Abstract
Here we report Drosophila Waharan (Wah), a 170-kD predominantly nuclear protein with two potential human homologues, as a newly identified regulator of endosomal trafficking. Wah is required for neuromuscular-junction development and muscle integrity. In muscles, knockdown of Wah caused novel accumulations of tightly packed electron-dense tubules, which we termed ‘sausage bodies’. Our data suggest that sausage bodies coincide with sites at which ubiquitylated proteins and a number of endosomal and lysosomal markers co-accumulate. Furthermore, loss of Wah function generated loss of the acidic LysoTracker compartment. Together with data demonstrating that Wah acts earlier in the trafficking pathway than the Escrt-III component Drosophila Shrb (snf7 in Schizosaccharomyces pombe), our results indicate that Wah is essential for endocytic trafficking at the late endosome. Highly unexpected phenotypes result from Wah knockdown, in that the distribution of ubiquitylated cargos and endolysosomal morphologies are affected despite Wah being a predominant nuclear protein. This finding suggests the existence of a relationship between nuclear functions and endolysosomal trafficking. Future studies of Wah function will give us insights into this interesting phenomenon.
Keywords: Drosophila, Nuclear proteins, Endosome, Muscle, Ubiquitin
Introduction
Intracellular trafficking through the endosome provides an essential means to dispose of or recycle transmembrane proteins, but it also forms an efficient platform for certain receptor-mediated signalling processes (Sorkin and von Zastrow, 2009). Different aspects of endosomal trafficking are associated with different vesicular compartments and core machineries (Sorkin and von Zastrow, 2009). In brief, on binding of ligands, cell-surface receptors might acquire a signal (such as ubiquitylation) that targets them for endocytosis and subsequent fusion into the early-endosomal compartment; this compartment is typically associated with the small GTPase Rab5. Here, cargo is sorted for several subcellular destinations such as the Golgi or nucleus, or moved into the gradually maturing Rab7-associated late endosome and multivesicular bodies. In multivesicular bodies, cargo becomes internalised, catalysed by the ESCRT-I to ESCRT-III complexes, and destined for lysosomal degradation. Apart from the core machineries of endocytic trafficking, rather little is known about regulatory factors and links to other cellular processes expected to regulate, refine and orchestrate events of endosomal trafficking in space and time. For example, an increasing number of factors have demonstrated functions in both the nucleus and endosome (Askree et al., 2004; Pilecka et al., 2007), but potential systemic links between both compartments are little explored. Here we report the 170-kD predominantly nuclear factor Drosophila Waharan (Wah) as a novel endosomal regulator in larval muscles. Loss of its function causes the appearance of a prominent aberrant tubular compartment and ubiquitylated inclusions of endolysosomal origin.
Results and Discussion
wah is synonymous with CG4699 and encodes a nuclear protein
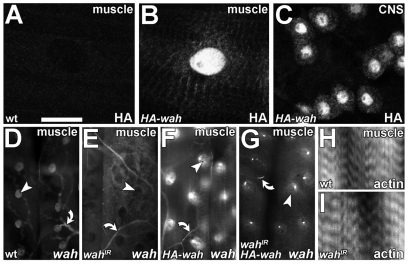
We selected the embryonic-lethal Drosophila mutation l(3)S009413 in a genetic screen owing to its neuromuscular-junction-overgrowth phenotype and named it waharan (wah; Kashmiri term for ‘to expand’). The wah mutation could be clearly assigned to the annotated locus CG4699 (supplementary material Figs S1, S2). Reciprocal sequence-homology searches suggest that wah shares homology in two blocks of the sequence with the human genes AAI26156 (LOC151050) and KIAA1267 (LOC284058; supplementary material Fig. S1B), of which KIAA1267 is one of a group of genes linked to the Tau H1 haplotype on chromosome 17 that confers risk of idiopathic Parkinson's disease (Oliveira et al., 2004). The only predicted functional regions of Wah comprise up to five nuclear localisation sequences and a potential histone fold motif (supplementary material Fig. S1B), typical of proteins that mediate DNA metabolism (Arents and Moudrianakis, 1995). This prediction was consistent with the previous identification of Wah and KIAA1267 as uncharacterized components of the dosage compensation complex (DCC) implicated in the regulation of chromatin conformation (Marin, 2003; Mendjan et al., 2006). Accordingly, haemagglutinin (HA)-tagged Wah (HA-Wah) was predominantly detected in nuclei of larval motorneurons and muscles (but also in small puncta in the cytoplasm; Fig. 1A-C).
