Abstract
Voting to determine one’s leaders is among the most important decisions we make, yet little is known about the brain’s role in how we come to these decisions. Behavioral studies have indicated that snap judgments of political candidates’ faces can predict election outcomes but that the traits that lead to these judgments differ across cultures. Here we sought to investigate the neural basis for these judgments. American and Japanese natives performed simulated voting judgments of actual American and Japanese political candidates while neural activity was measured using functional magnetic resonance imaging (fMRI). Candidates for whom participants chose to vote elicited stronger responses in the bilateral amygdala than candidates for whom participants chose not to vote. This was true regardless of either the participant’s culture or the target’s culture, suggesting that these voting decisions provoked the same neural response cross-culturally. In addition, we observed a participant culture by target culture interaction in the bilateral amygdala. American and Japanese participants both showed a stronger response to cultural outgroup faces than they did to cultural ingroup faces, however this was unrelated to their voting decisions. These data provide insight to the mechanisms that underlie our snap judgments of others when making voting decisions and provide a neural correlate to cross-cultural consensus in social inferences.
Keywords: culture, nonverbal behavior, face perception, politics, amygdala
INTRODUCTION
Voting is one of the most important decisions that individuals can make in a democratic society. Despite the weighty consequences that result from an electoral choice, research has shown that electoral outcomes can be predicted based on individuals’ snap judgments of politicians’ faces (Todorov et al., 2005; Chiao et al., 2008a). One study found that judgments of competence based on seeing American political candidates’ faces for only 100 milliseconds accurately predicted 59.6% of the actual electoral outcomes (Ballew & Todorov, 2007).
Culture may play an important role in determining for whom individuals choose to vote. Although much previous work has shown evidence for cultural universality in the perceptions of individuals for some personality and physiognomic traits (Zebrowitz et al., 1993; Albright et al., 1997), there is also research showing that culture has a substantial impact on the traits that people value in their leaders (Misumi & Peterson, 1985). For instance, leaders in Japan versus the USA are believed to vary markedly in character (e.g., Ayman, 1993; S. N. Kaplan, 1994; Yamagishi et al., 1998). Yet the majority of studies examining the relationship between trait inferences and electoral outcomes have examined Western cultures. Todorov and colleagues (Todorov et al., 2005; Ballew & Todorov, 2007) investigated Americans’ judgments of American candidates, Martin (1978) examined Australians’ judgments of Australian candidates, Poutvaara et al. (2009) conducted an online survey of mostly Western respondents’ judgments of Finnish candidates, and Antonakis and Dalgas (2009) studied Swiss children’s and adults’ judgments of French candidates. One recent study (Rule et al., in press), however, examined American and Japanese participants’ judgments of American and Japanese political candidates. This study showed that although both American and Japanese perceivers agreed across cultures in their judgments of individual candidates’ faces, they chose to elect different candidates. Specifically, traits related to power (dominance and facial maturity) were related to American participants’ electoral choices whereas traits related to warmth (likeability and trustworthiness) were related to Japanese participants’ electoral choices. The current study was therefore interested in exploring the basis for this cross-cultural convergence in perception but divergence in electoral choice. To do so, we measured American and Japanese perceivers’ brain activity while making electoral choices using fMRI.
One brain area believed to be critically involved in the processing of social information about others is the amygdala. Lesion studies have shown that patients with amygdala damage are severely impaired in their ability to evaluate others. For instance, individuals with bilateral amygdala damage are significantly more likely to perceive untrustworthy faces as trustworthy and to believe that these individuals are approachable. Additionally, patients with amygdala damage are impaired in reading others’ emotions and inferring their thoughts from nonverbal cues (Adolphs et al., 2002). Functional magnetic resonance imaging (fMRI) studies of healthy participants have shown that the amygdala is involved not only in evaluations of trustworthiness from faces (Winston et al., 2002) but also in the evaluation of facial attractiveness (Winston et al., 2007) and a host of other social traits (e.g. responsibility, intelligence, and competence, among others; Todorov & Engell, 2008). In addition, the amygdala has been implicated in the evaluative processing of social information (see Adolphs, 2003; Phelps, 2006; Schiller et al., 2009) and is sensitive to a stimulus’s motivational importance (Phelps & LeDoux, 2005; Cunnigham et al., 2008) or the degree to which it is arousing (Anderson et al., 2003; Sander et al., 2003). The amygdala therefore seemed like a probable structure to be involved in voting judgments and was the focus of the current investigation.
