Abstract
Fetal microchimerism occurs in normal human reproduction and is a relatively new discovery in biology. Recent data in the scientific and medical literature indicates that some of the autoimmune diseases that show a predilection for women in their child-bearing years and beyond are linked to fetal microchimerism from previous pregnancies. The pathological role of fetal microchimeric progenitor immature T cells in autoimmune disease in women is explored. Fetal microchimerism is increased in women who had a termination of pregnancy and may be associated with the development of autoimmune disease later on in life. Furthermore, the consistently rising incidence of autoimmune diseases in women over the past four decades may be attributed to the increase in the utilization of abortion.
Keywords: Microchimerism, autoimmune disease, abortion, pathophysiology
Introduction
Fetal microchimerism, which can be thought of as a form of trans-placental stem cell transplant, is becoming a plausible and unifying biological entity that helps to explain the etiology, the large diversity of tissue pathology, the predilection for females and the yearly increase in the incidence of autoimmune diseases in women. During their reproductive and post-reproductive years, women have a greater propensity than men to develop any one of a large variety of chronic autoimmune diseases. Autoimmune diseases are characterized by: (1) being the third most common category of chronic diseases after cancer and heart disease, (2) affecting 5% to 8% of the population, (3) having had for decades an unexplainable increasing incidence, (4) having the potential of affecting virtually any tissue in the body, (5) comprising over 80 different autoimmune diseases and (6) being predominately thyroid diseases and rheumatoid arthritis, which together comprise over 65 percent of the total incidence of all autoimmune diseases [1-6].
Materials and method
The following databases were searched for articles related to microchimerism, autoimmune disease, abortion and pathophysiology: Web of Science, PubMed, Google.
Fetal microchimerism
Initial publications of the relatively recent discovery of fetal microchimerism occurred in the late 1970's [7]. Fetal microchimerism is the transfer of intact living fetal cells from the fetal circulation into the maternal circulation and occurs in all pregnancies and increases with gestational age [8-10]. Microchimerism can be portrayed as a legacy of pregnancy that persists for decades via fetal cell engraftment in maternal bone marrow or other tissues [11-14]. The process of microchimerism is bidirectional and the transfer of maternal cells into the fetal circulation is known as maternal microchimerism [15,16]. Transfer of fetal hematopoietic pluripotent progenitor cells begins in the fourth or fifth week after fertilization and continues throughout the pregnancy [17-22]. The presence of fetal microchimeric cells can be detected for up to 30 days in the maternal postpartum blood stream [23]. Fetal microchimeric cells of male embryos/fetuses can be selectively detected and magnified by assaying for the presence of the Y-chromosome-containing cells among a large number of maternal cells marked by XX chromosomes [24,25]. More microchimeric cells are transferred after surgical abortions than after spontaneous abortions [23]. Male fetal cells have been demonstrated in both maternal synovial tissue and skin of patients with rheumatoid arthritis and in the skin and blood of women with systemic sclerosis [26-28]. Fetal cells were also shown to proliferate in consecutive cell cultures and were detected in maternal tissues as long as 27 years postpartum [29]. Fetal microchimerism occurs with either male and female embryos and fetuses but because of the uniqueness of the Y chromosome, detection of male microchimerism in maternal tissue is easier to detect. The extremely small number (5 to 10 embryo/fetal microchimeric cells) in pregnancies bearing male embryos/fetuses among millions of maternal cells can be detected [26,30]. The techniques of either polymerase chain reaction or fluorescent in situ hybridization passages demonstrated stem-cell-like properties of microchimeric cells from male embryos/fetuses [26,30,31].