Fig. 1.
Larval expression of wah and gross muscle morphology upon Wah knockdown. (A-C) Anti-HA staining in late-larval muscles of wild type (wt; A) and in animals with HA-wah expression in muscles (B; BG57-Gal4 driver) or CNS (C; D42-Gal4 driver). (D-G) In situ hybridisation using an N-terminal wah antisense probe (see supplementary material Fig. S1B) on late-larval muscles of wild type (D), and in muscles expressing wahIR (E), HA-wah (F) or wahIR together with HA-wah (G); arrowheads indicate representative nuclei, arrows indicate potential background staining in trachea. (H,I) Wild-type (H) and wahIR-expressing (I) late-larval muscles, stained for F-actin (phalloidin). Scale bar (in A): 50 μm (for A-D,H,I); 135 μm (for D-G).
Wah knockdown induces sausage-body formation
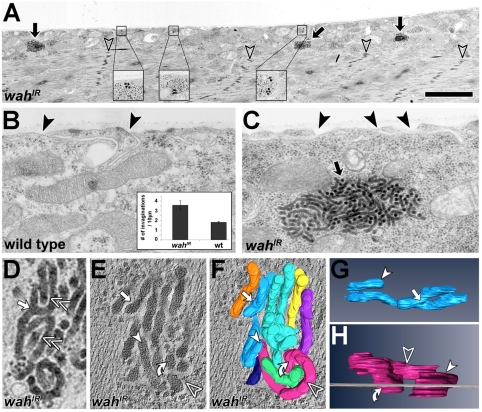
In wild-type larvae, muscles displayed high levels of wah transcripts; this expression was abolished upon knockdown of Wah through targeted expression of wahIR (double-stranded RNA construct) (supplementary material Fig. S1B, Table S1) and was enhanced upon targeted expression of a full-length HA-wah rescue construct (Fig. 1D-G; note that HA-wah was functionally validated in the context of our studies in the nervous system: supplementary material Fig. S2D). Specific knockdown of Wah in late-larval muscles (UAS-wahIR driven by BG57-Gal4) led to muscles that were easily overstretched and tended to snap upon dissection of larval specimens (not shown), although their sarcomere structure was not overtly affected (Fig. 1H,I; Fig. 2A; Fig. 3G). Ultrastructural analyses of wahIR-expressing late-larval muscles revealed an increase in surface invaginations, and the presence of prominent densely packed composite bodies, which we termed ‘sausage bodies’ (Fig. 2). They were composed of electron-dense tubules up to several μm long, with a constant diameter of 37±0.5 nm (mean ± s.e.m.; n=36). In spite of their dense packing, the tubules usually made no direct contacts but tended to be separated by a narrow gap of about 8 nm (Fig. 2D, double chevrons). Electron-tomography studies revealed that sausage bodies consist of elongated tubules, not discrete vesicles (Fig. 2E,F,H, curved arrows). Furthermore, tubules displayed branching points (Fig. 2, white arrows). All these features suggest that sausage bodies are membranous and might reflect aberrations of the intracellular trafficking machinery. Tubular endosomal aberrations reported previously were by contrast electron-lucent (Razi and Futter, 2006) or, if electron-dense, failed to display any structural similarity to sausage bodies (Kegel et al., 2000; Nakano et al., 2001). Therefore, we propose that sausage bodies represent novel structures.
Fig. 2.
