Abstract
Rationale
Kappa opioid receptors (KORs) have been implicated in depressive-like states associated with chronic administration of drugs of abuse and stress. Although KOR agonists decrease dopamine in the nucleus accumbens (NAc), KOR modulation of phasic dopamine release in the core and shell subregions of the NAc—which have distinct roles in reward processing—remains poorly understood.
Objectives
Studies were designed to examine whether the time course of effects of KOR activation on phasic dopamine release in the NAc core or shell are similar to effects on motivated behavior.
Methods
The effect of systemic administration of the KOR agonist salvinorin A (salvA)—at a dose (2.0 mg/kg) previously determined to have depressive-like effects—was measured on electrically evoked phasic dopamine release in the NAc core or shell of awake and behaving rats using fast scan cyclic voltammetry. In parallel, the effects of salvA on intracranial self-stimulation (ICSS) and sucrose-reinforced responding were assessed. For comparison, a threshold dose of salvA (0.25 mg/kg) was also tested.
Results
The active, but not threshold, dose of salvA significantly decreased phasic dopamine release without affecting dopamine reuptake in the NAc core and shell. SalvA increased ICSS thresholds and significantly lowered breakpoint on the progressive ratio schedule, indicating a decrease in motivation. The time course of the KOR-mediated decrease in dopamine in the core was qualitatively similar to the effects on motivated behavior.
Conclusions
These data suggest that the effects of KOR activation on motivation are due, in part, to inhibition of phasic dopamine signaling in the NAc core.
Keywords: Intracranial self-stimulation, Operant, Progressive ratio, Sucrose, Nucleus accumbens core, Nucleus accumbens shell, Depression, Fast scan cyclic voltammetry
Introduction
Drug dependence and stress are often associated with depression in humans (Kessler 1997; Markou et al. 1998) and with behaviors in rodents that are thought to reflect characteristics of depression, including a reduced ability to experience reward (anhedonia) and loss of motivation (Koob et al. 1989; Pittenger and Duman 2008). Drugs of abuse and stress have been shown to promote the synthesis and release of the neuropeptide dynorphin—an endogenous ligand at kappa opioid receptors (KORs; Chavkin et al. 1982)—in humans and animals that is coincident with the emergence of depressive-like effects (Chartoff et al. 2009; Cole et al. 1995; Hurd and Herkenham 1993; Shippenberg et al. 2007). Activation of KORs produces depressive-like behaviors in humans and rodents (Pfeiffer et al. 1986; Shippenberg and Herz 1987; Todtenkopf et al. 2004). Likewise, KOR antagonists or genetic deletion of KORs attenuates stress- and drug-induced depressive-like states (Carey et al. 2007; Carlezon et al. 1998; Mague et al. 2003; McLaughlin et al. 2003). Together these data suggest that activation of KORs underlies, in part, stress-and drug-induced depressive-like states. However, the mechanisms by which KORs decrease motivation are not fully understood.
Dopamine signaling within the NAc is important for the regulation of mood and motivation (Nestler and Carlezon 2006; Wise et al. 1978). The NAc includes two primary subregions: the core and shell (Zahm 2000), and increasing evidence supports distinct roles for these subregions in processing motivationally salient stimuli and subsequent behaviors (Bari and Pierce 2005; Ito et al. 2004; Kelley 1999; Meredith et al. 2008). The effects of KOR activation on dopamine transmission in the NAc core versus shell are not well characterized. KORs are highly expressed on dopamine terminals in the NAc and on dopamine cell bodies in the midbrain (Mansour et al. 1995; Svingos et al. 2001) where they inhibit transmitter release and neuronal excitability (Britt and McGehee 2008; Hjelmstad and Fields 2003). KOR agonists have been shown to reduce tonic dopamine levels in the striatum and NAc using microdialysis (Carlezon et al. 2006; Gehrke et al. 2008; Zhang et al. 2005), and KOR agonists microinjected directly into the NAc are aversive (Bals-Kubik et al. 1993). Microdialysis is a technique that captures slow changes in extracellular dopamine levels. Dopamine neurons typically fire action potentials at low frequencies (<5 Hz; tonic), but periodically exhibit brief, high frequency (20–60 Hz) periods of activity (phasic) (Hyland et al. 2002; Schultz 1998). Phasic activations of dopamine neurons lead to brief increases in NAc dopamine (Tsai et al. 2009) that are tightly correlated with motivated behavior for a variety of reinforcers including food (Roitman et al. 2004), brain stimulation reward (Cheer et al. 2005; Cheer et al. 2007), and drugs of abuse (Phillips et al. 2003). Indeed, phasic activation of dopamine neurons appears to be sufficient for reinforcement (Tsai et al. 2009).
Based on previous studies of the depressive-like effects of the potent and highly selective KOR agonist salvinorin A (salvA) (Carlezon et al. 2006; Chavkin et al. 2004; Roth et al. 2002), we chose to investigate the temporal effects of a maximally effective dose of salvA on phasic dopamine release in the NAc core and shell evoked by stimulation of the ventral tegmental area (VTA) using fast scan cyclic voltammetry (FSCV) in awake and behaving rats. In parallel, we measured the effects of salvA on motivated behavior using two operant conditioning procedures: intracranial self-stimulation (ICSS) and sucrose-reinforced responding. The goal of this study was to determine whether KORs exert distinct control of dopamine transmission in the NAc core versus shell that might correlate with effects on motivated behavior.
