Abstract
Dendritic cells (DC) pulsed with tumor-derived peptides, proteins, genes, or lysates have been studied as therapeutic cancer vaccines. However, the overall therapeutic efficacy of this approach has been limited, indicating a need to either enhance its potency or combine it with other treatment modalities. Photodynamic therapy (PDT) process consists of injecting a photosensitizer, which selectively accumulates at the lesion site, followed by local illumination of the tumor with a laser of the appropriate wavelength to activate the specific drug. PDT has the potential to create an environment at the tumor site that favors both tumor antigen loading and activation of DCs, key requirements for induction of antitumor immunity. Here, we report that PDT can induce IL-1 and IL-6 and reduce TNF-α expression from DCs. This finding has potentially broad clinical implications since these changes are mechanistically involved in the observed effects of PDT on host immune responses. Not all tumors are amenable to PDT, either because of size or location, and one could conceive of an adjuvant use for PDT vaccines in conjunction with other cancer modalities that do not enhance the host antitumor immune response.
Keywords: Photodynamic therapy, dendritic cells, tumor immunity, interleukin
Introduction
Dendritic cells (DC) are the most potent antigen-presenting cells. DCs pulsed with tumor-derived peptides, proteins, genes, or lysates, as well as DCs fused with tumor cells, have been studied as therapeutic cancer vaccines [1-9]. Although the methods are complex and costly to implement, promising results have been obtained in clinical trials in patients with advanced malignancies. These trials have shown DC-based vaccination to be well tolerated and capable of inducing tumor-specific T-cell responses and regression of metastatic disease. On the other hand, the overall therapeutic efficacy of this approach has been limited, indicating a need to either enhance its potency or combine it with other treatment modalities. Among the modalities that might be combined with DC-based immunotherapy are systemically administered antitumor drugs as well as locally targeted therapies such as radiation, radiofrequency ablation, and photodynamic therapy (PDT).
PDT has been approved in many countries as an anticancer therapy, mainly for the palliative treatment of surgically inaccessible tumors. The PDT process consists of injecting a photosensitizer, which selectively accumulates at the lesion site, followed by local illumination of the tumor with a laser of the appropriate wavelength to activate the specific photosensitizer [10]. Irradiation with light of the proper wavelength can lead to two outcomes. First, activation of the photosensitizer can transform the drug from its ground state to an excited singlet state; from this state, the drug may decay directly back to the ground state by emitting fluorescence, a property that can be used clinically for photodetection. Second, to generate a therapeutic photodynamic effect, the photosensitizer must undergo electron spin conversion to a triplet state. In the presence of oxygen, this excited molecule can react directly with its substrate, by proton or electron transfer, to form radicals or radical ions that interact with oxygen to produce oxygenated products (a type I reaction). Alternatively, the energy of the excited photosensitizer can be directly transferred to oxygen to form a singlet oxygen (a type II reaction), which is the most damaging species generated during PDT [12]. Tumor destruction after PDT results from direct cytotoxic effects as well as from the induction of a local inflammatory response [13]. Thus, preclinical studies have shown that PDT not only mediates apoptotic and necrotic killing of tumor cells but also alters the tumor microenvironment through the release of cytokines and interleukins. On the basis of its unique mechanism of tumor destruction, PDT has the potential to create an environment at the tumor site that favors both tumor antigen loading and activation of DCs, key requirements for induction of antitumor immunity [14]. Here, we report that PDT can induce the expression of IL-1 and IL-6 from DCs and propose that these changes are mechanistically involved in the observed effects of PDT on the host immune response.