Ultrastructural analysis of wahIR-expressing muscles. (A) wahIR-expressing late-larval muscle (transverse sections) display normal contractile sarcomeres (open arrowheads point at electron-dense Z-bands); sausage bodies (black arrows and close-ups) accumulate predominantly in the sarcoplasm. (B,C) Tubular surface invaginations (black arrowheads) are less abundant in wild-type (wt) than in wahIR-expressing muscles (inset in B). Black arrow in C indicates a sausage body. (D) Higher-resolution view of a sausage body showing branch points (white arrow) and even spacing between electron tubules (double chevrons). (E-H) EM tomogram of a sausage body (E) and its 3D reconstruction with individual tubules shown in different colours (F-H); see supplementary material Movies 1 and 2); tubules penetrating the image plane (grey line in H) perpendicularly (curved arrow in H) appear as pseudo-vesicular structures in horizontal view (curved arrow in E). Symbols are used consistently throughout the figure. Scale bar (in A): 3 μm (for A); 0.6 μm (for B,C); 120 nm (for D-F); 100 nm (for G); 50 nm (for H).
Fig. 3.
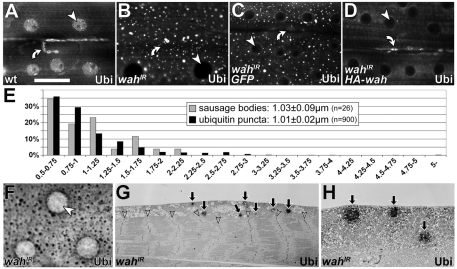
Characterisation of wahIR-induced ubiquitin puncta. (A-D) Anti-ubiquitin staining in the sarcoplasm of late-larval muscles in wild type (wt; A), and in muscles expressing wahIR (B), coexpressing wahIR with a GFP construct (C) or coexpressing wahIR with HA-wah (D); arrowheads point at nuclei, curved arrows at neuromuscular junctions (both of which lack ubiquitin upon muscular knockdown of Wah). (E) Distributions of diameters (larger than 0.5 μm) of sausage bodies and ubiquitin puncta; inset indicates the average diameters. (F-H) Pre-embedding immuno-EM for ubiquitin in wahIR-expressing late-larval muscles (diaminobenzidine staining catalysed by horseradish peroxidase); resin-embedded specimens show the same staining pattern as fluorescently labelled muscles (compare F and B); ubiquitin-positive patches localise predominantly in the sarcoplasm (G,H), as is similarly found for sausage bodies (compare Fig. 2A). Arrows indicate sausage bodies; arrowheads indicate nuclei. Scale bar (in A): 50 μm (for A-D,F); 12 μm (for G); 2.2 μm (for H).
Staining late-larval wahIR-expressing muscles for conjugated mono- and polyubiquitin revealed prominent patterns of evenly dispersed puncta; these patterns were never seen in control muscles (Fig. 3B,C,F). Sausage bodies and wahIR-induced ubiquitin aggregates were predominantly localised in the sarcoplasm and rarely in sarcomeres (Fig. 2A and Fig. 3G,H). Micrographs of transverse muscle profiles displayed 0.07±0.01 (mean ± s.e.m.; n=5 muscles) sausage bodies per μm muscle length. By comparison, lines drawn along the anteroposterior axis on confocal projections of muscles cut across 0.15±0.01 (n=10 muscles) ubiquitin puncta per μm muscle length. These frequencies were rather similar, when considering the differences in confocal and ultrastructural resolution [analyses of lines in confocal images would be expected to include more objects than displayed by thin (100 nm) electron microscopy (EM) sections]. When diameters of these sausage bodies and ubiquitin puncta (at positions at which anteroposterior lines cut across them) were filtered for size (⩾0.5 μm, i.e. above the resolution threshold of the confocal microscope), they displayed comparable means (≈1 μm) and size distributions (Fig. 3E). Post-embedding immuno-EM procedures as a means to confirm coincidence of ubiquitin puncta and sausage bodies could not be established. Instead, we used pre-embedding EM strategies, which revealed anti-ubiquitin-labelled patches in wahIR-expressing muscles; the distribution of these patches was similar to that of sausage bodies (Fig. 2A versus Fig. 3G,H); such stained patches were absent in parallel treated specimens in which the first antibody was omitted (not shown). Phenotypes of sausage bodies and ubiquitin puncta were completely rescued when HA-Wah, but not a GFP control construct, was coexpressed with wahIR, thus demonstrating their dependence on loss of Wah function (Fig. 3C,D and not shown).