Methods
Animals
For these studies, 48 male Sprague–Dawley rats (Charles River Laboratories) weighing approximately 350–400 g at the time of testing were used. Rats used for FSCV (n=29) and sucrose-reinforced responding (n=10) experiments were maintained at the University of Illinois at Chicago (UIC), and rats (n=9) used for ICSS were maintained at McLean Hospital. Rats were individually housed with a 12 h/12 h light/dark cycle, and all experiments were conducted during the light phase. Rats were maintained on ad libitum food and water, except where otherwise noted. All rats were treated according to the guidelines recommended by the Animal Care Committee at the University of Illinois at Chicago and Institutional Animal Care and Use Committee of McLean Hospital, in strict accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (1996).
Drugs
SalvA was provided by Dr. Cécile Béguin (McLean Hospital, Belmont, MA). The drug was extracted and purified according to established methods (Lee et al. 2005). The samples used for testing in this report were determined by high performance liquid chromatography to be >99% pure. SalvA was dissolved in a vehicle of 75% dimethyl sulfoxide plus 25% distilled water (vehicle). SalvA and vehicle were administered by IP injection in a volume of 1 ml/kg. Previous studies have shown that salvA (2.0 mg/kg) is maximally effective at increasing ICSS thresholds and increasing immobility time in the forced swim test (FST) without decreasing rates of responding or locomotor activity (Beguin et al. 2008; Carlezon et al. 2006). Therefore, we chose this active dose to examine how the depressive-like effects of KOR activation would modulate motivated behaviors and stimulated dopamine release in the NAc. The low dose of salvA (0.25 mg/kg) was used for comparison because it has previously been shown to be a threshold dose in both the ICSS test and FST (Carlezon et al. 2006).
Surgical procedures
FSCV Rats were anesthetized with ketamine hydrochloride (100 mg/kg, intraperitoneal (IP)) and xylazine hydrochloride (10 mg/kg, IP). A guide cannula (Bioanalytical Systems; West Lafayette, IN) was implanted dorsal to either the NAc core (1.3 mm anterior to bregma, +1.5 mm lateral to midline, and 2.5 mm ventral from skull surface) or NAc shell (1.3 mm anterior to bregma, +0.9 mm lateral to midline, and 2.5 mm ventral from skull surface). A chlorinated silver reference electrode was placed contralateral to the guide cannula in the left forebrain. Stainless steel skull screws and dental cement were used to secure the guide cannula and reference electrode to the skull. Once dry, the obdurator was removed from the guide cannula, and a micromanipulator containing a carbon fiber electrode was attached. The electrode was then lowered into the NAc. A bipolar-stimulating electrode (0.20 mm diameter; Plastics One, Roanoke, VA) was positioned dorsal to the VTA (5.2 mm posterior to bregma, +1.0 mm lateral to midline, and 7.0 mm ventral to skull surface) and lowered in 0.2-mm increments until electrically (60 pulses, 60 Hz, 120 μA, 4 ms/phase) evoked dopamine release was detected via the carbon fiber electrode (see Online Resource 2 and below for details). After optimizing evoked dopamine release, the stimulating electrode was cemented and the carbon fiber electrode was removed and replaced with the obdurator (Day et al. 2007; Heien et al. 2005; Roitman et al. 2004). Rats recovered when they reached pre-surgery body weight (~2–5 days).
ICSS Rats were anesthetized with sodium pentobarbital (65 mg/kg, IP; Abbott Laboratories, North Chicago, IL) supplemented with subcutaneous atropine (0.25 mg/kg) to minimize bronchial secretions and implanted with stainless steel monopolar electrodes (0.25-mm diameter; Plastics One, Roanoke, VA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, +1.7 mm lateral to midline, and 7.8 mm below dura). See Online Resource 3 for a representative histological section. The electrodes were coated with polyamide insulation except at the tip. A non-insulated stainless steel wire was used as the anode and wrapped around a stainless steel screw embedded in the skull, and the entire assembly was coated with acrylic cement.
Fast scan cyclic voltammetry
Recordings FSCV measures extracellular concentrations of electroactive species including dopamine with subsecond temporal resolution and has been described in detail elsewhere (Day et al. 2007). Briefly, a carbon fiber microelectrode is lowered into the NAc and held at −400 mV relative to the Ag/AgCl reaction at the reference electrode. Periodically, the voltage is increased to +1300 mV and decreased to −400 mV in a triangular fashion (400 V/s). This triangle waveform is repeated at 10 Hz. Chemical species that are electroactive within this voltage range will oxidize and reduce at various points along the triangle waveform, and the resultant currents are measured at the surface of the electrode and recorded on a computer using software written in LabView (National Instruments, Schaumburg, IL) (Heien et al. 2004). The current arising from the double-layer capacitance and electroactivity of surface functional groups on the carbon fiber was removed by background subtraction. The background period (1,000 ms) was obtained within 5 s before each stimulation of the VTA. Dopamine is electroactive within the applied voltage range, is identified by its oxidation (at ~0.6 V) and reduction (at ~−0.2 V versus Ag/AgCl) features, and is quantified by measuring its oxidative current, which is directly proportional to its concentration at the electrode surface (Anstrom et al. 2009; Day et al. 2007; Heien et al. 2004; Jones et al. 2009).
Following recovery of pre-surgical weight, each rat was placed in a standard experimental operant procedure chamber (Med Associates, Inc.; St. Albans, VT). The obdurator was removed from the guide cannula, and a micromanipulator containing a carbon fiber (recording) electrode was inserted. The recording electrode was lowered into the NAc and locked into place. A headstage containing electrical components necessary for application of the triangle waveform, measurement of resultant changes in current at the electrode surface as well as the delivery of current via the stimulating electrode, was connected to the animal. The VTA was stimulated (24 pulses, 60 Hz, 120 μA, 4 ms/pulse) to evoke dopamine release in the NAc every 5 min until the peak concentration of three consecutive measurements differed by no more than 10% (baseline). Upon establishing a stable baseline, rats were injected with vehicle or salvA (0.25, or 2.0 mg/kg). The VTA was stimulated, as above, every 5 min for the first hour following injection and every 15 min for the subsequent 2 h.