Fetal microchimerism and the increased incidence of auto-immune disease
In the late 1990's the discovery of fetal microchimeric cells in maternal tissues led to the finding of a positive association between fetal microchimerism and autoimmune diseases in women [27,32-43]. Progenitor cells of the fetal immune system, such as immature T cells, along with T and B lymphocytes, monocytes, macrophages and NK cells, are among the different fetal cell types that ultimately can be transferred to maternal tissues. Within maternal tissues the fetal microchimeric progenitor immature T cells, also known as CD4 cells, are capable of self-renewal, proliferation, differentiation and activation. Activation of progenitor cells can result in the production of paracrine and autocrine inflammatory cytokines and chemokines that are involved in autoimmune diseases. Clones of these types of hibernating cells are involved in a form of graft-vs-host reactions seen in some autoimmune diseases [44,24,45]. The role of fetal microchimerism in transplant tolerance has remained an enigma Activation of hibernating fetal microchimeric cells have been postulated to result in the initiation of an autoimmune disease. Unknown triggering agents that activate these fetal microchimeric immune cells to attack the maternal host cells resulting in an autoimmune disease, have not yet been definitely identified [48]. Viral, bacterial agents, drugs or abnormal local tissue proteins that can serve as an antigen are among the suspected triggers. Microchimerism may also contribute to the risk of an autoimmune disease by providing HLA susceptibility alleles [49]. Microchimerism in affected tissues is more likely to be demonstrable in women with autoimmune disease than in women with non-autoimmune diseases [50]. Fetal microchimerism has been demonstrated in Hashimoto's thyroiditis and Graves's Disease but found to be absent in normal thyroids [51-53].
Post-abortion and the incidence of fetal microchimerism
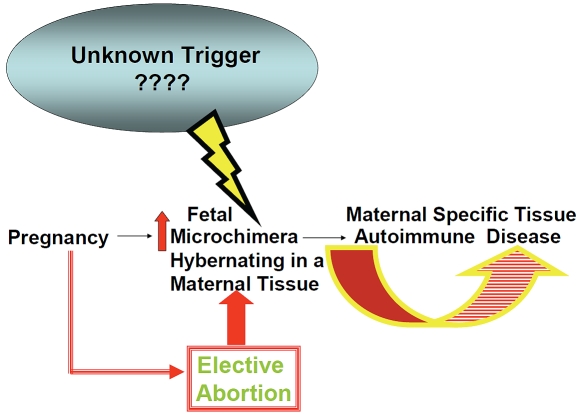
There is an increased fetal-to-maternal transfer of fetal undifferentiated progenitor cells during an abortion procedure as the placenta is being destroyed [54-56]. The amount of fetal DNA found in maternal circulation following a first-trimester abortion was found to be higher in women who underwent a surgical abortion than in women who had a chemical abortion [23]. The phenomenon of increased fetal cell trafficking following a medical abortion was also confirmed in a murine model [57]. Since the embryonic circulatory system is established in the first trimester of pregnancy, there is a greater probability for the transfer of a larger number of hematopoietic progenitor T cells during a first trimester termination of pregnancy [58,24]. Furthermore, fetal loss in elective abortions is accompanied with the loss of suppression of the maternal immune system by Early Pregnancy Factor, which may be another factor in the setting the stage for the future development of autoimmune disease [50]. Thus, women who had an elective abortion in either the first or second trimester have an greater risk for fetal microchimerism and the risk of developing an auto-immune disease for the rest of their lives (Figure 1). Animal experimentation and collection of human data will be necessary to sort out the underlying relationship between fetal microchimerism and specific autoimmune diseases in women.
Figure 1.
Elective abortion increases the degree of fetal microchimerism which favors the development of autoimmune disease in post-abortion women.
References
- 1.Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun Rev. 2008;7(8):626–30. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. 2004;10(11):2005–11. doi: 10.3201/eid1011.040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2(3):119–25. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 4.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2(9):777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 5.Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors. Autoimmunity and Autoimmune Diseases. Harrison's Online: Harrison's Principles of Internal Medicine. 17e, Chapter 312.
- 6.Pendergraft JS. Autoimmune diseases - causes, symptoms and treatments. http://ezinearticles.com/?Autoimmune-Diseases— Causes,-Symptoms-and treatments&id=1920321.