Materials and methods
PDT was performed as reported previously [15]. For stationary culture, mouse Lewis lung carcinoma (LLC) cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Sigma-Aldrich Inc., MO) with 10% fetal calf serum (FCS) in a Black with Clear Bottom 96-well Microtest™ Optilux™ Plate (BD bioscience Inc., CA) (1×104 cells/well). Twenty-four hours later, when the cells had adhered to the plate, the medium was removed and the cultures were washed three times with phosphate-buffered saline solution (PBS, pH 7.4, Sigma). Talaporfin sodium (Mono-L-aspartyl chlorin e6, Laserphyrin®, provided by Meiji Seika Co., Ltd., Tokyo, Japan) dissolved in FCS free DMEM was added to each well (50 μg/mL). After 2 hrs, the supernatant was removed and the cells were thoroughly washed three times with PBS. FCS free DMEM was added, and the cells were subjected to laser irradiation (wavelength; 664 nm, power density; 60 mW/cm2) for 30 sec at a dose equivalent to the lethal dose (LD100). Immediately after laser irradiation, the culture medium was replaced with complete growth medium. Twenty-four hours after PDT, cells and supernatants were collected and spun to clear cell debris (PDT-generated lysates). In parallel, freeze/thaw lysates were generated by subjecting LLC cells (FCS free DMEM) to three freeze/thaw cycles in liquid nitrogen and 37°C water bath, followed by centrifugation to remove the cell debris (freeze/thaw-generated lysates). Stationary culture media of LLC cells (FCS free DMEM) was used as a control.
The JAWSII mouse DC line was cultured in complete growth medium containing Minimum Essential Medium (MEM) alpha with ribonucleo-sides, deoxyribonucleosides, 4 mM L-glutamine, and 1 mM sodium pyruvate supplemented with 10% FCS and 5 ng/mL murine recombinant GM-CSF. JAWSII cells were incubated overnight in a 96-well microplate (l*104 cells/well), and the culture media was replaced with PDT-generated lysates or freeze/thaw-generated lysates (100 μL/well). After 24 hrs, the supernatants were collected and subjected to ELISA assay. The IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, Macrophage Inflammatory Protein (MIP) 1α, Transforming Growth Factor (TGF) β, Tumor Necrosis Factor (TNF) α, and Vascular Endothelial Growth Factor (VEGF) levels in the supernatant were quantified from each sample using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN).
All data are expressed as the relative ratio against the control (stationary LLC cultured media) and as the means ± standard derivation (n=6-12). Statistical significance (defined as P values <0.01) was evaluated using the unpaired Student's ttest (two-tailed).
Results and discussion
PDT has been shown to enhance the host antitumor immune response [13]. To determine whether this enhancement was at least in part a consequence of the effects of PDT on tumor cells, we tested the immunogenicity of tumor cell lysates generated by in vitro PDT treatment. To do so, PDT-generated tumor cell lysates were added to DC cultures and IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, MIP-1α, TGF-β, TNF-α, and VEGF levels were measured. Before the experiments, we performed the array test by using Proteome Profiler™ Array kit (R&D Systems, Minneapolis, MN) and picked up the cytokines or growth factors that seemed to be the differences between experimental group and negative control group (data not shown). IL-1α, IL-1β, and IL-6 were the most markedly increased, and TNF-α was decreased in DC culture supernatants following this treatment (Figure 1A). These cytokines must have been secreted from DCs because they were not detected in the tumor cell lysates. The concentrations of other cytokines, with the exception of IL-2 and IL-12, which were below the detection limit of ELISA, were not changed compared with those of control cells. In parallel, cytokine levels were also examined in the supernatants of DC cultures treated with freeze/thaw-generated tumor cell lysates (Figure 1B). In these experiments, the levels of cytokines and growth factors secreted to the supernatant were unchanged after treatment with the freeze/thaw -generated lysates.
Figure 1.
