Abstract
Traditionally, complex cultural symbols like brands are investigated with psychological approaches. Often this is done by using semantic differentials, in which participants are asked to rate a brand regarding different pairs of adjectives. Only recently, functional magnetic resonance imaging (fMRI) has been used to examine brands. In the current work we used fMRI in combination with a semantic differential to cross-validate both methods and to improve the characterization of the basic factors constituting the semantic space. To this end we presented pictures of brands while recording subject's brain activity during an fMRI experiment. Results of the semantic differential arranged the brands in a semantic space illustrating their relationships to other cultural symbols. FMRI results revealed activation of the medial prefrontal cortex for brands that loaded high on the factor ‘social competence’, suggesting an involvement of a cortical network associated with social cognitions. In contrast, brands closely related to the factor ‘potency’ showed decreased activity in the superior frontal gyri, possibly related to working memory during task performance. We discuss the results as a different engagement of the prefrontal cortex when perceiving brands as cultural symbols.
Keywords: cultural objects, prefrontal cortex, brands, fMRI
INTRODUCTION
When we try to describe the meaning of a cultural symbol, we sometimes have difficulties to find the right words. The reason for this seems to be that cultural symbols often have very complex meanings. Brands can be defined as such complex cultural symbols. They can be described as cultural symbols that promise certain advantages of a consumer good to the customer. These cultural symbols are complex because they may have different meanings or brand images even for slightly different cultures. For example, clothes of a brand that symbolizes value products for adult people often have completely different connotations for young people. Furthermore, the image of a brand sometimes changes dramatically over time. In addition, brands often form cultural symbols that are necessary for being a member in certain social groups, especially in groups of teenagers. Also, the opposite situation may occur. If a union leader drives a brand of a luxury car, the members of the union may be offended by using this ‘wrong’ cultural symbol, which may be seen as being more appropriate for the director of the company. Thus, the possession of goods from certain kinds of brands often is used to mark the social state of the owner and to distinguish him or her from other groups. In sum, brands are important complex cultural symbols that we skillfully use in our daily social life.
In particular for brands as cultural symbols the description of those symbols is of high interest. Semantic differentials have been proven to be very effective in providing detailed information about the perceived context when thinking at a well-known brand (e.g. Green and Tull, 1978). The technique of semantic differentials has been introduced by Osgood et al. (1957). It was designed to measure the connotative meaning of concepts, personalities, or symbols. In this method subjects are asked to rate a concept or term on a scale with the poles described by two contrary adjectives (e.g. ‘healthy’ and ‘sick’). The results provide information about the connotations of the term by revealing its relationships to a number of adjectives in a semantic space (Osgood et al., 1957; for a German sample: Hofstätter, 1957). For example, the term ‘safety’ may be close to the adjectives ‘peaceful’ and ‘cooperative’, but far away from the adjective ‘wild’. Based on a large collection of semantic differential scales, Osgood et al. (1957) performed factorial analyses and found three underlying determinants of semantic space that people use to assess concepts or phrases. Subsequent studies revealed that these three underlying dimensions are used by all subjects to evaluate concepts, values, or terms of their social environment, irrespective of language or culture. The first of these three factors are refered to ‘evaluation’ and loaded high on the adjective pair ‘good–bad’. A second factor was related to ‘strong–weak’ adjectives. This factor was named ‘potency’. Finally, the third factor described an ‘active–passive’ dimension and was labeled ‘activity’. However, other studies suggest different descriptions. Hofstätter (1957) labeled the ‘evaluation’ dimension in a psychoanalytic view as ‘mother’ and the factor ‘potency’ as ‘father’. Based on more recent data Dziobek and Hülser (2007) suggest to describe this factor as ‘social competence’ and to keep the description of the dimension ‘potency’.
