Abstract
The present research examined the interaction between genes and culture as potential determinants of individuals’ locus of attention. As the serotonin (5-HT) system has been associated with attentional focus and the ability to adapt to changes in reinforcement, we examined the serotonin 1A receptor polymorphism (5-HTR1A). Koreans and European Americans were genotyped and reported their chronic locus of attention. There was a significant interaction between 5-HTR1A genotype and culture in the locus of attention. Koreans reported attending to the field more than European Americans, and this cultural difference was moderated by 5-HTR1A. There was a linear pattern such that those homozygous for the G allele, which is associated with reduced ability to adapt to changes in reinforcement, more strongly endorsed the culturally reinforced mode of thinking than those homozygous for the C allele, with those heterozygous in the middle. Our findings suggest that the same genetic predisposition can result in divergent psychological outcomes, depending on an individual’s cultural context.
Keywords: culture, 5-HTR1A, attention
CULTURE, SEROTONIN RECEPTOR POLYMORPHISM AND LOCUS OF ATTENTION
People differ in how they process cognitive information. Some people are more likely to attend to a focal issue and object. Other people are more likely to attend to the field and the context. A large body of research in psychology has demonstrated that there are systematic factors that influence individuals’ modes of thinking. These cognitive differences, namely holistic vs analytic modes of thinking, are fostered by culture, as holistic thinking is more prevalent in Eastern cultural contexts, whereas analytic thinking is more prevalent in Western cultural contexts (Nisbett et al., 2001).
However, Easterners and Westerners vary not only in their socio-cultural context, but also in their genetic make-up (e.g. Chang et al., 1996; Gelernter et al., 1997). There is increasing empirical evidence suggesting a significant role for genes in influencing particular psychological outcomes, such as personality, social behaviors and proneness to psychological illnesses (e.g. Lesch et al., 1996; Bachner-Melman et al., 2005; Ben Zion et al., 2006). Thus, the possibility of a genetic basis for culturally variable psychological tendencies, such as holistic vs analytic mode of thinking is viable. Yet, research suggests that environmental input can significantly interact with the effect of genes (e.g. Caspi et al., 2003; Bakermans-Kranenburg and van IJzendoorn, 2006; Taylor et al., 2006; Eisenberg et al., 2008; Kim-Cohen and Gold, 2009; but see also Risch et al., 2009 for the case of 5-HTTLPR), and culture represents a potentially important source of environmental input. In the present study, we examine how a particular gene (5-HTR1A) and culture interact to produce specific modes of thinking that are known to vary systematically across cultures.
Culture and locus of attention
Cultural analyses of cognition have posited that particular cultural meanings and practices foster particular modes of thinking (Bruner, 1996; Greenfield, 1997). It has been well documented that there are reliable differences in the modes of thinking between people from Eastern cultural contexts and people from Western cultural contexts (Nisbett et al., 2001). People from east-Asian cultural contexts tend to adopt a holistic style of reasoning that is characterized by the tendency to attend to the entire field and attribute the causes of a social event to external situational factors. In contrast, people from Western cultural contexts tend to adopt an analytic style of reasoning that is characterized by the tendency to attend primarily to focal information, and attribute causes of a social event to internal and dispositional factors (Nisbett et al., 2001). Moreover, there is strong evidence that these differences are sensitive to cultural and environmental factors and are thus, culturally based. For example, Asian Americans with great exposure to American culture tend to show cognitive response patterns that resemble people from Western cultural contexts (e.g. Norenzayan et al., 2002).
Moreover, these cognitive styles develop in response to the particular socio-cognitive tasks that are required in different cultural and environmental contexts, and consequently cognitive differences are found even within the same region and among the same ethnic group (Nisbett et al., 2001; Uskul et al., 2008). For instance, in a study comparing different communities in Turkey’s eastern Black Sea region, members of farming and fishing communities, which emphasize social cooperation, exhibited greater holistic attentional tendencies (i.e., greater attention to the field than to the object) than members of herding communities, which emphasize individual decision-making (Uskul et al., 2008). In short, holistic vs analytic mode of thinking—locus of attention in particular—is a strategy to direct cultural participants’ attention to culturally important tasks posed by their environment. In the present study, we examined the interaction between a genetic factor and culture as potential determinants of individuals’ chronic locus of attention.