Changes in phasic dopamine signaling can be due to alterations in dopamine release and/or changes in the number or function of the dopamine transporter (DAT). FSCV during electrical stimulation of the VTA permits the dissociation of changes in phasic NAc dopamine signaling due to changes in dopamine release from changes due to the rate of dopamine reuptake via the DAT (Stamford et al. 1989). Peak dopamine concentration was measured for each stimulation. The decay phase of the dopamine spike evoked by stimulation of the VTA is mainly governed by dopamine reuptake dynamics (Giros et al. 1996;Senior et al. 2008). Thus, we examined the falling phase of the dopamine spike as described previously (Cheer et al. 2004;Senior et al. 2008). Rate of dopamine reuptake by the DAT is concentration dependent, so we analyzed the portion of the falling phase that was similar in concentration across time.
Intracranial self-stimulation procedures
After one week of recovery from surgery, rats were trained to respond for brain stimulation using a continuous reinforcement schedule (FR1) at 141 Hz, where each lever press earned a 500-ms train of square wave cathodal pulses (100 μs per pulse), as described (Carlezon and Chartoff 2007). The delivery of the stimulation was accompanied by illumination of a 2-W house light. The stimulation current was adjusted (final range, 110–210 μA) for each rat to the lowest value that would sustain a reliable rate of responding (at least 40 responses per 50 s). After the minimal effective current was found for each rat, it was kept constant throughout training and testing.
Rats were then trained on a series of 15 descending stimulation frequencies (141–28 Hz, in 0.05 log10 Hz increments). Each series of frequencies comprised 1-min trials at each frequency. Each 1-min trial included a 5-s period during which non-contingent stimuli were given (1/s) at the next available stimulation frequency, a 50-s test period during which the number of responses was counted, and a 5-s period during which no stimulation was available. Each stimulation train was followed by a 500-ms time out. All responses were counted, regardless of whether they earned stimulation or occurred during a time out. After responding had been evaluated at each of the 15 frequencies, another series of identical frequencies was started. To characterize the functions relating response strength to reward magnitude (rate-frequency function), a least-squares line of best fit was plotted across the frequencies that sustained responding at 20, 30, 40, 50, and 60% of the maximum rate using customized analysis software. The stimulation frequency at which the line intersected the X axis (theta 0) was defined as the ICSS threshold (see Carlezon and Chartoff 2007). This is considered the theoretical point at which the stimulation becomes reinforcing. Rats were trained for an average of 3–4 weeks until mean ICSS thresholds remained stable (±10% for four consecutive days).
For drug testing, three rate-frequency functions (3× 15 min) were determined for each rat immediately before drug injection. The first function served as a “warm-up” and was discarded because it tends to be variable. The ICSS thresholds and maximum rates for the second and third functions were averaged and served as baseline parameters. Each rat then received an IP injection of drug or vehicle, and 12 more 15-min rate-frequency functions were obtained (3 h of testing). Each rat was treated with either vehicle or salvA (0.25, 2.0 mg/kg) over the course of the experiment, administered in random order. Drug treatment days were separated by 2–3 non-drug days in which the rats were exposed to six 15-min rate-frequency functions to ensure that they had recovered from prior treatment and their ICSS thresholds remained within 10% of their original baseline values. After each rat had received the three experimental treatments, it was treated again with vehicle to ensure that no conditioned drug effects were evident. It has previously been shown that multiple salvA treatments administered in this manner do not result in sensitization or tolerance to the aversive effects of the drug (Carlezon et al. 2006).
Operant responding for sucrose reward
Progressive ratio (PR) responding for sucrose was employed to test the effects of salvA on motivated behavior. This schedule assesses motivation to respond by progressively increasing the effort rats must expend to receive a fixed reward. PR responding is sensitive to dopamine manipulations (Aberman et al. 1998; Hamill et al. 1999). Alterations in PR can be attributed to either changes in motivation or motor capability. To dissociate effects on motivation from potential motor deficits, rats were also tested on a fixed ratio (FR) 5 schedule for sucrose. The FR5 paradigm typically results in higher rates of responding that are less sensitive to changes in dopamine activity (Aberman and Salamone 1999).
After a one-week acclimation period following arrival, rats were food restricted to approximately 90% of their free-feeding weight. For 2 days, rats underwent magazine training in standard operant procedure chambers equipped with two levers, two cue lights, a pellet dispenser, and a house light (Med Associates, Inc.; St. Albans, VT). During magazine training, two levers were constantly available with one (active) lever yielding a single 45-mg sucrose pellet when pressed. The other (inactive) lever had no programmed response when pressed. In addition, sucrose pellets were delivered on average every 60 s. On subsequent days, rats were shaped to press the active lever. Following 3 days of responding at least 100 times on the active lever, rats were shifted to a series of FR requirements with each ratio being administered for three consecutive days. Rats were trained on FR1, then FR3 and finally FR5. In all FR paradigms, a single sucrose pellet was delivered upon completion of the ratio on the active lever. All behavioral sessions lasted 30 min. Following FR5, rats were exposed to 2 days of the PR paradigm. The PR sessions were 2 h in length and involved escalating requirements for the rats to obtain sucrose pellets. The following are the first 23 sequences of lever presses required to obtain sucrose pellets in the exponential PR paradigm: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, and 492. No animal completed more than 23 ratios during a PR session. Testing began following 2 days of PR training.
