Abstract
Cultural neuroscience is an interdisciplinary field of research that investigates interrelations among culture, mind and the brain. Drawing on both the growing body of scientific evidence on cultural variation in psychological processes and the recent development of social and cognitive neuroscience, this emerging field of research aspires to understand how culture as an amalgam of values, meanings, conventions, and artifacts that constitute daily social realities might interact with the mind and its underlying brain pathways of each individual member of the culture. In this article, following a brief review of studies that demonstrate the surprising degree to which brain processes are malleably shaped by cultural tools and practices, the authors discuss cultural variation in brain processes involved in self-representations, cognition, emotion and motivation. They then propose (i) that primary values of culture such as independence and interdependence are reflected in the compositions of cultural tasks (i.e. daily routines designed to accomplish the cultural values) and further (ii) that active and sustained engagement in these tasks yields culturally patterned neural activities of the brain, thereby laying the ground for the embodied construction of the self and identity. Implications for research on culture and the brain are discussed.
Keywords: culture, self, brain, independence/interdependence
INTRODUCTION
… familiar categories of behavior—marriage customs, food taboos, folk superstitions and so on—certainly do vary across cultures, but the deeper mechanisms of mental computation that generate them may be universal and innate (Pinker, 2002, p. 39).
What Steve Pinker is referring to in this quote is a view of the human mind as an autonomous computational machine. Now a half century old, this view became a dominant metaphor of the mind around the 1950s when a computer was invented. This metaphor presented an appealing possibility that the human mind might also have a set of algorithms that enable it to receive an input and perform intelligent transformations on it (see Posner, 1989 for this history). Ever since then, the computer metaphor has held sway on social and behavioral sciences in general and on psychology in particular. It was central in cognitive psychology (Neisser, 1967), which had important influences on social cognition and social psychology in general (Fiske and Taylor, 1991; Wyer and Srull, 1994).
Any powerful metaphors can highlight issues and research agendas. Such metaphors can therefore contribute to the development of empirical and theoretical knowledge. The computer metaphor is not an exception. The enormous progress the above-noted fields have undergone over the last half century may owe importantly to this single metaphor. At the same time, however, any powerful metaphors, including this one, can also hide and background some other aspects that are equally important and fundamental. We believe that useful as it obviously is, the computer metaphor is limiting in some important ways especially if taken too literally, thereby hampering a further development of the related fields. In particular, the computer metaphor would portray the mind as fixed, bounded and housed neatly in the head, and but for sensory receptors nearly completely insulated from the external environment. However, recent demonstrations of neural plasticity and epigenesis emphasize the significance of experience in brain development by suggesting that non-genetic, environmental factors can lead to dramatic changes in gene expression (e.g. Suomi, 1999; Gunnar et al., 2001; Meaney and Syzf, 2005; Lee et al., 2006). Given this emerging evidence, it has become increasingly clear that ‘the mind itself’ is significantly influenced by socio-cultural contexts insofar as experience is powerfully organized by culture. This possibility, however, is often back-grounded, underappreciated and thus under-researched within the general theoretical framework informed by the metaphor endorsed by Pinker and many contemporary researchers of the human mind.
A new theoretical framework of cultural neuroscience, which we will suggest later in this article, is intended to restore a much needed balance in emphasis (see Ambady and Bharucha, 2009; Dominguez et al., 2010; Kitayama and Uskul, in press; Kitayama and Tompson, 2010; Losin et al., in press; Malafouris, in press; Seligman and Brown, in press for related perspectives). It seeks to establish an alternative view of the human mind as biologically prepared and, yet, supplemented, transformed and fully completed through active participation and engagement in the eco-symbolic environment called culture. In our view, then, cultural neuroscience is an interdisciplinary field of research that investigates interrelations among culture, mind and the brain (Kitayama & Tompson, 2010; Kitayama & Uskul, in press). Drawing on both the growing body of scientific evidence on cultural variation in psychological processes and the recent development of social and cognitive neuroscience, this emerging field of research aspires to understand how culture as an amalgam of values, meanings, conventions and artifacts that constitute daily social realities might interact with—that is, both constructing and being constructed by, the mind and its underlying brain pathways of each individual member of the culture. As we will see, this new field has potential of bridging social and biological sciences, thereby contributing to a new integrative theoretical framework for the study of the human mind.
WHY ADD ‘NEURO’ TO THE STUDY OF CULTURE?
Over the last two decades, a number of researchers have argued that psychological processes are malleably shaped to a degree that is far greater than was previously considered possible by exposure to, and active engagement in, socio-cultural environments. This thesis has received considerable support from the last two decades of research in cultural psychology (e.g. Markus and Kitayama, 1991, 1994; Nisbett et al., 2001; Kitayama et al., 2006; Heine, 2008, for reviews). Nevertheless, increasingly more compelling evidence has begun to emerge from recent adoption of neuroscience measures in the field. In fact, the present special issue of Social, Cognitive, and Affective Neuroscience is a testament to this observation.
Before the modern rebirth of cultural psychology, a number of cross-cultural studies in psychology had been largely based on survey methods (e.g. Hofstede, 2001; Schwartz, 1992). Although powerful and capable of demonstrating a broad bird’s eye view of world cultures (e.g. Inglehart and Baker, 2001), the methods had left open the question of how culture might influence psychological processes, mechanisms, and structures of each individual. One important strength of the cultural psychology literature was that, unlike its predecessors, it took advantage of a variety of experimental paradigms and tasks to investigate underlying processes and mechanisms (see Kitayama and Cohen, 2007, for a review). Although important and crucial in theory development in psychology in general, there is an important limitation in this endeavor as well because any psychological paradigms or tasks necessarily involve observations of downstream outcomes of hypothesized processes or mechanisms, such as response time, recall or recognition, and judgment.
Neuroscience measures have enabled researchers to observe neural processes underlying the psychological processes more rapidly and concurrently than was ever before possible with traditional behavioral measures alone. For example, the processing of socially significant stimuli (e.g. one’s own face) can be enhanced. Moreover, this enhancement can be detected as early as one tenth of a second. With traditional psychological measures, a phenomenon such as this is simply unobservable. Yet, with neuroscience measures, especially with event-related potentials (ERPs; which have extremely high time resolution), a variety of hypotheses regarding early visual processing or early spontaneous attention can be tested with relative ease. Initial neural evidence is indicative of strong cultural effects on such processing (Park et al., 2009; Sui et al., 2009; Ishii et al., in press).
Moreover, with functional magnetic resonance imaging (fMRI), it is possible to identify specific brain regions that are recruited in a variety of psychological operations such as perception, judgment, and decision making (see e.g. Adolphs, 2009; Lieberman, 2010, for reviews). With putative functions of these regions of the brain reasonably specified, the technique enables researchers to specify the nature of brain mechanisms underlying such psychological operations. Again, culture has proven to be quite powerful in modulating psychological processes. As we will see, when solving simple arithmetic problems, native English speakers engage the left perisylvian cortices—areas that are typically involved in linguistic processing. Surprisingly, however, native Chinese speakers show very little activation in this area. Instead, they show marked activation in a pre-motor association area (Tang et al., 2006). A finding like this is especially powerful because it demonstrates that the same behavioral outcome is accomplished by different brain pathways. This suggests that people carry out the same tasks by recruiting varying component neural operations depending on their social or cultural backgrounds.
In what follows, we will present a brief review of recent cultural neuroscience evidence that demonstrates the degree to which brain pathways are shaped by culture. We will document that cultural tools and cultural practices have powerful influences on brain pathways. Importantly, there is a growing body of literature demonstrating that symbolic aspects of culture, including certain normative mandates of culture such as independence and interdependence, also influence brain pathways. We will then ask a more fundamental question of exactly how culture might influence the brain. Our answer is premised on the hypothesis that recurrent, active, and long-term engagement in scripted behavioral sequences (called cultural tasks) can powerfully shape and modify brain pathways. Relying on this idea, we will propose a theoretical framework for understanding the culture–mind interaction. We will then examine some implications of the framework to suggest several future directions of research in cultural neuroscience.
CULTURE AND THE BRAIN: NEW EVIDENCE
Plasticity of the brain
A growing body of research in cognitive and social neuroscience has begun demonstrating the substantial degree by which connectivities and functions of different areas of the brain change as a result of experience in general and of repeated engagement in some specific cultural practices in particular. This literature has been extensively reviewed elsewhere (Chiao, in press; Fiske, 2009; Han and Northoff, 2008; Park and Gutchess, 2006; Wilson, in press; Chiao and Ambady, 2007). So, here it will suffice to highlight a few recent examples.
Abacus experts in Japan
In East Asian countries, it is quite customary, at least traditionally, for children to learn how to use the abacus. Still today, abacus is often taught in China, Taiwan and Japan as part of arithmetic classes in elementary schools. Abacus users learn to move an array of beads to represent numbers, perform arithmetic operations on them and thus to generate answers. Experts can perform complex operations with apparent ease. There is a small literature in cognitive and developmental psychology examining what might be happening in abacus experts as they solve arithmetic problems. Early on, a Japanese developmental psychologist, Giyoo Hatano, proposed that abacus experts acquire a mental representation of abacus and operate on the mental abacus (Hatano and Osawa, 1983; see also Stigler, 1994). One implication of this idea is that abacus experts represent numbers spatially in terms of locations of relevant beads on the mental abacus (Hatano et al., 1987).
Recently, Hanakawa et al. (2003) conducted an fMRI study, investigating some neural implications of the mental abacus hypothesis: if experts use a mental abacus in mental computation, they should engage parietal regions of the brain because they are linked to spatio-visual processing (Mellet et al., 1998). The researchers asked both abacus masters and novices (all Japanese) to solve a variety of arithmetic problems and observed patterns of brain activations. Their data suggest that novices tend to show activations in motor cortices as well as areas involved in linguistic processing (e.g. Broca’s area) during mental computation. However, for intermediate abacus users, a much more prominent activation was found in the left parietal lobe and, moreover, for abacus masters this parietal activation was found bi-laterally. One additional and crucial piece of data came from an analysis of how brain activations might increase as a function of difficulty of mental computation. The parietal activation was systematically greater as a function of the number of digits involved in the mental computation, thus indicating the crucial involvement of the parietal lobe in arithmetic processing.
Spatial navigation and the hippocampus
In all animals including humans, spatial memory and navigation is a skill that is essential for survival. One area of the brain that plays a fundamental role in this regard is the hippocampus (O'Keefe and Nadel, 1978). The hippocampus is a seahorse-shaped structure that forms part of the limbic system that surrounds the thalamus.
In humans, located inside of the medial temporal lobe, the hippocampus has long been known to serve a critical function in memory formation and retrieval (Squire and Schacter, 2002). People with extensive hippocampal damage are known to show substantial memory loss (Scoville and Milner, 1957). Recent work by Schacter and colleagues (Schacter et al., 2007; Schacter and Addis, 2008) has shown that the hippocampus is part of the system that forms and retrieves episodic memory representations as well as crafting representations of future episodic events. Furthermore, the hippocampus is known to retain the ability to produce new neurons throughout much of the life span (Eriksson et al., 1998). These neurons are likely to form new connections, which in turn could be culled and pruned out, to modify neural pathways.
Spatial navigation, especially, navigation of the self in a complex, everchanging environment, would require retrieving previous episodes, simulating possible future courses of action, and then deciding which routes to take to reach given destinations. It may not be a coincidence, then, that the hippocampus is also heavily implicated in spatial navigation. Numerous studies with rodents have identified neurons that respond specifically to particular places in space. These neurons, called place cells, are located primarily in the hippocampal areas (O'Keefe and Nadel, 1978). As may be expected, one neuroimaging study finds that while navigating the self in a virtual environment, subjects exhibit enhanced activations in the right hippocampus as well as in the right inferior parietal regions, again demonstrating the crucial role of the hippocampus in spatial navigation (Maguire et al., 1998).