- 7.Nelson JL. Your cells are my cells. Sci Am. 2008;298(2):64–7. [PubMed] [Google Scholar]
- 8.Nelson JL. Microchimerism and Autoimmune Disease. JAMA. 1998;338(17):1223–1225. doi: 10.1056/NEJM199804233381711. [DOI] [PubMed] [Google Scholar]
- 9.Lo YM, Lau TK, Chan LY, Leung TN, Chang AM. Quantitative analysis of the bidirectional fetomaternal transfer of nucleated cells and plasma DNA. Clin Chem. 2000;46(9):1301–9. [PubMed] [Google Scholar]
- 10.IMAMURA S. SATO A. OTO H. Pregnancy-Induced Microchimerism. Fukushima Med J. 2001;51(2):113–119. http://sciencelinks.jp/j-east/article/200123/000020012301A0695384.php. [Google Scholar]
- 11.O'Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, Roberts IA, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364(9429):179–82. doi: 10.1016/S0140-6736(04)16631-2. Jul 10-16. [DOI] [PubMed] [Google Scholar]
- 12.Adams Waldorf KM, Nelson JL. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37(5):631–44. doi: 10.1080/08820130802205886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rood JJ, Roelen DL, Claas FHJ. The effect of noninherited maternal antigens in allogeneic transplantation. Semin Hematol. 2005;42(2):104–111. doi: 10.1053/j.seminhematol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Evans PC, Lambert N, Maloney S, Furst DE, Moore JM, Nelson JL. Long-term fetal microchimerism in peripheral blood mononuclear cell subsets in healthy women and women with scleroderma. Blood. 1999;93(6):2033–7. [PubMed] [Google Scholar]
- 15.Stevens AM. Do maternal cells trigger or perpetuate autoimmune diseases in children? Pediatr Rheumatol Online J. 2007;5:9. doi: 10.1186/1546-0096-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88(11):4390–5. [PubMed] [Google Scholar]
- 17.Gilliam AC. Microchimerism and skin disease: true-true unrelated? J Invest Dermatol. 2006;126(2):239–41. doi: 10.1038/sj.jid.5700061. [DOI] [PubMed] [Google Scholar]
- 18.Loubière LS, Lambert NC, Flinn LJ, Erickson TD, Yan Z, Guthrie KA, Vickers KT, Nelson JL. Maternal microchimerism in healthy adults in lymphocytes, monocyte/macrophages and NK cells. Lab Invest. 2006;86(11):1185–92. doi: 10.1038/labinvest.3700471. [DOI] [PubMed] [Google Scholar]
- 19.Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, Lee TH. Kinetics of fetal cellular and cell-free DNA in the maternal circulation during and after pregnancy: implications for noninvasive prenatal diagnosis. Transfusion. 2001;41(12):1524–30. doi: 10.1046/j.1537-2995.2001.41121524.x. [DOI] [PubMed] [Google Scholar]
- 20.Shields LE, Andrews RG. Gestational age changes in circulating CD34+ hematopoietic stem/progenitor cells in fetal cord blood. Am J Obstet Gynecol. 1998;178(5):931–7. doi: 10.1016/s0002-9378(98)70526-5. [DOI] [PubMed] [Google Scholar]
- 21.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 22.Aractingi S, Uzan S, Dausset J, Carosella ED. Microchimerism in human diseases. Immunol Today. 2000;21(3):116–8. doi: 10.1016/s0167-5699(99)01580-7. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Fujimori K, Sato A, Ohto H. Microchimerism after induced or spontaneous abortion. Obstet Gynecol. 2008;112(3):593–7. doi: 10.1097/AOG.0b013e31818345da. [DOI] [PubMed] [Google Scholar]