One group of rats (n=5) was injected with either vehicle or salvA (0.25, 2.0 mg/kg) and immediately placed into the operant procedure chamber for a 3-h PR session. Rats were then returned to their home cages. After 4 days without testing or training, two consecutive days of PR training were administered followed by another test day with a different dose of salvA. This procedure continued until all doses of salvA had been administered. The other group of rats (n=5) was injected with either vehicle or salvA (0.25, 2.0 mg/kg) and immediately placed into the operant procedure chamber for a 2-h FR5 session. After 4 days without testing or training, two consecutive days of PR were administered followed by another FR5 test day with a different dose of salvA. This procedure continued until all doses of salvA had been administered. For both groups, the order of drug dose was randomized and counterbalanced across rats. Upon completion of salvA injections, each group of rats began the other operant procedure. During PR and FR5 sessions, the number of lever presses on active and inactive levers was recorded. Breakpoints were also calculated by assessing the number of ratios completed prior to 15 min of inactivity in a session, as established by Hodos (Hodos 1961).
Histology
Upon completion of the FSCV and ICSS experiments, histological assessment of stimulating and recording electrodes was performed (see Supplementary Methods and Online Resources 1, 2, and 3).
Statistical analysis
The effects of salvA on ICSS thresholds, maximum rates of responding in ICSS, and electrically evoked phasic dopamine release in the NAc core and shell were analyzed using two-way ANOVAs (treatment × time) with repeated measures on time. Significant effects were analyzed using post hoc Fisher's protected t tests. Dopamine reuptake curves were analyzed with repeated measures ANOVA (stimulation time × decay time).
To determine the acute effects of salvA on PR and FR5 responding for sucrose, cumulative active lever responding was divided into 5-min epochs over the course of the test session and analyzed using a two-way, within-subjects ANOVA (treatment × time) with Tukey's post hoc comparisons. Inactive PR and FR5 lever responding was analyzed using a one-way (treatment) within-subjects ANOVA with Tukey's post hoc comparisons. Breakpoint was analyzed by comparing the final ratio completed prior to 15 min of inactivity in a session and analyzed using a one-way, within-subjects ANOVA with Tukey's post hoc comparisons.
Results
SalvA decreased evoked phasic dopamine release in the NAc without affecting reuptake
This study examined the temporal effects of acute salvA on phasic dopamine release and subsequent reuptake in either the NAc core or shell. All data were normalized for each subject to the average of the baseline measurements (percent baseline) to control for differences in the initial magnitude of evoked dopamine release across rats. Dopamine release evoked in the NAc core (Figs. 1 and 2a) by VTA stimulation depended on an interaction between drug treatment and time (F44,330=2.94, p<0.01), with dopamine release decreasing across groups as the 3-h session progressed. Post hoc analysis revealed that salvA (2.0 mg/kg) significantly decreased dopamine release in the NAc core compared to vehicle from 5 to 135 min post-injection (p<0.01) with the exception of 60 min post-injection. Similarly, salvA (2.0 mg/kg) significantly decreased dopamine release in the NAc core compared to salvA (0.25 mg/kg) from 5 to 40, 50 and 75 min post-injection (p<0.01). SalvA (0.25 mg/kg) did not significantly alter dopamine release in this region. The effects of salvA on stimulated dopamine release in the NAc core were maximal at 15 min (37.3±2%, mean±SEM of baseline dopamine release).
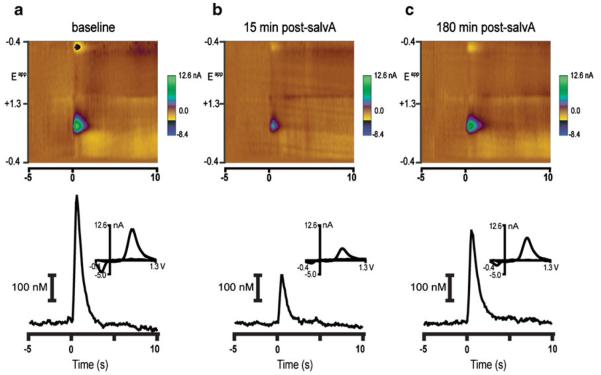
Fig. 1.
A representative example of salvA (2.0 mg/kg) effects on electrically evoked phasic dopamine. a) Electrical stimulation of the VTA evokes a phasic increase of dopamine in the NAc core prior to injection (baseline). Top: Background-subtracted color plot indicates changes in dopamine concentration in response to stimulation (time=0; 24 pulses, 60 Hz, 120 μA, 4 ms/phase, monophasic). Time is the abscissa, the electrode potential is the ordinate, and current changes are encoded in color. Dopamine concentration [identified by its oxidation (~0.6 V; green) and reduction (~−0.2 V; blue on the negative going scan) features] increased in response to the stimulation. Bottom: dopamine concentration as a function of time is extracted from the color plot above. Inset: Current changes due to the presence of dopamine at the electrode surface are confirmed by plotting current as a function of electrode potential (cyclic voltammogram). b) The same stimulation train evokes a smaller change in dopamine concentration 15 min and c) 180 min post-salvA (2.0 mg/kg) injection. Data in b and c were plotted and analyzed as in a
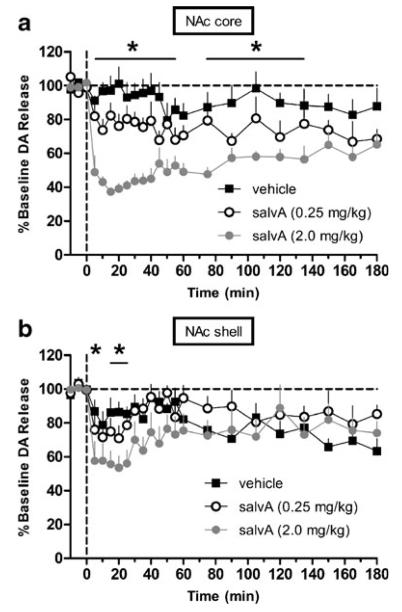
Fig. 2.