Another study by Maguire and colleagues has demonstrated that extensive ‘training’ in spatial navigation can result in structural changes in the hippocampus (Maguire et al., 2000). The researchers tested London cab drivers who varied widely in the number of years of experience. Like in many other major European cities, London streets are highly complex. Moreover, cab driving, unlike, say, driving of a bus on regular routes, requires considerable ‘improvisations’ in finding short cuts, avoiding traffic jams, changing routes as a function of the day of the week, and the like. Driving as a cab driver in big, complex cities, then, would require continuously retrieving relevant episodic memories, simulating possible routes, and operating on the mental map of the place while making numerous driving decisions. In short, the cab drivers may constantly make heavy use of the navigating functions of their hippocampi.
To assess the validity of this analysis, the researchers obtained structural magnetic resonance images of the brains of the London cab drivers and analyzed the volume of the anterior, the middle, and the posterior parts of the hippocampus and observed that, relative to the hippocampi of matched control subjects, the hippocampi of the cab drivers showed a substantial enlargement in the posterior part and an equally noticeable, although unanticipated and currently somewhat puzzling, reduction in size in the anterior part. This structural difference, moreover, was attributable to experience because it was significantly correlated with the number of years of experience as a cab driver.
Arithmetic processing in China
Another important demonstration of malleability of brain functions as a function of experience comes from a recent study by Tang and colleagues on arithmetic processing (2006). It has long been known that when one mentally solves simple arithmetic problems, say, ‘12 + 32’, linguistic processing areas of the brain including the Broca’s and Wernicke’s areas are strongly engaged (Dehaene and Cohen, 1995; Dehaene, et al., 1999). It appears that individuals use linguistic codes in carrying out mental computation. In fact, the aforementioned study by Hanakawa and colleagues on abacus experts showed that novice Japanese do show a pattern that is consistent with this general conclusion.
Building on this previous work, Tang and colleagues investigated patterns of brain activation linked to mental computation among European Americans and Chinese and found strong evidence for the involvement of linguistic processing areas in mental computation. Interestingly, however, the evidence was at best very weak for the Chinese, who instead showed a strong activation in the pre-motor cortex during mental computation—another finding consistent with the data from Japanese abacus novices. Tang and colleagues attribute this surprising association of mental computation with the brain regions involved in motor responses to the fact that during training of Chinese language, a strong emphasis is placed on writing. The argument is that Chinese also use linguistic processing in mental computation, but the linguistic processing implicates motor movement much more prominently in Chinese than in English. This interpretation, however, seems somewhat inconsistent with the observation that Chinese participants did not show much activation in the traditional linguistic processing areas including the Broca’s and Wirnicke’s areas during mental computation.
At this point, then, it is not entirely clear why Chinese participants show strong activations in the pre-motor areas during mental computation. One conjecture comes from an observation that in traditional Confucian societies, particularly in China, Japan, and Korea, abacus training is very common. It would seem possible, then, that computation involved finger movements that are typical in the use of abacus. Alternatively, in these countries, arithmetic computation is typically taught in school with an emphasis placed on writing down intermediate steps on a sheet of paper and, importantly, extensive drills of this way of calculation are quite typical (as in the Kumon training that has become increasingly popular in the US in the recent years). For these reasons, Chinese participants in the Tang et al. experiment might have represented numbers spatio-visually by literally writing them down on the mental sheet of paper while solving arithmetic problems.
Summary
We presented a few examples that illustrate how repeated performance in the same routines can result in systematic differences in brain pathways that are engaged. This appears to be the case whether the routines at issue pertain to cultural tools such as abacus, cultural routines such as driving in a city that is complex to navigate, or cultural or educational practices such as arithmetic computation. The main point is that brain pathways can change as long as they are fired in certain scripted ways over an extended period of time. When fired together, the brain neurons begin to be wired together. The mind, then, becomes ‘retooled’ as a result (Wilson, in press).
Cultural views of the self and the brain
Studies reviewed above examine the degree to which specific cultural tools, practices, and tasks might foster certain brain changes. Given the positive findings obtained in these studies, it is reasonable to anticipate systematic differences in mental processes and underlying brain pathways in different cultural regions. Different cultural regions have been characterized in terms of different sets of cultural tools, practices, and tasks. Importantly, the cultural tools, practices, and tasks are not randomly assembled or distributed. To the contrary, they are organized by certain themes or values, including (but not limited to) independence or individualism and interdependence or collectivism (Markus and Kitayama, 1991; Nisbett et al., 2001; Triandis, 1995; Kitayama et al., 2007). Effects of culture, therefore, are likely to go beyond the effects that are attributable to each individual tool, practice or task. Instead, culture is organized by meanings, folk beliefs and values that tie together the relevant tools, practices, and tasks. Cultural influences are likely to be reinforced and determined by the layers of specific tools, practices, and tasks that are integrated into a more or less coherent, interconnected network.
For a long time, anthropologists have documented highly diverse cultural practices and institutions (e.g. Shweder, 2003). It is assumed that these cultural practices and institutions can foster very different notions of the self and well-being. They may also invite very different styles of cognition and emotion (e.g. DeVos, 1973). Following these earlier contributions, the last two decades of research in cultural psychology examined a number of psychological tendencies related to independence or interdependence of the self, and provided convincing evidence that these psychological tendencies show remarkable cultural variations (Markus and Kitayama, 1991, 1994; Nisbett, et al., 2001; Kitayama et al., 2006).
Importantly, this behavioral research on culture has recently incorporated neuroscience measures such as functional magnetic resonance imaging (fMRI) and electroencephalography, providing initial evidence that cultural variations in behavioral responses are accompanied by corresponding differences in brain functions (e.g. Chiao, in press; Fiske, in press; Han and Northoff, 2008; Kitayama and Uskul, in press). This emerging literature on culture and the brain is briefly reviewed in this section.
The general working hypothesis in this literature is based on the notion of cultural views of the self as independent (which is more prominent in the west) and as interdependent (which is more prominent in the east) (Markus and Kitayama, 1991; Nisbett et al., 2001; Markus and Kitayama 2004; Kitayama et al., 2006). Specifically, people engaged in Western cultures (including North American middleclass cultures as well as their Western European counterparts) are more independent in the sense that (i) they keep their personal self highly accessible and place a greater value on it, (ii) they use this schema of independence and apply it to social perception, with consequences in term of nonsocial basic attention biases, (iii) their emotional life is grounded more on personal goals, desires, and needs, and (iv) they are strongly motivated internally by such goals and concerns. In contrast, people engaged in Eastern cultures (including East Asian countries such as China, Korea, Japan and Taiwan, as well as North Americans with such Asian heritage) are more interdependent in the sense that (i) they keep their interpersonal or social self relatively more accessible and place a greater value on it, (ii) they use this schema of interdependence and apply it to social perception, with consequences in attention biases, (iii) their emotional responses are grounded more in social goals, agendas and concerns, and (iv) they are strongly motivated by such social goals and concerns. Each of these points can be illustrated with numerous research examples that use both behavioral and neural dependent variables.
Neural representations of the self
Recent work by Zhu and colleagues (2007) has shown that the structure of the self varies systematically across cultures at the level of brain representations. Previous work provides abundant evidence that while the self is thought about and elaborated on, the medial prefrontal cortex (mPFC) is engaged (Craik et al., 1999; Kelley et al., 2002; Lieberman et al., 2004; Gutchess, et al., 2006). Zhu and colleagues hypothesized that the mPFC should also be engaged by close others among people in China—an interdependent culture. One reason is that the self and close others are supposedly tightly connected for Chinese. Another possible reason is that interdependent selves are defined, at least within the Chinese context, by relational attributes, which are shared among close others. Closely connected selves are likely to have highly overlapping representations. Yet another reason is that in interdependent contexts close others are just as relevant to the self as the self itself is. In contrast, for those with independent self, the self might still be quite distinct from others no matter how close the two might be, as the relationship is based on the assumption of mutual separation. Furthermore, these selves tend to be defined by unique, idiosyncratic features. The findings were consistent with this reasoning, with mPFC strongly engaged when one’s mother was thought about and elaborated on among Chinese, but not among people of Western cultural origin (Zhu et al., 2007).1
It is interesting to note that when people think about the self from others’ point of view (e.g. ‘Does my teacher think that I am lazy?’), the mPFC activation disappears. Instead, a more dorsal and posterior part of the PFC receives prominent activations (D’Argembeau et al., 2007). A recent, intriguing series of imaging studies by Han and colleagues have shown that when Chinese with strong religious commitments to Christianity and Buddhism think about the self, they show activations in the dorsal regions of the PFC rather than in the rostal and ventral mPFC that is typically linked to the self (Han et al., 2008; Han et al., in press). One interpretation is that religious people take the perspective of the God or Buddha and draw a judgment on the self. Perspective taking, however, might be more common as a cultural practice in interdependent cultures including Chinese culture (Leung and Cohen, 2007; Wu and Keysar, 2007). It is thus important to find out whether the hypothesized linkage between religiosity and perspective taking would generalize to other cultures, such as the USA, that value independence more.
Two decades ago, Kitayama et al. (1990, described in Markus and Kitayama, 1991) hypothesized that the self would be more salient, elaborated, and/or accessible than others including close others for independent selves, but this effect might be weaker or even reversed for interdependent selves. Drawing on work by Tversky and colleagues (see Tversky, 1977 for a review) on asymmetry of similarity judgment between elaborate vs impoverished concepts (i.e. North Korea is more similar to Russia than Russia is similar to North Korea because typically people know much more on Russia than on North Korea), Kitayama and colleagues tested the hypothesis and found, as predicted, that for North Americans one’s friends are judged to be more similar to the self than the self is to the friends. Importantly, however, the pattern was non-significantly reversed for Indian subjects.
Recent work with ERPs has validated this initial observation, showing that when shown a facial photo and asked to make a simple judgment of the face orientation (i.e. looking to the right or the left), people exhibit an ERP component that is sensitive to covert orienting processes to target stimuli (negative peak approximately 200 ms after the presentation of the photo, called N200 or N2). As may be expected, this N2 response has been shown to be reliably stronger for the self-face than for the faces of one’s colleagues among the British participants. Importantly, this self-face advantage in N2 response was attenuated among Chinese participants (Sui et al., 2009). Another relevant neuroimaging study shows that this effect can be influenced by priming of independence, demonstrating the causal significance of independence and interdependence in modulating the salience of the personal self. Specifically, when independence is primed with a manipulation that highlights the personal self (Gardner et al., 1999), the salience of the self-face becomes quite prominent even among Chinese (Sui and Han, 2007). Along with a recent imaging study by Chiao and colleagues (2009), which shows a remarkable degree of within-culture variation in the self, this work highlights the significance of examining individual differences and situation-dependencies of the self within a cross-cultural framework.
Cognitive biases linked to schemas of independence vs interdependence
One notable consequence of independence (vs interdependence) can be found in person perception. When people apply a cultural schema of independence, they tend to focus on dispositional attributes of another person in lieu of available situational constraints. The resulting cognitive bias of highlighting dispositional factors in person perception (called the dispositional bias) has proven to be highly robust. In fact, Lee Ross (1977) coined the term, the fundamental attribution error, to refer to this bias. However, what if an alternative schema of interdependence were more salient or more accessible as should be the case in East Asia? Under such conditions, the emphasis on disposition (vis-à-vis situation) in person perception may be greatly attenuated. Miller (1984) provided initial evidence for this prediction in her comparative work involving North Americans and Indians. The basic finding has since been replicated and extended by subsequent researchers (Morris and Peng, 1994; Chua et al., 2005b; Kitayama et al., 2006; Kitayama et al., 2009; see Choi et al., 1999; Mason and Morris, in press, for reviews).