Moreover, previous work in cultural neuroscience has implicated the amygdala in social judgments from faces. Chiao et al. (2008b) found that American and Japanese perceivers showed significant amygdala responses compared to baseline when evaluating emotional fear faces from both cultures. Critically, however, the participants showed greater amygdala responses to same-culture versus other-culture faces that were expressing fear but statistically equivalent responses to both same-culture and other-culture faces that were angry, happy, or neutral. These data show evidence of cultural tuning, whereby the evolutionary importance of accurately detecting fear leads to a greater response to these expressions among similar versus dissimilar others, perhaps because threats to the ingroup may be more relevant to threats to the self than to the outgroup.
These findings differ slightly, however, from previous intergroup work which has reported that the amygdala often responds more strongly to outgroup versus ingroup members. One early study showed that both Black and White Americans exhibited a greater bilateral amygdala response to faces of the other race than to faces of their own race (Hart et al., 2000). A more recent study extended this work by showing that the faces of learned outgroup members elicited a stronger amygdala response regardless of the targets’ races (Van Bavel et al., 2008). Interestingly, Chiao et al. (2008b) did not observe an overall outgroup effect for culture but showed an opposite ingroup effect (though only during perceptions of fear faces). Thus, rather than finding an increase in amygdala response, potentially because of a negative bias towards outgroup members (e.g. Phelps et al., 2001), Chiao et al. (2008b) found an increase related to a positive bias that may have evolved to help ingroup members. Given that these studies differ not only in terms of the type of group studied but also in the nature of their tasks, we were curious to see whether we might also observe a group-based difference in amygdala response to the Japanese and American faces during voting judgments.
Several recent studies have examined neural responses to political stimuli, yet each has explored a different question and not all are relevant to the context of electoral choice (Mitchell et al., 2005; Westen et al., 2006; Kaplan et al., 2007; Amodio et al., 2008; Spezio et al., 2008). In one very relevant study, however, Knutson et al. (2006) observed greater activation in the amygdala in response to the faces of politicians with whom the participants held congruent political beliefs (e.g. Democrats responding to Democrat politicians’ faces) as opposed to those politicians for whom the participants’ political beliefs were incongruent (e.g. Democrats responding to Republican politicians’ faces). In essence, the amygdala responded more to the faces of individuals who the perceivers were likely to politically support versus those who they were likely to not support. Thus, we would expect to see a greater amygdala response to American participants’ choice of winners (consistent with Knutson et al., 2006). It is not clear whether the same pattern will hold for Japanese participants or for judgments of outgroup political candidates, however.
Thus, to examine both (i) the processes involved in choosing candidates and (ii) the role of culture in voting behavior, the current work investigated the neural substrates relating to American and Japanese individuals’ electoral choices employing targets from both cultures. Participants from both cultures were shown a series of American and Japanese politicians’ faces and asked to indicate whether they would vote for each person while being scanned with fMRI. The study therefore had a 2 (participant culture) × 2 (target culture) × 2 (voted for/not voted for) design. Given previous work that has implicated the amygdala in impressions of others’ social traits from the face (e.g. Winston et al., 2002) and its role in processing faces of politicians (Knutson et al., 2006), we selected the left and right amygdalae as a priori regions of interest (ROIs) in voting decisions.