When calculating semantic differentials or creating semantic spaces, Osgood et al. (1957) [as well as Hofstätter (1957)] used statistical approaches that reduce the amount of data to the underlying determinants, e.g. factorial analyses. Those approaches may successfully reduce data to a minimum of underlying factors. However, unfortunately factorial analyses do not tell us exactly what the extracted single factor is about. Often, different researchers used different descriptions for the same factors, as for example, Hofstätter (1957) and Osgood et al. (1957). Thus, other methodological approaches may provide valuable contributions to understand brand associations and to help to better characterize the extracted factors.
Only recently, fMRI has been used as a new technique to investigate the neural correlates of brands as cultural symbols (e.g. McClure et al., 2004). The results revealed different activations in the prefrontal cortex (Erk et al., 2002; McClure et al., 2004; Schaefer et al., 2006, 2007b) as well as in limbic areas when perceiving different brands (McClure et al., 2004; Schaefer et al., 2007a). For example, Erk et al. (2002) reported significantly more activation in ventral striatum and other reward-related brain areas when subjects had to rate sports cars compared with ratings of limousines or small cars. Schaefer et al. (2007a) showed that not only the perception of pictures of sports cars involve reward-related brain areas, but even the mere perception of logos of personally favorite brands compared with non-favorite brands. Furthermore, McClure et al. (2004) demonstrated the activation of the dorsolateral part of the prefrontal cortex (DLPFC), midbrain and hippocampi when the participants consumed a sugared drink brand-cued by the favorite brand (compared to the non-favorite brand and control conditions). They further reported activation of the ventromedial prefrontal cortex (VMPFC) that correlated with subjects’ behavioral preferences for those beverages. Schaefer et al. (2006, 2007b) examined brand logos of different categories of brands and suggested differential engagement of the prefrontal cortex depending on the attributed characteristic of a brand. In particular, they report an activation of the anterior medial prefrontal cortex (AMPFC) for prestigious brands (compared with other brand categories), pointing to an active network known to be associated with self-relevant processing.
Here, we aimed examining the neural representation of brands in order to improve the characterization of the basic factors of the semantic space and to cross-validate both methods. Although semantic differentials have been investigated for decades, the correct description of these factors remains an issue. Thus, different researchers have been using very different labels for the underlying factors. This is particularly true for the factor ‘evaluation’ (Osgood et al., 1957), which also has been described as ‘mother’ [in a psychoanalytic view (Hofstätter, 1957)] and more recently as ‘social competence’ (Dziobek and Hülser, 2007). Here we aimed to employ the fMRI technique to show what cortical regions are associated with the perception of brands that are strongly linked to that factor. According to the recent suggestions to describe this factor as ‘social competence’, we hypothesized that brands that load high on this dimension involve activation of brain areas which are known to be related to social perceptions, in particular regions in the medial prefrontal cortex (MPFC) (e.g. Krueger et al., 2009). In contrast, if older descriptions of this factor are more appopriate and this dimension is more related to cognitive processes like ‘evaluation’ (Osgood et al., 1957), we would expect active regions in the anterior cingulum (ACC) or DLPFC, that are known for being involved in evaluation tasks, error processing and ambiguous (‘cool’) decisions rather than for social processes (e.g. Krain et al., 2006).
In order to test our hypotheses we conducted an fMRI experiment in which subjects viewed different goods of well-known brands. We used brands of freely available pharmaceutical products (e.g. Aspirin). Pilot studies revealed that these brands are suitable cultural objects that can be described by semantic differentials. Subjects had to assess these goods regarding their attitude towards them. After the experiment, we asked the participants to complete a questionnaire (including semantic differentials) for each of the presented brands. The results of these questionnaires were used to arrange the brands into a semantic space. We then used the factors that determined this semantic space to compare brain responses to brands that loaded high on one factor to brands that loaded high on another.