The cross-cultural variation calls into question the degree to which the dispositional bias is ubiquitous across cultures or truly fundamental in that sense. It has been argued, however, that the cultural variation can be explained without challenging the key assumption that dispositional bias is still universal and ‘fundamental’. The argument is based on the hypothesis that there are two stages in person perception, with a first stage of automatic dispositional inference followed by a second stage of deliberate situational adjustment (Gilbert and Malone, 1995). Whereas the dispositional inference is automatic, quick, and obligatory, the situation adjustment is deliberate, slow, and optional. This theoretical framework can accommodate the cultural variation in dispositional bias by assuming that cultures vary in terms of the second optional process of situational adjustment: Relative to European Americans, Asians are more likely to attend and cognitively elaborate on situational constraints, thus showing an attenuated dispositional bias (Gilbert et al., 1988). The validity of this analysis, however, rests on the claim that the initial dispositional inference is equally automatic and obligatory across cultures.
In a series of studies, Na and Kitayama (2009) tested if cultural variation exists not only in situational adjustment, but also in spontaneous dispositional inference. They hypothesized that given the interdependent model of person, dispositions are less important in accounting for another person’s behavior. As a consequence, interdependent individuals are less likely to routinely engage in dispositional inferences, and hence less likely automatically producing dispositional inferences. To address this issue, the researchers presented European Americans and Asian Americans with many pairs of a facial photo and a simple behavior. Participants were asked to merely memorize the pairs. Subsequently, participants performed a lexical judgment task. Immediately before a stimulus word (or non-word) was presented, they were briefly shown each of the facial photos used in the first phase of the study. On some trials, the stimulus word referred to the trait that was associated with the face (the congruent trials). On some other trials, the word had no semantic relation with the trait associated with the face (the neutral trials). On the remaining trials, a word-like sequence of alphabetical letters was shown. To the extent that the trait was inferred and then assigned to the face, the lexical judgment should be more efficient, as revealed in shorter response time, on the congruent than on neutral trials. This facilitation effect served as an index of dispositional inference.
Na and Kitayama found a reliable cultural difference: Whereas European Americans showed a facilitation effect, thereby demonstrating spontaneous trait inference, the effect completely vanished for Asian Americans. The implication is both clear and important: cultures vary not only in the salience of situational information (as shown in previous studies), but also in the degree to which dispositions are automatically inferred and ascribed to the target person. Na and Kitayama conceptually replicated the initial behavioral evidence with an ERP measure, by showing that an ERP marker for the detection of semantic incongruity (N400) is reliably greater for incongruous traits than for congruous traits in the lexical judgment task for European Americans. But, this effect was vanished for Asian Americans.
Recent neuroimaging studies have demonstrated that when dispositions of another person are inferred, a network of the brain defined by the mPFC, temporal parietal junction (TPJ), and temporal pole is reliably activated (Harris et al., 2005; see also Mason and Morris, in press). Extrapolating from the Na and Kitayama finding, we may expect that this network will be less likely to be spontaneously recruited by Asians. In another recent study, Kobayashi and colleagues (2007) scanned the brains of Japanese and North American children as they read either stories that encourage mentalizing (i.e. mind-reading) of protagonists or stories that do not have any mentalizing elements. The participants were not explicitly instructed to infer the mental states of the protagonists. The researchers found a reliable activation of the mentalizing network (mPFC and TPJ) for both Japanese and Americans. Importantly, however, the activation was reliably more pronounced for European Americans than for Japanese, thereby corroborating the thesis of Na and Kitayama.
One basic pattern that can be gleaned from the cross-cultural studies on person perception is that person as a figural object (vis-à-vis situational information) stands out in social perception of North Americans. For Asians and Asian Americans, however, the figural object seems more embedded because it is tied more closely to situational constraints or affordances. Extrapolating from this literature, it may be anticipated that nonsocial attention mechanisms can also be influenced by culture. Specifically, attention may be more focused for North Americans and it is more diffused or more holistic for Asians and Asian Americans. Recent studies by Masuda and Nisbett (2001), Kitayama and colleagues (2003), Chua and colleagues (2005a), and many others have provided converging evidence for this anticipation with a variety of behavioral measures including response time, task performance, and eye-tracking.
Some recent imaging studies provide additional evidence. For example, Hedden and colleagues (2008) have shown in their fMRI study that European Americans recruit an attention network of the brain (defined by prefrontal cortex and parietal cortex) when they attend holistically to both an object and its context than when they focus exclusively on the object. However, Asians show a reversed pattern, recruiting the same attention network more in the focused attention task than in the holistic attention task. It appears that people use intentional and effortful attention to compensate for their ‘cultural cognitive deficits’.
In another relevant study, Gutchess and colleagues (2006) found that when processing focal objects, areas involved in object-processing (the bilateral middle temporal gyrus, the left superior parietal and the right superior temporal) were recruited more for Americans than for Chinese, particularly in elderly populations. In this study, however, the two cultural groups did not differ in the brain activity involved in background processing. More recent fMRI studies that tested a similar idea with somewhat different procedures have obtained consistent findings (Goh et al., 2007; Jenkins et al., in press).
One important ambiguity in interpretation of the two lines of studies noted above stems from the fact that whereas Hedden and colleagues used the observed brain activation to suggest that people compensate for the culturally conditioned lack of fluency of processing, Gutchess and colleagues use the observed brain activation to suggest the cultural fluency of processing (see also Gutchess et al., in press, for a related issue). The findings therefore must be carefully interpreted in light of other available data, both behavioral and neural. In future work, some behavioral or self-report data may be quite useful to test the Hedden et al. interpretation that the brain activation observed by their study is related to conscious effort. It will also be important to use additional measures to show that the activation observed by Gutchess and colleagues is related exclusively to fluency in automatic processing.
The hypothesized sensitivity or attentional attunement to contextual information for Asians has also been investigated with ERPs. Goto et al. (in press) demonstrated that an ERP marker corresponding to the detection of semantic incongruity (N400; Ganis and Kutas, 2003) is enhanced when a background scene in a picture is incongruent with an object featured in the picture. As may be expected, this incongruity response was significantly more pronounced for Asians than for European Americans. Importantly, this response was observed to become weaker for both cultural groups as a function of independent self-construal as assessed by Singelis’ (1994) measure. Ishii and colleagues (in press) reported a compatible finding in an ERP study that looked at the processing of word meaning as a function of background vocal tone.
The context sensitivity in attention may also involve a temporal dimension. When observing an object (say, a car), individuals may vary in their propensity to holistically attend to other related objects that are observed in the past. These objects constitute a ‘mnemonic context’ for the perception of the impinging object. Extrapolating from the existent work on spatial attention, Duffy and Kitayama (2007) hypothesized that the mnemonic context effect would also be greater for Asians than for European Americans. The researchers presented subjects with a series of circles that varied in size. Subjects were to judge the diameter of each circle. Within this task, a context effect is typically found such that the size judgment of a target circle is a weighted average of the objective size of the target and the average size of all the circles that have been presented in the series. Duffy and Kitayama (2007) replicated this context effect. As predicted, however, this context effect was greater (i.e. a greater weight given to the previous circles) for Japanese than for European Americans. This behavioral finding has been conceptually replicated with an ERP measure (Lewis et al., 2008). They used an oddball paradigm, in which a target stimulus and an unanticipated, distracting stimulus is presented amongst more frequent standard non-target stimulus, and found that a novelty P3 is significantly greater for Asians than for European Americans. The novelty P3 is an index of a ‘surprise response’ to an unanticipated stimulus. It therefore is likely to increase as a function of weight given to a default expectation that is formed by repeatedly presented, anticipated stimuli. Importantly, this brain response (novelty P3 within an oddball paradigm) was reliably predicted by interdependence as assessed by a questionnaire measure.
In sum, the emerging body of neuroscience studies on cultural variation in cognition has begun to demonstrate that (i) as compared to interdependent Asians independent European Americans tend to engage in processing of focal social objects in lieu of background contextual information and, moreover, (ii) analogous cultural differences can be found even in non-social domains. Both fMRI and ERP methods have been quite instrumental in showing specific brain mechanisms that are involved.
Emotions and emotion regulation
There are very few systematic cross-cultural neuroscience investigations on emotion. Thus, there is very little to report in this section. Nevertheless, a few promising areas of research may be suggested (see also Seligman and Brown, in press for a related discussion). In the current cultural psychology literature, one highly consistent behavioral finding involves emotions that are derived from independence such as pride in the self, feelings of superiority, anger, and frustration (called socially disengaging emotions) and emotions that are derived from interdependence such as friendly feelings, respect, guilt and shame (called socially engaging emotions) (Kitayama et al., 2006; Kitayama and Park, 2007). An empirical generalization is that the socially engaging emotions are experienced more for Asians and socially disengaging emotions are experiencec more for European Americans. Systematic cross-cultural variations might be expected on brain areas recruited to yield experiences of social engagement and social disengagement (see Schaefer et al., in press, for a similar approach applied to the evaluation vs potency dimensions of the semantic differential).
Another extension would test cross-culturally divergent emotional responses to conditions of adversity. For example, in a recent study, Kimel et al. (2009) adopted a cyber-ball paradigm (Williams and Jarvis, 2006) to manipulate social exclusion among European Americans and Japanese. In a cyber-ball paradigm, participants are either ostracized or included during an online ball tossing game by two or three other players who are, in fact, controlled by an experimenter. As may be predicted by the supposition that Japanese are relatively more interdependent, Japanese reportedly experienced more negative emotions after social exclusion than Americans did. Importantly, however, this cross-cultural difference was found in sadness, but not in anger. Sadness is typically linked to interpersonal loss and a resulting motivation to restore the original relationship; whereas anger is unequivocally self-oriented, expressing frustrations associated with interfered goals of the self.
It is possible that pain areas (e.g. the ACC and the anterior insula) are implicated in social exclusion across cultures (Eisenberger et al., 2003); yet given the Kimel et al., finding, the somatic sensation of pain might be construed in different fashion to produce divergent emotional experience across cultures. This possibility is consistent with Damasio’s (2003) somatic marker hypothesis, which holds that evolutionarily grounded somatic arousals are differentially perceived and interpreted to produce subjective feelings. Also consistent is an observation by Levenson et al. (1992). These researchers commented that relational contexts might be required for Asians to experience emotions, but probably not for North Americans. Indeed, a recent study by Uchida and Kitayama (2009) has shown that unlike Americans, Japanese experience interpersonal harmony as an integral constituent of happiness. It is of interest to see if this cross-culturally divergent emotion construction might entail activation of different areas of the brain.
Yet another important area of fruitful cross-cultural exploration involves emotion regulation. A recent review of self-report measures of emotion regulation shows that Asians are far more likely to suppress their emotions than European Americans (Matsumoto et al., 2008). This finding goes hand-in-hand with a Confucian idea that emotions often hinder ever-important social relations. If Asians routinely try to suppress their emotions and, moreover, if Asian culture provides them with some effective means to do so, they may become more capable in emotion regulation. One possibility, suggested by a recent study by Grossmann and Kross (in press), is that interdependent people might be more capable than independent people to take a third person perspective even to their own emotional distress, thereby accomplishing a greater degree of self-detachment, which could allow them to regulate their emotions.
