Abstract
Background
The distribution of a syndrome in space and time may suggest clues to its etiology. The cause of Kawasaki syndrome, a systemic vasculitis of infants and children, is unknown, but an infectious etiology is suspected.
Methods
Seasonality and clustering of Kawasaki syndrome cases were studied in Japanese children with Kawasaki syndrome reported in nationwide surveys in Japan. Excluding the years that contained the 3 major nationwide epidemics, 84,829 cases during a 14-year period (1987–2000) were analyzed. To assess seasonality, we calculated mean monthly incidence during the study period for eastern and western Japan and for each of the 47 prefectures. To assess clustering, we compared the number of cases per day (daily incidence) with a simulated distribution (Monte Carlo analysis).
Results
Marked spatial and temporal patterns were noted in both the seasonality and deviations from the average number of Kawasaki syndrome cases in Japan. Seasonality was bimodal with peaks in January and June/July and a nadir in October. This pattern was consistent throughout Japan and during the entire 14-year period. Some years produced very high or low numbers of cases, but the overall variability was consistent throughout the entire country. Temporal clustering of Kawasaki syndrome cases was detected with nationwide outbreaks.
Conclusions
Kawasaki syndrome has a pronounced seasonality in Japan that is consistent throughout the length of the Japanese archipelago. Temporal clustering of cases combined with marked seasonality suggests an environmental trigger for this clinical syndrome.
Kawasaki syndrome is an acute, self-limited vasculitis of infants and children with unknown etiology. Seasonality of this condition was first reported by Tomisaku Kawasaki in 1967.1 He described an increase in cases in the spring and summer and a decrease in the fall and winter during a 5-year period (1961–1966). An increased incidence of Kawasaki syndrome in the winter-spring months has been reported from many different geographic regions.2-6 This syndrome is recognized by a constellation of clinical signs (including fever, rash, and conjunctival injection) lasting 1 to 2 weeks. An infectious etiology is suspected on the basis of the clinical similarities to other infectious diseases. Peak incidence occurs in the infant/toddler age group, with only rare cases in infants younger than 3 months of age and in adults. This suggests that transplacental antibodies may confer protection and also that most infections are asymptomatic and result in the development of protective antibodies. The major complication of Kawasaki syndrome is damage to the coronary arteries, which can be prevented in most cases with a high dose of intravenous gamma globulin administered within the first 10 days of fever. Kawasaki syndrome is now the leading cause of acquired heart disease in children in the United States and Japan. The rising incidence in Japan and perhaps the United Kingdom lends a sense of urgency to finding the cause.7,8 At current rates, 1 of every 150 children in Japan will suffer from Kawasaki syndrome. Clear documentation of seasonality and clustering of Kawasaki syndrome cases would lend further weight to the hypothesis that Kawasaki syndrome has an environmental trigger.
METHODS
Nationwide Survey
Nationwide surveys in Japan conducted from 1979 through 2000 documented 150,321 cases of Kawasaki syndrome during this 22-year period.9-13 Briefly, the surveys were conducted through questionnaires sent to all pediatric departments in hospitals with more than 100 beds. The questionnaires asked for patient information, including sex, birth date, date of hospitalization, illness day of hospitalization (illness day 1 defined as the first day of fever), and prefecture of residence. Government-sponsored health insurance provides equal access to health care for all children in Japan, where all cases of Kawasaki syndrome are hospitalized. Although it is impossible to ascertain all cases of Kawasaki syndrome in the absence of a diagnostic test, the high incidence in Japan coupled with the nationwide out breaks in 1979 –1986 have resulted in a high level of awareness of the syndrome among pediatricians. Thus, the majority of clinically typical cases in Japan are presumably recognized and hospitalized.
Response rates for the questionnaires from the 16 nationwide surveys varied from 62% to 76%.7 Incomplete records for 361 patients were excluded from analysis, leaving 149,960 cases. For the analysis of seasonality and clustering, the years that contained the 3 major nationwide epidemics in Japan (1979,1982, and 1986) were excluded, leaving 84,829 cases during the 14-year period from 1987 through 2000. The hospital admission date of the 84,829 cases was used to determine the daily incidence for each of the 5,114 days.