Wah knockdown impairs endosomal-membrane traffic
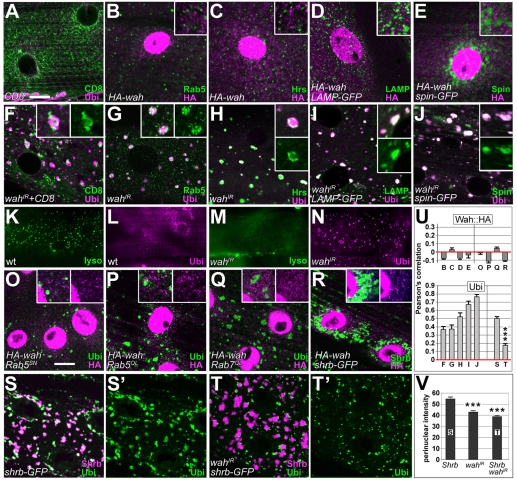
Using ubiquitin puncta as readout, we obtained further support for the hypothesis that Wah knockdown affects the intracellular trafficking machinery. First, upon knockdown of Wah, the integral-membrane-protein marker mCD8-GFP (normally highlighting cell surfaces) strongly accumulated at intracellular sites of ubiquitin clusters (Fig. 4A,F), suggesting that membrane traffic to or from the surface was blocked. Second, in wahIR-expressing muscles, the early-endosome marker Rab5 or the late-endosome and lysosomal markers Hrs, LAMP1-GFP and Spin-GFP all formed aberrant accumulations that tended to be closely associated with ubiquitin puncta (Figs 4B-E versus G-J). By contrast, Hrs failed to enrich when ubiquitin accumulations were caused by other means (blocking proteasome function via dominant-negative DTS5, or tau-myc-induced impairment of microtubule-dependent transport) (Mudher et al., 2004; Schweisguth, 1999) (supplementary material Fig. S3). Third, the fluorescent LysoTracker probe for acidic organelles failed to stain in wahIR-expressing muscles, whereas wild-type muscles reliably displayed prominent LysoTracker puncta (Fig. 4K-N). Finally, ubiquitin accumulations were a common feature upon interference with endocytic trafficking, because they were likewise found when Rab5SN (inhibitory Rab5), Rab5QL (activated Rab5), Rab7QL (activated Rab7) or Shrb-GFP (a dominant-negative form of the ESCRT-III component snf7) were targeted to late-larval muscles (Fig. 4O-S). Of these, Shrb-GFP in particular caused ubiquitin accumulations that were significantly more enriched around the nucleus than were wahIR-induced puncta (Fig. 3B,C and Fig. 4S,V). When coexpressed with wahIR, the intensity of Shrb-GFP-induced perinuclear ubiquitin puncta was reduced and ubiquitin puncta tended to localise to the fringes of Shrb-GFP-labelled compartments rather than distribute throughout (Fig. 4T,V). This result further demonstrated that Wah knockdown affected endosomal function; it suggested that the presumptive block in endocytic trafficking occurs at the late-endosomal–lysosomal stage, earlier in the degradative pathway than ESCRT-III activity.
Fig. 4.