A recent study by Goldin et al. (2008) shows that European Americans exhibit an even greater amygdala activation when they try to suppress their emotions to pictures that evoke negative emotions. This paradoxical effect is likely due to a need to pay close attention to an emotion even when the goal of doing so is to suppress the emotion (Wegner et al., 1993). In contrast, another recent imaging study by Ohira and colleagues (2006) tested Japanese and found that their Japanese subjects were entirely capable of eliminating the amydgala activation when asked to do so (although they showed some evidence of autonomic arousal).2
Yet another domain for fruitful exploration relates to emotional responses to ingroup vs outgroup members. Recent work by Xu and colleagues (2009) suggests that empathetic responses to another person’s distress are much stronger if the person is from an ingroup than if he/she is from an outgroup. Adams and colleagues (in press) showed that the amygdala activation to a fear face depends on both gaze and group membership of the stimulus face. In particular, the amygdala responds especially strongly to an ingroup fear face of averted gaze (indicative of a common threat in the direction of the gaze) and an outgroup fear face of direct gaze (indicative of a potential threat to the self) (see also Chiao et al., 2008; Moriguchi et al., 2005). Interestingly, in these studies, there is no cross-cultural difference in the responses to the ingroup vs outgroup stimuli (see also Rule et al., in press, for a similar point). Ethnocentrism and an assortment of psychological and neural processes involved in it might prove to be widespread and relatively uniform across cultures although part of this is likely to be due to the relative familiarity of ingroup (as opposed to outgroup) members (Adams et al., 2009).
Personal versus social motivation
Given the general significance of personal or private attributes for independent selves and social or public attributes for interdependent selves (Kim and Drolet, 2009), the nature of social motivations may also vary cross-culturally. A general hypothesis has been implied by Taiwanese psychologists, Yu and Yang (1994), who observed that achievement motivation in Taiwan is ‘socially oriented’. In their view, what appears to be a purely personal striving of achievement is in fact anchored in expectations of significant others and the sense of social obligations and duties to the others in Taiwan. This observation is also echoed in an earlier investigation by DeVos on achievement motivation in Japan (DeVos, 1965).
Recent behavioral studies have provided abundant evidence for this prediction. Iyengar and Lepper (1999), for example, tested intrinsic motivation and showed that European American children are motivated to perform a task they have chosen. In contrast, Asian American children did not show this effect at least to the same degree. Instead, these children were motivated more by a choice made by their mothers, underscoring the hypothesis that interdependent selves are more strongly motivated when one’s motivation is anchored in others’ expectations. A more recent series of studies on cognitive dissonance by Kitayama and colleagues (2004) are consistent with this hypothesis. Replicating a large number of previous Western studies, these researchers have found a choice justification effect among Asians and Asian Americans. However, the conditions in which the effects are obtained vary systematically across cultures. Whereas European Americans (or Canadians) show a strong justification effect when the choice is perceived as private and personal, Asians and Asian Americans show the choice justification effect only when the choice is perceived as public. From this pattern of data it has been proposed that European Americans justify their choice when the choice is private and thus it threatens the personal sense of the self (e.g. ‘I might be irrational and stupid’), whereas Asians and Asian Americans justify their choice when the choice is public and, thus, it threatens the public sense of the self (e.g. ‘They might think that I am irrational and stupid’).
Notably, the ‘public’ choice in some of the studies cited above is no more than a choice that subjects make while being exposed to a set of schematic faces. Evidently, when one is unobtrusively exposed to a schematic face while making a decision, the person spontaneously categorizes the decision to be public, as being made under others’ scrutiny, and treats it as such. It is also possible that if the situation is categorized as public, pertinent social norms are activated (Haley and Fessler, 2005; Rigdon et al., 2009).
Extrapolating from this body of evidence, we may hypothesize that when European Americans are exposed to watching faces, they are reminded of a strong norm for independence. At the same time, the watching faces give rise to the impression of their decision as public. Decisions made under public scrutiny are perceived as less than independent and, as a consequence, they may be seen as counter-normative and, thus, as irrelevant to the self. Likewise, when Asians are exposed to watching faces, they may be reminded of a strong norm for interdependence. At the same time, the watching faces give rise to the impression of their decision as public. Decisions made under public scrutiny are perceived as quite relevant to their interdependent status and, as a consequence, they are seen as highly relevant to the self. The pattern observed in the foregoing set of studies is consistent with the general hypothesis that private decisions are more significant for European Americans, but public decisions are more significant for Asians and Asian Americans.
The foregoing hypothesis implies that whenever Asians (or European Americans) make decisions in public (or in private), they tend to treat the decisions as more important. Because this contingency is likely to be repeated and thus overlearned, we may expect that cues indicating the public (or private) nature of the decisions will automatically alert the brain of the individuals that the decisions are significant to the self. In a recent ERP study, Park, Gehring, and Kitayama (2009) tested this possibility by examining the error-related negativity (ERN), an event-related brain potential component that is associated with error commission in speeded choice reaction time tasks (Falkenstein et al., 1991; Gehring et al., 1993). Previous work showed that the ERN magnitude is sensitive to the motivational significance of the errors. For example, ERNs are stronger as a function of monetary incentives associated with correct decisions (Hajcak, et al., 2005; Pailing and Segalowitz, 2004).
In the Park et al., study, participants were asked to perform a flanker task where they were asked to judge the direction of the center arrow that was surrounded by two arrows on each side (e.g. >><>> or <<><<). As a cue indicating the publicity of a decision, Park et al. briefly exposed the participants to a facial photo or a control image (i.e. a scrambled face or a house) right before the presentation of the stimulus arrows. If the face cue were sufficient to alert Asians to potential significance of the decision, it would enhance the ERN for Asians. For European Americans, however, the publicity of a decision can diminish the significance of the decision. If so, their ERNs might be weaker in the face priming trials relative to the control trials. The results were consistent with these predictions. Asians showed a stronger ERN in the face priming trials than in the control trials. In contrast, European Americans showed a pattern that is completely opposite, exhibiting a weaker ERN in the face priming trials than in the control trials. Importantly, individuals’ self-beliefs of interdependence (vs independence), as assessed with Singelis scale (1994), reliably predicted the ERN magnitude in the face priming trials. In fact, the cultural difference in the face priming effect was completely mediated by the cross-culturally differential level of interdependence (vs independence).
Summary
When Chiao and Ambady (2007) published a chapter on cultural neuroscience, there was not much to be reviewed in the literature. Now only in a matter of a few years, this new field of research has produced a fair number of empirical findings. Although some of the empirical claims may have to be qualified by future studies, the rapid rate at which the new body of knowledge on culture and the brain has grown is impressive. It seems quite clear that the brain and various neural processing pathways it contains are influenced, sometimes quite substantially, by culture and, as a consequence, when two or more vastly different cultures are compared, highly systematic differences in brain responses can be observed. While the basic anatomic structure of the brain will never be influenced by culturally organized experience, patterns of connectivities among various structural elements or areas of the brain as well as parameters associated with such connectivities appear to be influenced substantially.
We believe that these cross-cultural findings may best be understood and interpreted in light of the evidence for brain plasticity as a function of cultural tools and practices reviewed in the preceding section of this article. This evidence strongly suggests that repeated engagement in conventionalized behavioral patterns (as in abacus use or in cab driving) results in highly systematic changes in pertinent brain responses. By extrapolating from this observation, it would be safe to hypothesize that culture’s influences on the brain result from repeated participation and engagement in culture’s conventions, routines, and socially shared scripts for action. This point leads us to the next section, in which we present a theoretical model designed to bridge culture and the brain by emphasizing the critical role of ‘cultural tasks’ as a middle range layer connecting culture to the brain and vice versa.
CULTURALLY PATTERNED NEURAL ACTIVITIES: TOWARD A COMPREHENSIVE THEORETICAL FRAMEWORK
How can we begin to understand cultural differences and similarities in brain and behavioral responses, individual differences in these, as well as a variety of priming effects within a single theoretical framework? Admittedly, much more data are required before making any sweeping generalizations or any detailed theoretical accounts on cultural influences at all levels of analysis. If it were to be successful, however, this empirical effort must proceed hand in hand with an attempt to develop and elaborate a general theoretical framework that is informed by all known facts about culture, mind, and the brain. Such a framework would be indispensable in navigating the research effort through this emerging new frontier.
Layers of culture
There is a general consensus that culture is organized by ideas (Kroeber and Kluckhohn, 1952). However, researchers vary widely in the emphasis they give to different types of ideas as essential ingredients of culture. Some researchers have defined culture in terms of explicitly held beliefs and values. For example, Triandis once operationalized shared cultural ideas as those sentiments that a group of four or so people could agree on (Triandis et al., 1990; see also Schwartz, 1992, for a similar approach). Given this premise on the nature of culture, it is sensible to use self-report survey questions on cultural values and attitudes as a primary research tool and use answers to these questions as face-valid manifestations of culture (Oyserman et al., 2002).
In contrast, some other researchers place a far greater emphasis on implicit aspects of culture. As noted by Durkheim (1964), culture to humans may be analogous to what water is to fish. Typically, cultural anthropologists argue that culture is composed of layers of assumptions that are hidden from the surface because they are inscribed in daily practices and institutionalized in mundane routines, conventions, and societal norms (e.g. Shweder, 1991; D'Andrade, 1995; Shore, 1996). It is very clear, however, that both of these two aspects do exist in culture. In fact, there is a growing consensus that culture is a very complex process that is composed of some distinct structural components. Some of the components might be quite explicit, but some others might be highly tacit. A far more important, and potentially productive, route to take would be to start theorizing on the dynamic interplay among some of the key components of culture [see Dominguez et al., (2010), for an anthropological critique of the culture concept].
Motivated in part by this theoretical concern, Kitayama and colleagues (Kitayama, et al., in press; Kitayama et al., 2009; Kitayama and Imada, 2010) have distinguished among three key constituents of culture, and describes some different ways in which they are related to one another. A first key component of culture is explicit values that are emphasized in a given cultural group. For example, values related to independence are emphasized in Western cultures; whereas those related to interdependence are emphasized in Eastern cultures.
Second, culture contains a number of conventions, routines, or shared scripts for action. These are called cultural tasks. Kitayama and colleagues hypothesize that cultural tasks are typically designed or intended to achieve the culture’s primary values. For example, independent values, such as autonomy, freedom, enjoyment, uniqueness, and equality that are emphasized in North America are reflected in a variety of cultural tasks such as adversarial interpersonal argumentation, anti-elitist attitudes and practices, self-expression, equal treatment of people regardless of ranks, and the like. Likewise, values on interdependence, such as social harmony, filial piety, mutual understanding, and empathy that are sanctioned in Asia are reflected in corresponding cultural tasks, say practices designed to achieve social consensus, Confucian practices of paying respect to senior members of one’s own group, hierarchical ways in which to address one another, and the like.
A third component of culture is each individual’s implicit psychological and neural tendencies. These tendencies are typically aligned with the culture’s values. The last two decades of research in cultural psychology has provided convincing evidence for certain psychological tendencies that are typically associated with cultural values of either independence or interdependence (e.g. Markus and Kitayama, 1991; Kitayama et al., 2006). More recently, it has become increasingly clear that the psychological tendencies are culturally aligned precisely because underlying neural activities are also culturally patterned.
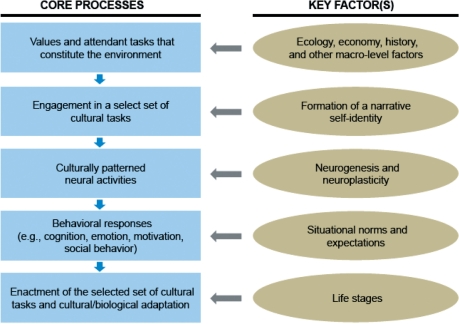
Note that cultural values are the most explicit of the three and the neural activities are the most covert or implicit. Cultural tasks fall right in the middle. Extending this analysis, we will propose a theoretical account that seeks to connect culture and the brain. We suggest that culture induces neural activities, often modifying and fostering neural processing pathways, by providing cultural tasks designed to achieve the culture’s values. Individuals routinely engage in such tasks in their effort to achieve cultural adaptation. Through this repeated engagement in cultural tasks, new neural activities are induced, reinforced, and established. These culturally patterned neural activities enable the person to seamlessly perform his or her own significant cultural tasks, thereby solidly anchoring the self and identity in the cultural world. This theoretical framework is schematically illustrated in Figure 1.