METHODS
Participants
Thirty-two American (n = 16; eight females) and Japanese (n = 16; nine females) university students provided informed consent in a manner approved by the Massachusetts Institute of Technology’s Committee On the Use of Humans as Experimental Subjects and the Tufts University Institutional Review Board, and participated in exchange for monetary compensation. One American participant was removed from analysis because of excessive movement and three participants (two Japanese, one American) were removed because they identified one or more same-culture targets. The final sample therefore consisted of 28 participants, half of whom were Japanese.
All participants were screened for neurological history, MRI compatibility, handedness, and use of psychoactive medications. Participants were recruited via online message-board postings or paper advertisements. All of the Japanese participants were students at a Japanese university who were in the USA participating in a language study-abroad program at Tufts University. These participants were screened for their exposure to US culture, including any previous time spent visiting the USA. None had spent more than a month in the USA, including the time between their arrival for the foreign study program and the date of scanning.
Stimuli
Photos of 58 (29 election winners, 29 election losers) candidates from the 2004 and 2006 American Senate elections and photos of 58 (29 winners, 29 losers) candidates from the 2000 election of the Japanese Diet were downloaded from databases on internet websites; all targets were male. Each image was cropped to show only the candidate’s face, converted to grayscale, and standardized for size and image resolution. Pre-testing showed that the targets did not differ in affect or attractiveness according to culture [F 's < 0.86, p’s > 0.52] or electoral success [F 's < 1.11, p’s > 0.48]; nor were there interactive effects between culture and electoral success [F 's < 2.16, p’s > 0.14].
Procedure
Participants were instructed in their native language. Native Japanese research assistants translated and back-translated the instructions and other materials for the experiment. Participants were instructed that they would be seeing a series of faces for 2 s and that they should indicate via button-press whether they would or would not vote for each person (participants’ average number of vote decisions = 50.1%, SD = 17.6%; Cronbach’s α = 0.79). Participants were informed that the face would remain on the screen for the entire 2 s regardless of when they made their response (thereby controlling for stimulus duration and looking time) but that they must make their judgment while each face was present, as responses made after the 2 s presentation had elapsed would not be recorded (2% of all trials). Participants were given several practice trials to ensure that they understood the task and to acquaint them with the timing of the stimulus presentation before the scan began. Response times occurred on an average of 1,076 (SD = 267) milliseconds after stimulus onset and did not differ by participant culture, target culture, electoral success, or participant response: F 's < 1.79, p’s > 0.19.
The faces were presented in random order and the order of targets versus null trials of fixation (i.e. the presentation of a fixation cross) was pseudo-randomized into 3 orders using the Counter script designed for Matlab, which spaces each condition’s trials so as to optimize the efficiency of blood-oxygenation-level-dependent (BOLD) signal estimation (Dale and Buckner, 1997). Participants were presented with equal numbers of American faces, Japanese faces, and fixation trials. Null (fixation) trials were not modeled so as to serve as a baseline.
fMRI acquisition
Data were acquired using a Siemens Magnetom Tim Trio 3-T scanner located at the Athinoula A. Martinos Brain Imaging Center at the Massachusetts Institute of Technology in Cambridge, MA. Anatomical images were acquired using a T1-weighted protocol (256 × 256 matrix, 192 1.33-mm sagittal slices). Functional images consisted of 32 (5 mm thick with 1 mm slice gap) oblique-axial slices, parallel to the AC-PC line and acquired in interleaved order (voxel dimensions: 3.125 mm2). Single-shot gradient echo EPI imaging was used with a TR of 2 seconds, TE of 30 ms, and flip angle of 90°. Data were collected in a single run consisting of 174 volumes (58 American faces, 58 Japanese faces, and 58 null fixation trials).
