Abstract
Genes and culture are often thought of as opposite ends of the nature–nurture spectrum, but here we examine possible interactions. Genetic association studies suggest that variation within the genes of central neurotransmitter systems, particularly the serotonin (5-HTTLPR, MAOA-uVNTR) and opioid (OPRM1 A118G), are associated with individual differences in social sensitivity, which reflects the degree of emotional responsivity to social events and experiences. Here, we review recent work that has demonstrated a robust cross-national correlation between the relative frequency of variants in these genes and the relative degree of individualism–collectivism in each population, suggesting that collectivism may have developed and persisted in populations with a high proportion of putative social sensitivity alleles because it was more compatible with such groups. Consistent with this notion, there was a correlation between the relative proportion of these alleles and lifetime prevalence of major depression across nations. The relationship between allele frequency and depression was partially mediated by individualism–collectivism, suggesting that reduced levels of depression in populations with a high proportion of social sensitivity alleles is due to greater collectivism. These results indicate that genetic variation may interact with ecological and social factors to influence psychocultural differences.
Keywords: 5-HTTLPR, serotonin transporter, polymorphism, MAOA-uVNTR, A118G
Over the last 30 years, social psychologists have documented an impressive array of psychocultural differences. For example, in East Asian cultures the self tends to be defined in relationship to the group, or collective, whereas in Western cultures (e.g. Europe and the nations of the former British Commonwealth) there is a greater proclivity for the self to be viewed as unique, stable and independent of the social group (Markus and Kitayama, 1991). A critical question raised by such findings is how do such cultural differences arise? Why do some groups tend towards collectivism, while others tend towards individualism?
Answering this complex question will require integrating many levels of analysis including ecological, sociological, demographic, economic, psychological and biological. A helpful means of integrating these diverse influences is to adopt a cultural neuroscience perspective (Chiao and Ambady, 2007), because the brain is the central hub where each of these influences converge. Accordingly, genes affecting brain function are likely to influence the adoption and formation of cultural norms and, conversely, culture may also shape the expression and selection of genes. Although the study of psychological genetics is in its infancy and much is still to be learned, in this article, we present data suggesting that variation in several genes known to affect brain function appear to influence the degree to which one is emotionally responsive to the social environment. We then extend this social sensitivity hypothesis to the cultural realm and present evidence indicating that it may be of relevance to the cultural construct of individualism–collectivism. Although the vast majority of genetic variation exists within populations (Lewontin, 1972), a measurable proportion of human genetic variation does exist between populations of different ancestral origins. Therefore, we examine below the relationship between population differences in cultural orientation and the relative frequency of several genetic variants thought to affect sensitivity to the social environment. In addition, we also explore potential psychological processes that may explain the effect.
GENETIC VARIATION AND SOCIAL SENSITIVITY
Serotonin transporter and social sensitivity
We begin our discussion of genetics with a focus on variation in the serotonin transporter gene (SLC6A4) and, in particular, a polymorphism within this gene that is probably the most studied polymorphism in psychiatry. The serotonin transporter is best known as the site of action for the drug Prozac and related antidepressants (Wong et al., 1995). Within a portion of the serotonin transporter gene, there is a segment of DNA that is longer in some individuals than others (Lesch et al., 1994). In straightforward fashion, the short version is called the short allele, whereas the long version is called the long allele. An individual can then have one of three possible genotypes at this location in the DNA (which is identified by the acronym 5-HTTLPR): short/short, short/long, or long/long. (For a primer on contemporary molecular genetic terminology and concepts, see: Way and Gurbaxani, 2008).