Fig. 1.
The theoretical framework proposed here is designed to understand how culture and the brain might influence one another in dynamic fashion. The key idea is that the influence of culture on brain activities is mediated by repeated long-term engagement in a select set of cultural tasks (scripted behaviors designed to accomplish the primary cultural values such as independence and interdependence). Behavioral responses produced by the culturally patterned neural activities facilitate cultural and biological adaptation by enabling the person to seamlessly perform the cultural tasks of his or her own choosing. Each stage involved in the core process of the culture-mind interaction (depicted on the left) is influenced by a set of factors described on the right.
Values, tasks and the brain: dynamic influences
Both the primary values of culture and the corresponding set of cultural tasks are historically developed under the influence of ecology, economy, and various other macroscopic societal conditions and organized into a loose whole that constitutes the cultural environment (the first layer of Figure 1). This environment represents a cumulative change the cultural group has undergone over generations. We literally stand on the shoulders of our ancestors (Tomasello, 1999). Culture, then, is a socio-historical process that must be analyzed on its own before being operationalized in any behavioral or neuroscience research projects.
Individuals are born into the cultural environment that is historically constituted by the pertinent values and the tasks designed to address and accomplish the values. Throughout their lives, they strive to adapt to the environment, achieve a degree of well-being, and accomplish biological adaptation (as assessed by reproduction of the self and its kin). Because the cultural tasks define the criterion by which to judge the degree to which each individual member of the culture lives up to the upmost values of the culture, the person can recognize himself or herself as a decent member of the culture as long as he or she performs at least some of the available cultural tasks in satisfactory fashion. Equally importantly, by successfully performing such cultural tasks, the person will be recognized as decent, respectable, and even admirable by people in the same cultural group. This public recognition will boost the identity of the self as a full-fledged member of the culture. Moreover, it will eventually enhance his or her likelihood of success in the cultural group, including a success in finding a desirable mate and reproducing offspring. For these reasons, individuals are likely to adopt some small set of available cultural tasks as their own and repeatedly engage in them (see the second layer of Figure 1). As we will note later, which cultural tasks are chosen and pursued is likely to be an important part of the construction of the self-narrative—a story (or the ‘stream of consciousness’ of William James) that defines the current self on the basis of previous experiences, while projecting it to the future (McAdams, 2006). This narrative solidly anchors the self in the culture and society, thereby affording the person to construct a stable identity.
The sustained participation in scripted behavioral routines (here referred to as cultural tasks) is important for our present theoretical concern precisely because we know from the emerging evidence for brain plasticity reviewed earlier in this article that depending on which tasks are pursued and practiced, very different patterns of neural activities are likely to be induced. These neural activities will eventually be over-learned, thus, becoming well connected and, thus, automatic. In other words, brain pathways will be modified and neural activities will be culturally patterned as a result. As we will note later, neurogenesis is likely to play some important role in the induction of culturally patterned neural activities (see the third layer of Figure 1).
Although the culturally patterned neural activities are fostered and formed through repeated engagement in cultural tasks, once formed, they will be the primary agent by which to express and embody one’s cultural values in his or her overt behaviors, judgments, and other cognitive, emotional, and motivational responses. As illustrated in the fourth layer of Figure 1, specific psychological responses are influenced by the culturally patterned neural activities. However, they are also influenced by numerous extraneous variables, most notably, fluctuating norms and expectations available in the immediate social situation (Asch, 1951).
Importantly, however, performance of cultural tasks, especially if it were finely attuned to life stages, gender, and other socio-demographic categories, would be normatively expected and uniformly sanctioned in virtually every given setting of the cultural world at issue. We may therefore anticipate that the culturally patterned neural activities will enable the person to perform, both seamlessly and automatically, the pertinent cultural tasks (see the last layer of Figure 1). Because it is spontaneous, this act is likely to be perceived as internally motivated, self-endorsed, and thus genuine by both the self and other community members alike (Ryan and Deci, 2006). Together, the successful engagement in the cultural tasks reinforces one’s cultural identity while maintaining or even enhancing one’s social standing in the cultural community. The self that emerges out of this process is fully embodied (Niedenthal, 2007) and, as such, revealed not just in the internal representations of the self, whether cognitive or neural, but more importantly reflected in neural mechanisms involved in sensory, motor, and affective processing (Wilson, 2002).
Accounting for cultural neuroscience evidence
The present analysis is consistent with the growing body of literature (reviewed earlier) that demonstrates the power of cultural tools and practices in shaping brain processes (see also Wilson, in press). Going beyond this literature, however, it also defines culture as an amalgam of such tools and practices, which is organized in terms of cultural tasks that are recruited to accomplish cultural values. As noted, this organization is nothing less than the behavioral environment—namely, the setting in which each and every individual who engages in the culture thinks, feels, and acts.
By conceptualizing culture as an organized set of tools and practices designed to achieve cultural values, the present theory accounts for systematic cultural variations in brain responses as a result of long-term engagement of each individual in tasks of the respective cultures. It also recognizes systematic differences among individuals within any given culture both (i) in terms of their commitment and endorsement of the culture’s primary values such as independence and interdependence and (ii) in terms of their idiosyncratic choices of cultural tasks as a means for achieving the cultural values and thus establishing their cultural identities.
The present analysis is also consistent with a variety of cultural priming effects (e.g. Hong et al., 2000; Kuhnen et al., 2001; Miyamoto et al., 2006; see Oyserman and Lee, 2008, for a review). For example, when some aspects of independence (or Western culture) are temporarily activated, individuals often show psychological tendencies that are linked to the independence (or Western culture) or vice versa for interdependence (or Eastern culture). Moreover, recent neuroscience work has begun to show that this is true not only in behavioral markers of independence (or interdependence), but also in their neural counterparts (e.g. Sui and Han, 2007; but also see e.g. Mok and Morris, 2009, for an important qualification to the general pattern). This body of evidence demonstrates that various environmental cues, cultural symbols, and culturally relevant concepts or constructs can temporarily activate the primary values of culture, specific cultural tasks, and/or component psychological processes required to carry out specific cultural tasks.
In short, our theoretical framework (Figure 1) is consistent with the exiting cultural neuroscience evidence. Yet, it goes much further, offering some new predictions and implications. As we already noted, the theory provides a systematic account of (i) both between-culture differences and within-culture variations. Moreover, it has implications for (ii) neurogenesis and cultural acquisition. It is also noteworthy that the theory regards (iii) the culturally patterned neural activities as the primary agent to enact and embody cultural values in behavior, with major implications for the correlates of neural activities, while emphasizing (iv) the socially distributed nature of culturally patterned neural activities. Taken as a whole, the present theoretical framework can provide a coherent platform upon which to map out the multi-layered processes linking culture to the brain.
Neurogenesis and cultural acquisition
The present analysis assumes that culture provides a means for humans to achieve biological adaptation. In other words, acquiring culture is a prerequisite for biological adaptation in general and for competing in the ‘reproductive market’. Recent work on neurogenesis offers important insights. This work demonstrates that production of new neurons in many parts of the brain occur not only in the first few years of life, but also later in life as well. Neurogenesis can continue virtually throughout life in some small areas of the brain including the olfactory bulb and the hippocampus (Eriksson et al., 1998). In most other areas this is not the case, but for some areas such as parietal lobes, neurogenesis is quite active until the late 20s. Most notably, Giedd and colleagues (1999, 2006) suggest that neurogenesis has a ‘second peak’ at around puberty, right around the time when individuals become ready for sexual reproduction. Summarizing their finding, Gieldd (quoted in Schwartz, 2002) observe:
What is most surprising is that you get a second wave of overproduction of gray matter, something that was thought to happen only in the first 18 months of life. Then there is a noticeable decline. It looks like there is a second wave of creation of gray matter at puberty, probably related to new connections and branches, followed by pruning (Schwartz, 2002, p.128).
The biological design noted here may reflect the fact that culture is humans’ way to achieve biological adaptation. It makes very good sense, then, to acquire the most up-to-date culture when one enters the reproductive market. Only those individuals who are capable of flexibly acquiring the most current culture and practicing the culture’s tools and tasks would have the maximal chance of achieving a full-fledged status as a cultural member and, thus, attracting mates.
By emphasizing the ‘second peak’ of neurogenesis, we do not wish to imply that cultural learning does not happen until puberty. To the contrary, evidence is very strong that enculturation begins very early in life and proceeds gradually (Greenfield et al., 2003 for a review). Nor do we want to equate neurogenesis with cultural acquisition. Most likely, cultural acquisition is only one of many consequences of neurogenesis that is made possible in conjunction with numerous other factors. We do propose, however, that the acquisition of culture might be accelerated especially at puberty and over some years afterwards. Consistent with this analysis, an anthropological observation of Japanese sojourners in the USA suggested that for them to be fully adjusted in the USA, they have to come back to Japan before puberty (Minoura, 1992). Moreover, Choudhury (in press) underscores the significance of adolescence as a period of cultural acquisition. McAdams (2006) makes a similar point on the basis of the fact that only when individuals reach late adolescence do they become capable of selecting among different identities (and different cultural tasks, we should add) and constructing an overarching self-narrative. Thus, he argues
It is not until the period of emerging adulthood … that people begin to arrange their entire lives … into broad and self-defining life narratives. …The [emerging] story ties together the many different aspirations you have and roles you play into a meaningful narrative framework (pp. 83–84).
Altogether, our theoretical framework is consistent with the important link, mentioned above, that has been uncovered between culture and biology. The ‘second peak’ of neurogenesis enables each individual to organize his or her cultural mind once again right before the person starts his or her reproductive career. The domains of culture and biology are not separate—much less antagonistic (see also Way and Lieberman, in press, for a similar point). To the contrary, biology prepares individuals for culture, which in turn is instrumental for them to achieve biological fitness in the long run.
Culturally patterned neural activities as the agent to enact cultural values
The current theoretical analysis implies that psychological processes and attendant neural pathways are recruited and thus controlled both by cultural tasks and by the cultural values these tasks are designed to address. This implies that there should be some degree of association between endorsement of explicit values of culture such as independence and interdependence and brain activities that enable the person to perform cultural tasks. Initial evidence reviewed in this article in fact shows that explicit cultural values as assessed, for example, by the Singelis scale of self-construal are systematically associated with neural activations that are theoretically linked to the respective cultural tasks (e.g. Lewis et al., 2008; Hedden et al., 2008; Park et al., 2009; Chiao et al., 2010; Goto et al., in press; Ishii et al., in press). This association, however, might be expected to be even greater once the specific cultural tasks that are important for each individual can be identified.
Recently, Zou and colleagues (2009) have proposed that judgments, decisions, and various social behaviors are regulated more in reference to perceived social norms rather than to one’s personal values or attitudes. Asians, for example, are more likely than European Americans to be interdependent (vs independent), not because they are personally committed to the corresponding values of interdependence (vs independence), but because they perceive strong normative expectations for interdependence (vs independence). Perceived norms might be correlated with personal values, but this correlation need not be strong. At first glance, then, the Zou et al., hypothesis might look inconsistent with an implication of the present analysis.
The inconsistency, however, seems more apparent than is real. The large body of literature on conformity (e.g. Asch, 1951) suggests that the effort to regulate one’s responses in reference to perceived norms and expectations—the kind of process that obviously exists and that is highlighted by Zou and colleagues—occurs primarily when the responses are clearly observable. In fact, evidence reported by Zou and colleagues is based exclusively on self-report measures of one’s own personality and dispositional bias in attribution. In contrast to these verbal or quasi-verbal responses, neural responses rarely ‘spill out’ of the skull. They are completely private. Thus, even when observable behaviors are strongly influenced by perceived norms that are activated in a given situation (as proposed by Zou and colleagues), there might still be a clear effect of personal values ‘within the skull’, at the level of neural responses.