Functional data were analyzed in an event-related design using BrainVoyagerQX. Preprocessing of the data consisted of 3D motion correction, slice scan time correction (sinc interpolation), spatial smoothing using a 3D Gaussian filter (7-mm FWHM), and voxelwise linear detrending and high-pass filtering of frequencies (above three cycles per time course). Structural and functional data for each participant were transformed to standard Talairach stereotaxic space (Talairach and Tournoux, 1988) and coregistered both mathematically and manually to assure precise matching between the functional and structural images.
fMRI analysis
Each participant’s BOLD signals were modeled in an event-related design using two separate design matrices, where individual predictors were modeled as boxcar functions convolved with a two-gamma hemodynamic response function. First-level general linear model (GLM) analyses conducted on individual participants’ fMRI signal were submitted to a second-level random effects analysis, treating participants as a random factor.
The first design matrix contained the predictors based on participants’ actual subjective voting behavior: Japanese-voted, Japanese-not voted, American-voted and American-not voted. The second design matrix contained predictors based on objective election outcomes of target faces: Japanese winner, Japanese loser, American winner, and American loser.
Given our a priori hypothesis of amygdala involvement, we extracted parameter estimates (beta values) of BOLD signal from anatomical ROIs of the left and right amygdalae, based on a hypothesis-blind research assistant’s drawings of each participant’s individual anatomy; tracing criteria were based on standard neuroanatomical definitions of the amygdala’s boundaries. Once extracted, these mean signal values were subjected to statistical analysis using SPSS.
RESULTS
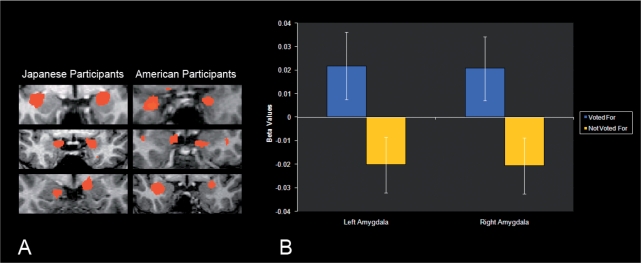
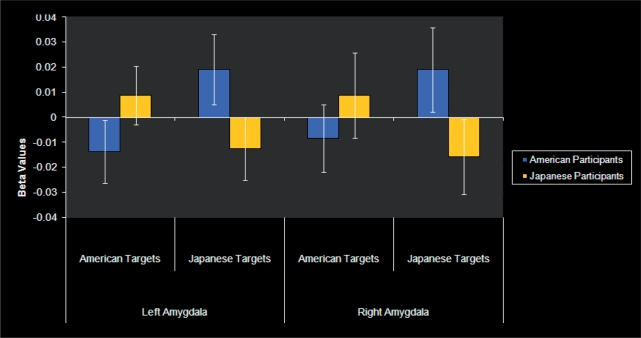
A 2 (participant culture: American, Japanese) × 2 (target culture: American, Japanese) × 2 (outcome: voted, not voted) × 2 (amygdala: left, right) ANOVA (repeated measures on all but the first factor) was conducted on the beta values extracted from each participant’s anatomically defined bilateral amygdalae. This analysis showed a main effect of outcome and the direction of the means showed significantly more amygdala response for the targets for whom participants voted as compared to the targets for whom the participants did not vote: F(1, 26) = 4.81, p = 0.037, r = 0.40; see Figure 1. In addition, the analysis showed a significant participant culture × target culture interaction: F(1, 26) = 5.86, p = 0.025, r = 0.43; see Figure 2. Examination of the means indicated that individuals showed greater bilateral amygdala response to cultural outgroup faces than they did to cultural ingroup faces. No other effects were significant, all F’s < 1.63, all p’s > 0.21.
Fig. 1.
Contrast of activation of targets for whom participants chose to vote over targets for whom participants chose not to vote in the left and right amygdala. Panel A shows activations in individual participants, representative of both cultural groups (displayed in neurological convention). Panel B shows mean extracted beta values from participant-specific ROIs of the left and right amgydala; error bars represent the standard errors of the means.