SalvA decreases phasic dopamine concentration evoked by electrical stimulation of the VTA. a) Time course of salvA effects on the peak concentration of dopamine in the NAc core evoked by electrical stimulation. Compared to vehicle (n=6 rats), salvA (2.0 mg/kg; n=6 rats) significantly decreased evoked dopamine concentration in the NAc core from 5–135, but not 60, min post-injection. Compared to vehicle, salvA (0.25 mg/kg; n=6 rats) had no effect on peak dopamine concentration. b) Time course of salvA effects on the peak concentration of dopamine in the NAc shell evoked by electrical stimulation. Compared to vehicle (n=6 rats), salvA (2.0 mg/kg; n=6 rats) significantly decreased evoked dopamine concentration in the NAc shell from 5 to 25 min with the exception of 10-min post-injection. Compared to vehicle, salvA (0.25 mg/kg; n=5 rats) had no effect on peak dopamine concentration. In both graphs, each point represents the mean (+sem) percent change from pre-injection baseline. *p<0.01 compared to vehicle at each time point, Fisher's protected t tests
Dopamine release evoked in the NAc shell (Fig. 2b) by VTA stimulation depended on an interaction between drug treatment and time (F44,308=1.73, p<0.01) with dopamine release decreasing across groups as the 3-h session progressed. Post hoc analysis revealed that salvA (2.0 mg/kg) significantly decreased dopamine release in the NAc shell compared to vehicle from 5 to 25 min post-injection with the exception of 10 min post-injection (p<0.01). SalvA (0.25 mg/kg) did not significantly alter dopamine release in this region. The effects of salvA on stimulated dopamine release in the NAc shell were maximal at 20 min (53.5±7%, mean±SEM of baseline dopamine release). Interestingly, we found that vehicle injection produced a transient decrease in the shell. Previous work has demonstrated that aversive (Roitman et al. 2008) or novel stimuli (Aragona et al. 2009) suppress dopamine release in the shell. Thus, it is likely that at least part of the suppression of phasic dopamine release in the shell is due to the aversive properties of the injection procedure itself.
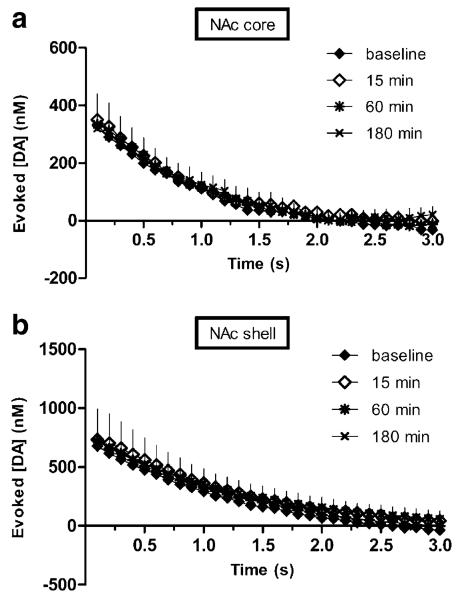
While salvA decreased phasic dopamine, it had no impact on reuptake. The time course of salvA's effects on reuptake was analyzed separately for each dose. SalvA (2.0 mg/kg) had no effect on the rate of reuptake in either the NAc core (Fig. 3a; F116,580=0.66; ns) or shell (Fig. 3b; F116,580=0.28; ns). In addition, rate of reuptake was unchanged in both regions following either vehicle or salvA (0.25 mg/kg; data not shown).
Fig. 3.
SalvA does not affect the rate of dopamine reuptake in the NAc. The descending portions of dopamine concentration curves in the NAc core a) and shell b) evoked at baseline and 15, 60, and 180-min post-salvA (2.0 mg/kg) injection are overlaid. Comparisons of the descending curves of concentration-matched, electrically evoked dopamine indicate that dopamine reuptake rates are not different. In both graphs, each point represents the mean (+sem) dopamine concentration evoked by stimulation of the VTA (n=6 rats). ANOVAs (stimulation time × decay time) revealed no main effects
SalvA increased ICSS thresholds
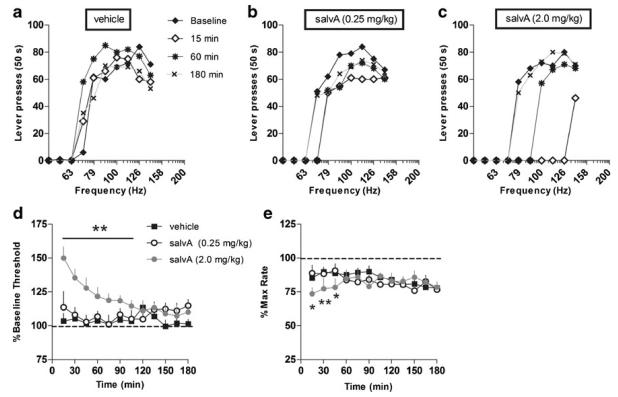
SalvA caused rightward and downward shifts of the rate-frequency functions (Fig. 4a, b, c). Data from a representative rat show that vehicle does not affect rate-frequency functions at 15, 60, or 180 min post-injection compared with the baseline rate-frequency function (Fig. 4a). However, data from the same rat show that 15 and 60 min after administration of the active dose of salvA (2.0 mg/kg; Fig. 4c), higher frequencies are required to sustain responding. The maximum rate of responding is depressed at 15 but not at 60 min compared with baseline values, suggesting that by 60 min after salvA, motivation to respond is decreased (increases in ICSS thresholds), but motor capacity is unaffected. Administration of a threshold dose of salvA (0.25 mg/kg; Fig. 4b) results in a slight right shift in thresholds and decrease in maximum response rates, but on average (Fig. 4d, e) these differences are not significant.