Based on previous studies we hypothesized a differential engagement of regions in the prefrontal cortex for different factors (e.g. Watanabe, 1996; Erk et al., 2002; Goel and Dolan, 2003; Knutson et al., 2003; O’Doherty et al., 2003; Paulus and Frank, 2003; McClure et al., 2004; Krain et al., 2006; Schaefer et al., 2006, 2007a, b). More in detail, we expected activations in the MPFC for brands loading high on the factor ‘social competence’ [labeled as ‘evaluation’ by Osgood et al. (1957) and as ‘mother’ by Hofstätter (1957) and Hofstätter and Lübbert (1958) in a psychoanalytic view]. Recent studies have suggested that enduring dispositions of others and the self, or interpersonal norms and scripts engages the medial prefrontal cortex (MPFC) (e.g. Amodio and Frith, 2006; Overwalle, 2008; Krueger et al., 2009). Since adjectives and concepts like ‘clear’, ‘open’, or ‘ideal self-image’ are close to the factor ‘social competence’ (and, in contrast, concepts like ‘loneliness’, ‘depression’ or ‘reserved’ load highly negatively on this dimension), we hypothesized that this factor may be at least in part be based on an engagement of the MPFC. Previous studies demonstrated an involvement of the MPFC when perceiving brands (e.g. Deppe et al., 2005; Schaefer et al., 2006, 2007b), but the concrete function of this brain area for brand perception remained an issue.
Since both factors were orthogonal, we expected for brands loading high on the factor ‘potency’ less activation in MPFC compared to the other brands. In contrast, we here expected neural activations of the DLPFC, which has been related to cognitive control (e.g. Watanabe, 1996). Several studies suggested a role of the DLPFC when perceiving brands or beeing cued by brand related information (e.g. McClure et al., 2004; Schaefer et al., 2006, 2007a, b).
MATERIALS AND METHODS
Participants
Twelve healthy right-handed native German volunteers with no neurological diseases participated in the study (three males, mean age 33.09 years). The study adhered to the Declaration of Helsinki. Ethical approval and written informed consent from all subjects were obtained prior to the fMRI experiment.
Procedure
We selected a set of 18 pictures of freely available pharmaceutical brand products for visual presentation (e.g. Aspirin). All products were well known to the German participants due to intensive advertising efforts. A questionnaire completed by the subjects prior to the study confirmed that subjects were familiar with the presented brands (4.5 on a 5-point-scale ranging from 1 to 5). The pictures presented the typical and most representative product for each brand and contained the name of the brand. Furthermore, the pictures were compatible regarding the size of the image (Figure 1).
Fig. 1.
Two examples of stimuli used for visual presentation in the fMRI scanner.
The fMRI experiment consisted of four scanning sessions (runs). Each run included all 18 pictures. The picture stimuli were presented sequentially in central vision on a computer monitor for duration of 9 s in a randomized order. One second after the picture has disappeared, participants were asked to rate the presented stimuli on a 5-point-scale regarding their personal attractiveness during a time interval of 10 s. Thus, presentation and rating of the brands took 20 s, followed by breaks of 4 s during which a fixation asterisk was displayed. During the presentation of the fixation asterisk, subjects were told to relax and stop performing the task. Prior to the fMRI experiment, subjects were made familiar with the task.
Semantic differential
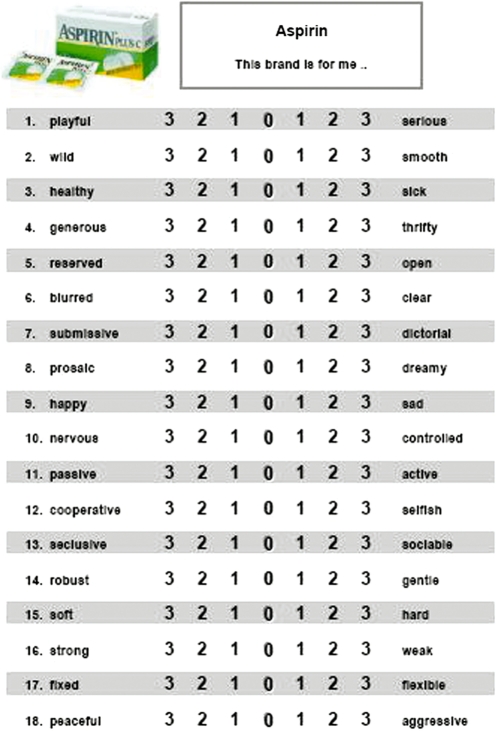
After the fMRI experiment had ended, we asked the participants to complete a questionnaire (semantic differential). Subjects were required to rate the brands according to their relationships to 18 pairs of contrary adjectives like ‘strong’ vs ‘weak’, ‘healthy’ vs ‘sick’, or ‘wild’ vs ‘smooth’ (see Hofstätter, 1957; Osgood et al., 1957). They indicated their response on a seven-point scale (ranging from +3 to −3) (see Figure 2).