Fig. 2.
Mean beta values showing the participant culture by target culture interaction, plotted separately for the left and right amygdalae. Participants showed a greater response to opposite-culture, outgroup targets.
We conducted a second ANOVA to compare targets’ actual electoral success, as opposed to participants’ choices about for whom to vote. The results of this 2 (participant culture: American, Japanese) × 2 (target culture: American, Japanese) × 2 (outcome: elected, not elected) × 2 (amygdala: left, right) ANOVA with repeated measures on all but the first factor showed no effects for outcome but repeated the participant culture × target culture interaction [F(1, 26) = 4.72, p = 0.039, r = .39]. No other effects were significant, all F 's < 2.74, all p’s > 0.11. Participants’ voting choices were uncorrelated with the outcomes of the actual elections, regardless of participant or target culture: all r’s < |0.24|, all p’s > 0.07. However, there was high cross-cultural agreement between American and Japanese participants’ average voting decisions (r = 0.58, p < 0.001) for both American (r = 0.40, p < 0.001) and Japanese (r = 0.79, p < 0.001) targets.
We also examined these effects at the whole-brain level (voxelwise p < 0.001; minimum cluster size > five functional voxels) for all of the 2 (participant culture: American, Japanese) × 2 (target culture: American, Japanese) × 2 (outcome: voted, not voted; or outcome: elected, not elected) main effects and interactions. Only two contrasts revealed significant clusters of activation at this threshold. Comparisons of election winners and election losers showed clusters of significantly greater activation to losers over winners in the right (Talairach coordinates: 17, −52, −38; 1019 mm3) and left (Talairach coordinates: −8, −54, −38; 148 mm3) tonsils of the cerebellum and comparisons of Japanese over American targets revealed a significant cluster of activation in the right inferior parietal lobule (Talairach coordinates: 43, −38, 41; BA 40; 224 mm3) and right superior frontal gyrus (Talairach coordinates: 26, 23, 49; BA 8; 225 mm3). However, it should be acknowledged that signal loss due to imaging artifacts might have prevented the observation of additional effects, particularly in areas such as the orbitofrontal cortex.
We also conducted an exploratory whole-brain analysis of our primary contrasts of interest (voted versus not-voted targets and the target × participant interaction) at a more liberal threshold: voxelwise p < 0.005, minimum cluster size > five functional voxels. These contrasts showed significant clusters of activation for voted over not-voted targets in the right (Talairach Coordinates: 22, −2, −23; 192 mm3) and left (Talairach Coordinates: −22, −6, −20; 275 mm3) amygdalae, as well as in the left superior temporal gyrus (Talairach Coordinates: −41, 2, −15; BA 38; 449 mm3) and right inferior frontal gyrus (Talairach Coordinates: −29, 10, −15; BA 47; 409 mm3). The target culture × participant culture interaction showed two clusters of significant activation: one in the left superior frontal gyrus (Talairach Coordinates: −19, 39, 36; BA 9; 175 mm3) and a second in the left culmen of the cerebellum (Talairach Coordinates: −9, −34, −17; 293 mm3). Mean extracted beta values from these clusters showed an outgroup effect, similar to what we observed in the amygdala ROI analyses above. Specifically, Japanese participants showed a greater response to outgroup, American targets and American participants showed a greater response to outgroup, Japanese targets in both the cerebellar culmen and the superior frontal gyrus.
DISCUSSION
The bilateral amygdala was significantly more responsive to the faces of politicians for whom participants chose to vote versus those for whom they chose not to vote. Importantly, this effect occurred independent of both the participants’ and the targets’ cultural group membership, showing evidence of cross-cultural universality. Yet we also found evidence for cultural specificity that was unrelated to voting choice in the form of a target culture by participant culture interaction in which participants showed a greater response to other-culture targets than they did to same-culture targets.