Fig. 4.
Effects of salvA on ICSS thresholds. Rats were treated systemically with vehicle or salvA (0.25, 2.0 mg/kg, IP) and immediately placed in the ICSS chambers for 12 rate-frequency determinations over the course of the 180-min test session. (a, b, and c) Rate of bar pressing (per 50 s) as a function of stimulation frequency for a representative rat at indicated times after vehicle (a), salvA (0.25 mg/kg) (b), or salvA (2.0 mg/kg) (c) injection. Time course of drug effects on thresholds (d) and maximal rates of responding (e). Each point in d and e represents the mean (+sem) percent change from pre-injection baseline thresholds. SalvA (2.0 mg/kg) significantly increased thresholds for 105 min and decreased maximal rates of responding for 45 min compared to vehicle (N=9 rats). *p<0.05, **p<0.01 compared to vehicle at each time point, Fisher's protected t tests
The effects of salvA on ICSS thresholds depended on an interaction between dose and time (F22,264=3.63, p<0.0001): the active dose of salvA significantly increased ICSS thresholds compared with vehicle control for 105 min, whereas the threshold dose did not significantly alter thresholds (Fig. 4c). ICSS thresholds remained quite stable compared to baseline values over the 3-h test session in vehicle-treated rats, although there was a general downward drift in maximum rates in all treatment groups (Fig. 4d), suggesting a decrease in motor output with no change in reward function. The effects of salvA on maximum rates of responding also depended on an interaction between dose and time (F22,264=1.76, p<0.05): the high dose of salvA significantly decreased the maximum rate of responding for the first 45 min, whereas the low dose did not alter rates (Fig. 4d). Treatment with vehicle caused a slight decrease in maximum response rates compared to pretreatment baseline levels, suggesting nonspecific effects of vehicle.
SalvA reduces motivation to work for sucrose reward
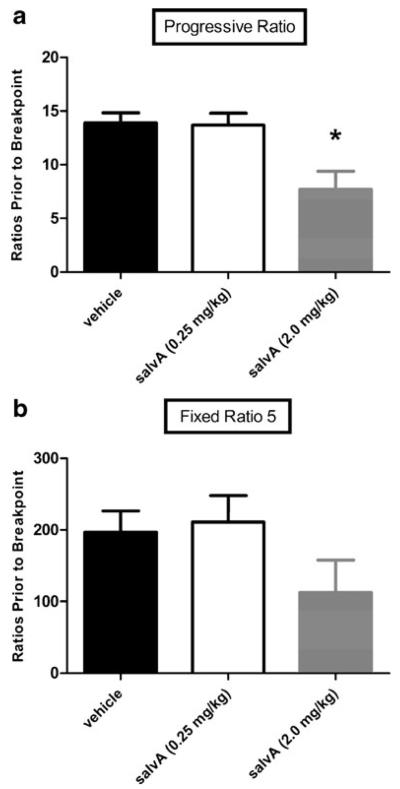
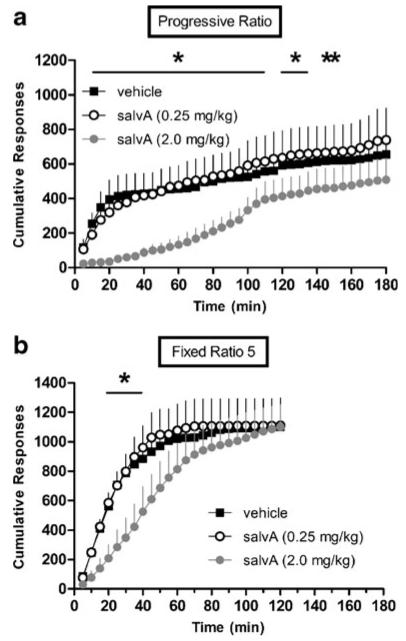
The PR paradigm has been used extensively as an assay of motivation for reward and is sensitive to changes in NAc dopamine (Aberman et al. 1998; Hamill et al. 1999). Here, salvA (2.0 mg/kg) lowered breakpoint in PR responding for sucrose reward. Figure 5a shows the number of ratios completed prior to 15 min of inactivity (breakpoint) as a function of salvA dose in the 3-h PR session. There was a significant effect of salvA dose on breakpoint (F2,18=7.97, p<0.05). Post hoc analyses revealed that the active dose of salvA (2.0 mg/kg) decreased breakpoint compared with vehicle and salvA (0.25 mg/kg) (p<0.05). The threshold dose of salvA (0.25 mg/kg) did not alter breakpoint. Responding on an FR5 ratio is less sensitive to changes in NAc dopamine (Cousins and Salamone 1994; Salamone et al. 2001). As shown in Fig. 5b, there was a trend towards lower breakpoint following salvA (2.0 mg/kg), but it was not significant (F2,18=1.94, ns). SalvA suppressed the rate of responding under both PR and FR5 conditions. Figure 6a shows the effects of salvA on cumulative active lever responding over the 3-h PR session. The effects of salvA on PR depended on an interaction between treatment and time (F70,630=3.39, p<0.05). Post hoc analyses revealed that the active dose of salvA (2.0 mg/kg) significantly decreased responding compared to vehicle between 10 and 150 min post-injection—but not at 115 or 140 min (p<0.05). Similarly, salvA (2.0 mg/kg) decreased responding compared to salvA (0.25 mg/kg) beginning 10 min post-injection and lasting the remainder of the behavioral session (p<0.05). Figure 6b shows cumulative active lever responding as a function of salvA dose across the 2-h FR5 session. The effects of salvA on FR5 responding on the active lever depended on an interaction between treatment and time (F46,414=2.33, p<0.05). Post hoc analyses revealed that rats treated with vehicle or salvA (0.25 mg/kg) did not differ in active lever responding throughout the behavioral session. Rats treated with 2.0 mg/kg salvA responded less than animals treated with vehicle beginning 20 min post-injection and lasting until 40 min post-injection (p<0.05). Similarly, rats treated with salvA (2.0 mg/kg) responded less than animals treated with salvA (0.25 mg/kg) beginning at 20 min post-injection and lasting until 50 min post injection (p<0.05). There were no differences in responding on the inactive lever across drug treatment during neither PR nor FR5 responding (data not shown). Importantly, at equivalent post-drug time points, salvA-injected rats (2.0 mg/kg) responded in the FR5 paradigm at rates comparable to vehicle-injected rats in the PR paradigm.