Refining insights into endosomal functions of Wah. All images show horizontal views of late-larval muscles at the level of the sarcoplasm; genotypes or expressed constructs are indicated bottom left, detected labels bottom right. (A-J) Localisation of the membrane marker mCD8-GFP (CD8) and the endosomal and/or lysosomal markers Rab5, Hrs, LAMP-GFP (LAMP) and Spinster-GFP (Spin) in control animals (A-E), and upon knockdown of Wah (F-J); none of the endosomal markers colocalised with HA-Wah (B-E); upon knockdown of Wah, CD8-GFP and endosomal markers accumulate and closely correlate with ubiquitin puncta (F-J). (K-N) Puncta of LysoTracker staining detected by live imaging of wild-type muscles (lyso; K) is abolished upon wahIR expression (M); L and N show specimens from the same batch subsequently stained for ubiquitin. (O-R) Targeted expression of constructs functionally interfering with endosomal trafficking: dominant-negative and activated Rab5 (Rab5SN and Rab5QL, respectively), activated Rab7 (Rab7QL), and dominant-negative snf7 (shrb-GFP); these conditions cause accumulations of ubiquitin (O,P,Q,S), but do not affect the localisation of cytoplasmic HA-Wah puncta (O-R). (S,T) Shrb-GFP co-clusters with ubiquitin around nuclei (S,S′); coexpression of wahIR suppresses perinuclear build-up of ubiquitin (T,T′; see also V) and its localisation to the Shrb-GFP compartment (see also U). (U) Pearson's colocalisation correlation coefficients (1=perfect colocalisation, 0=random distribution, −1=perfect exclusion) for all double-stained images of this plate, as indicated by letters below bars; HA-Wah fails to colocalise with endosomal markers (upper graph, left) and with ubiquitin accumulations induced by endosomal manipulations (upper graph, right); ubiquitin puncta induced by wahIR (lower graph, left) or Shrb-GFP (lower graph, right) colocalise with endosomal markers. ***P≤0.001, Mann-Whitney Rank Sum Test. (V) Pixel intensities of ubiquitin staining measured in circular ROIs of 35-μm diameter concentrically around nuclei; bars represent the means of respective histograms. ***P≤0.001, Mann-Whitney Rank Sum Test. Scale bar (in A): 30 μm (for K-N); 15 μm (for all other images; insets are 200% enlarged).
Conclusion and future directions
Taken together, our data suggest that knockdown of Wah causes the formation of sausage bodies and a block in late-endosomal trafficking, leading to the appearance of prominent ubiquitylated puncta, presumably reflecting the accumulation of proteins that fail to be stripped of their ubiquitin tags; if sausage bodies are the ultrastructural correlate of these ubiquitin puncta, their electron-dense nature might reflect content that is destined for electron-dense lysosomal compartments (Baudhuin et al., 1965), which would also be in agreement with the loss of LysoTracker staining. Given these unusual phenotypes, examination of the function of Wah might offer insight into novel mechanisms of the regulation of endocytic trafficking. It remains to be determined whether the endocytic trafficking defects are the indirect consequence of the role of Wah in the nucleus (see supplementary material Fig. S4) or represent a direct function of Wah (in this regard, note that a fraction of HA-Wah localises outside the nucleus; Fig. 1B). An increasing number of factors are reported to execute such dual functions in both the nucleus and at the endosome (Askree et al., 2004; Berndsen et al., 2008; Pilecka et al., 2007; Stauffer et al., 2001; Weiss et al., 2008; Wu et al., 2005; Xu et al., 2009; Yeghiayan et al., 1995), and we propose that this is also true for Wah. So far, our immunohistochemical attempts failed to localise cytoplasmic HA-Wah to the endosomal machinery (top graph in Fig. 4U). However, yeast two-hybrid screens reported elsewhere (http://flybase.org/reports/FBgn0038364.html) identified components of the endocytic-trafficking machinery as binding partners of Wah, including TSG101 (ESCRT-I component), GEFMeso (encoded by CG30115; a membrane-bound regulator of GTPases) and Sec6 (exocyst component). The repertoire of phenotypes reported here in the genetically amenable Drosophila muscle system provides a versatile means to assess potential functional links of Wah to these factors.
