 (1.35–2.79) | <0.001 | 1.08 (1.04–1.12) | <0.001 |
| Diastolic, HR (95%CI) | Ref. | 1.46 (1.02–2.10) | 1.06 (0.80–1.40) | 1.61 (1.25–2.06) | 0.001 | 1.06 (1.03–1.10) | <0.001 |
| Adjusted for non-BP variables* | |||||||
| Rest BP, range, mmHg | ≤120/80 | 120–139/80–89 | 140–159/90–99 | ≥160/100 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 1.68 (1.25–2.26) | 2.61 (1.88–3.62) | 2.86 (1.89–4.32) | <0.001 | 1.21 (1.14–1.27) | <0.001 |
| Diastolic, HR (95%CI) | Ref. | 1.21 (0.94–1.57) | 2.36 (1.79–3.12) | 2.28 (1.53–3.39) | <0.001 | 1.20 (1.14–1.26) | <0.001 |
| Bruce stage 2 BP, range, mmHg | ≤146/73 | 147–160/74–80 | 161–180/81–90 | >180/90 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 0.91 (0.56–1.48) | 1.31 (0.85–2.02) | 1.88 (1.23–2.87) | <0.001 | 1.09 (1.04–1.14) | 0.001 |
| Diastolic, HR (95%CI) | Ref. | 1.09 (0.74–1.60) | 1.24 (0.85–1.81) | 1.63 (1.14–2.32) | 0.004 | 1.09 (1.05–1.13) | <0.001 |
| Maximum BP during exercise, range, mmHg | ≤160/80 | 161–180/81–85 | 181–200/86–95 | >200/95 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 1.20 (0.82–1.76) | 1.37 (0.93–2.02) | 1.66 (1.14–2.40) | 0.002 | 1.06 (1.01–1.10) | 0.008 |
| Diastolic, HR (95%CI) | Ref. | 1.54 (1.07–2.22) | 1.03 (0.77–1.37) | 1.40 (1.08–1.81) | 0.037 | 1.04 (1.01–1.08) | 0.02 |
| Adjusted for non-BP variables and baseline BP┼ | |||||||
| Bruce stage 2 BP, range, mmHg | ≤146/73 | 147–160/74–80 | 161–180/81–90 | >180/90 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 0.81 (0.50–1.32) | 1.03 (0.66–1.59) | 1.22 (0.78–1.91) | 0.14 | 1.01 (0.96–1.07) | 0.69 |
| Diastolic, HR (95%CI) | Ref. | 1.05 (0.71–1.53) | 1.06 (0.72–1.53) | 1.11 (0.77–1.61) | 0.58 | 1.03 (0.99–1.08) | 0.15 |
| Maximum BP during exercise, range, mmHg | ≤160/80 | 161–180/81–85 | 181–200/86–95 | >200/95 | P, trend | Per 10/5mmHg | P |
| Systolic, HR (95%CI) | Ref. | 1.07 (0.73–1.57) | 1.13 (0.76–1.67) | 1.14 (0.77–1.69) | 0.50 | 1.00 (0.95–1.04) | 0.83 |
| Diastolic, HR (95%CI) | Ref. | 1.40 (0.97–2.02) | 0.90 (0.68–1.21) | 1.03 (0.78–1.34) | 0.79 | 0.99 (0.96–1.03) | 0.74 |
Models adjusting for non-BP variables adjusted for age, sex, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, smoking, body mass index, and family history
Models adjusting for non-BP variables and baseline BP additionally included baseline systolic and diastolic BP.
When exercise capacity was added to the models (not shown), the HR for ExSBPlow was further attenuated (1.52, 95% CI 0.98–2.36, for ExSBPlow>180mmHg) but remained unchanged for ExSBPmax (1.65, 95% CI 1.13–2.40, for ExSBPmax>200mmHg). Additional adjustment for exercise-induced ST-segment ischemic changes, chest pain terminating the test, and achieving target heart rate did not significantly change these results.