Fig. 5.
Effects of salvA on the number of ratios completed for sucrose pellets prior to 15 min of inactivity (breakpoint). a) Compared to vehicle and salvA (0.25 mg/kg), salvA (2.0 mg/kg) significantly lowered breakpoint in the PR schedule. b) There were no significant differences in FR5 breakpoints across salvA dose. In both graphs, each bar represents the mean (+sem) breakpoint during the session (n=10 rats). *p<0.05 compared to vehicle and salvA (0.25 mg/kg), Tukey's Honestly Significant Difference tests
Fig. 6.
Effects of salvA on cumulative active lever responding for sucrose pellets. a) Compared to vehicle, salvA (2.0 mg/kg) significantly decreased active lever responding in the PR schedule between 10 and 150 min post-injection—but not at 115 or 140 min. b) Compared to vehicle, salvA (2.0 mg/kg) significantly decreased active lever responding in the FR5 schedule between 20 and 40-min post-injection. In both graphs, each point represents the mean (+sem) number of cumulative lever presses made on the active lever (n=10 rats). *p<0.05 compared to vehicle at each time point, Tukey's Honestly Significant Difference tests
Discussion
This study shows that a behaviorally active dose of the KOR agonist salvA decreases phasic dopamine release in the NAc elicited by electrical stimulation of the VTA. This is consistent with recent ex vivo work (Britt and McGehee 2008) but importantly extends the findings to awake and behaving animals. Furthermore, salvA has discrete effects on dopamine release in the NAc shell and core: salvA-mediated decreases in stimulated dopamine release in the core were prolonged compared with the shell and were temporally similar to the inhibitory effects of salvA on motivated behavior. SalvA increased ICSS thresholds, which reflects a decrease in the rewarding impact of—and consequently the motivation to work for—the medial forebrain bundle stimulation (Carlezon and Chartoff 2007). This confirms and extends prior work demonstrating reward-decreasing effects of KOR agonists in the ICSS paradigm (Beguin et al. 2008; Carlezon et al. 2006; Todtenkopf et al. 2004; Tomasiewicz et al. 2008). SalvA also decreased operant responding for sucrose pellets, which is indicative of a decrease in motivation to obtain a natural reinforcer.
The NAc core and shell subregions have distinct connectivity and reward-processing abilities that result in unique, though not mutually exclusive, functions (Ikemoto 2007; Meredith et al. 2008; Zahm 2000). The mechanisms underlying the differences between salvA's effects on dopamine release in the core versus shell are not known. The NAc shell appears to have higher levels of KORs than the core under normal conditions (Mansour et al. 1995), suggesting that the effects of a KOR agonist would be more robust in the shell. Given that we observed the opposite trend, it is possible that there are differences in coupling of KORs to downstream effectors between the core and the shell. It has also been shown that activation of KORs can modulate the dopamine transporter (DAT) (Gehrke et al. 2008; Thompson et al. 2000), raising the possibility that salvA has differential effects on the number or function of the DAT in the core and the shell. Using FSCV, which directly assesses the rate of dopamine reuptake (Cheer et al. 2004; Senior et al. 2008), we found that salvA had no effect on reuptake in either the core or the shell over the course of the 3-h test period. Finally, it is possible that the extended effects of salvA observed uniquely in the core are due to activation of secondary molecular processes such as modulation of dopamine synthesis or release.
The finding that salvA initially suppresses phasic dopamine release in both the NAc shell and the core but has a prolonged effect in the core that is temporally similar to effects on motivated behavior highlights the complexity of NAc function. Unconditioned rewarding, aversive, and novel stimuli preferentially elicit dopamine concentration changes in the shell (Aragona et al. 2009; Bassareo and Di Chiara 1999; Pontieri et al. 1995; Roitman et al. 2008). For example, it has been shown using FSCV that an aversive taste stimulus elicits a decrease in phasic dopamine release in the NAc shell (Roitman et al. 2008). Interestingly, the predominant response of NAc neurons to an aversive stimulus is an increase in firing rate (Roitman et al. 2005; Wheeler et al. 2008), raising the possibility that some aversive stimuli suppress phasic dopamine activity through increased activity of dynorphin-releasing NAc neurons projecting back to the midbrain. In contrast to the shell, the NAc core is most often associated with goal-directed behavior and the motivational value of conditioned stimuli (Aragona et al. 2009; Bari and Pierce 2005; Ito et al. 2000; Roitman et al. 2004). Dopamine release in the core precedes and peaks at the time of operant responses for a variety of reinforcers including ICSS (Cheer et al. 2005) and food (Roitman et al. 2004). Microinjection of a dopamine receptor antagonist into the NAc core decreases ICSS of the medial forebrain bundle without affecting motor performance (Stellar and Corbett 1989). Finally, microinjections of dopamine receptor antagonists into the NAc core, but not shell, reduce operant responding for food reinforcement on a progressive ratio schedule (Bari and Pierce 2005).