Gene X culture interaction and modes of thinking
Much research has found that the serotonin (5-HT) system is implicated in an array of cognitive effects including attention, cognitive flexibility and long-term memory (see Schmitt et al., 2006 for review). More specifically, these findings come from human studies, all of them conducted in Western cultures that utilize acute tryptophan (TRP) depletion (ATD). ATD involves orally administering an amino acid suspension without L-TRP, the only precursor for 5-HT in the brain, which reduces TRP transport into the brain by increasing competition for active transport sites across the blood−brain barrier. ATD results in temporarily lower levels of 5-HT activity in the brain. ATD studies demonstrate that under the condition of 5-HT depletion, people show an increased ability to attend to relevant stimuli while ignoring irrelevant information (Schmitt et al., 2000; Ahveninen et al., 2002), and also show an increased ability to direct and focus cognitive activity on specific stimuli over a prolonged period of time (Ramaekers et al., 1995). At the same time, animal studies as well as ATD human studies report that 5-HT depletion tends to impair cognitive flexibility, such as the ability to adapt one’s behavior to changes in reinforcement once learning of a particular reinforcement system has taken place (Clarke et al., 2004; Park et al., 1994).
Given the findings regarding cognition and the 5-HT system, we examined the role of a specific 5-HT system polymorphism that is known to influence the transmission of 5-HT in individuals’ chronic modes of thinking. In particular, we examined the gene by culture interaction focusing on the C(-1019)G 5-HTR1A gene. 5-HTR1A is an autoinhibitor of 5-HT release. The G allele of the C(−1019)G polymorphism prevents binding of putative repressor proteins, leading to enhanced gene expression and reduced serotonergic neurotransmission (Lemonde et al., 2003; Huang et al., 2004), and has been tied to neuroticism and depression (Lemonde et al., 2003; Strobel et al., 2003; Huang et al., 2004).
In the present research, we investigated the extent to which cultural factors play a role in the association between different genotypes of 5-HTR1A and cognitive functioning. The rationale for this investigation is twofold. First, one cognitive function associated with 5-HT regards locus of attention, and this cognitive function is also known to systematically vary across cultures (Nisbett et al., 2001). Second, another cognitive association of 5-HT is cognitive flexibility (Clarke et al., 2004; Park et al., 1994), which may influence the degree to which individuals primarily adhere to a culturally reinforced cognitive style. Increased cognitive flexibility, we reasoned, would be associated with reduced adherence to cultural styles of thinking because, at least among adults, once the learning of culturally dominant mode of thinking occurs, cognitively inflexible individuals may be less likely to switch cognitive modes depending on specific situations and the nature of the tasks. Then, it is probable that the largest cultural difference in cognitive styles is among people homozygous for the G allele of 5-HTR1A. Thus, we speculated that rather than increasing attention to relevant and focal stimuli, 5-HTR1A might shape the degree to which individuals rely on the culturally dominant mode of thinking and locus of attention in particular. Perhaps the previous studies (e.g. Schmitt et al., 2000; Ahveninen et al., 2002), conducted only with Western samples, exhibited the pattern of increased attention on focal objects among those with the G allele because that is the dominant cognitive style in Western cultural contexts. Thus, locus of attention might differ in Eastern cultural contexts among people with G allele and G/G genotype, in particular, as individuals within those cultures may pay greater attention to the field.
In the present study, we assessed people’s mode of thinking using the Analysis-Holism Scale (AHS) (Choi et al., 2007), a validated scale that has been shown to predict cognitive differences. We predicted that the cognitive flexibility shaped by 5-HTR1A could manifest itself in different ways in different cultures. That is, we hypothesized that the cultural differences in locus of attention would be most pronounced among those homozygous for the G allele. Further, we predicted a linear pattern such that those homozygous for the G allele would more strongly endorse the culturally reinforced mode of thinking (i.e. European Americans paying greater attention to a focal object and Koreans paying more attention to the field) than those homozygous for the C allele, with those heterozygous in the middle, producing a gene by culture interaction.