Annual Incidence
Patients with Kawasaki syndrome usually are diagnosed and hospitalized in Japan within the first week of illness. The annual incidence was determined as the total number of hospitalizations in a given year.
Monthly Incidence and Anomalies
Monthly incidence was expressed as an average number of cases per day in a given month to account for the variation in the number of days per month. Anomalies in incidence were calculated by subtracting the mean number of cases per day for a given month from the observed number of cases per day for that month. We conducted linear regression analysis to eliminate the effect of the increasing incidence during the 14-year period and to obtain the detrended anomalies (cases per day) for each month. Correlation coefficients for the monthly time series were determined by Pearson’s test.
Daily Incidence
In Japan, only the most emergent cases are admitted to the hospital on weekends and holidays. This leads to 2 types of bias in the distribution of hospital admissions. The first is a day-of-the-week bias, in which the average number of cases admitted to hospital on Sundays is much lower than the other days of the week. The second type of bias is a holiday effect, with admissions dropping during observed public holidays, often followed by a compensatory increase in cases on the 1 or 2 days immediately after the holiday. To obtain daily incidence for the analysis of clustering, 2 adjustments were made. For the day-of-the-week bias, the observed number of hospitalizations for any given day was adjusted as follows:
where radj = the adjusted rate for a particular day, robs = the observed rate on that particular day, rav = average rate for that particular day of the week, and rall = the average daily rate for the entire time series. The holiday effect was corrected for the 11 fixed Japanese national holidays by averaging the number of hospitalizations during holidays together with the number of cases in the 2 days after each holiday.
Geographic Comparisons
To assess geographic patterns of disease, the country was arbitrarily divided into 2 regions with 32 western and 15 eastern prefectures. Tokyo, Kanagawa, Saitama, Gunma, and Nigata formed the western border of the eastern group of prefectures. Mean monthly incidence for the 14-year period was calculated for each prefecture and ranked on a 1–12 color scale with red and blue representing the highest and lowest incidence for each prefecture, respectively. This representation of disease activity for each month was projected onto maps of Japan. Because of the continuous color algorithm, the color variations do not precisely correspond to prefecture boundaries.
Temporal Clustering of Cases
We used the adjusted daily incidence and arbitrarily chose 4 days as the time interval for our cluster definition. The minimum number of cases in nonoverlapping 4-day periods that defined a cluster was chosen such that only 5% of the cases were included in clusters. Cases for the 14-year period were divided into 4 groups: 6-month blocks (January to June and July to December) and eastern and western Japan (Table 1). Following this algorithm, thresholds for clusters were determined separately for each group. A cluster was defined as >35– 46 cases per 4-day period, depending on the time block and geographic region. Simulation of cases was performed by a Monte Carlo experiment, which distributed the same number of cases as in the Kawasaki syndrome series with equal probability during the 14 years (1987–2000) using a random number generator and repeated 10,000 times. The number of clusters was compared with the mean number of simulated clusters in the Monte Carlo distribution. Confidence limits of the mean, using an assumed normal distribution to represent the distribution of the Monte Carlo clusters, were determined to evaluate the significance of the difference.
TABLE 1.
Comparison of Clustering in Kawasaki Syndrome and Simulated Data Sets
| Kawasaki Syndrome |
||||
|---|---|---|---|---|
| Geographic Region/Time Period | Cluster Size* | Percent of Cases in Clusters |
No. Clusters | No. Simulated Clusters (Mean ± SD) |
| Eastern Japan; January to June (n = 20,085) | ≥39 | 4.9 | 162 | 129.8 (±6.7) |
| Eastern Japan; July to December (n = 18,743) | ≥36 | 5.1 | 166 | 136.9 (±6.8) |
| Western Japan; January to June, (n = 24,089) | ≥46 | 4.9 | 164 | 128.9 (±6.7) |
| Eastern Japan; July to December (n = 21,912) | ≥41 | 5.6 | 179 | 150.7 (±6.7) |
The cluster size was arbitrarily defined as the number of cases during a 4-day period that represented approximately 5% of the Kawasaki syndrome sample for 2 time-periods and 2 geographic regions.