Fig. 2.
Example of a questionnaire used to form a semantic differential for one particular brand. Subjects had to rate the brands according to their relationships to 18 pairs of contrary adjectives.
The results of the semantic differential were integrated in a semantic space based on the results of semantic differentials by a more comprehensive study on a German sample (Dziobek and Hülser, 2007). In this previous study 1300 subjects assessed 52 global terms and values like ‘love’, ‘coziness’, etc. according to 18 pairs of contrary adjectives. The study aimed to provide a more updated database compared to the older studies of Hofstätter (1957) and Osgood et al. (1957). A factorial analysis (principal component analysis) with orthogonal factors and subsequent orthogonal VARIMAX rotation of this database resulted in two main factors, which explained together 87% of variance (45% for the first and 42% for the second factor, after rotation). A third factor explained only little variance (6%) and was omitted for further analysis. For this analysis only factors with Eigenvalues >1 were considered (Kaiser–Guttman-criteria, for further results see Supplementary Table S1) (Dziobek and Hülser, 2007). According to the model of Osgood et al. (1957) the two resulting factors can be labeled as ‘evaluation’ and ‘potency’. However, based on their results the authors preferred to interpret the first factor as ‘social competence’ instead of ‘evaluation’. The description of the second factor ‘potency’ was used as Osgood suggested.
In the current study, we used this database of the previous study to relate the different brands with more terms than that of the scales and thus providing the opportunity to cross-validate our results with the fMRI data. Hence, we were able to arrange the brands of the current study in a semantic space with 18 pairs of adjectives (semantic differential) and 52 global terms of the previous study. Furthermore, we followed the more appropriate description of the factor ‘social competence’.
fMRI data acquisition
fMRI data were acquired with a 3 T Magnetom Trio Siemens scanner for T2-weighted functional MR images using axially oriented echo-planar imaging (TR = 1.5 s, TE = 30 ms, flip angle = 75°, 26 slices, 5 mm thickness, resolution 3.5 ×3.5 × 5 mm). For each subject, data were acquired in four scanning sessions. Due to T1 equilibration effects, the first four volumes of each session were discarded. For anatomical reference, a T1-weighted anatomical image was obtained (3D-SPGR, TR = 24 ms, TE = 8 ms). Visual images were back-projected onto a screen at the end of the scanner bed close to the subject’s feet. Subjects viewed the images through a mirror mounted on the birdcage of the receiving coil. In addition to a head strap, foam cushions were placed tightly around the side of the subject’s head to minimize head motion. Data preprocessing and statistical analyses were carried out using SPM5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, University College London, London, UK). Individual functional images were realigned to correct for inter-scan movement using sinc interpolation and subsequently were normalized into a standard anatomical space (MNI, Montreal Neurological Institute template), resulting in isotropic 3 mm voxels as described previously (Friston et al., 1995). Data were then smoothed with a Gaussian kernel of 6 mm full-width half maximum.
Statistical parametric maps were calculated using multiple regression with the hemodynamic response function modeled in SPM5. Data analyses were performed at two levels. First, we examined data on the individual subject level by using a fixed effects model. The results of the semantic differential were used to create subgroups of brands loading positively (and negatively, respectively) on the factor ‘social competence’ and ‘potency’, respectively. We used these groups as regressors for the fixed effect model. Second, the resulting parameter estimates for each regressor at each voxel were then entered into a second-level analysis with a random effects model. We used a block-design model with a boxcar regressor convolved with the hemodynamic response function to compare brain responses elicited by brands that loaded high (positively) vs low (negatively) on the extracted factors of the semantic differential. Furthermore, we compared brain responses to brands that loaded high on one factor to brands that loaded high on another.