Exercise Diastolic Blood Pressure
Analyses were also performed for diastolic BP measured at rest, ExDBPlow and ExDBPmax (Table 3). In age- and sex-adjusted as well as multivariate-adjusted analyses, ExDBPlow>90mmHg and ExDBPmax>95mmHg were significantly associated with CVD death. Based on the HRs per 5 mmHg- increments in diastolic BP, the magnitude of association was stronger for rest diastolic BP, followed by ExDBPlow, then ExDBPmax, and similarly when analyses were repeated per 1-standard deviation increments (not shown). After adjustment for non-BP variables, corresponding HRs for ExDBPlow>90mmHg and ExDBPmax>95mmHg were 1.63 (95% CI 1.14–2.32), and 1.40 (95% CI 1.08–1.81), respectively. With further adjustment for exercise capacity, the HRs were not substantially changed (1.60, 95% CI 1.12–2.29, and 1.42, 95% CI 1.10–1.83, respectively, not shown).
When rest systolic and diastolic BP were added to the multivariate models, neither exercise systolic nor diastolic BP attained at any level of exercise was significantly associated with CVD death in the population as a whole.
Exercise Blood Pressure in Non-Hypertensive Individuals
Since ExSBPlow and ExDBPlow were the strongest exercise BP variables associated with CVD death, further analyses at Bruce stage 2 exercise were performed across prespecified subcategories of baseline BP. When examining only non-hypertensive individuals (untreated baseline BP <140/90mmHg), Bruce stage 2 BP >180/90mmHg was associated with a significant 1.5- to 2-fold increased risk of CVD death (Table 4). Importantly, with further adjustment for rest BP, baseline non-hypertensive participants continued to have significantly higher risk of CVD death associated with ExSBPlow>180 vs. ≤180mmHg (adjusted HR 1.81, 95% CI 1.28–2.55, p=0.001).
Table 4.
Association of Bruce stage 2 exercise BP with CVD death according to baseline BP categories
| Normal BP <120/<80mmHg N=2338 | Prehypertension 120–139/80–89mmHg N=2506 | Non-Hypertension (Normal BP or Prehypertension) <140/<90mmHg N=4844 | Hypertension ≥140/90mmHg Or treatment N=1734 | |
|---|---|---|---|---|
| Bruce stage 2 SBP | 2.44 (1.27–4.69) | 1.64 (1.11–2.44) | 1.96 (1.40–2.74) | 1.32 (0.95–1.83) |
| >180 vs. ≤ 180mmHg | P=0.007 * | P=0.01 * | P<0.001 * | P=0.09 |
| Bruce stage 2 SBP | 1.07 (0.91–1.26) | 1.05 (0.96–1.16) | 1.08 (1.00–1.17) | 1.00 (0.93–1.06) |
| per 10 mmHg increase | P=0.41 | P=0.27 | P=0.04 | P=0.89 |
| Bruce stage 2 DBP | 2.07 (1.07–3.99) | 1.25 (0.86–1.83) | 1.48 (1.06–2.06) | 1.51 (1.13–2.03) |
| >90 vs. ≤ 90 mmHg | P=0.03 * | P=0.25 | P=0.02 ┼ | P=0.006 * |
| Bruce stage 2 DBP | 1.04 (0.90–1.21) | 1.02 (0.95–1.10) | 1.04 (0.98–1.11) | 1.06 (1.01–1.12) |
| per 5 mmHg increase | P=0.58 | P=0.59 | P=0.21 | P=0.02 |
Values shown are HRs (95% CI) adjusted for non-BP risk factors
After additionally adjusting for rest systolic and diastolic BP plus non-BP risk factors:
P <0.05 and
P=0.06 after additionally adjusting for rest systolic and diastolic BP plus non-BP risk factors.
Participants with Normal Blood Pressure
Furthermore, the subcategory of non-hypertensive individuals with normal BP (<120/80mmHg) and ExSBPlow>180 vs. ≤180mmHg saw the greatest increase in risk of CVD death compared to individuals with prehypertension or hypertension (Table 4), and risk persisted after adjusting for rest BP (adjusted HR 2.39, 95% CI 1.26–4.51, p=0.007).