METHOD
Participants
Participants were 149 Koreans (74 females and 75 males; 57 community members and 92 college students; mean age = 24.91 years) and 140 European Americans (85 females, 54 males and 1 unspecified; 51 community members and 89 college students; mean age = 26.97 years). The Korean participants were recruited in Korea and their ethnicity was confirmed by their indication of Korea as the country of birth as well as by their name at the recruitment. Ethnicity of participants recruited in the United States was determined based on self-categorization. Participants were allowed to choose only one ethnic category out of six ethnic groups (e.g. Asian American, European American, African American, Latino American, Native American, Native Pacific Islander), but ‘Other’ category was provided for those who do not clearly fit into these categories or for those with mixed ethnicities. European American participants both chose European American as their ethnic group and indicated that they were born in the United States. Student participants were recruited through class announcements and campus flyers, and community participants were recruited among campus employees and from adult classes in both countries. Participants received either course credit or payment ($10 or 10 000 for students and $20 or 20 000
for students and $20 or 20 000 for community members) for their participation.
for community members) for their participation.
Assessment of cognitive mode
Participants completed a packet of questionnaires. All the questionnaires were translated into Korean for Korean participants using the backtranslation method (one Korean−English bilingual translator translated the original materials in English into Korean and another independent bilingual translator translated the Korean-translated materials back to English to ensure the accuracy of Korean translation). They indicated their self-reported mode of thinking on the Analysis-Holism Scale (Choi et al., 2007). The scale includes four sub-components: causality (e.g. ‘Everything in the world is intertwined in a causal relationship’; α = 0.78), attitudes toward contradiction (e.g. ‘We should avoid going to extremes’; α = 0.62), perception of change (e.g. ‘Current situations can change at any time’; α = 0.64) and locus of attention (e.g. ‘It is more important to pay attention to the whole context rather than the details’; α = 0.75). This last component was our key dependent variable. Each component was measured with six items on a 1 (‘strongly disagree’) to 7 (‘strongly agree’) scale, with higher values indicating more holistic thinking. Participants also completed demographic questionnaires.
Genotyping
After the completion of questionnaire measures, participants provided saliva or cheek swab samples collected with the Orasure oral specimen collection device (Orasure; for cheek swab samples) or Oragene collection device (Genotek; for saliva samples). The Oragene samples were kept at room temperature, and the Orasure samples were stored at −20°C for 3−4 months until processed. DNA was extracted using the Puregene DNA purification kit (Gentra Systems, Inc., Minneapolis, MN). Concentrations were determined on a spectraphotometer and equalized across samples by diluting the high-concentration samples with water. The genotype of the 5-HTR1A C(-1019)G polymorphism was then assessed using a commercially available TaqMan SNP Genotyping assay. The SNP assay contains forward and reverse PCR primers as well as two allele-specific probes conjugated with either VIC or FAM fluorescent marker. Each PCR mixture consisted of DNA templates, the SNP-specific Genotyping assay, and Taqman Genotype master mix (ABI). PCR amplification was carried out on an ABI 7500 real time PCR machine following the PCR conditions recommended by the manufacture of the SNP probe. Following PCR reactions, the allelic discrimination program (ABI) generated a genotype plot in which samples were separated into four clusters, representing the CC, GG, CG and undetermined genotypes.
All samples were run in duplicate which in all cases were confirmed to be consistent.
RESULTS
Genotype
Koreans and European Americans differed in their allelic distribution. Among Koreans, there was a higher proportion of G alleles (7 C/C, 52 C/G and 90 G/G) than among European Americans (34 C/C, 65 C/G and 41 G/G), χ2(2, N = 289) = 37.31, P < 0.001. This difference is consistent with distributions found in previous observations (Lemonde et al., 2003 for European Americans and Yu et al., 2006 for Asians). Both groups resulted in Hardy−Weinberg equilibrium, χ2(2, N = 149) = 0.01, P = 0.99 for Koreans and χ2(2, N = 140) = 0.58, P = 0.75 for European Americans.