SD indicates standard deviation.
Clustering was also assessed by an alternative method. Cases were divided into eastern and western Japan and the distribution of cases per day was compared with the distribution of simulated cases/day (Monte Carlo distribution). Similarly, the distribution of Kawasaki syndrome and simulated cases was compared between 6-month blocks (January to June and July to December).
Analysis of Weather Variables
We assessed daily temperature and precipitation over Japan and atmospheric circulation patterns in the Asia-western North Pacific sector (inclusive Japan) to determine whether there were reproducible weather patterns that occurred 0 to 14 days before Kawasaki syndrome clusters (as defined in Table 1). Temperature and precipitation were taken from daily weather station observations at 29 locations across Japan. Atmospheric circulation was represented by the height of the 700 kilopascal surface. Weather anomalies for each day were determined as a deviation from the climatological daily mean (1950 –1990).
RESULTS
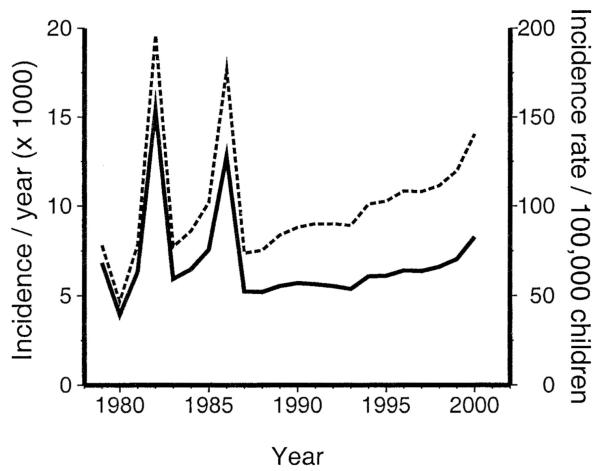
The number of patients diagnosed with Kawasaki syndrome per year for the 22-year time series increased from 5256 in 1987 to 8267 in 2000 (Fig. 1). Because of the decreasing birthrate in Japan coupled with the increasing number of cases, the incidence rate per 100,000 children younger than 5 years increased by 90% from 74 in 1987 to 140 in 2000. The mean incidence for the 14-year period from 1987–2000 was 17 cases per day, 505 cases per month, and 6059 cases per year.
FIGURE 1.
Incidence of Kawasaki syndrome in Japan, 1979–2000. Incidence (solid line): number of hospitalizations (cases per year). Incidence rate (dashed line): number of Kawasaki syndrome hospitalizations per 100,000 children younger than 5 years of age per year. Note the epidemics in 1982 and 1986 and the rising attack rate during the decade of the 1990s.
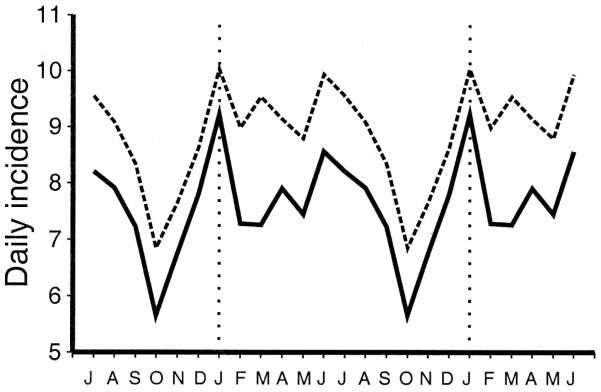
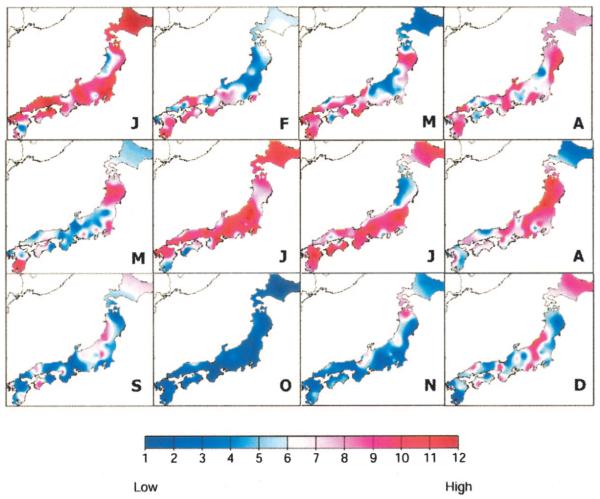