Participants with Prehypertension
The presence of prehypertension at baseline incurred an increased risk of CVD death. Subcategorization of participants into those with baseline BP <120/80mmHg (reference) followed by prehypertension values 120–129/80–84 (n=1488) and 130–139/85–89mmHg (n=1018), yielded adjusted HRs of 1.00, 1.18 (0.79–1.76), and 1.65 (1.11–2.46), respectively; p trend=0.01. Furthermore, among prehypertensive individuals, having ExSBPlow>180 vs. ≤180mmHg imparted a significantly higher risk of CVD death (Table 4), which persisted after further adjustment for rest BP (adjusted HR 1.56, 95% CI 1.05– 2.32, p=0.03).
Tests for Interactions
Statistically significant interactions were seen between baseline BP and exercise systolic BP in relation to CVD death. There was significant interaction between baseline BP categorized as hypertension/non-hypertension and ExSBPlow>180 vs. ≤180mmHg for CVD death (p, interaction=0.03). Similarly, significant interaction was seen when baseline BP was categorized as normal BP/prehypertension/hypertension. No significant interactions were noted for exercise diastolic BP.
Incremental Value of Bruce Stage 2 Exercise BP Over Baseline BP and Risk Factors
Finally, we compared measures of model discrimination, goodness-of-fit, and reclassification (Supplementary Table) for models with and without ExSBPlow. The referent model was comprised of baseline BP and non-BP risk factors, and compared with two models: one that incorporated ExSBPlow>180 vs. ≤180mmHg, and the other incorporated ExDBPlow>90 vs. ≤90mmHg. There was no substantial change in the c-index. ExBPlow improved goodness-of-fit, especially for non-hypertensive individuals. When subjects were classified according to 3 categories of risk (≤2.5%, 2.5% to 10%, and 10%), there was borderline significant reclassification improvement (p=0.05) with adding ExSBPlow>180mmHg to the referent model (NRI 12.0%, 95% CI [−0.1–24.2%]), or ExDBPlow>90mmHg (NRI 9.9%, 95% CI [−0.3– 20.0%]). Repeat analysis using 4 categories of risk resulted in respective NRI values of 18.0% (3.1–32.9%, p=0.02) and 12.0% (−1.4–125.3%, p=0.08). The IDI was significantly improved with adding either ExSBPlow or ExDBPlow to the referent model (respective relative IDIs of 14.3% and 12.0%, p=0.02 and 0.03).
Discussion
In this, prospective North American cohort of asymptomatic individuals followed for 20 years, we found that BP measured at rest, at Bruce stage 2, and during exercise were all significantly associated with CVD death independent of non-BP risk factors. The association was strongest for rest BP, followed by Bruce stage 2 BP, then maximal BP attained during exercise. Further analysis revealed exercise BP was no longer significant after accounting for rest BP overall. However, baseline hypertension status modified the risk associated with exercise BP. Specifically, among non-hypertensives with either normal BP or prehypertension at rest, elevated exercise BP at Bruce stage 2 >180/90mmHg identified individuals with up to 2.4-fold higher risk of future CVD death and added predictive value to rest BP and risk factors, possibly warranting more aggressive treatment than currently recommended.
Prior studies were mixed regarding the independent association of exercise BP and CVD death. Fagard and colleagues4 demonstrated that BP attained at submaximal or maximal workloads was not predictive of future CVD death once the model controlled for baseline BP. Although their study population was small and included only baseline hypertensive participants, these finding are in accordance with the present study which included a larger and healthier baseline population.
The idea that systolic exercise BP independently predicts CVD death came from two studies from the early 1990s. Filipovsky and colleagues studied nearly 5,000 subjects and found an association between submaximal exercise systolic BP and death after controlling for baseline BP.6 However, they did not find an association between baseline hypertension and CVD death. Mundal and colleagues examined >2,000 subjects and found submaximal exercise systolic BP was significantly related to CVD death independent of baseline BP.7 However, levels of significance diminished once baseline BP was added to the model, consistent with our findings and suggesting that the relative contribution of baseline BP may have been underestimated when initially reported.