Previous work has shown that individuals agree in their perceptions of traits from faces across cultures (Zebrowitz et al., 1993; Albright et al., 1997). The current data parallel those findings. American and Japanese participants’ behavioral responses showed significant cross-cultural agreement. Moreover, no main effect was observed between American and Japanese participants’ neural responses, neither in the amygdala ROI nor in the whole-brain analysis. Thus, participants from both cultures showed high agreement in their voting judgments and in their neural responses to the candidates from both cultures.
Importantly, this does not mean that perceivers from both cultures were considering the same traits when making their judgments. Previous work has observed that American and Japanese voters value different traits in their leaders (e.g. Misumi and Peterson, 1985), the former favoring traits related to power and the latter favoring traits related to warmth (Rule et al., in press). One goal of the current study was to seek evidence for this dissociation. For instance, previous work has suggested that the amygdala may not encode specific social traits from faces but, instead, may be evaluating the overall valence of the face, with lower valence faces being associated with a greater amygdala response (Todorov and Engell, 2008). In such a case, we might have expected that Japanese perceivers would show a lower amygdala response for the candidates for whom they chose to vote and that American perceivers would show a greater amygdala response for the candidates for whom they chose to vote. Rather, we observed that both groups of perceivers showed the latter effect. This could be because the amygdala simply serves as the perceptual or evaluative tool by which the perceivers form their impressions, not a reflection of the outcome of their electoral decision. Thus, although the perceivers might have been valuing different traits, this evaluation may be reflected in the amygdala response, rather than its outcome.
Given that the amygdala is important for social evaluation (e.g. Winston et al., 2002; Schiller et al., 2009), it is not surprising that it was found to be involved in participants’ voting choices. Other studies have suggested that the amygdala is involved in processing the relevance of stimuli (see Sander et al., 2003 for a review). Similarly, the amygdala is known to respond more to stimuli that are more arousing (Anderson et al., 2003) and to social information that is subjectively valued (Schiller et al., 2009). Thus, the amygdala response observed in the current work might well reflect participants’ inclination to vote for candidates whom they found to be more salient or arousing. Indeed, related work examining amygdala response to politicians found that participants expressed a greater amygdala response to the faces of candidates who agreed with their political ideology over the faces of candidates who did not agree with their political ideology (Knutson et al., 2006). Those authors interpreted their effects as possibly reflecting a relevance-based response whereby salient stimuli provoked a greater response in the amygdala. Indeed, this may serve to explain the basis for individuals’ preferences for candidates based on perceptions of their faces in the current study, as well.
Although participants from both cultures showed a similar amygdala response to the candidates for whom they chose to vote versus those for whom they chose not to vote, they showed a different overall response according to the target’s culture, independent of their electoral choice. The results of the participant culture by target culture interaction presented evidence for an outgroup effect such that participants had a stronger amygdala response to opposite-culture faces than they did to same-culture faces. This effect is consistent with a host of previous studies that have found increased amygdala activation to the faces of outgroup versus ingroup members. Hart et al. (2000), for example, found that Black and White individuals showed a stronger bilateral amygdala response to racial outgroup (White and Black, respectively) faces (see also Cunningham et al., 2004; Richeson et al., 2008). Similarly, Fischer et al. (2004) found that the left amygdala responded more when men viewed outgroup (female) faces than ingroup (male) faces. One explanation offered for these outgroup effects is that the amygdala is highly responsive to novel stimuli, such as the faces of outgroup members (Dubois et al., 1999; Phelps et al., 2001; Van Bavel et al., 2008). This may be particularly applicable to the current study, in which the outgroup faces differed not only in race but also in nationality and culture (Chiao and Ambady, 2007).