One implication of the analysis here is noteworthy: Brain activities ought to be related to one’s endorsement of cultural values to a greater extent than the corresponding overt behaviors are. At first glance this prediction would seem surprising: How can it be that your personal values are related to your neural activities while barely related to your behaviors? This does not make sense if the values directly influence the behaviors. The present analysis suggests otherwise. It maintains that neural activities serve as the primary agent by which to reflect one’s personal values of culture. Behaviors, however, are determined jointly by the neural activities and numerous other factors including perceived social norms and expectations (Ajzen, 1985; the forth layer of Figure 1). Thus, the tight link between personal values and neural activities, along with fragile relations between the values and behavioral responses, would provide strong evidence for the present theoretical framework.
Socially distributed nature of neural markers of culture
Last, but not least, our analysis implies that different individuals perform different cultural tasks that are available in their culture. Moreover, which brain pathways are formed and maintained—and to what extent for that matter—would depend on the specific cultural tasks that are chosen. This means that although there may be numerous brain markers that are associated with primary cultural values such as independence and interdependence these brain markers will never be neatly packed into any single person’s brain. To the contrary, they may be socially distributed. Any single person cannot be fully independent or interdependent because individuals choose to be independent or interdependent in their idiosyncratic ways (the second layer of Figure 1). Yet, when aggregated, these individuals as a whole would show what their culture fosters in their brains. In its entirety, the cultural mind is more collective than personal. It may literally exist at the group level (see Caporael, 2003 for a related discussion).
One important consequence is that when assessed across individuals, the associations among the brain markers of culture would be limited at best. That is to say, from the knowledge that John is independent (or interdependent) with respect to a given brain marker X one may barely be able to predict that he would also be independent (or interdependent) with respect to another brain marker Y even though the markers X and Y are known to be equally good markers of independence (or interdependence). At present, no neural evidence is available. Yet at the level of behavior, evidence for the predicted lack of within-culture correlations is solid and replicable (Kitayama et al., 2009; Na et al., in press). Future work should specify particular cultural tasks for independence and interdependence and test the degree to which within-culture individual differences might be systematically understood in terms of the cultural tasks of different individuals.
CONCLUSIONS
Nearly two decades ago, cultural psychology as a distinct field of empirical inquiry was born. The exact date can be disputed, but the birth was marked by some landmark books by Bruner (1992), Cole (1996), Shweder (1991) and Nisbett and Cohen (1996) as well as by theoretical reviews by Markus and Kitayama (1991) and Triandis (1989). The field of cultural psychology was motivated and in fact defined by the following three guiding questions:
How does culture influence the human mind?
Is culture a superficial overlay on the basic, universal computational machine called the mind? Alternatively, is culture a crucial constitutive element of the mind? If so, what are specific mechanisms underlying this constitutive process?
What theoretical framework do we need in order to make visible progress in answering these questions?
In subsequent years, cultural psychology has prospered and provided great insights into each of these questions by establishing a solid body of theoretical and empirical knowledge. At the same time, however, some limitations have lingered on. In particular, one urgent issue is to find better ways for talking about mind and body, culture and biology, and nurture and nature. No longer is it possible to demarcate the domain of culture as separate from biology and ignore the latter in the analysis of the former. It is in this junction that we see the greatest potential of cultural neuroscience. In fact, the brain is the quintessential biological organ. And cultural neuroscience aspires to understand ways in which culture might be implicated in this central biological organ of the human species. We thus suggest that cultural neuroscience can make important contributions at least in three important ways.
First, cultural neuroscience can make an important contribution to basic neuroscience by providing a much richer and elaborate conception of ‘context’ in analyzing brain plasticity and formation. The development of research on spatial navigation aptly illustrates the power of basic neuroscience for an analysis of culture. Without previous work on place cells by O'Keefe and colleagues (1978), the imaginative study by Maguire and colleagues on London cab drivers (2000) would have been utterly impossible. At the same time, however, it also illuminates the power of culture to inform basic neuroscience. Without the work of Maguire and colleagues, it would have still been an open question of how generalizable the studies on rats might be to humans.
Secondly, cultural neuroscience can also make important contributions to the study of culture by providing important insights about how ‘deep’ culture can go into the human brain. If nothing else, it would be very hard to maintain, with the currently available behavioral evidence alone, the position that culture is no more than a superficial overlay that may be best stripped out in the study of the human mind. The emerging body of cultural neuroscience evidence, reviewed here, has begun to establish that culture is a centrally important element that constitutes some important aspects of mental functioning and thus brain pathways. Moreover, because the brain reflects culture, brain activation patterns can provide important information about the very characteristics of the cultures themselves that are compared.
Third and foremost, because of its focus on the quintessential biological organ, the brain, cultural neuroscience lends itself to a variety of issues and questions that are located at the interface between nature and nurture, biology and culture, and genes and memes. Ambady and Bharucha (2009) concur with this assessment by noting that this emerging field will ‘provide the exciting opportunity to examine the mutual interplay of culture and biology across multiple levels of analysis, from genes and brain to mind and behavior, across the life span (p. 345)’.
Recent work on epigenesis suggests that it is not genes alone, but it is intricate interactions between genetic potentials and environments that ultimately give concrete shapes to behavior. Epigenesis is a term in biology that implies development of an organism that unfolds through neuro-chemical mechanisms of cell differentiations. Given its biological origin it should not come as any surprise that epigenesis was long assumed to be under genetic control. Importantly, however, a number of recent studies (e.g. Suomi, 1999; Gunnar et al., 2001; Meaney and Szyf, 2005; Lee et al., 2006) have demonstrated how experience (which becomes patterned by culture in human societies) ‘gets under the skin’ during the developmental process to influence the brain and genetic expressions as well as behavior.
This is surely true in non-human primates. Suomi (2009) has found that one-fifth of the entire rhesus monkey genome is differentially methylated in both brain and blood cells as a function of early social experience. Similar gene−environment interactions are common in humans too. For example, Sheese and colleagues (2007) report that DRD4—a dopamine receptor gene that is implicated in attention-deficit hyperactivity disorder and other hyperactivity disorders—functions very differently depending on quality of parenting (see also Nikolaidis and Gray, in press). Long-allelic versions of the gene were associated with sensation seeking, high-intensity pleasure, and impulsivity only for the children who receive poor quality parenting. Analogous interactions between genes (and corresponding temperamental dispositions) and culture have received intensive research effort in the recent years (Aron et al., in press; Kim et al., in press; Nikolaidis and Gray, in press).
It would seem likely that various genetic polymorphisms that are unevenly distributed across cultures interact with local ecological environments (e.g. population density) and cultural practices (e.g. parenting and dominant social norms) to yield some of the cultural variations in psychological processes and underlying brain pathways (Chiao and Blizinsky, in press; Kim et al., in press; Way and Lieberman, in press). Thus, hidden ‘behind’ the cultural variations in mentality and associated brain pathways there might be an important set of mechanisms by which culture/ecology and genetics bi-directionally influence one another over time. Although much has yet to be learned, this hypothesis deserves serious scholarly attention.
This brings us back to the quote by Pinker at the very beginning of this article. In his quote, Pinker conceptualized the human mind as fundamentally bounded and independent of its external environment. Throughout this article, however, we have presented a case for strong formative influences culture can have on various component processes of the mind. Through investigating this general idea with cutting-edge methods, cultural neuroscience will have the potential of contributing a new view of person as biologically prepared and, yet, fully completed only through cultural participation.
To conclude, culture has become a frontier for neuroscience and, conversely, neuroscience has also become a frontier for cultural psychology. It makes a perfect place for intellectual challenges and aspirations if the two frontiers are brought together. The truth involved in this premise, we believe, has been abundantly demonstrated in this landmark special issue in Social, Cognitive, and Affective Neuroscience and its companion volume, both edited by Joan Chiao. They represent an important milestone for the field of cultural neuroscience. It marks the successful birth of the field. It seeks to draw out what the future agendas are for cultural neuroscientists and how researchers might approach them. We hope that this article provides some theoretical ideas and insights that will be useful in illuminating the winding roads toward the ultimate goal of understanding the cultural constitution of the human brain.
Acknowledgments
The writing of this paper was facilitated by a National Science Foundation grant (BCS 0717982) and a National Institute of Aging grant (RO1 AG029509-01). We thank Hannah Chua, Keiko Ishii, Ethan Kross, Jinkyung Na, Steve Tompson, Oscar Ybarra and Carolyn Yoon for their helpful comments on an earlier draft.
Footnotes
1The theoretical meaning of the Zhu et al. finding may deserve a careful analysis. This finding might suggest that the ways in which social relations are constructed vary across cultures. In particular, relations that emphasize ‘relational unity’ (supposedly more typical in cultures that emphasize familial unity) and relations that are grounded in unique intentions, preferences, and individual choices of participating parties (supposedly more typical in cultures that emphasize individual freedom and autonomy) may cause very different patterns of brain activation such that people committed to the unity view of relations show a merger of representation of mother with the self; whereas those who are committed to the choice-based view of relations may show a quite prominent activation of the self (vis-à-vis mother). The same point might prove important in understanding the nature of within-culture variations. Interdependent individuals in China, for example, may show an especially strong propensity toward the relational unity (as exemplified by the Zhu et al. finding). These individuals may then show what may be regarded as an interdependent pattern of brain activation in self-reference type tasks. How about within-culture variations in an independent culture? Here, more interdependent individuals might be more strongly committed to what we referred to above as the choice-based view of interpersonal relations. If so, more interdependent individuals in independent cultures might show what Zhu et al. would regard as a more independent pattern of brain activation in the self-reference type tasks. Preliminary findings (Ray et al., in press) appear consistent with this conjecture. There is no reason to believe that between-culture variation and within-culture variation are isomorphic (Na et al., 2010; Shweder, 1973). This issue requires more concerted research attention in the future.
2The moderation effect of culture on the rebound effect has been demonstrated in the domain of stereotype. Zhang and Hunt (2008) had participants write two stories about a stereotyped group. Half of the participants were instructed not to use the stereotype about the group in the first story (the suppression condition). The remaining half were not given this instruction. As would be predicted, and consistent with the Goldin et al., finding, European Americans showed increased stereotype use in the second story in the suppression condition (relative to the control condition). However, consistent with the Ohira et al., finding, Chinese exhibited no such effect.