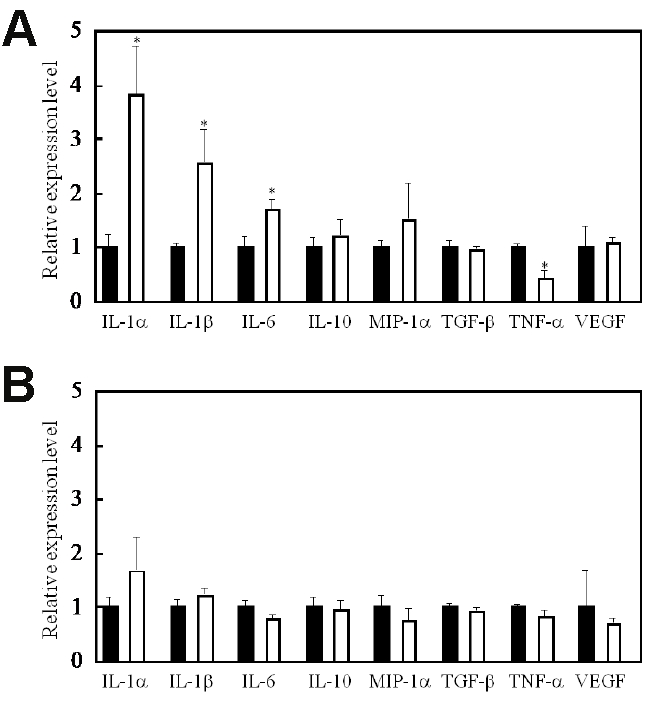
PDT-generated cell lysates activate DCs. IL-1α, IL-1β, and IL-6 were most markedly increased and TNF-α was decreased following the addition of PDT-generated lysates to DC cultures (a). In contrast, cytokine levels did not change after addition of freeze/thaw-generated tumor cell lysates to DC cultures (b). IL-2 and IL-12 secretion levels were below the detection limit of ELISA. *, p<0.01: significant difference between the addition of PDT-generated lysates and addition of stationary culture media from LLC cells.
IL-1α, IL-1β, and TNF-α were investigated in parallel because they are recognized IL-6 inducers and actsynergistically with IL-6 to induce antitumor responses in mice [16,17]. We confirmed the enhancement of IL-6 secretion from cells after PDT in vitro described earlier by Kick et al. [18]. Further, as suggested by Kick et al., TNF-α does not seem to play a role in IL-6 induction by PDT because the changes in IL-6 are neither preceded nor accompanied by similar changes in TNF-α. PDT induces TNF-α in murine peritoneal macrophages in vitro [19], and a recent study by Anderson et al. [20] has demonstrated up-regulation of TNF-α in keratinocytes in vitro by PDT using a phthalocyanine-derived photo-sensitizer. The decreased levels of TNF-α observed in our study might be related to the DCs used, as the regulatory region of the TNF-α gene has been shown to have allelic differences [21].
It remains to be determined whether the enhanced generation of IL-6 plays a role in the PDT tumor response. Intratumoral injection of IL-6 or transduction of the IL-6 gene into tumor cells can enhance tumor immunogenicity and inhibit tumor growth in experimental murine tumor systems [17,22,23]. Thus, PDT may enhance local antitumor immunity by up-regulating IL-6 production in DCs. The mechanisms by which this is achieved are not yet clear. Dougherty et al. [23] have suggested that IL-6 may further the recruitment of tumoricidal macrophages into the tumor bed. On the other hand, Mule et al. [17] have shown that IL-6-mediated tumor regression could be abrogated by in vivo depletion of either CD4+ or CD8+ T-cell subsets. Although this study did not examine T-cell responses, changes in T-cell function might occur, and we are presently analyzing this using co-culture methods. Luna et al. [24] have shown in murine RIF cells in vitro that the early-response genes c-fos and c-jun are induced by Photofrin; these gene products form the AP-1 transcription factor, which induces IL-6 expression [16,18,25].
Gollnick et al. [26] reported that vaccination with PDT-generated tumor cell lysates elicits a tumor-specific immune response as demonstrated by protection against subsequent tumor inoculation, induction of tumoricidal activity in the spleen, and increased numbers of IFN-g-secreting splenic cells. These studies demonstrate that PDT is able to enhance the inherent immunogenicity of at least some tumor cells. The nature of the “activation” factor in PDT-generated tumor cell lysates is unknown, although there are several promising candidates. Although recent studies have focused on the use of genetically engineered cancer vaccines or tumor-associated antigen-primed DCs [27,28], there is no convincing evidence that these vaccines have an overwhelming advantage over crude vaccines [27]. The finding that PDT-generated tumor cell lysates were effective antitumor vaccines has potentially broad clinical implications. Not all tumors are amenable to PDT, either because of size or location, and one could conceive of an adjuvant use for PDT vaccines in conjunction with other cancer modalities that do not enhance the host antitumor immune response, such as surgery and/or chemotherapy.