An initial clue to the psychological role of the 5-HTTLPR comes from seminal work examining the interaction of the 5-HTTLPR with stressful life events (Caspi et al., 2003). This study found that individuals with the short allele (particularly those with the short/short genotype) were at greater risk for depression when exposed to life stressors such as divorce or the death of a loved one than were long/long individuals. An adverse social environment during childhood had similar effects. In other words, short/short individuals were more sensitive to the depression-inducing effects of social stress than were long/long individuals. This interaction between the 5-HTTLPR and stress extends to other phenotypes associated with the serotonin system as well, including post-traumatic stress disorder (Xie et al., 2009), antisocial behavior (Li and Lee, in press), substance use (Brody et al., 2009a), suicidality (Roy et al., 2007), sleep quality (Brummett et al., 2007) and anxiety sensitivity (Stein et al., 2007). The multiple phenotypes affected by this interaction attests to the robustness of the effect. According to reviews of the role of the 5-HTTLPR in moderating the effects of stress upon depression, the effect is most reproducible when objective measures of stress are used (Uher and McGuffin, 2010), rather than subjective measures, for which the interaction has not universally replicated (Risch et al., 2009). Clearly, further research is needed to identify the molecular, and particularly psychological, moderators of this interaction effect.
One important variable potentially influencing the relationship between the 5-HTTLPR, stress and psychological state may be the positivity of the social environment. Thus, the association of the short allele with sensitivity to negative events appears to be only half the story. In a study of depressive symptomatology, when short/short individuals had experienced more positive than negative events over the last 6 months, they had the lowest levels of depressive symptomatology in the sample (Taylor et al., 2006), indicating that short/short individuals are more sensitive to positive life events as well as negative ones. Subsequent research has shown that this relationship between life events and affect for individuals with the short/short genotype was primarily driven by the social events, as the nonsocial events were not significantly related to affect (Way and Taylor, 2010). Other groups have found heightened sensitivity to positive social influences amongst short allele carriers as well, which has even been documented using neurochemical measures (Manuck et al., 2004). Thus, these results suggest that the 5-HTTLPR moderates sensitivity to social influence regardless of its valence.
Because short/short individuals are more sensitive to the social realm, social support appears to be more important for maintaining their well-being. In support of this claim, short/short individuals exposed to a natural disaster (a hurricane) were at no higher risk for depression than long/long individuals provided they perceived that they had good social support (Kilpatrick et al., 2007). However, if short/short individuals exposed to this disaster perceived that they did not have good social support they had a 4.5 times greater risk for depression. Similarly, a randomized control trial designed to improve nurturant and involved parenting reduced adolescent risky behavior, but only amongst those with the short allele (Brody et al., 2009b). A similar differential sensitivity was seen among adolescents in foster care. If the short/short individuals had a reliable mentor present in their life they were at no higher risk for depression than adolescents with the other genotypes. However, if they did not have such support they were at a high risk for depression (Kaufman et al., 2004). Thus, being embedded in a richly interconnected social network, as is present in collectivistic cultures, might be particularly important for maintaining the well-being of short/short individuals.
The µ-opioid receptor and social rejection
Another system that appears to be involved in social sensitivity, particularly sensitivity to social rejection, is the opioid system. Original evidence linking the opioid system to social processes comes from animal studies of the drug morphine, which acts on a receptor in the opioid system, the µ-opioid receptor. Panksepp (1998) found that low, nonsedative doses of the physical pain-killer morphine quelled the distress of separation from the caregiver in infants of multiple mammalian species (Herman and Panksepp, 1978; Panksepp et al., 1978a, 1978b). This data indicates that the endogenous opioid system is involved in signaling the distress of separation from conspecifics.
According to recent neuroimaging data in humans, this role for the opioid system appears to apply to the distress associated with the severance of a social bond in adulthood as well. In a positron emission tomography study, women exhibited decreased µ-opioid mediated neurotransmission when recalling the death of a loved one or the breakup of a romantic relationship (Zubieta et al., 2003). Hence, it appears that the level of µ-opioid receptor dependent signaling within the brain is a reflection of an individual’s current inclusion status.