Materials and Methods
Fly genetics
Fly stocks used are listed in supplementary material Table S1. The UAS-HA-wah construct was assembled as follows: since no cDNA clones were available that covered the entire wah coding region, we assembled a suitable clone in steps. The 5′ untranslated region (UTR) and 5′ end of the wah coding region were cloned in a NotI-XhoI fragment from cDNA clone SD06860 in pOT2 (obtained from the Drosophila Genome Resource Center, Bloomington, IN) into pUAST. SD06860 seems to be an artefactual genomic clone that covers 5240 bp (11620471-11625710; DNA sequence accession number FM867599; supplementary material Fig. S5). The 5′ UTR and the 5′ end of the coding region were replaced with 748 bp amplified from the N-terminal coding region of cDNA clone LD46639 in pOT2 (DGRC; supplementary material Fig. S5) using a sense primer with EcoRI and NotI restriction sites (TAATATAGAATTCGCGGCCGC-11620629-ATGGCCCCAGCGCTCACAGC-11620648; in all primers, sequences that anneal to wah are bold and restriction sites are italicised) and an anti-sense primer with a genomic KpnI restriction site (11621451-CCTGCACGGGTACCGGAACA-11621432). The 3′ end of wah was PCR amplified from genomic DNA using a sense primer (11625698-AGCTCTTCTCGAGGAGCGACGCCGCTTCGAGACTTTCCTTAAGTTC-11625743) and an anti-sense primer that annealed to the 3′ end of the coding region (TAATATACTCGAG-11626743-TTAGATGCGTCTGCTGCGAA-11626724) and was introduced into the rest of the construct in pUAST using XhoI restriction sites, to make pUAST-wah. Finally, the N-terminal HA tag including a start codon was PCR amplified from another construct (EB1aff-2HA in pUAST) (Bottenberg, 2004) using a sense primer with an EcoRI site (5′-TCGCGGGGAATTCCACCGACGGAGAAGCATGGGC-3′) and an antisense primer with a Not1 site (5′-TAATATAGCGGCCGCTATAGTTCTAGAGGCTCGAGAGGCCTTGA-3′) and introduced in the 5′ end of wah in pUAST-wah using EcoRI and NotI restriction enzymes. Transgenic flies were generated by Best Gene (http://www.thebestgene.com/).
Immunohistochemistry, LysoTracker stains and image analysis
Late third-instar larvae were dissected and stained following standard procedures (Budnik et al., 2006), using Cy5- or Cy3-labelled phalloidin (10 minutes at 1:100 or 1:500, respectively), and primary and fluorescently tagged secondary antibodies (supplementary material Table S1). For staining with LysoTracker RED DND-99 (diluted 1:20,000 in Schneider's medium), larvae were incubated at 26°C for 1.5 hours, washed and imaged live. Analyses of pixel densities and of Pearson's colocalisation correlation coefficient were carried out in MBF ImageJ (http://www.macbiophotonics.ca/imagej/).
In situ hybridisation
Larvae were dissected and fixed with 4% paraformaldehyde, quickly washed and stored at −20°C in methanol until hybridisation was performed according to standard protocols (Cornell et al., 1999) using Digoxigenin-labelled probes (supplementary material Fig. S1B).
EM, and analysis of ubiquitin aggregates and sausage bodies
Procedures followed protocols published in detail elsewhere (Budnik et al., 2006). For immuno-EM, larvae were fixed with 4% paraformaldehyde, incubated with anti-ubiquitin, biotinylated anti-mouse secondary antibodies (donkey, 1:200), Vector Elite ABC kit (1:100 in PBT), stained in 0.7 mg/ml diaminobenzidine plus 2.0 mg/ml H2O2 in PTB, post-fixed in 1% glutaraldehyde, then contrasted and embedded (TAAB LV; TAAB Laboratories Equipment, Berkshire, UK) following standard procedures (Budnik et al., 2006). For three-dimensional (3D) reconstruction, tilted series were taken from 120-nm sections every 2° (−60° to 64°), and tomograms generated using the eTomo interface in IMOD 3.13 (Boulder Laboratory) and reconstructed in 3D via AMIRA 5.2.0 (Visage Imaging GmbH, Berlin).