The current findings may therefore contribute towards reconciling the previously observed difference between the intergroup amygdala differences found in some work and the intercultural amygdala differences reported by Chiao et al. (2008b). Specifically, although Chiao et al. (2008b) observed a greater amygdala response to ingrounp faces, this was specific to the context of fearful emotional expressions. The authors interpreted this effect as an evolutionary adaptation whereby signals of threat elicit stronger responses from similar (ingroup) individuals than from dissimilar (outgroup) individuals. The outgroup effect observed here during a voting task, then, may reflect a more general intergroup response that was not captured in Chiao et al.’s (2008b) study, which showed no intercultural differences for neutral, angry, or happy faces. Methodological differences might also contribute to these differences, as Chiao et al.’s (2008b) participants were scanned at two different locations, at different times, and in different countries; whereas the participants in the intergroup (e.g. Van Bavel et al., 2008), interracial (e.g. Hart et al., 2000), and current studies employed participants at one scanning site, in one country, at the same time.
Thus, the current findings raise some new conceptual and methodological questions worth exploring with additional work. Another area worthy of future study is to examine or improve the ecological validity of the current voting task. In particular, in the present study (like many of the hypothetical voting studies conducted to date; e.g. Todorov et al., 2005) participants were unfamiliar with all of the targets and were therefore basing their impressions on appearances. This is typically not how voting decisions are believed to be made in actual elections and it is therefore uncertain how much these results may generalize to real-world voting behavior. Although it is nevertheless interesting that some of these judgments have been found to predict the actual outcomes of elections, future efforts may wish to extend this work using more realistic methodological contexts.
In sum, the current study presents two novel findings of interest to cultural neuroscience. First, participants’ voting decisions about candidates from two different cultures were reflected in an amygdala response to preferred political candidates’ faces. These findings suggest that the amygdala may be important in evaluating candidates for political office. Moreover, these effects were independent of either the perceiver’s culture or the candidate’s culture, suggesting that the neural basis for electoral decisions may extend across cultures. Second, participants showed a greater overall amygdala response to the faces of outgroup candidates than they did to the faces of ingroup candidates. This extends the work that has been previously done examining amygdala response across cultures and helps to reconcile what is known about the neural response to group members in intercultural contexts with what is known about the neural response to group members in intracultural contexts.
Acknowledgments
The authors would like to thank Mariko Shimada, Ryutaro Kawabata and Kana Okano for their assistance with this study. This research was supported in part by National Science Foundation grant (BCS-0435547) to Nalini Ambady, a National Science Foundation graduate research fellowship to Nicholas O. Rule, and a Social Sciences Research Institute grant from the Pennsylvania State University to Reginald B. Adams, Jr.
REFERENCES
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews Neuroscience. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–74. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Albright L, Malloy TE, Dong Q, et al. Cross-cultural consensus in personality judgments. Journal of Personality and Social Psychology. 1997;72:558–69. doi: 10.1037//0022-3514.72.3.558. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Jost JT, Master SL, Yee CM. Neurocognitive correlates of liberalism and conservatism. Nature Neuroscience. 2008;10:1246–7. doi: 10.1038/nn1979. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stapin I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Antonakis J, Dalgas O. Predicting elections: Child’s play! Science. 2009;323:1183. doi: 10.1126/science.1167748. [DOI] [PubMed] [Google Scholar]
- Ayman R. Leadership perception: The role of gender and culture. In: Chemers RM, Ayman R, editors. Leadership Theory and Research. San Diego, CA: Academic Press; 1993. pp. 137–165. [Google Scholar]
- Ballew CC, II, Todorov A. Predicting political elections from rapid and unreflective face judgments. Proceedings of the National Academy of Sciences. 2007;104:17948–53. doi: 10.1073/pnas.0705435104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of Cultural Psychology. NY: Guilford Press; 2007. pp. 237–54. [Google Scholar]