Acknowledgments
This research was carried out with a Grant-in-Aid for Scientific Research (Young Scientists A), Japan Society for the Promotion of Science (JSPS).
References
- 1.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccina tion of patients with B-cell lymphoma using autologous antigen pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Palucka K. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 4.Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:9482–9487. doi: 10.1073/pnas.95.16.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells geneticallymodifiedwith an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent anti-genpresenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 8.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 9.Figdor CG, DeVries JM, Lesterhuis WJ, Melief CJM. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 10.Lightdale CJ, Heier SK, Marcon NE, McCaughan JS, Jr, Gerdes H, Overholt BF, Sivak MV, Jr, Stiegmann GV, Nava HR. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc. 1995;42:507–512. doi: 10.1016/s0016-5107(95)70002-1. [DOI] [PubMed] [Google Scholar]
- 11.Verma S, Watt GM, Mai Z, Hasan T. Strate gies for enhanced photodynamic therapy effects. Photochem Photobiol. 2007;83:996–1005. doi: 10.1111/j.1751-1097.2007.00166.x. [DOI] [PubMed] [Google Scholar]
- 12.Buytaert E, Dewaele M, Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim Biophys Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engleman EG, Brody J, Soares L. Using signaling pathways to overcome immune tolerance to tumors. Sci STKE. 2004 doi: 10.1126/stke.2412004pe28. pe28. [DOI] [PubMed] [Google Scholar]
- 15.Kushibiki T, Sakai M, Awazu K. Differential effects of photodynamic therapy on morphologically distinct tumor cells derived from a single precursor cell. Cancer Lett. 2008;18:244–251. doi: 10.1016/j.canlet.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 16.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 17.Mule JJ, Custer MC, Travis WD, Rosenberg SA. Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J Immunol. 1992;148:2622–2629. [PubMed] [Google Scholar]
- 18.Kick G, Messer G, Goetz A, Plewig G, Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-l but not NF-kB DNA binding. Cancer Res. 1995;55:2373–2379. [PubMed] [Google Scholar]
- 19.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J Natl Cancer Inst. 1990;82:34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 20.Anderson C, Hrabovsky S, McKinley Y, Tubesing K, Tang H, Dunbar R, Mukhtar H, Elmets CA. Phthalocyanine photodynamic therapy: disparate effects of pharmacologic inhibitors on cutaneous photosensitivity and on tumor regression. Photochem Photobiol. 1997;65:895–901. doi: 10.1111/j.1751-1097.1997.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 21.Vincek V, Kurimoto I, Medema JP, Prieto E, Streilein JW. Tumor necrosis factor a polymorphism correlates with deleterious effects of ultraviolet B light on cutaneous immunity. Cancer Res. 1993;53:728–732. [PubMed] [Google Scholar]
- 22.Mullen CA, Coale MM, Levy AT, Stetler-Stevenson WG, Liofta LA, Brandt S, Blaese RM. Fibrosarcoma cells transduced with the JL-6 gene exhibit reduced tumorigenicity, increased immunogenicity, and decreased metastatic potential. Cancer Res. 1992;52:6020–6024. [PubMed] [Google Scholar]
- 23.Dougherty GJ, Thacker JD, Lavey RS, Belldegrun A, McBride WH. Inhibitory effect oflocaily produced and exogenous interleukin-6 on tumor growth in vivo. Cancer Immunol Immunother. 1994;38:339–345. doi: 10.1007/BF01525513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lana MC, Wong S, Gomer CJ. Photodynamic therapy mediated induction of early response genes. Cancer Res. 1994;54:1374–1380. [PubMed] [Google Scholar]
- 25.Kick G, Messer G, Plewig G, Kind P, Goetz AE. Strong and prolonged injunctionof c-jun and c-fos proto-oncogenesby photodynamictherapy. Br J Cancer. 1996;74:30–36. doi: 10.1038/bjc.1996.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. [PubMed] [Google Scholar]
- 27.Sinkovics JG, Horvath JC. Vaccination against human cancers. Int J Oncol. 2000;16:81–96. doi: 10.3892/ijo.16.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–274. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]