- 24.Klonisch T, Drouin R. Fetal-maternal exchange of multipotent stem/progenitor cells: microchimerism in diagnosis and disease. Trends Mol Med. 2009;15(11):510–8. doi: 10.1016/j.molmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Gannagé M, Amoura Z, Lantz O, Piette JC, Caillat-Zucman S. Feto-maternal microchimerism in connective tissue diseases. Eur J Immunol. 2002;32(12):3405–13. doi: 10.1002/1521-4141(200212)32:12<3405::AID-IMMU3405>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Hromadnikova I, Zlacka D, Hien Nguyen TT, Sedlackova L, Zejskova L, Sosna A. Fetal cells of mesenchymal origin in cultures derived from synovial tissue and skin of patients with rheumatoid arthritis. Joint Bone Spine. 2008;75(5):563–6. doi: 10.1016/j.jbspin.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Johnson KL, Bianchi DW. Fetal cells in maternal tissue following pregnancy: what are the consequences? Hum Reprod Update. 2004;10(6):497–502. doi: 10.1093/humupd/dmh040. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JL, Furst DE, Maloney S, Gooley T, Evans PC, Smith A, Bean MA, Ober C, Bianchi DW. Microchimerism and HLA-compatible relationships of pregnancy in scleroderma. Lancet. 1998;351(9102):559–62. doi: 10.1016/S0140-6736(97)08357-8. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93(2):705–8. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissauer D, Piper KP, Moss PA, Kilby MD. Persistence of fetal cells in the mother: friend orfoe? BJOG. 2007;114(11):1321–5. doi: 10.1111/j.1471-0528.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 31.Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA. 2004;292(1):75–80. doi: 10.1001/jama.292.1.75. [DOI] [PubMed] [Google Scholar]
- 32.Apari P, Rózsa L. The tripartite immune conflict in placentals and a hypothesis on fetal->maternal microchimerism. Med Hypotheses. 2009;72(1):52–4. doi: 10.1016/j.mehy.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Nelson JL. Maternal-fetal immunology and autoimmune disease: is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39(2):191–4. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JL. Naturally acquired microchimerism: for better or for worse. Arthritis Rheum. 2009;60(1):5–7. doi: 10.1002/art.24217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson Lee J. Research interests. http:// www.gs.washington.edu/faculty/jnelson.htm.
- 36.Sarkar K, Miller FW. Possible roles and determinants of microchimerism in autoimmune and other disorders. Autoimmun Rev. 2004;3(6):454–63. doi: 10.1016/j.autrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Adams KM, Nelson JL. Microchimerism: an investigative frontier in autoimmunity and transplantation. JAMA. 2004;291(9):1127–31. doi: 10.1001/jama.291.9.1127. [DOI] [PubMed] [Google Scholar]
- 38.Nelson JL. Microchimerism in human health and disease. Autoimmunity. 2003;36(1):5–9. doi: 10.1080/0891693031000067304. [DOI] [PubMed] [Google Scholar]
- 39.Lambert N, Nelson JL. Microchimerism in autoimmune disease: more questions than answers? Autoimmun Rev. 2003;2(3):133–9. doi: 10.1016/s1568-9972(02)00149-0. [DOI] [PubMed] [Google Scholar]
- 40.Nelson JL. Pregnancy and microchimerism in autoimmune disease: protector or insurgent? Arthritis Rheum. 2002;46(2):291–7. doi: 10.1002/art.501. [DOI] [PubMed] [Google Scholar]
- 41.Stevens A, Nelson J. Maternal and Fetal Microhhimerism: Implications for Human Diseases. NeoReviews. 2002;3(11):e11–e19. [Google Scholar]
- 42.Nelson JL. Microchimerism and human autoimmune diseases. Lupus. 2002;11(10):651–4. doi: 10.1191/0961203302lu271oa. [DOI] [PubMed] [Google Scholar]
- 43.Nelson JL. Microchimerism: incidental byproduct of pregnancy or active participant in human health? Trends Mol Med. 2002;8(3):109–13. doi: 10.1016/s1471-4914(01)02269-9. [DOI] [PubMed] [Google Scholar]