Gene X culture interactions
We conducted 5HTR1A (C/C, C/G vs G/G) X culture (Koreans vs European Americans) ANOVAs to examine the gene by culture interactions with different components of the AHS as dependent variables (Table 1). Gender did not have any effect on the results, and did not interact with any other variables, and thus, we will not mention it further.
Table 1.
Means and standard deviations for the AHS
| AHS | Culture | 5-HTR1A | M | s.d. | Culture F(p) | 5-HTR1A F(p) | Interaction F(p) |
|---|---|---|---|---|---|---|---|
| Attention | Koreans | C/C | 5.1 | 0.46 | 33.74 (<0.001) | 1.56 (0.21) | 5.55 (0.004) |
| C/G | 5.44 | 0.75 | |||||
| G/G | 5.55 | 0.67 | |||||
| European Americans | C/C | 4.76 | 0.99 | ||||
| C/G | 4.72 | 0.9 | |||||
| G/G | 4.23 | 0.98 | |||||
| Contradiction | Koreans | C/C | 5.41 | 0.62 | 9.59 (0.002) | 4.86 (0.008) | 2.45 (0.09) |
| C/G | 5.14 | 0.84 | |||||
| G/G | 5.12 | 0.8 | |||||
| European Americans | C/C | 5.13 | 0.78 | ||||
| C/G | 4.87 | 0.89 | |||||
| G/G | 4.36 | 1.05 | |||||
| Change | Koreans | C/C | 4.34 | 1.03 | 4.98 (0.03) | .65 (0.52) | 0.01 (0.99) |
| C/G | 4.45 | 0.76 | |||||
| G/G | 4.55 | 0.86 | |||||
| European Americans | C/C | 4.64 | 0.72 | ||||
| C/G | 4.76 | 0.82 | |||||
| G/G | 4.82 | 0.77 | |||||
| Causality | Koreans | C/C | 5.91 | 0.8 | 32.90 (<0.001) | 1.70 (0.18) | 4.26 (0.02) |
| C/G | 5.47 | 0.93 | |||||
| G/G | 5.6 | 0.83 | |||||
| European Americans | C/C | 4.8 | 0.78 | ||||
| C/G | 5.09 | 0.95 | |||||
| G/G | 4.58 | 0.93 |
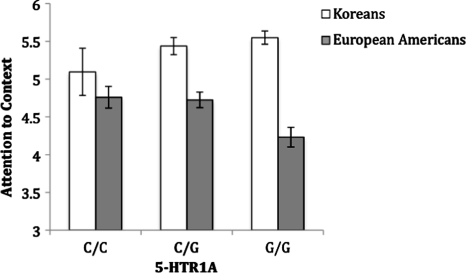
The ANOVA on locus of attention showed that there was a significant main effect of culture, F(1, 280) = 33.74, P < 0.001, such that Koreans (M = 5.49, s.d. = 0.70) reported attending more to the context than European Americans (M = 4.59, s.d. = 0.97) did. There was no main effect of 5-HTR1A, F(2, 280) = 1.56, P = 0.21. There was the predicted interaction between culture and 5-HTR1A, F(2, 280) = 5.55, P = 0.004 (Figure 1). We separated by genotype and conducted planned pair-wise comparisons between the two cultural groups. There was a clear linear pattern regarding the magnitude of the cultural difference. The cultural difference was greatest among the G/G group with a very large effect size (M = 5.55, s.d. = 0.67 for Koreans and M = 4.23, s.d. = 0.98 for European Americans, P < 0.001, d = 1.57) and smallest among the C/C group with a medium effect size (M = 5.10, s.d. = 0.46 for Koreans and M = 4.76, s.d. = 0.99 for European Americans, P = 0.33, d = 0.44). The C/G group fell between the two groups with a large, but considerably smaller effect size compared to the G/G group (M = 5.44, s.d. = 0.75 for Koreans and M = 4.72, s.d. = 0.90 for European Americans, P < 0.001, d = 0.87).