REFERENCES
- Adams R.B., Jr, Flanklin R.G., Jr, Rule NO, et al. Culture, gaze and the neural processing of fear expressions. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.B., Jr., Rule NO, Franklin R.G., Jr, et al. Cross-cultural reading the mind in the eyes: An fMRI investigation. Journal of Cognitive Neuroscience. 2009;22(1):97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajzen I. From intentions to actions: a theory of planned behavior. In: Kuhi J, Beckmann J, editors. Action-control: From Cognition to Behavior. Heidelberg: Springer; 1985. pp. 11–39. [Google Scholar]
- Ambady N, Bharucha J. Culture and the brain. Current Directions in Psychological Science. 2009;18:342–345. [Google Scholar]
- Aron A, Ketay S, Hedden T, Aron EN, Markus HR, Gabrieli JDE. Temperament trait of sensory processing sensitivity moderates cultural differences in neural response. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch SE. Effects of group pressure upon the modification and distortion of judgment. In: Guetzkow H, editor. Groups, Leadership and Men. Pittsburgh, PA: Carnegie Press; 1951. [Google Scholar]
- Bruner J. Another look at New Look 1. American Psychologist. 1992;47:780–783. doi: 10.1037//0003-066x.47.6.780. [DOI] [PubMed] [Google Scholar]
- Chiao JY. Cultural neuroscience: Visualizing culture-gene influences on brain function. In: Decety J, Cacioppo J, editors. Handbook of Social Neuroscience. Oxford, UK: Oxford University Press; in press. [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: Parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of Cultural psychology. New York: Guilford Press; 2007. pp. 237–254. [Google Scholar]
- Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proceedings of the Royal Society B: Biological Sciences. in press doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, et al. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22(1):1–11. doi: 10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, et al. Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience. 2008;20:2167–2174. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- Choi I, Nisbett RE, Norenzayan A. Causal attribution across cultures: Variation and universality. Psychological Bulletin. 1999;125:47–63. [Google Scholar]
- Choudhury S. Culturing the adolescent brain: What can neuroscience learn from anthropology? Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Boland JE, Nisbett RE. Cultural variation in eye movements during scene perception. Proceedings of the National Academy of Sciences USA. 2005a;102:12629–12633. doi: 10.1073/pnas.0506162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua HF, Leu J, Nisbett RE. Culture and diverging views of social events. Personality and Social Psychology Bulletin. 2005b;31:925–934. doi: 10.1177/0146167204272166. [DOI] [PubMed] [Google Scholar]
- Cole M. Cultural Psychology: A Once and Future Discipline. Cambridge: MA: Cambridge University Press; 1996. [Google Scholar]
- Caporael LR. Repeated assembly. In: Schur S, Rauscher F, editors. Alternative Approaches to Evolutionary Psychology. Kluwer: 2003. pp. 71–90. [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Damasio AR. Looking for Spinoza: Joy, Sorrow, and the Feeling Brain. 2003 [Google Scholar]
- D'Andrade RG. The Development of Cognitive Anthropology. Cambridge: UK: Cambridge University Press; 1995. [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Towards an anatomical and functional model of number processing. Mathematical Cognition. 1995;1:83–120. [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284(5416):970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- DeVos G. Achievement orientation, social self-security, and Japanese economic growth. Asian Survey (alternative title Far Eastern Survey) 1965;5(12):575–589. [Google Scholar]
- DeVos G. Socialization for Achievement: Essays on the Cultural Psychology of the Japanese. Berkeley: University of California Press; 1973. [Google Scholar]
- Domínguez JF, Turner R, Lewis ED, Egan G. Neuroanthropology: a humanistic science for the study of the culture-brain nexus. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Kitayama S. Mnemonic context effect in two cultures: attention to memory representations? Cognitive Science. 2007;31:1009–1020. doi: 10.1080/03640210701703808. [DOI] [PubMed] [Google Scholar]
- Durkheim E. The Rules of Sociological Method. 1964. (Translated from the French by S.S. Solovay and J.H. Mueller edited by George E.G. Catlin). New York, London, the Free Press. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodeal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fiske ST. Cultural processes. In: Berntson G, Cacioppo JP, editors. Handbook of Neuroscience for the Behavioral Sciences. New York: Wiley; 2009. [Google Scholar]
- Fiske ST, Taylor SE. Social Cognition. 2nd. New York: McGraw-Hill; 1991. [Google Scholar]
- Ganis G, Kutas M. An electrophysiological study of scene effects on object identification. Brain Research: Cognitive Brain Research. 2003;16(2):123–144. doi: 10.1016/s0926-6410(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Gardner WL, Gabriel S, Lee AY. “I” value freedom, but “we” value relationships: Self-construal priming mirrors cultural differences in judgment. Psychological Science. 1999;10:321–326. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4(6):385–390. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development durting childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Malone PS. The correspondence bias. Psychological Bulletin. 1995;117:21–38. doi: 10.1037/0033-2909.117.1.21. [DOI] [PubMed] [Google Scholar]
- Gilbert DT, Pelham BW, Krull DS. On cognitive busyness: when person perceivers meet persons perceived. Journal of Personality and Social Psychology. 1988;54:733–740. [Google Scholar]
- Goh JO, Chee MW, Tan JC, et al. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cognitive, Affective and Behavioral Neuroscience. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto SG, Ando Y, Huang C, Yee A, Lewis RS. Cultural differences in the visual processing of meaning: Detecting incongruities between background and foreground objects using the N400. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield PM, Keller H, Fuligni A, Marnard A. Cultural pathway through universal development. Annual Review of Psychology. 2003;54:461–490. doi: 10.1146/annurev.psych.54.101601.145221. [DOI] [PubMed] [Google Scholar]
- Grossmann I, Kross E. The impact of culture on adaptive vs maladaptive self-reflection. Psychological Science. in press doi: 10.1177/0956797610376655. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Developmental Psychopathology. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Hedden T, Ketay S, Aron A, Gabrieli JDE. Neural differences in the processing of semantic relationships across cultures. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC. Cultural differences in neural function associated with object processing. Cognitive, Affective and Behavioral Neuroscience. 2006;6:102–109. doi: 10.3758/cabn.6.2.102. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Fessler DMT. Nobody's watching? Subtle cues affect generosity in an anonymous economic game. Evolution and Human Behavior. 2005;26(3):245–256. [Google Scholar]
- Han S, Gu X, Mao L, Ge J, Wang G, Ma Y. Neural substrates of self-referential processing in Chinese Buddhists. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Mao L, Gu X, Zhu Y, Ge J, Ma Y. Neural consequences of religious belief on self-referential processing. Social Neuroscience. 2008;3(1):1–15. doi: 10.1080/17470910701469681. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H. Neural correlates underlying mental calculation in abacus experts: functional magnetic resonance imaging study. Neuroimage. 2003;19:296–307. doi: 10.1016/s1053-8119(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Harris LT, Todorov A, Fiske ST. Attributions on the brain: Neuro-imaging dispositional inferences, beyond theory of mind. NeuroImage. 2005;28:763–769. doi: 10.1016/j.neuroimage.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Hatano G, Amaiwa S, Shimizu K. Formation of a mental abacus for computation and its use as a memory device for digits: a developmental study. Developmental Psychology. 1987;23(6):832–838. [Google Scholar]
- Hatano G, Osawa K. Digit memory of grand experts in abacus-derived mental calculation. Cognition. 1983;15:95–110. doi: 10.1016/0010-0277(83)90035-5. [DOI] [PubMed] [Google Scholar]
- Hedden T, Ketay S, Aron A, Markus HR, Gabrieli JDE. Cultural influences on neural substrates of attentional control. Psychological Science. 2008;19:12–17. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- Heine SJ. Cultural Psychology. New York: W.W. Norton; 2008. [Google Scholar]
- Hofstede G. Culture's Consequences: Comparing Values, Behaviors, Institutions, and Organizations Across Nations. 2nd. Thousand Oaks, CA: SAGE Publications; 2001. [Google Scholar]
- Hong YY, Morris MW, Chiu CY, Benet-Martinez V. Multicultural minds: A dynamic constructivist approach to culture and cognition. American Psychologist. 2000;55:709–720. doi: 10.1037//0003-066x.55.7.709. [DOI] [PubMed] [Google Scholar]
- Inglehart R, Baker WE. Modernization, cultural change, and the persistence of traditional values. American Sociological Review. 2000;65(1):19–51. [Google Scholar]
- Ishii K, Kobayashi Y, Kitayama S. Interdependence modulates the brain response to word-voice incongruity. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar SS, Lepper MR. Rethinking the role of choice: a cultural perspective on intrinsic motivation. Journal of Personality and Social Psychology. 1999;76:349–366. doi: 10.1037//0022-3514.76.3.349. [DOI] [PubMed] [Google Scholar]
- Jenkins LJ, Yang YJ, Goh J, Hong YY, Park DC. Cultural differences in the lateral occipital complex while viewing incongruent scenes. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI Study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H, Drolet A. Express your social self: Cultural differences in choice of brand-name versus generic products. Personality and Social Psychology Bulletin. 2009;35(12):1555–1566. doi: 10.1177/0146167209348641. [DOI] [PubMed] [Google Scholar]
- Kim H, Sherman D, Taylor S, et al. Culture, serotonin receptor polymorphism (5-HTR1A), and locus of attention. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimel S, Uchida Y, Kitayama S. Social exclusion is more painful in an interdependent (vs. independent) culture: A Japan-US comparison. Unpublished manuscript. University of Michigan; 2009. [Google Scholar]
- Kitayama S, Cohen D. Handbook of Cultural Psychology. New York: Guilford Press; 2007. [Google Scholar]
- Kitayama S, Conway L.G., III, Pietromonaco PR, Park H, Plaut VC. Ethos of independenceacross regions in the U.S.: the production-adoption model of cultural change. American Psychologist. in press doi: 10.1037/a0020277. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Duffy S, Kawamura T, Larsen JT. A cultural look at New Look: Perceiving an object and its context in two cultures. Psychological Science. 2003;14:201–206. doi: 10.1111/1467-9280.02432. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Duffy S, Uchida YK. Self as cultural mode of being. In: Kitayama S, Cohen D, editors. The Handbook of Cultural Psychology. New York: Guilford Press; 2006. [Google Scholar]
- Kitayama S, Imada T. Implicit independence and interdependence: a cultural task analysis. In: Feldman Barrett L, Smith E, Mesquita B, editors. The Mind in Context. New York: Guilford Press; 2010. pp. 174–200. [Google Scholar]
- Kitayama S, Markus H, Tummala P, Kurokawa M, Kato K. Culture and self-cognition. 1990. Unpublished manuscript. [Google Scholar]
- Kitayama S, Mesquita B, Karasawa M. The emotional basis of independent and interdependent selves: Socially disengaging and engaging emotions in the US and Japan. Journal of Personality and Social Psychology. 2006;91:890–903. doi: 10.1037/0022-3514.91.5.890. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Park H. Cultural shaping of emotion and well-being: How does it work? Social and Personality Psychology Compass. 2007;1:202–222. [Google Scholar]
- Kitayama S, Park H, Sevincer AT, Karasawa M, Uskul AK. A cultural task analysis of implicit independence: comparing North America, Western Europe, and East Asia. Journal of Personality and Social Psychology. 2009;97:236–255. doi: 10.1037/a0015999. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Snibbe AC, Markus HR, Suzuki T. Is there any ‘‘free’’ choice? Self and dissonance in two cultures. Psychological Science. 2004;15(8):527–533. doi: 10.1111/j.0956-7976.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Tompson S. Envisioning the future of cultural neuroscience. Asian Journal of Social Psychology. 2010;13:92–101. doi: 10.1111/j.1467-839X.2010.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S, Uskul A. Culture, mind, and the brain: Current evidence and future directions. Annual Review in Psychology. in press doi: 10.1146/annurev-psych-120709-145357. [DOI] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E. Cultural and linguistic effects on neural bases of ‘‘theory of mind’’ in American and Japanese children. Brain Research: Cognitive Brain Research. 2007;1164:95–107. doi: 10.1016/j.brainres.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeber AL, Kluckhohn CK. Culture: A Critical Review of Concepts and Definitions. New York: Random House; 1952. [Google Scholar]