- 44.Leduc M, Aractingi S, Khosrotehrani K. Fetal-cell microchimerism, lymphopoiesis, and autoimmunity. Arch Immunol Ther Exp (Warsz) 2009;57(5):325–9. doi: 10.1007/s00005-009-0044-7. [DOI] [PubMed] [Google Scholar]
- 45.Adams KM, Lambert NC, Heimfeld S, Tylee TS, Pang JM, Erickson TD, Nelson JL. Male DNA in female donor apheresis and CD34-enriched products. Blood. 2003;102(10):3845–7. doi: 10.1182/blood-2003-05-1570. [DOI] [PubMed] [Google Scholar]
- 46.Artlett CM. Pathophysiology of fetal microchimeric cells. Clin Chim Acta. 2005;360(1-2):1–8. doi: 10.1016/j.cccn.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H. Fetal-maternal microchimerism: impact on hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17(5):546–52. doi: 10.1016/j.coi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Davidson A. Diamond B. Autoimmune Diseases. N Engl J Med. 2001;345(5):340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 49.Rak JM, Maestroni L, Balandraud N, Guis S, Boudinet H, Guzian MC, Yan Z, Azzouz D, Auger I, Roudier C, Martin M, Didelot R, Roudier J, Lambert NC. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum. 2009;60(1):73–80. doi: 10.1002/art.24224. [DOI] [PubMed] [Google Scholar]
- 50.Khosrotehrani K, Johnson KL, Lau J, Dupuy A, Cha DH, Bianchi DW. The Influence of Fetal Loss on the Presence of Fetal Cell Microchimerism. Arthritis Rheum. 2003;48(11):3237–41. doi: 10.1002/art.11324. [DOI] [PubMed] [Google Scholar]
- 51.Klintschar M, Immel UD, Kehlen A, Schwaiger P, Mustafa T, Mannweiler S, Regauer S, Kleiber M, Hoang-Vu C. Fetal microchimerism in Hashimoto's thyroiditis: a quantitative approach. Eur J Endocrinol. 2006;154(2):237–41. doi: 10.1530/eje.1.02080. [DOI] [PubMed] [Google Scholar]
- 52.Ando T, Davies TF. Clinical Review 160: Postpartum autoimmune thyroid disease: the potential role of fetal microchimerism. J Clin Endocrinol Metab. 2003;88(7):2965–71. doi: 10.1210/jc.2002-021903. [DOI] [PubMed] [Google Scholar]
- 53.Jameson JL. Evidence for Fetal Microchimerism in Autoimmune Thyroid Disease. Harrison's Online: Harrison's Principles of Internal Medicine. 17e, Chapter 320 9/4/2002. [Google Scholar]
- 54.Bianchi DW, Farina A, Weber W, Delli-Bovi LC, Deriso M, Williams JM, Klinger KW. Significant fetal-maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184(4):703–6. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- 55.Nemescu D, Onofriescu M. The impact of fetal cells persistence in maternal organism. GINECO RO. 2008;4(4):212–216. [Google Scholar]
- 56.Yan Z, Lambert NC, Guthrie KA, Porter AJ, Loubiere LS, Madeleine MM, Stevens AM, Hermes HM, Nelson JL. Male microchimerism in women without sons: quantitative assessment and correlation with pregnancy history. Am J Med. 2005;118(8):899–906. doi: 10.1016/j.amjmed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Johnson KL, Tao K, Stroh H, Kallenbach L, Peter I, Richey L, Rust D, Bianchi DW. Increased fetal cell trafficking in murine lung following complete pregnancy loss from exposure to lipopolysaccharide. Fertil Steril. 2010;93(5):1718–1721. doi: 10.1016/j.fertnstert.2009.08.042. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGrath H., Jr Elective pregnancy termination and microchimerism: comment on the article by Khosrotehrani etal. Arthritis Rheum. 2004;50(9):3058–9. doi: 10.1002/art.20650. author reply 3059. [DOI] [PubMed] [Google Scholar]