Fig. 1.
The locus of attention as a function of culture separated by 5-HTR1A genotype (error bars represent standard errors).
We also conducted within-culture comparisons to examine predicted linear tendencies across the three genotypes in both cultures. We conducted a planned contrast assigning weights of −1, 0, 1 to C/C, C/G and G/G groups, respectively, separated by culture. Among European Americans, the contrast was significant, t(136) = 2.41, P = 0.02, indicating that there was the predicted linear relationship among different genotypes. Among Koreans, the predicted linear relationship among different genotypes was opposite of European Americans, although this contrast was marginally significant, t(144) = −1.66, P = 0.099 (for other sub-components of the AHS, see Table 1). In summary, our predictions were supported by the results demonstrating that cultural differences in locus of attention were most pronounced among G/G genotype participants, showing the gene by culture interaction effect.
DISCUSSION
As predicted, we found significant interactions between 5-HTR1A and culture on locus of attention. The results among European American participants were quite consistent with those of previous research. European Americans with the G allele, which is associated with reduced 5-HT transmission, showed an increased attention to the focal object, similar to ATD studies with experimentally reduced 5-HT activity (Schmitt et al., 2000; Ahveninen et al., 2002). However, Koreans, in contrast, showed increased attention to the context, the culturally consistent mode of thinking and this was particularly the case for Koreans with the G allele.
In our theoretical framework, we examined whether culture influences the phenotypic expression of genotypes of the 5-HTR1A. Culture provides a context that affords opportunities and constraints for the development of psychological tendencies by providing reinforcements via specific norms, rules and guidelines for where to direct one’s attention and how to conduct actions in given situations (Shweder, 1990; Kim and Markus, 1999; Uskul et al., 2008). The present results show that the increased focused attention due to lower 5-HT activity might be culture-specific, and in a culture in which attention to the field and context is fostered, lower 5-HT activity does not lead to such a cognitive outcome. The present findings support the assertion that culture is not only constrained by genes, but also may influence the phenotypic manifestation of genes. Moreover, given the findings concerning the relationship between 5-HT and cognitive flexibility to adapt to changes in reinforcement (Park et al., 1994; Clarke et al., 2004), it is possible that the 5-HT system is linked to the degree to which people adhere to psychological tendencies that are modal and reinforced in their cultural contexts.
Although the present study examined cultural difference in the self-reported use of cognitive modes, we believe that the results introduce an important issue that should be further developed in future research, examining actual cognitive processes. Thus, it would be very interesting to see whether 5-HTR1A moderates the effect of culture on visual attention. For example, Japanese are more likely than European Americans to attend to and recall contextual factors when viewing underwater or nature scenes, whereas European Americans are more likely to attend to and recall aspects of the focal object (Masuda and Nisbett, 2001). We would predict that these cultural differences would be magnified amongst those with the G/G allele. It is also important to consider the role of genetic predispositions for recent cross-cultural findings examining the neural correlates of these attentional differences (Hedden et al., 2008). Increased activation in frontal and parietal brain regions associated with attentional control was observed when participants engaged in culturally incongruent tasks, i.e. context-dependent visual attention tasks for European Americans and context-independent visual attention tasks for Asian Americans (Hedden et al., 2008). The integration of genetics with cultural psychological and social neuroscience approaches is thus an important direction for future research (Han and Northoff, 2008; Sherman et al., in press).
One potential confound in the current finding is that Koreans and European Americans differ from each other, not only in their cultural experiences, but also in their genetic make-up. That is, it is possible that the current findings are due to a gene by gene interaction, rather than gene by culture interaction. In order to address this issue, we included a small group of Korean Americans (N = 21; all born in the United States; 5 C/G and 16 G/G) who should be quite similar to Koreans in their genetic make-up but who grew up within American culture. Our results show that within each genotype (both C/G and G/G groups as our sample did not include any C/C Korean American participants), the Korean Americans’ responses were between those of the Koreans and the European Americans. Moreover, among the GG participants, the Korean Americans (M = 4.81 s.d. = 0.96) were somewhat closer to the European Americans (pair-wise comparison, P = 0.53) than the Koreans (P = 0.23). Among the C/G participants, the Korean Americans (M = 4.97 s.d. = 0.55) were also closer to the European Americans (P = 0.02) than the Koreans (P < 0.001). These results, although based on a very small sample size, are consistent with the idea that culture plays the key role in shaping the psychological outcomes of different 5-HTR1A genotypes.