Analysis of the mean monthly incidence revealed a bimodal seasonality in both western and eastern Japan (Fig. 2). Peaks were observed in January and again in June and July. The lowest incidence was noted in October and November. The higher incidence in western Japan is likely related to the greater number of children at risk based on a 10-year (1990 –2000) population average: 3,384,000 versus 2,717,000 children younger than 5 years of age in western versus eastern Japan, respectively. The seasonal variation in Kawasaki syndrome incidence was further explored by analyzing the mean monthly incidence for each prefecture (Fig. 3). Again, there was strong consistency in Kawasaki syndrome activity across the entire country. The peaks in incidence in January and June/July were in striking contrast to the nadir in October—a pattern that was consistent for all 47 prefectures.
FIGURE 2.
Geographic patterns of Kawasaki syndrome cases. Average incidence (cases per day) by month, averaged over time period from July 1987 to June 2000, in eastern Japan (solid line) and western Japan (dashed line).
FIGURE 3.
Monthly Kawasaki syndrome activity, 1987–2000. The mean hospitalizations per month were calculated for each prefecture during the 14-year period and ranked on a 1–12 scale (see color bar), with red and blue representing the highest and lowest means for each prefecture, respectively. Months of the year are designated by single letter. Note the peaks in January and June/July with a nadir in October. Because of the continuous color algorithm, the color variations do not precisely correspond to prefecture boundaries.
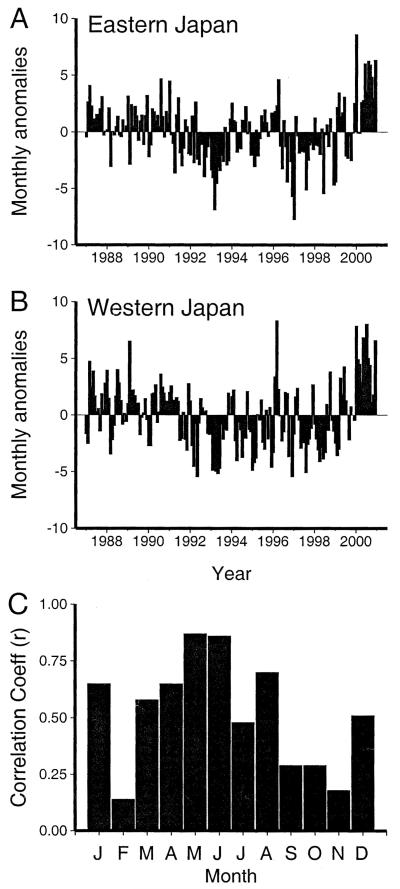
The monthly anomalies in incidence also were remarkably similar between western and eastern Japan (Fig. 4). A positive correlation coefficient was observed between the monthly anomalies in western and eastern Japan throughout the year, although the correlation was relatively weaker in months with low Kawasaki syndrome activity (October and November). Months with the highest incidence showed the highest correlation between the 2 halves of the country.
FIGURE 4.
Monthly anomalies, 1987–2000. Average number of Kawasaki syndrome hospitalizations per day expressed as a deviation from the mean number of hospitalizations per day for (A) eastern Japan and (B) western Japan after linear regression analysis to eliminate the effect of increasing incidence during the 14-year period. C, Correlation coefficient for detrended monthly anomalies for eastern versus western Japan.