Since we had a priori hypotheses on active regions in the prefrontal cortex we thresholded the resulting images at P < 0.001 (uncorrected for multiple comparisons). The anatomical interpretation of the functional imaging results was performed with the Talairach Daemon (http://www.talairach.org/daemon.html) using a cube search range of 3 mm and the SPM Anatomy toolbox (Eickhoff et al., 2005).
RESULTS
Behavioral results
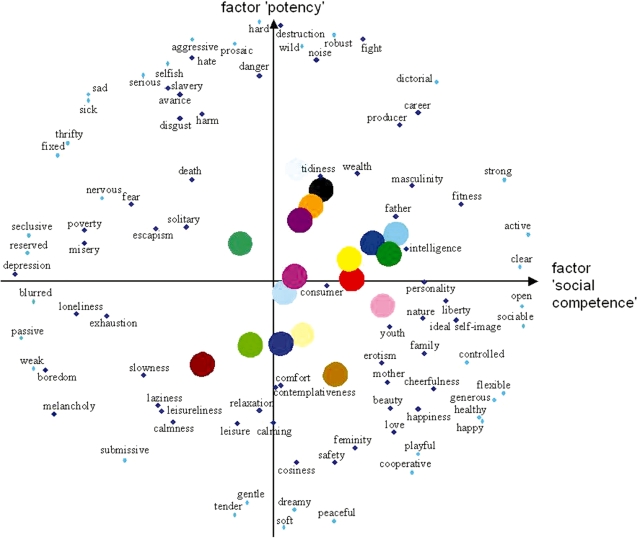
Analysis of the semantic differentials resulted in different positions for the brands in the semantic space (see Figure 3 and Supplementary Table S2). For further analysis, we chose brands that loaded high on the factor ‘potency’ (positively and negatively) and brands that loaded high on the factor ‘social competence’. However, almost no brands revealed negative loadings on the factor ‘social competence’ (see Figure 3). Thus, for this dimension we only used brands that loaded positively on this factor.
Fig. 3.
Semantic space built by the results of the semantic differentials. Brands and concepts are displayed on a 2D schema (factors ‘social competence’ and ‘potency’; factors were orthogonal). Colored circles depict the different brands.
fMRI results
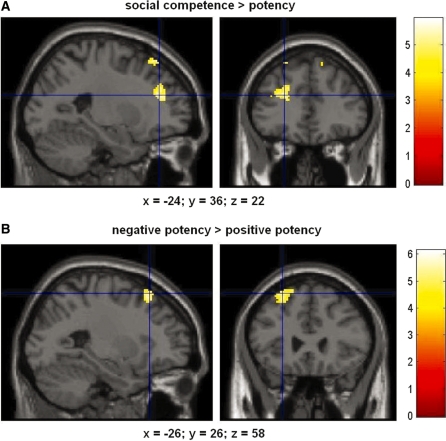
None of the subjects reported any problems or expressed feelings of being fatigue while performing the task in the scanner. To cross-validate our behavioral results with the results of the fMRI experiment, we formed groups of brands that loaded high (positively) on the factor ‘potency’ and compared the elicited neural activation with the brain responses to a group of brands that loaded low (negatively) on this factor (see Figure 3). For the contrast ‘potency positive loading’ vs ‘potency negative loading’ no voxel survived the threshold of significance. However, the contrast ‘potency negative loading’ minus ‘potency positive loading’ revealed active regions bilaterally in the superior frontal gyrus (SFG) (x, y, z: −38, 20, 56, peak z-score 3.97; 12, 34, 58, peak z-score 3.47; uncorrected, at P < 0.001; see Figure 4, Table 1 and Supplementary Table S3).
Fig. 4.