In addition, in the present research, we examined only the polymorphism of 5-HTR1A. However, it will be important to examine the role of other polymorphisms in 5-HT system, such as the 5-HT transporter promoter polymorphism (5-HTTLPR) and TRP hydroxylase 2 gene (TPH2), in order to test if the pattern found with 5-HTR1A would generalize to other 5-HT genes or is uniquely associated with 5-HTR1A.
The present study underscores the importance of extending gene studies and studies revealing gene-by-environmental interactions beyond a search for factors that predispose to psychopathology. For example, we have found that genes, 5-HTR1A and oxytocin receptor gene (OXTR) and culture interact with each other and moderate the relationship between stress level and use of social support in a sample of non-clinical participants (H.S. Kim et al., Manuscript in preparation1). The cultural difference in how much people used social support as a function of stress level was particularly pronounced among G/G genotype. These studies point to the importance of examining the genetic, environmental and cultural basis of normal variations in ways of thinking and behaving.
The present research essentially addresses the nature-nurture question, focusing on the constituent influence of culture and thus, proposes a different perspective to examine gene−environment interactions. Much of the public discourse on genes centers on the notion that there is a clear gene that can be directly linked to specific psychological or behavioral tendencies. This simplistic understanding of the role of genes can be particularly problematic when it is associated with group differences, such as cultural and racial differences, as such a view can lead to thinking that many observed psychological and behavioral differences are fixed. Yet, the influence of genes on everyday behaviors is far from simple and the factors that moderate their behavioral and psychological consequences remain largely unknown. The present research suggests that socio-cultural factors may shape the phenotypic expression of particular genetic predispositions by leading to different modes of thinking. By demonstrating how the genetic predisposition can lead to different modes of thinking as a function of culture, our approach aims to illustrate both the impact and limitations of genetic analyses. In so doing, we hope to provide a framework to further the understanding of the complex and joint roles of genes and culture in the shaping of modes of thinking.
Conflict of Interest
None declared
Footnotes
1These study results come from the same dataset that was used in the current article.
REFERENCES
- Ahveninen J, Kahkonen S, Pennanen S, Liesivuori J, Ilmoniemi RJ, Jaaskelainen IP. Tryptophan depletion effect on EEG and MEG responses suggest serotonergic modulation of auditory involuntary attention in humans. Neuroimage. 2002;16:1052–61. doi: 10.1006/nimg.2002.1142. [DOI] [PubMed] [Google Scholar]
- Bachner-Melman R, Gritsenko, Nemanov L, Zohar AH, Dina C, Ebstein RP. Dopaminergic polymorphisms associated with self-report measures of human altruism: A fresh phenotype for the dopamine D4 receptor. Molecular Psychiatry. 2005;10:333–335. doi: 10.1038/sj.mp.4001635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychology. 2006;48:406–9. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Ben Zion IZ, Tessler R, Cohen L, Lerer E, Raz Y, Bachner-Melman R, et al. Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differences in human sexual behavior: Desire, . arousal and sexual function. Molecular Psychiatry. 2006;11:782–6. doi: 10.1038/sj.mp.4001832. [DOI] [PubMed] [Google Scholar]
- Bruner JS. The culture of education. Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang FM, Kidd JR, Livak KJ, Pakstis AJ, Kidd KK. The world-wide distribution of allele frequencies at the human dopamine D4 receptor locus. Human Genetics. 1996;98:91–101. doi: 10.1007/s004390050166. [DOI] [PubMed] [Google Scholar]