Our study additionally extends the knowledge of exercise diastolic BP and its association with CVD death. Until recently, there have been correlative findings between exercise diastolic BP and cholesterol levels and insulin resistance,17 but little on its capability to predict death. The Framingham Offspring study recently noted an association between low-level exercise diastolic BP and CVD events.8 The present study suggests that both Bruce stage 2 and maximum diastolic BP predict future CVD death. However, like its systolic counterpart, the predictive capability of diastolic BP during exercise is attenuated once rest BP is added to the model.
Interestingly, we found that baseline hypertension status significantly modified the risk associated with exercise BP. Furthermore, there was significant reclassification of risk (up to 18.0%) among non-hypertensives with adding Bruce stage 2 BP >180/90mmHg to baseline risk factors. Among non-hypertensives, and particularly those with normal rest BP (<120/80mmHg), having an elevated Bruce stage 2 BP >180/90mmHg imparted increased risk for CVD death, even after accounting for baseline BP and risk factors. Physiologically, increased cardiac output, decreased peripheral vascular resistance, and the interaction therein determines blood pressure during exercise. When cardiac output is not balanced by increased compliance from peripheral muscle vasculature dilation, the result is a sharp increase in systolic BP.8 This aberrant physiology, which may relate to early vascular stiffness or an exaggerated sympathetic response, may account for the increased risk in this population, particularly for those at Bruce stage 2 who had normal baseline BP, as they experienced the steepest change in BP at this early stage of exercise, incurring a higher risk compared to those with higher resting BP values or higher BP at later stages of exercise. The stronger relationship of Bruce stage 2 BP and future death compared to maximum exercise BP may also reflect that participants were asked to stop exercising once target heart rate was achieved. The association of maximum exercise BP with death may have been stronger if participants were allowed to exercise to exhaustion. However, inability to standardize maximal effort given different participant stature, size, and level of conditioning may be an insurmountable challenge of exercise testing.
Similar to individuals with normal BP, prehypertensive individuals with Bruce stage 2 exercise BP >180/90mmHg had higher risk after accounting for baseline BP and risk factors. Data from NHANES 2005–2006 estimate that ~25–35% of the US population ≥20 years of age has prehypertension, including >32 million men and >25 million women.18 Increased CVD,19 thickening of the carotid intima and media,20 and diastolic ventricular dysfunction,21 have been demonstrated in prehypertensive individuals, including those with BP as low as 115/75mmHg.22 Recent work noted increased CVD events in prehypertensives across the age spectrum23 and consistent with our own findings, in the prehypertensive group 130–139/85–89mmHg.24–25 As there is little evidence regarding pharmacologic treatment and outcomes in this population, current treatment recommendations are lifestyle modifications.
There are potential limitations to the present study. Despite participant recruitment in various geographic sites from a large range of socioeconomic and occupational populations, >95% of participants were Caucasian. Additionally, ~40% of the study population was recruited on the basis of lipid abnormalities. However, similar Framingham Risk Scores and death rates were previously reported in populations less saturated with lipid abnormalities, with no significant interaction between high LDL cholesterol and death.26 The exercise test protocol was a submaximal Bruce protocol, with termination at ≥90% of maximum predicted heart rate. It remains to be seen if our results would be applicable to other exercise test protocols and if results would change if participants were allowed to exercise to exhaustion. Additionally, our primary study end point was CVD death, a hard endpoint, and it is unclear if similar results would be noted for non-fatal “softer” cardiovascular events, such as angina or non-fatal myocardial infarction.
In conclusion, rest BP, low-level (Bruce stage 2) exercise BP, and maximum exercise BP were associated with higher CVD death. Exercise-related BP, regardless of workload, was not significant after accounting for rest BP in the overall population. However, baseline hypertension status significantly modified the risk associated with exercise BP. Among non-hypertensives with either normal BP or prehypertension, Bruce stage 2 BP >180/90mmHg identified those at significantly higher risk of CVD death and added predictive value to rest BP and other risk factors, a finding that may warrant more aggressive treatment in those individuals than is currently recommended.