Supplementary Material
Acknowledgments
We thank colleagues and fly stock centres (Mishima, Japan; Bloomington, US) for fly strains or antibodies; Tobi Starborg and David Holmes for training in EM tomography and reconstruction; Georg Vogler, Matthew Ronshaugen and Marian Wilkin for experimental advice; and Philip Woodman, Martin Baron, Barbara Ciani and Marcos González-Gaitán for discussion. EM and Bioimaging Facilities at FLS are supported by equipment grants from the Wellcome Trust and the University of Manchester Strategic Fund. This work was supported by funds of the EU (QLG3-CT-2001-01181) to A.P. and C.J.O'K., the Wellcome Trust (078593/Z/05/Z) and the BBSRC (BB/E009085/1) to A.P., and a BBSRC PhD studentship to R.P.A. Deposited in PMC for release after 6 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/14/2369/DC1
References
- Arents G., Moudrianakis E. N. (1995). The histone fold: a ubiquitous architectural motif utilized in DNA compaction and protein dimerization. Proc. Natl. Acad. Sci. USA 92, 11170-11174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askree S. H., Yehuda T., Smolikov S., Gurevich R., Hawk J., Coker C., Krauskopf A., Kupiec M., McEachern M. J. (2004). A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. USA 101, 8658-8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M. (1993). The mesoderm and its derivatives. In The development of Drosophila melanogaster, vol. 2 (eds Bate M., Martínez Arias A.), pp. 1013-1090 Cold Spring Harbor: CSH Laboratory Press; [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. (1965). Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J. Cell Biol. 26, 219-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndsen C. E., Tsubota T., Lindner S. E., Lee S., Holton J. M., Kaufman P. D., Keck J. L., Denu J. M. (2008). Molecular functions of the histone acetyltransferase chaperone complex Rtt109-Vps75. Nat. Struct. Mol. Biol. 15, 948-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenberg W. (2004). Neuronal differentiation and epithelial integrity: The role of Drosophila Short stop. In Inst. Genetics, pp. 166 Mainz: Johannes Gutenberg-University; [Google Scholar]
- Budnik V., Koh Y. H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. (1996). Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 17, 627-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V., Gorczyca M., Prokop A. (2006). Selected methods for the anatomical study of Drosophila embryonic and larval neuromuscular junctions. Int. Rev. Neurobiol. 75, 323-365 [DOI] [PubMed] [Google Scholar]
- Cornell M., Evans D. A., Mann R., Fostier M., Flasza M., Monthatong M., Artavanis-Tsakonas S., Baron M. (1999). The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics 152, 567-576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deák P., Omar M. M., Saunders R. D., Pal M., Komonyi O., Szidonya J., Maroy P., Zhang Y., Ashburner M., Benos P., et al. (1997). P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E-87F. Genetics 147, 1697-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudu V., Bittig T., Entchev E., Kicheva A., Julicher F., González-Gaitán M. (2006). Postsynaptic mad signaling at the Drosophila neuromuscular junction. Curr. Biol. 16, 625-635 [DOI] [PubMed] [Google Scholar]
- Entchev E. V., Schwabedissen A., González-Gaitán M. (2000). Gradient formation of the TGF-beta homolog Dpp. Cell 103, 981-991 [DOI] [PubMed] [Google Scholar]
- Kegel K. B., Kim M., Sapp E., McIntyre C., Castano J. G., Aronin N., DiFiglia M. (2000). Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J. Neurosci. 20, 7268-7278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf M., Sánchez-Soriano N., Technau G. M., Urban J., Prokop A. (2003). Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev. Biol. 260, 207-225 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461 [DOI] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261-269 [DOI] [PubMed] [Google Scholar]
- Marin I. (2003). Evolution of chromatin-remodeling complexes: comparative genomics reveals the ancient origin of “novel” compensasome genes. J. Mol. Evol. 56, 527-539 [DOI] [PubMed] [Google Scholar]
- Mendjan S., Taipale M., Kind J., Holz H., Gebhardt P., Schelder M., Vermeulen M., Buscaino A., Duncan K., Mueller J., et al. (2006). Nuclear pore components are involved in the transcriptional regulation of dosage compensation in Drosophila. Mol. Cell 21, 811-823 [DOI] [PubMed] [Google Scholar]