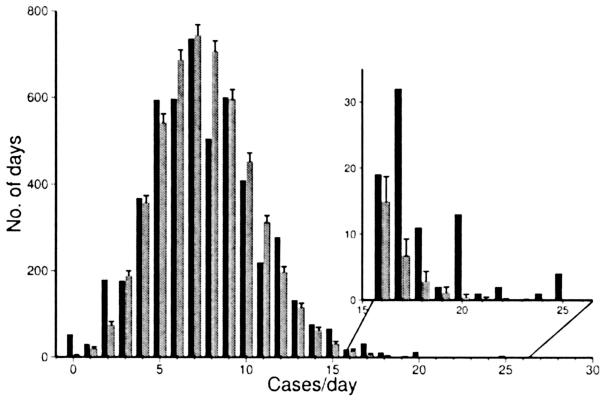
Two methods were used to examine clustering of cases during the 14-year period. In the first, we compared the number of clusters between the Kawasaki syndrome data set and a simulated distribution of the same number of cases over the same number of days. A higher number of clusters of cases was observed for both the January to June and July to December half-year periods and for both eastern and western Japan (Table 1). In the second method, we compared the daily incidence of Kawasaki syndrome (hospitalizations per day adjusted for day-of-week and holiday biases) to the simulated number of cases per day in the Monte Carlo distribution. More days with a high incidence were noted in the Kawasaki syndrome data set compared with the simulated data set, as shown in Figure 5 for eastern Japan. The distribution of daily incidence showed a similar increased number of high incidence-days in both eastern and western Japan and in both the January-June and July-December time periods (data not shown). Examination of weather anomalies revealed no consistent pattern for deviations in temperature, precipitation, or atmospheric circulation during the 15-day period leading up to a Kawasaki syndrome cluster (data not shown).
FIGURE 5.
Comparison of the observed distribution of daily Kawasaki syndrome incidence (black bars) with the simulated distribution of the same number of synthetic cases created by a random number generator and repeated 10,000 times (Monte Carlo distribution, gray bars) for eastern Japan, January-December. Data are for 1987–2000 (5114 days). The inset shows a detail of the number of days on which there were 16–26 cases per day.
DISCUSSION
Striking seasonal variation, temporal clustering, and a 90% increase in incidence of Kawasaki syndrome cases were observed during a 14-year period in Japan. Analysis of a large cohort of cases over the time period (1987–2000), during which there were no major epidemics, revealed a remarkably consistent bimodal seasonality of Kawasaki syndrome with a marked nadir of cases in October that occurred throughout the entire country of Japan. Consistent with this observation, the large nationwide epidemics in Japan in 1979, 1982, and 1986 (preceding our period of analysis) all occurred during the winter-spring months and peaked in March-May, April, and February-March, respectively.10 Previous reports of Kawasaki syndrome cases from the United States and Japan using much smaller data sets have described a unimodal distribution of cases with a winter/spring peak.1-6,10
The bimodal seasonality of Kawasaki syndrome reported here may be an epidemiologic clue to the etiologic agent. Seasonal variation is a characteristic of many pediatric viral and bacterial infections, each of which has a characteristic timing and duration of its annual cycle that is consistent from year to year in a given region. Notable examples include influenza virus, respiratory syncytial virus, and rotavirus in the winter and enteroviruses in the summer and fall.14 The seasonal occurrence of various infectious diseases in Japan is monitored by the Japan National Institute of Infectious Disease.15 The epidemic patterns of 12 different diseases of childhood are monitored by this surveillance system.16 Several serotypes of Group A β-hemolytic streptococci follow a bimodal distribution, with peaks in December-February and again in May-June, and a prominent nadir of infections in September. In addition, some adenovirus serotypes (type 1, 2, 3, 5, and 7) have a bimodal incidence in some years. However, we could not detect a dramatic increase in numbers of these infections to match the 90% increase in Kawasaki syndrome cases over the study period. Viral and bacterial cultures from patients with Kawasaki syndrome have failed thus far to yield a consistent organism,17,18 nor have molecular methods identified a causative agent.19 The observed bimodal seasonal peaks in Kawasaki syndrome may suggest that different infectious diseases trigger the disease in winter/spring and in summer.