Supplementary Material
Acknowledgments
The investigators, staff, and participants of the Lipid Research Clinics (LRC) are gratefully acknowledged. The LRC is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) with LRC investigator collaboration. This manuscript was prepared using a limited access dataset and does not necessarily reflect opinions of the LRC Study or the NHLBI.
Funding
None.
Footnotes
Conflict of Interest Disclosures
Dr. Mora has received research grant support from the NHLBI (K08 HL094375).
References
- 1.Benbassat J, Froom P. Blood pressure response to exercise as a predictor of hypertension. Arch Intern Med. 1986;146:2053–2055. [PubMed] [Google Scholar]
- 2.Dlin RA, Hanne N, Silverberg DS, Bar-Or O. Follow-up of normotensive men with exaggerated blood pressure response to exercise. Am Heart J. 1983;106:316–320. doi: 10.1016/0002-8703(83)90198-9. [DOI] [PubMed] [Google Scholar]
- 3.Singh JP, Larson MG, Manolio TA, O’Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 4.Fagard R, Staessen J, Thijs L, Amery A. Prognostic significance of exercise versus resting blood pressure in hypertensive men. Hypertension. 1991;17:574–578. doi: 10.1161/01.hyp.17.4.574. [DOI] [PubMed] [Google Scholar]
- 5.Allison TG, Cordeiro MA, Miller TD, Daida H, Squires RW, Gau GT. Prognostic significance of exercise-induced systemic hypertension in healthy subjects. Am J Cardiol. 1999;83:371–375. doi: 10.1016/s0002-9149(98)00871-6. [DOI] [PubMed] [Google Scholar]
- 6.Filipovsky J, Ducimetiere P, Safar ME. Prognostic significance of exercise blood pressure and heart rate in middle-aged men. Hypertension. 1992;20:333–339. doi: 10.1161/01.hyp.20.3.333. [DOI] [PubMed] [Google Scholar]
- 7.Mundal R, Kjeldsen SE, Sandvik L, Erikssen G, Thaulow E, Erikssen J. Exercise blood pressure predicts cardiovascular mortality in middle-aged men. Hypertension. 1994;24:56–62. doi: 10.1161/01.hyp.24.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O’Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2008;101:1614–1620. doi: 10.1016/j.amjcard.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Jr, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC., Jr ACC/AHA 2002 guideline update for exercise testing: Summary article: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (committee to update the 1997 exercise testing guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 10.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic north american men. The Lipid Research Clinics Mortality Follow-Up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 11.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 12.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res. 1971;3:323–332. [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 14.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 15.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 17.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000;101:611–615. doi: 10.1161/01.cir.101.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 20.Toikka JO, Laine H, Ahotupa M, Haapanen A, Viikari JS, Hartiala JJ, Raitakari OT. Increased arterial intima-media thickness and in vivo LDL oxidation in young men with borderline hypertension. Hypertension. 2000;36:929–933. doi: 10.1161/01.hyp.36.6.929. [DOI] [PubMed] [Google Scholar]
- 21.Kimura Y, Tomiyama H, Nishikawa E, Watanabe G, Shiojima K, Nakayama T, Yoshida H, Kuwata S, Kinouchi T, Doba N. Characteristics of cardiovascular morphology and function in the high-normal subset of hypertension defined by JNC-VI recommendations. Hypertens Res. 1999;22:291–295. doi: 10.1291/hypres.22.291. [DOI] [PubMed] [Google Scholar]
- 22.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: Current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 24.Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, LaCroix AZ, Black HR. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115:855–860. doi: 10.1161/CIRCULATIONAHA.106.656850. [DOI] [PubMed] [Google Scholar]
- 25.Mainous AG, 3rd, Everett CJ, Liszka H, King DE, Egan BM. Prehypertension and mortality in a nationally representative cohort. Am J Cardiol. 2004;94:1496–1500. doi: 10.1016/j.amjcard.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.