The effects of salvA on ICSS and sucrose responding may be due, in part, to motor deficits rather than a reduction in drive per se. Although prior studies showed that salvA (2.0 mg/kg) was maximally effective at increasing ICSS thresholds and immobility time in the FST without significantly affecting motor activity (Beguin et al. 2008; Carlezon et al. 2006), we found that this dose of salvA had rate-decreasing effects in the ICSS paradigm for the first 45 min after drug injection. However, salvA significantly increased thresholds for 105 min, suggesting that KOR-mediated motivational deficits can be dissociated from motor effects and are longer lasting. Furthermore, the measure of reward threshold used in this study (theta 0) has been shown to be minimally sensitive to treatment-induced alterations in response capabilities (Miliaressis et al. 1982). SalvA did not eliminate the capacity to respond for sucrose reward because rats treated with salvA were able to press on an FR5 schedule of reinforcement at rates similar to vehicle treated rats on a PR schedule at equivalent times post-treatment. The effects of salvA on PR are similar to dopamine-depleting lesions of the NAc and dopamine antagonist treatment (Aberman et al. 1998; Hamill et al. 1999). Higher ratio requirements are more sensitive to disruption of dopamine signaling (Aberman and Salamone 1999), suggesting that the prolonged suppression of phasic dopamine release in the NAc core in the present study is important for the effects of salvA on operant response measures. Collectively, the effects of salvA in these operant conditioning procedures are consistent with a transient decrease in motor ability and a prolonged decrease in motivation.
KOR-mediated decreases in motivated behavior and NAc dopamine are similar to effects often observed after chronic administration of drugs of abuse and stress (Goussakov et al. 2006; Markou and Koob 1991). These effects are distinct from acute responses to drugs of abuse and many types of stress, in which dopamine activity in the NAc is increased and reward systems are activated (Imperato et al. 1989; Piazza and Le Moal 1996; Sorg and Kalivas 1991; for review see Yap and Miczek 2008). In fact, activation of endogenous KOR systems may be a compensatory response to drugs of abuse and stress, such that after the acute effects have passed, dynorphin tone remains elevated and contributes to the subsequent decrease in NAc dopamine activity and emergence of depressive-like states (see Knoll and Carlezon 2009). Although this hypothesis requires further testing, our current findings confirm the idea that KORs regulate complex mood states by simultaneously decreasing dopamine release, reward function and motivation, and they extend our knowledge by demonstrating discrete KOR-mediated effects on phasic dopamine release in the NAc core and shell.
Supplementary Material
Acknowledgements
This work was supported by the Morris D. Braun Foundation (EHC) and by National Institutes of Health grants DA023094 (to EHC) and DA025634 (to MFR) and NARSAD (to MFR).
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Beguin C, Potter DN, Dinieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, Liu-Chen LY, Carlezon WA, Jr, Cohen BM. N-methylacetamide analog of salvinorin A: a highly potent and selective kappa-opioid receptor agonist with oral efficacy. J Pharmacol Exp Ther. 2008;324:188–195. doi: 10.1124/jpet.107.129023. [DOI] [PubMed] [Google Scholar]
- Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28:1672–1681. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Heien ML, Aragona BJ, Kim M, Carelli RM, Wightman RM. Society for Neuroscience. Washington: 2005. Simultaneous measurements of fast dopamine release and coincident accumbal firing patterns at the same electrode during goal-directed behavior. Abstract. [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Gehrke BJ, Chefer VI, Shippenberg TS. Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berl) 2008;197:509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goussakov I, Chartoff EH, Tsvetkov E, Gerety LP, Meloni EG, Carlezon WA, Jr, Bolshakov VY. LTP in the lateral amygdala during cocaine withdrawal. Eur J Neurosci. 2006;23:239–250. doi: 10.1111/j.1460-9568.2005.04538.x. [DOI] [PubMed] [Google Scholar]
- Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav. 1999;64:21–27. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Heien ML, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–5704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005 doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2009 doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Lee DY, Karnati VV, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, Jr, Cohen B. Synthesis and in vitro pharmacological studies of new C(2) modified salvinorin A analogues. Bioorg Med Chem Lett. 2005;15:3744–3747. doi: 10.1016/j.bmcl.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Mansour A, Watson SJ, Akil H. Opioid-receptor mRNA expression in the rat CNS: Anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Durivage A. Psychophysical method for mapping behavioral substrates using a moveable electrode. Brain Res Bull. 1982;8:693–701. doi: 10.1016/0361-9230(82)90097-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience. 2001;105:863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J. Dissociation of the actions of uptake blockers upon dopamine overflow and uptake in the rat nucleus accumbens: in vivo voltammetric data. Neuropharmacology. 1989;28:1383–1388. doi: 10.1016/0028-3908(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Stellar JR, Corbett D. Regional neuroleptic microinjections indicate a role for nucleus accumbens in lateral hypothalamic self-stimulation reward. Brain Res. 1989;477:126–143. doi: 10.1016/0006-8993(89)91400-5. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse. 2001;42:185–192. doi: 10.1002/syn.10005. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69, 593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA. Stress and rodent models of drug addiction: Role of VTA-accumbens-PFC-amygdala circuit. Drug Discov Today Dis Models. 2008;5:259–270. doi: 10.1016/j.ddmod.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.