In Japan, the Kawasaki syndrome peaks in January (the coldest month) and June/July (the months with highest precipitation) suggest that environmental factors or behaviors related to these conditions may contribute to disease incidence. However, positive anomalies in incidence (clusters) did not correlate with daily anomalies in temperature, precipitation, or atmospheric circulation during the 15 days preceding a cluster.
Temporal clustering of cases was originally reported in large nationwide epidemics in Japan.11 The present study indicates that temporal clustering continues to occur despite the absence of nationwide or regional epidemics. An epidemic curve with large nationwide outbreaks followed by a steady endemic rate with clustering of cases is consistent with the hypothesis that a new agent was introduced into a highly susceptible population.20 Temporal clustering of cases that are geographically unrelated is consistent with the lack of evidence for person-to-person spread.
The temporal clustering documented in the present study might suggest an environmental trigger or infectious agent that acts over a large area and causes the syndrome in previously unexposed, genetically susceptible individuals, with asymptomatic infection leading to protective immunity in the majority of the population. A genetic contribution to Kawasaki syndrome susceptibility is suspected based on the high prevalence among certain Asian populations,6,7 the 10-fold increased relative risk in siblings of an index case,21,22 and the emerging recognition of this syndrome in successive generations within the same family.23 Thus, Kawasaki syndrome may manifest only in young, susceptible individuals with a genetic predisposition who are exposed to an unknown environmental trigger or infectious agent. The fact that Kawasaki syndrome is a self-limited vasculitis strongly suggests a definitive immune response that terminates the inflammatory process in affected children. New hypotheses for the cause of this syndrome in Japan should focus on the bimodal seasonality as a potential clue. Studies of Kawasaki syndrome on other continents should also explore whether the bimodal seasonality is observable in other regions or is peculiar to Japan.
ACKNOWLEDGMENTS
We thank Mary Tiree for expert assistance in climate analysis and computer graphics.
Supported in part by the NOAA Office of Global Programs through the California Applications Program (to D.R.C. and G.T.) and NIH-RO1HL69413 (to J.C.B. and H.S.).
Footnotes
Current address of Garrick Tong: Albany Medical College, Albany, NY 12208.
REFERENCES
- 1.Kawasaki T. Pediatric acute febrile mucocutaneous lymph node syndrome with characteristic desquamation of fingers and toes: my clinical observation of 50 cases. Jpn J Allergol. 1967;16:178–222. in Japanese. English translation by Shike H, Shimizu C, Burns JC. Pediatri Infect Dis J. 2002;21:993–1096. doi: 10.1097/00006454-200211000-00002. Article Plus.
- 2.Bell DM, Morens DN, Holman RC, Hurwitz MK. Kawasaki syndrome in the United States. Am J Dis Child. 1983;137:211–214. doi: 10.1001/archpedi.1983.02140290003001. [DOI] [PubMed] [Google Scholar]
- 3.Belay ED, Holman RC, Clarke MJ, et al. The incidence of Kawasaki syndrome in west coast health maintenance organizations. Pediatr Infect Dis J. 2000;19:828–32. doi: 10.1097/00006454-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Chang R-KR. [accessed December 14, 2004];Hospitalizations for Kawasaki disease among children in the United States, 1988–1997. Pediatrics. 2002 :109. doi: 10.1542/peds.109.6.e87. Available at http://www.pediatrics.org/cgi/content/full/109/6/e87; Internet. [DOI] [PubMed]