- Kuhnen U, Hannover B, Schubert B. The semantic-procedural interface model of the self: the role of self-knowledge for context-dependent versus context-independent mode of thinking. Journal of Personality and Social Psychology. 2001;38:397–407. doi: 10.1037/0022-3514.80.3.397. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Cohen D. The soft embodiment of culture: Camera angles and motion through time and space. Psychological Science. 2007;18(9):824–830. doi: 10.1111/j.1467-9280.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Heider K, Friesen WV. Emotion and autonomis nervous system activity in the Minangkabau of West Sumatra. Journal of Personality and Social Psychology. 1992;53:898–908. doi: 10.1037//0022-3514.62.6.972. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Goto SS, Kong LL. Culture and context: East Asian and European American differences in P3 event-related potentials and self-construal. Personality and Social Psychology Bulletin. 2008;34:623–634. doi: 10.1177/0146167207313731. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 5th. New York, NY: McGraw-Hill; 2010. [Google Scholar]
- Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an fMRI study. Journal of Personality and Social Psychology. 2004;87:421–435. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- Losin EAR, Dapretto M, Iacoboni M. Culture and neuroscience: additive or synergistic? Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams D. The Redemptive self: Stories Americans Live By. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Firth CD, O'Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafouris L. The Brain-Artefact Interface (BAI): a challenge for archaeology and cultural neuroscience. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological Review. 1991;98:224–253. [Google Scholar]
- Markus HR, Kitayama S. The cultural construction of self and emotion: Implications for social behavior. In: Kitayama S, Markus HR, editors. Emotion and Culture: Empirical Studies of Mutual Influence. Washington, DC: American Psychological Association; 1994. pp. 98–130. [Google Scholar]
- Mason MF, Morris MW. Culture, attribution and automaticity: a social cognitive neuroscience view. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Nisbett RE. Attending holistically vs. analytically: comparing the context sensitivity of Japanese and Americans. Journal of Personality and Social Psychology. 2001;81:922–934. doi: 10.1037//0022-3514.81.5.922. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Yoo SH, Nakagawa S, et al. Culture, emotion regulation, and adjustment. Journal of Personality and Social Psychology. 2008;94(6):925–937. doi: 10.1037/0022-3514.94.6.925. [DOI] [PubMed] [Google Scholar]
- Meaney MK, Syzf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neuroscience. 2005;28:456–462. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mellet E, Petit L, Mazoyer B, Denis M, Tzourio N. Reopening the mental imagery debate: lessons from functional anatomy. Neuro-Image. 1998;8:129–139. doi: 10.1006/nimg.1998.0355. [DOI] [PubMed] [Google Scholar]
- Miller JG. Culture and the development of everyday social explanation. Journal of Personality and Social Psychology. 1984;46:961–978. doi: 10.1037//0022-3514.46.5.961. [DOI] [PubMed] [Google Scholar]
- Minoura Y. A sensitive period for the incorporation of a cultural meaning system: a study of Japanese children growing up in the United States. Ethos. 1992;20:304–339. [Google Scholar]
- Miyamoto Y, Nisbett RE, Masuda T. Culture and the physical environment: holistic versus analytic perceptual affordances. Psychological Science. 2006;17:113–119. doi: 10.1111/j.1467-9280.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- Mok A, Morris MW. Cultural chameleons and iconoclasts: Assimilation and reactance to cultural cues in biculturals' personalities as a function of identity conflict. Journal of Experimental Social Psychology. 2009;45:884–889. [Google Scholar]
- Morris MW, Peng K. Culture and cause: American and Chinese attributions for social and physical events. Journal of Personality and Social Psychology. 1994;67:949–971. [Google Scholar]
- Moriguchi Y, Ohnishi T, Kawachi T, et al. Specific brain activation in Japanese and Caucasian people to fearful faces. Neuroreport. 2005;16:133–136. doi: 10.1097/00001756-200502080-00012. [DOI] [PubMed] [Google Scholar]
- Na J, Grossmann I, Varnum MEW, Kitayama S, Gonzalez R, Nisbett RE. Cultural differences are not always reducible to individual differences. Proceedings of the National Academy of Sciences USA. 2010;107(14):6192–6197. doi: 10.1073/pnas.1001911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Kitayama S. Cultural differences in spontaneous trait inference. Paper presented at the annual meeting of the Society for Personality and Social Psychology. 2009 [Google Scholar]
- Neisser U. Cognitive Psychology. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Nikolaidis A, Gray JR. ADHD and the DRD4 exon III 7-repeat polymorphism: an international meta-analysis. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Cohen D. Men, honor and murder. Scientific American Presents. 1996;10:16–19. [Google Scholar]
- Nisbett RE, Peng K, Choi I, Norenzayan A. Culture and systems of thought: holistic vs. analytic cognition. Psychological Review. 2001;108:291–310. doi: 10.1037/0033-295x.108.2.291. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Oyserman D, Coon HM, Kemmelmeier M. Rethinking individualism and collectivism: Evaluation of theoretical assumptions and meta-analyses. Psychological Bulletin. 2002;128:3–72. [PubMed] [Google Scholar]
- Oyserman D, Lee SWS. Does ulture influence what and how we think? Effects of priming individualism and collectivism. Psychological Bulletin. 2008;134:311–342. doi: 10.1037/0033-2909.134.2.311. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;40:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. The cognitive neuroscience of aging and culture. Current Directions in Psychological Science. 2006;15(3):105–108. [Google Scholar]
- Park J, Gehring WJ, Kitayama S. 2009, November. Cultural difference in the effects of “watching eyes” on the error-related negativity (ERN). Paper presented at the joint workshop between the University of Michigan and Kwansei Gakuin University. [Google Scholar]
- Pinker S. The Blank Slate: The Modern Denial of Human Nature. New York: Viking; London: Penguin: 2002. [Google Scholar]
- Posner MI. Foundations of Cognitive Science. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, et al. Interdependent self-construal and neural representations of the self and mother. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon M, Ishii K, Watabe M, Kitayama S. Minimal social cues in the dictator game. Journal of Economic Psychology. 2009;30(3):358–367. [Google Scholar]
- Ross L. The intuitive psychologist and his shortcomings: distortions in the attribution process. In: Berkowitz L, editor. Advances in Experimental Social Psychology. vol. 10. New York: Academic Press; 1977. pp. 173–220. [Google Scholar]
- Rule N, Freeman J, Moran J, Gabrieli J, Adams R, Ambady N. Voting behavior is reflected in amygdala response across cultures. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-regulation and the problem of human autonomy: Does psychology need choice, self-determiniation, and will? Journal of Personality. 2006;74:1557–1586. doi: 10.1111/j.1467-6494.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. In: Driver J, Haggard P, Shallice T, editors. Mental Processes in the Human Brain. Oxford: Oxford University Press; 2008. pp. 27–47. [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. The Prospective Brain: Remembering the Past to Imagine the Future. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Dziobek E, Rotte M. Combining a semantic differential with fMRI to investigate brands as cultural symbols. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JM. The Mind and the Brain: Neuroplasticity and the Power of Mental Force. New York, NY: Harper Collins; 2002. [Google Scholar]
- Schwartz SH. Universals in the content and structure of values: Theoretical advances and empirical tests in 20 countries. In: Zanna M, editor. Advances in Experimental Social Psychology. San Diego: Academic Press; 1992. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman R, Brown RA. Anthropology meets neuroscience: A transdisciplinary approach to culture, brain, and embodiment. Social Cognitive and Affective Neuroscience. in press [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Shore B. Culture in Mind: Cognition, Culture and the Problem of Meaning. New York: Oxford University Press; 1996. [Google Scholar]
- Shweder RA. The between and within of cross-cultural research. Ethos. 1973;1:531–545. [Google Scholar]
- Shweder RA. Thinking Through Cultures: Expeditions in Cultural Psychology. Cambridge: Harvard University Press; 1991. [Google Scholar]
- Shweder RA. Anti-postculturalism (or, the view from manywheres) In: Shweder RA, editor. Why Do Men Barbecue?: Recipes for Cultural Psychology. Cambridge, MA: Harvard University Press; 2003. [Google Scholar]
- Singelis TM. The measurement of independent and interdependent self-construals. Journal of Personality and Social Psychology. 1994;20:580–591. [Google Scholar]
- Squire LR, Schacter DL. The Neuropsychology of Memory. Guilford Press; 2002. [Google Scholar]
- Stigler J. ‘‘Mental abacus’’: the effect of abacus training on Chinese children's mental calculation. Cognitive Psychology. 1984;16:145–176. [Google Scholar]
- Sui J, Han S. Self-construal priming modulates neural substrates of self-awareness. Psychological Science. 2007;18:861–866. doi: 10.1111/j.1467-9280.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- Sui J, Liu CH, Han S. Cultural difference in neural mechanisms of self-recognition. Social Neuroscience. 2009;4(5):402–411. doi: 10.1080/17470910802674825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Developmental trajectories, early experiences, and community consequences: lessons from studies with rhesus monkeys. In: Keating D, Hertzman C, editors. Developmental Health and the Wealth of Nations. New York: Guilford Press; 1999. pp. 185–200. [Google Scholar]
- Suomi SJ. How gene-environment interactions shape biobehavioural development: Lessons from studies with rhesus monkeys. In: Tremblay RE, van Aken MAG, Koops W, editors. Development and Prevention of Behaviour Problems: From Genes to Social Policy. New York, NY: Psychology Press; 2009. pp. 7–23. [Google Scholar]
- Tang Y, Zhang K, Chen S, et al. Arithmetic processing in the brain shaped by cultures. Proceedings of the National Academy of Sciences, USA. 2006;103:10775–10780. doi: 10.1073/pnas.0604416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The Cultural Origins of Human Cognition. Harvard University Press; 1999. [Google Scholar]
- Triandis HC. The self and social behavior in differing cultural contexts. Psychological Review. 1989;96:269–289. [Google Scholar]
- Triandis HC, Bontempo R, Leung K, Hui CH. A method for determining cultural, demographic, and personal constructs. Journal of Cross-Cultural Psychology. 1990;21:302–318. [Google Scholar]
- Triandis HC. Boulder, CO: Westview Press; 1995. Individualism and collectivism. [Google Scholar]
- Tversky A. Features of similarity. Psychological Review. 1977;84:327–352. [Google Scholar]
- Uchida Y, Kitayama S. Happiness and unhappiness in east and west: Themes and variations. Emotion. 2009;9(4):441–456. doi: 10.1037/a0015634. [DOI] [PubMed] [Google Scholar]
- Way BM, Lieberman MD. Is there a genetic contribution to cultural differences? Collectivism, individualism, and genetic markers of social sensitivity. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner DM, Erber R, Zanakos S. Ironic processes in the mental control of mood and mood-related thought. Journal of Personality and Social Psychology. 1993;65:1093–1104. doi: 10.1037//0022-3514.65.6.1093. [DOI] [PubMed] [Google Scholar]
- Williams KD, Jarvis B. Cyberball: A program for use in research on ostracism and interpersonal acceptance. Behavior Research Methods, Instruments, and Computers. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- Wilson M. The re-tooled mind: how culture re-engineers cognition. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nsp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. Six views of embodied cognition. Psychonomic Bulleting & Review. 2002;9(4):625–636. doi: 10.3758/bf03196322. [DOI] [PubMed] [Google Scholar]
- Wu S, Keysar B. The effect of culture on perspective taking. Psychological Science. 2007;18(7):600–606. doi: 10.1111/j.1467-9280.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- Wyer RSJ, Srull TK. Handbook of social cognition. Hillsdale, NJ: Erlbaum; 1994. [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience. 2009;29(26):8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AB, Yang KS. The nature of achievement motivation in collectivist societies. In: Kim U, Triandis HC, Kagitcibasi C, et al., editors. Individualism and Collectivism: Theory, Method, and Applications. Thousand Oaks, CA: Sage; 1994. pp. 239–250. [Google Scholar]
- Zhang S, Hunt JS. The stereotype rebound effect: Universal or culturally bounded process? Journal of Experimental Social Psychology. 2008;44:489–500. [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self representation. Neuroimage. 2007;34:1310–1317. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Zou X, Tam KP, Morris MW, Lee SL, Lau IYM, Chiu CY. Culture as common sense: Perceived consensus versus personal beliefs as mechanisms of cultural influence. Journal of Personality and Social Psychology. 2009;97(4):579–597. doi: 10.1037/a0016399. [DOI] [PubMed] [Google Scholar]