- Chiao JY, Bowman NE, Gill H. The political gender gap: gender bias in facial inferences that predict voting behavior. PLoS ONE. 2008a;3:3666. doi: 10.1371/journal.pone.0003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Iidaka T, Gordon HL, et al. Cultural specificity in amygdala response to fear faces. Journal of Cognitive Neuroscience. 2008b;20:2167–74. doi: 10.1162/jocn.2008.20151. [DOI] [PubMed] [Google Scholar]
- Cunnigham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16:1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychological Science. 2008;19:152–60. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–40. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Dubois S, Rossion B, Schiltz C, et al. Effect of familiarity on the processing of human faces. NeuroImage. 1999;9:278–89. doi: 10.1006/nimg.1998.0409. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Herlitz A, Fransson P, Wright C, Backman L. Sex-differential brain activation during exposure to female and male faces. NeuroReport. 2004;15:235–8. doi: 10.1097/00001756-200402090-00004. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, Rauch SL. Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. NeuroReport. 2000;11:2351–5. doi: 10.1097/00001756-200008030-00004. [DOI] [PubMed] [Google Scholar]
- Kaplan JT, Freedman J, Iacoboni M. Us versus them: political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia. 2007;45:55–64. doi: 10.1016/j.neuropsychologia.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Kaplan SN. Top executive rewards and firm performance: a comparison of Japan and the United States. Journal of Political Economy. 1994;102:510–46. [Google Scholar]
- Knutson KM, Wood JN, Spampinato MV, Grafman J. Politics on the brain: an fMRI investigation. Social Neuroscience. 2006;1:25–40. doi: 10.1080/17470910600670603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DS. Person perception and real-life electoral behaviour. Australian Journal of Psychology. 1978;30:255–62. [Google Scholar]
- Misumi J, Peterson MF. The performance-maintenance (PM) theory of leadership: review of a Japanese research program. Administrative Science Quarterly. 1985;30:198–223. [Google Scholar]
- Mitchell JP, Macrae CN, Banaji M. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Conner KJ, Cunningham WA, et al. Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience. 2000;12:729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Poutvaara P, Jordahl H, Berggren N. Faces of politicians: babyfacedness predicts inferred competence but not electoral success. Journal of Experimental Social Psychology. 2009;45:1132–5. [Google Scholar]
- Richeson JA, Todd AR, Trawalter S, Baird AA. Eye-gaze direction modulates race-related amygdala activity. Group Processes & Intergroup Relations. 2008;11:233–46. [Google Scholar]
- Rule NO, Ambady N, Adams R.B., Jr, et al. Polling the face: prediction and consensus across cultures. Journal of Personality and Social Psychology. in press doi: 10.1037/a0017673. , DOI: 10.1037/a0017673. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neuroscience. 2009;12:508–14. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Rangel A, Alvarez RM, et al. A neural basis for the effect of candidate appearance on election outcomes. Social Cognitive and Affective Neuroscience. 2008;3:344–52. doi: 10.1093/scan/nsn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Social Cognitive and Affective Neuroscience. 2008;3:303–312. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Mandisodza A N, Goren A, Hall CC. Inferences of competence from faces predict election outcomes. Science. 2005;308:1623–6. doi: 10.1126/science.1110589. [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychological Science. 2008;19:1131–9. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Westen D, Blagov PS, Harenski K, Kilts C, Hamann S. Neural bases of motivated reasoning: an fMRI study of emotional constraints on partisan political judgment in the 2004 U.S. presidential election. Journal of Cognitive Neuroscience. 2006;18:1947–58. doi: 10.1162/jocn.2006.18.11.1947. [DOI] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5:277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Cook KS, Watabe M. Uncertainty, trust, and commitment formation in the United States and Japan. American Journal of Sociology. 1998;104:165–94. [Google Scholar]
- Zebrowitz LA, Montepare JM, Lee HK. They don’t all look alike: individuated impressions of other racial groups. Journal of Personality and Social Psychology. 1993;65:85–101. doi: 10.1037//0022-3514.65.1.85. [DOI] [PubMed] [Google Scholar]