- Mudher A., Shepherd D., Newman T. A., Mildren P., Jukes J. P., Squire A., Mears A., Berg S., MacKay D., Asuni A. A., et al. (2004). GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry 9, 522-530 [DOI] [PubMed] [Google Scholar]
- Nakano Y., Fujitani K., Kurihara J., Ragan J., Usui-Aoki K., Shimoda L., Lukacsovich T., Suzuki K., Sezaki M., Sano Y., et al. (2001). Mutations in the novel membrane protein spinster interfere with programmed cell death and cause neural degeneration in Drosophila melanogaster. Mol. Cell. Biol. 21, 3775-3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira S. A., Scott W. K., Zhang F., Stajich J. M., Fujiwara K., Hauser M., Scott B. L., Pericak-Vance M. A., Vance J. M., Martin E. R. (2004). Linkage disequilibrium and haplotype tagging polymorphisms in the Tau H1 haplotype. Neurogenetics 5, 147-155 [DOI] [PubMed] [Google Scholar]
- Pilecka I., Banach-Orlowska M., Miaczynska M. (2007). Nuclear functions of endocytic proteins. Eur. J. Cell Biol. 86, 533-547 [DOI] [PubMed] [Google Scholar]
- Plickert G., Gajewski M., Gehrke G., Gausepohl H., Schlossherr J., Ibrahim H. (1997). Automated in situ detection (AISD) of biomolecules. Dev. Genes Evol. 207, 362-367 [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Akbar M. A., Ray S., Sevrioukov E. A., Haberman A. S., Rohrer J., Kramer H. (2005). Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663-3673 [DOI] [PubMed] [Google Scholar]
- Razi M., Futter C. E. (2006). Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 17, 3469-3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth F. (1999). Dominant-negative mutation in the beta2 and beta6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proc. Natl. Acad. Sci. USA 96, 11382-11386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., von Zastrow M. (2009). Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Stern D., Beaton A., Rhem E. J., Laverty T., Mozden N., Misra S., Rubin G. M. (1999). The Berkeley Drosophila genome project gene disruption project. Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153, 135-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer D. R., Howard T. L., Nyun T., Hollenberg S. M. (2001). CHMP1 is a novel nuclear matrix protein affecting chromatin structure and cell-cycle progression. J. Cell Sci. 114, 2383-2393 [DOI] [PubMed] [Google Scholar]
- Sutherland D. J., Li M., Liu X. Q., Stefancsik R., Raftery L. A. (2003). Stepwise formation of a SMAD activity gradient during dorsal-ventral patterning of the Drosophila embryo. Development 130, 5705-5716 [DOI] [PubMed] [Google Scholar]
- Sweeney N. T., Brenman J. E., Jan Y. N., Gao F. B. (2006). The coiled-coil protein shrub controls neuronal morphogenesis in Drosophila. Curr. Biol. 16, 1006-1011 [DOI] [PubMed] [Google Scholar]
- Sweeney S. T., Davis G. W. (2002). Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 36, 403-416 [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Itoh S., ten Dijke P., Tabata T. (2000). Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell 5, 59-71 [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. (1989). A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98, 81-85 [DOI] [PubMed] [Google Scholar]
- Thor S., Andersson S. G., Tomlinson A., Thomas J. B. (1999). A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature 397, 76-80 [DOI] [PubMed] [Google Scholar]
- Vogler G., Urban J. (2008). The transcription factor Zfh1 is involved in the regulation of neuropeptide expression and growth of larval neuromuscular junctions in Drosophila melanogaster. Dev. Biol. 319, 78-85 [DOI] [PubMed] [Google Scholar]
- Weiss P., Huppert S., Kolling R. (2008). ESCRT-III protein Snf7 mediates high-level expression of the SUC2 gene via the Rim101 pathway. Eukaryotic Cell 7, 1888-1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. H., Alami S., Luk E., Wu C. H., Sen S., Mizuguchi G., Wei D., Wu C. (2005). Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat. Struct. Mol. Biol. 12, 1064-1071 [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T., Wilsch-Brauninger M., González-Gaitán M. (2003). Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J. Cell Biol. 161, 609-624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Gong Q., Xia B., Groves B., Zimmermann M., Mugler C., Mu D., Matsumoto B., Seaman M., Ma D. (2009). A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking. J. Cell Biol. 186, 343-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeghiayan P., Tu J., Vallier L. G., Carlson M. (1995). Molecular analysis of the SNF8 gene of Saccharomyces cerevisiae. Yeast 11, 219-224 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.