(A) Contrasts of brands loading high on the factor ‘social competence’ compared with brands loading high on the factor ‘potency’. This contrast showed significant activation in the MPFC and the SFG (for details see Table 1). (B) Contrasts of brands loading negatively on the factor ‘potency’ compared with brands loading positively on this factor. Results revealed activation of the SFG for brands loading negatively on this factor. Areas of significant fMRI signal change are shown as color overlays on the T1-MNI reference brain. The colored bar indicates the T-statistic of the activation (random effects analysis, thresholded at P < 0.001).
Table 1.
Brain areas activated in the random effects analysis
| Contrast | Brain region | Peak MNI coordinates | Peak T-statistic | z-score | Brodmann area |
|---|---|---|---|---|---|
| Social competence > potency | L MPFC | −24, 36, 22 | 5.94 | 3.98 | 9/10 |
| R MPFC | 14, 44, 32 | 4.60 | 3.43 | 9 | |
| 20, 48, 32 | 4.27 | 3.27 | 9 | ||
| L medial SFG | −22, 34, 58 | 5.11 | 3.66 | 8 | |
| R medial SFG | 14, 42, 54 | 4.80 | 3.52 | 8 | |
| Potency > social competence | Cerebellum | −4, −62, −26 | 5.13 | 3.66 | – |
| −4, −64, −10 | 4.62 | 3.44 | – | ||
| Potency (positive loadings) > potency (negative loadings) | – | – | – | – | – |
| Potency (negative loadings) > potency (positive loadings) | L lateral SFG | −38, 20, 56 | 5.90 | 3.97 | 8 |
| R medial SFG | 12, 34, 58 | 4.70 | 3.47 | 8 | |
| Lingual gyrus | 14, −94, −2 | 6.13 | 4.05 | 17 | |
| Thalamus | 12, −10, 8 | 4.82 | 3.53 | — |
Note: P < 0.001; L, left hemisphere, R, right hemisphere.
For the factor ‘social competence’ none of the brands showed negative loadings. We compared brain responses to brands that loaded high on the factor ‘social competence’ with those of brands that loaded high (positively) on the factor ‘potency’ (both factors were orthogonal in the principal component analysis). For the contrast ‘social competence positive loading’ minus ‘potency positive loading’ we found active regions bilaterally in the MPFC (−24, 36, 22, peak z-score 3.98; 14, 44, 32, peak z-score 3.43; uncorrected, at P < 0.001; see Figure 4, Table 1 and Supplementary Table S3). Furthermore, the SFG showed significant activations bilaterally (−22, 34, 58, peak z-score 3.66; 14, 42, 54, peak z-score 3.52; uncorrected, at P < 0.001, see Table 1 and Supplementary Table S3). The contrast ‘potency positive loading’ minus ‘social competence positive loading’ failed to show any significant voxels (uncorrected, at P < 0.001) in the cerebrum.
DISCUSSION
This study aimed to combine two different methods for the investigation of brands as cultural objects. Whereas the first approach used the well-established method of a semantic differential to create a semantic space that showed the brand in its relationships to other brands and concepts, the second approach employed the fMRI technique to acquire information about the neural representation of brands. The results revealed that brands loading high on the ‘social competence’ factor elicited significant activation in the MPFC, whereas brands related to the factor ‘potency’ were associated with reduced activation of the SFG.
The results of the semantic differential revealed the connotations of each brand by showing its relationships to other brands or concepts. Thus, a certain brand may be very close to the term ‘healthy’ but far away of the term ‘aggressive’. Semantic differentials are analyzed with factorial analyses (Osgood et al., 1957). However, the problem remains of assigning proper names to the extracted factors. The examination of the neural representations of brands may help to better characterize the underlying factors of the semantic space.