- 5.Holman RC, Belay ED, Clarke MJ, Kaufman SF, Schonberger LB. Kawasaki syndrome among American Indian and Alaska Native children, 1980 through 1995. Pediatr Infect Dis J. 1999;18:451–455. doi: 10.1097/00006454-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bronstein DE, Dille AN, Austin JP, Williams CM, Palinkas LA, Burns JC. Relationship of climate, ethnicity, and socioeconomic status to Kawasaki disease in San Diego County, 1994–1998. Pediatr Infect Dis J. 2000;19:1087–91. doi: 10.1097/00006454-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Yanagawa H, Yashiro M, Oki I, Nakamura Y, Zhang T. Thirty-year observation of the incidence rate of Kawasaki disease in Japan. Pediatr Res. 2002;53:4A. [Google Scholar]
- 8.Harnden A, Alves B, Sheikh A. Rising incidence of Kawasaki disease in England: Analysis of hospital admission data. Br Med J. 2002;324:1424–1425. doi: 10.1136/bmj.324.7351.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagawa H, Shigematsu I. Epidemiological features of Kawasaki disease in Japan. Acta Paediatr Jpn. 1983;25:94–107. [Google Scholar]
- 10.Yanagawa H, Yashiro M, Nakamura Y, Kawasaki T, Kato H. Results of 12 nationwide epidemiological incidence surveys of Kawasaki disease in Japan. Arch Pediatr Adolesc Med. 1995;149:779–783. doi: 10.1001/archpedi.1995.02170200069011. [DOI] [PubMed] [Google Scholar]
- 11.Yanagawa H, Nakamura Y, Ojima T, Yashiro M, Tanihara S, Oki I. Changes in epidemic patterns of Kawasaki disease in Japan. Pediatr Infect Dis J. 1999;18:64–66. doi: 10.1097/00006454-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Yanagawa H, Kawasaki T, Shigematsu I. Nationwide survey on Kawasaki disease in Japan. Pediatrics. 1987;90:58–62. [PubMed] [Google Scholar]
- 13.Yanagawa H, Nakamura Y, Yashiro M, Oki I, et al. [accessed December 14, 2004];Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics. 2001 :107. doi: 10.1542/peds.107.3.e33. Available at: http://www.pediatrics.org/cgi/content/full/107/3/e33; Internet. [DOI] [PubMed]
- 14.Dowell S. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerging Infect Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute of Infectious Disease and Japan Ministry of Health and Welfare [accessed December 14, 2004];Infectious Agents Surveillance Reports. Available at: http://idsc.nih.go.jp/iasr/; Internet.
- 16.Nakamura Y, Yanagawa H, Nagai M. Epidemic patterns of infectious diseases from the results of the surveillance of infectious disease in Japan. Pediatr Infect Dis J. 1988;7:262–266. [PubMed] [Google Scholar]
- 17.Leung DYM, Meissner C, Shulman ST, et al. Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J Pediatr. 2002;140:742–746. doi: 10.1067/mpd.2002.123664. [DOI] [PubMed] [Google Scholar]
- 18.Marchette NJ, Melish ME, Hicks R, Kihara S, Sam E, Ching D. Epstein-Barr virus and other herpesvirus infections in Kawasaki syndrome. J Infect Dis. 1990;161:680–684. doi: 10.1093/infdis/161.4.680. [DOI] [PubMed] [Google Scholar]
- 19.Rowley AH, Wolinsky SM, Relman DA, et al. Search for highly conserved viral and bacterial nucleic acid sequences corresponding to an etiologic agent of Kawasaki disease. Pediatr Res. 1994;36:567–571. doi: 10.1203/00006450-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kushner HI, Bastian JF, Turner CL, Burns JC. Rethinking the boundaries of Kawasaki Disease. Perspect Biol Med. 2003;46:216–233. doi: 10.1353/pbm.2003.0024. [DOI] [PubMed] [Google Scholar]
- 21.Fujita Y, Nakamura Y, Sakata K, Hara N, Kobayashi M, Nagai M, Yanagawa J, Kawasaki T. Kawasaki disease in families. Pediatrics. 1989;84:666–669. [PubMed] [Google Scholar]
- 22.Yanagawa H, Nakamura Y, Yashiro M, et al. [accessed December 14, 2004];Results of the nationwide epidemiologic survey of Kawasaki disease in 1995 and 1996 in Japan. Pediatrics. 1998 :102. doi: 10.1542/peds.102.6.e65. Available at http://www.pediatrics.org/cgi/content/full/102/6/e65; Internet. [DOI] [PubMed]
- 23.Uehara R, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease in parents and children. Acta Paediatr. 2003;92:694–697. doi: 10.1080/08035320310002768. [DOI] [PubMed] [Google Scholar]