- Choi, Koo M, Choi JA. Individual differences in analytic versus holistic thinking. Personality and Social Psychology Bulletin. 2007;33:691–705. doi: 10.1177/0146167206298568. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crogts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–80. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, Campbell B, Gray PB, Sorenson MD. Dopamine receptor genetic polymorphisms and body composition in undernourished pastoralists: an exploration of nutrition indices among nomadic and recently settled Ariaal men of northern Kenya. BMC Evolutionary Biology. 2008;8:173. doi: 10.1186/1471-2148-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–6. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Greenfield PM. You can't take it with you: Why ability assessments don't; cross cultures. American Psychologist. 1997;52:1115–1124. [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–54. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hedden T, Ketay S, Aron A, Markus HR, Gabrieli JDE. Cultural influences on neural substrates of attentional control. Psychological Science. 2008;19:1, 12–16. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, et al. Human 5-HT1A receptor C (-1019)G polymorphism and psychopathology. International Journal of Neuropsychopharmacology. 2004;7:441–51. doi: 10.1017/S1461145704004663. [DOI] [PubMed] [Google Scholar]
- Kim H, Markus HR. Deviance or uniqueness, harmony or conformity: A cultural analysis. Journal of Personality and Social Psychology. 1999;77:785–800. [Google Scholar]
- Kim-Cohen J, Gold A. Measured gene-environment interactions and mechanisms promoting resilient development. Current Directions in Psychological Science. 2009;18:138–42. [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown DD, et al. Impaired repression at the 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003;24:8788–99. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Masuda T, Nisbett RE. Attending holistically versus analytically: Comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology. 2001;81:922–34. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Peng K, Choi, Norenzayan A. Culture and systems of thought: Holistic versus analytic cognition. Psychological Review. 2001;108:291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- Norenzayan A, Smith EE, Kim B, Nisbett RE. Cultural preferences for formal versus intuitive reasoning. Cognitive Science. 2002;26:653–84. [Google Scholar]
- Park SB, Coull JT, McShane RH, Young AH, Sahakian BJ, Robbins TW, et al. Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology. 1994;33:575–88. doi: 10.1016/0028-3908(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Muntjewerff ND, O’Hanlon JF. A comparative study of acute and subchronic effects of dothiepin, fluoxetine and placebo on psychomotor and actual driving performance. British Journal of Clinical Pharmacology. 1995;39:397–404. doi: 10.1111/j.1365-2125.1995.tb04468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K.-Y, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression. Journal of the American Medical Association. 2009;301:2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JA, Jorissen BL, Sobczak S, van Boxtel MP, Hogervorst E, Deutz NE, et al. Tryptophan depletion impairs memory consolidation but improves focused attention in healthy young volunteers. Journal of Psychopharmacology. 2000;14:21–9. doi: 10.1177/026988110001400102. [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Wingen M, Ramaekers JG, Evers EAT, Riedel WJ. Serotonin and human cognitive performance. Current Pharmaceutical Design. 2006;12:2473–86. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Kim HS, Taylor SE. Cultural and social support: Neural bases and biological impact. Progress in Brain Research (in press) [DOI] [PubMed] [Google Scholar]
- Shweder R. Cultural psychology: what is it? In: Stigler J, Shweder R, Herdt G, editors. Cultural Psychology. Cambridge, England: Cambridge University Press; 1990. [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, et al. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. Journal of Neural Transmission. 2003;110:1445–53. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger N., I. Early family environment, current adversity, the serotonin transporter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–6. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Uskul AK, Kitayama S, Nisbett RN. Ecocultural basis of cognition: farmers and fishermen are more holistic than herders. Proceedings of the National Academy of Sciences of the USA. 2008;105:8552–6. doi: 10.1073/pnas.0803874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YWY, Tsai SJ, Liou YJ, Hong CJ, Chen TJ. Association study of two serotonin 1A receptor gene polymorphisms and fluoxetine treatment response in Chinese major depressive disorders. European Neuropsychophamacology. 2006;16:498–503. doi: 10.1016/j.euroneuro.2005.12.004. [DOI] [PubMed] [Google Scholar]