The first factor of the semantic space was called ‘social competence’ (Dziobek and Hülser, 2007), earlier described as ‘evaluation’ by Osgood et al. (1957) or ‘mother’ by Hofstätter (1957). FMRI results for brands loading high on this factor compared with brands loading high on the factor ‘potency’ in the semantic differential yielded activation bilaterally in the MPFC. Several studies suggest that the MPFC is associated with social cognitions. In particular, this brain area has been related to the capacity to recognize people’s behavioral intentions and also to understand enduring dispositions of others and the self (e.g. Amodio and Frith, 2006; D'A;rgembeau et al., 2007; Chiao et al., 2008; Overwalle, 2008; Krueger et al., 2009). For example, it has been proposed that areas in the MPFC support social functions like mentalizing or self-reflection (Gilbert et al., 2007). Here, we show that brands related to the factor ‘social competence’ elicited activations in the MPFC similar to those reported in the above-mentioned studies. The factor ‘social competence’ is close to concepts like ‘sociable’, ‘open’, or ‘active’ in the semantic space. These terms point to socially relevant aspects of behavior. Thus, the neuroimaging data confirm our hypothesis by suggesting that social aspects seem to be crucial in the description of the factor labeled as ‘evaluation’ by Osgood et al. (1957), ‘mother’ by Hofstätter et al. (1957) or ‘social competence’ by Dziobek and Hülser (2007).
Brands loading high on the factor ‘social competence’ elicited also bilateral activations in the SFG. Activations in the SFG have been related to higher cognitive functions like working memory (e.g. Wager and Smith, 2003). The active regions in the SFG in the current study may be related to task performance. Previous studies on brand perception similarly reported an activation of the SFG when participants were asked to rate brands (Deppe et al., 2005) or to imagine using typical products of brands (Schaefer et al., 2007b).
A second factor of the semantic space was called ‘potency’ (Osgood et al., 1957). In our hypotheses we assumed that this factor engaged the DLPFC and should lead to a reduced activation of the MPFC. FMRI results of brands positively related to this factor in contrast to brands loading negatively on it failed to show any significant areas. Nevertheless, brands loading negatively on this factor compared with brands loading positively on it yielded activation bilaterally in the SFG. In contrast, for the factor ‘social competence’ results yielded activations in the SFG for brands loading high (positively) on this factor. As noted above, this area has been associated with higher cognitive functions like working memory (e.g. Wager and Smith, 2003), possibly related to task performance. The factor ‘potency’ is described through adjectives like ‘wild’, ‘hard’, ‘danger’, or ‘aggressive’ (see Figure 3). Thus, we speculate that low (or negative) activation on the factor ‘potency’ in this study was associated with enhanced cognitive effort or working memory (Wager and Smith, 2003). It might have been difficult for the subjects to assess brands regarding their personal attractiveness when they have low or negative values on the ‘potency’ dimension. Here, the participants may have needed more cognitive effort to perform the task. In contrast, brands loading high on the factor ‘potency’ seem to be easier to assess regarding their personal attractiveness. Hence, it seems that brands loading strongly and positively on the factor ‘potency’ and which therefore are related closely to connotations like ‘danger’ or ‘aggressive’ are more salient or dominant and thereby ease the assessment of those brands. In contrast, brands loading negatively on this factor are linked to less salient connotations like ‘slowness’ or ‘comfort’, therefore making it more difficult to rate them according their personal attractiveness. A similar prefrontal inactivation in the SFG when subjects are focused on a demanding sensory categorization task has been reported by Goldberg et al. (2006).
Can the activation of the SFG for the factor ‘social competence’ be explained in an analog way? The results in Table 1 report slightly different peak activations in the SFG for both variables. Recent studies suggest that the SFG can be functionally dissociated into a lateral and medial part, whereas only the left lateral part seems to be critical for working memory (Boisgueheneuc et al., 2006). However, although in the current study the peak activations in the SFG were different, activations for both variables covered the left medial part of the SFG. Thus, we assume that the activations in the SFG we report here relay on similar mechanisms related to a working memory network. However, since the SFG has been related not only to working memory but also to a variety of different cognitive functions, these explanations remain speculative. Further studies are necessary to link these results with behavioral data and provide additional support for this interpretation.
The present study employed the approach of fMRI to enhance our knowledge of underlying factors of the semantic space. Given that this complex method is expensive and time-consuming, what does the neuroimaging results tell us that we cannot deduce from the behavioral factorial analysis alone? fMRI enable us to link the factors driven out of the behavioral data with the activation of certain cortical areas. Since the functional meaning of those areas (the MPFC and the SFG) are known from previous studies, the results can tell us what the extracted factors are about. More in detail, the results suggest to mark the factor originally described as ‘evaluation’ now as being mainly characterized by social perceptions. Thus, the description ‘social competence’ for this factor seems to be much more appropriate. Hence, the fMRI results provide important improvements for the factorial model of semantic space, which would not have been possible by looking on the behavioral data alone.
The outcome of this study also emphasizes the possibility of combining classical behavioral approaches such as factorial analyses of questionnaire data (e.g. semantic differentials) with advanced neuroimaging methods. This may encourage future studies on cultural objects (such as brands) to make use of these techniques in order to verify classical models or methodological approaches. The possible combination of both approaches (behavioral analysis and neuroimaging) provides an opportunity for neuroeconomics to link information on how the brain processes brand related information to established models, e.g. the model of semantic differentials by Osgood et al. (1957).
In conclusion, although the results of this combination of a semantic differential approach with neuroimaging techniques need to be confirmed by additional studies, we believe that this kind of analysis provides intriguing possibilities for future research on the questions how brands might be represented in the brain and how brand related behavior can be explained.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
Deutsche Forschungsgemeinschaft and the Bayer Vital GmbH.
Acknowledgments
The authors would like to thank Oliver Hülser, GfK, and Eberhard Dziobek, Bayer Vital GmbH, for helpful discussions.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Review Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, et al. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009;30:2813–20. doi: 10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Boisgueheneuc F, Levy R, Volle E, et al. Functions of the left superior frontal gyrus in humans: a leson study. Brain. 2006;129:3315–28. doi: 10.1093/brain/awl244. [DOI] [PubMed] [Google Scholar]
- D'A;rgembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Deppe M, Schwindt W, Kugel H, Plassmann H, Kenning P. Nonlinear responses within the medial prefrontal cortex reveal when specific implicit information influences economic decision making. Journal of Neuroimaging. 2005;15:171–182. doi: 10.1177/1051228405275074. [DOI] [PubMed] [Google Scholar]
- Dziobek E, Hülser O. Hofstätters Polaritätenprofil—neu entwickelt. Planung & Analyse. 2007;3:50–4. [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Erk S, Spitzer M, Wunderlich AP, Galley L, Walter H. Cultural objects modulate reward circuitry. NeuroReport. 2002;18:2499–503. doi: 10.1097/00001756-200212200-00024. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley K, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gilbert SJ, Williamson ID, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive and Affective Neuroscience. 2007;2:217–26. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage. 2003;20:2314–21. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–39. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Green PE, Tull DS. Research for Marketing Decisions. New Jersey, IL: Prentice-Hall; 1978. [Google Scholar]
- Hofstätter PR. Gruppendynamik. Reinbek: Rowohlt; 1957. [Google Scholar]
- Hofstätter PR, Lübbert H. Die Untersuchung von Stereotypen mit Hilfe des Polaritätsprofils. Zeitschrift für Markt- und Meinungsforschung. 1958;3:127–38. [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–84. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn Sci. 2009;13:103–9. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–87. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23:7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood CE, Suci G, Tannenbaum P. The Measurement of Meaning. Urbana, IL: University of Illinois Press; 1957. [Google Scholar]
- Paulus PM, Frank LR. Ventromedial prefrontal cortex activation is criticial for preference. Neuroreport. 2003;10:1311–5. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Rotte M. Favorite brands as cultural objects modulate reward circuit. Neuroreport. 2007a;18:141–5. doi: 10.1097/WNR.0b013e328010ac84. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Rotte M. Thinking on luxury or pragmatic brand products: Brain responses to different categories of culturally based brands. Brain Research. 2007b;1165:98–104. doi: 10.1016/j.brainres.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Berens H, Heinze H.-J, Rotte M. Neural correlates of culturally familiar brands of car manufacturers. Neuroimage. 2006;31:861–5. doi: 10.1016/j.neuroimage.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2008;30:829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;381:629–32. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]