In line with this hypothesis, a functional polymorphism (A118G) in the µ-opioid receptor gene was recently associated with self-reported dispositional sensitivity to rejection (Way et al., 2009), as measured by Mehrabian’s (1976) Sensitivity to Rejection Scale. (The A118G polymorphism is a slightly different type of polymorphism than the 5-HTTLPR. In this case, there is a single letter in the DNA code that is changed, an A to a G, rather than a stretch of DNA that is different between the two alleles, as is the case with the 5-HTTLPR). In particular, it was the G allele that was associated with greater sensitivity to rejection. Additionally, this group also assessed the relationship of the A118G polymorphism to neural response during an actual episode of rejection in which the participant was excluded from an online ball-tossing game (Cyberball) with two supposed others. Consistent with the findings using the trait measure of sensitivity to rejection, individuals carrying the G allele also had greater levels of neural response to this rejection episode within multiple brain areas known to be involved in the processing of physical pain (dorsal anterior cingulate cortex and anterior insula). Thus, according to both self-report and neural data, genetic variation in the µ-opioid receptor is associated with sensitivity to social rejection.
Monoamine oxidase A and social rejection
In addition to the opioids, other neurochemical systems are also likely to influence the experience of social rejection. One such candidate is monoamine oxidase A (MAOA), an enzyme that breaks down neurochemicals such as serotonin and dopamine (Shih et al., 1999), and that is present in high concentrations within the anterior cingulate (Ginovart et al., 2006), a brain region closely associated with the distress of social rejection (Eisenberger et al., 2003).
Within the gene coding for MAOA, there is a particular form of variation (referred to as the MAOA-uVNTR) that is associated with differences in the expression of the MAOA gene (Sabol et al., 1998). Using the previously described social exclusion task in the scanner (Cyberball), our group found that the MAOA-uVNTR was associated with the degree of exclusion-related neural activation, such that the individuals with the low expressing alleles had the greatest response within the portion of the anterior cingulate associated with self-reported distress to exclusion (Eisenberger et al., 2007).
This greater neural response to social rejection within brain areas that positively correlate with the acute distress of the rejection experience may be reflective of this polymorphism influencing sensitivity to social input in general. In gene–environment interaction studies, exposure to abuse or other maltreatment during childhood significantly increases the likelihood of engaging in antisocial behavior in adulthood amongst men with a low expressing allele, as first identified in the study of Caspi et al. (2002) and confirmed in a recent meta-analysis (Kim-Cohen et al., 2006).
Accumulating evidence indicates that the MAOA-uVNTR also affects differential sensitivity to the environment (Belsky et al., 2009), particularly the social environment. In five separate studies documenting the greater responsivity to negative social influences among individuals with the low expression allele, carriers of this same allele who did not have adverse childhood experiences had the lowest levels of later antisocial or violent behavior (Caspi et al., 2002; Kim-Cohen et al., 2006; Nilsson et al., 2006; Widom and Brzustowicz, 2006; Ducci et al., 2007). Presumably, if the degree of warmth and nurturance were assessed (only categorical measures of the presence or absence of maltreatment were used in these studies) even more robust support for this polymorphism affecting sensitivity to positive social influences would be found. Recent evidence using depression as the dependent measure supports this assertion (Kinnally et al., 2009). Thus, like the 5-HTTLPR and A118G polymorphisms, the MAOA-uVNTR also appears to affect sensitivity to social experiences.
Summary
Each of these polymorphisms (5-HTTLPR, A118G, MAOAuVNTR) appear to affect the degree to which well-being is dependent on the quality of the social environment. Being part of a closely knit and dependable social network appears to particularly benefit those with the putative social sensitivity forms (e.g. 5-HTTLPR short allele) of these polymorphisms. Conversely, for those with these social sensitivity alleles the experience of social loss can precipitate psychopathology. Particularly for the sensitivity alleles of the A118G and MAOA polymorphisms, it appears that the experience of being excluded from the social interactions of others can be particularly aversive. As all of the aforementioned research has focused at level of the individual, the question arises as to what effects might these alleles be having on the societal level? If a population had more or less of these alleles, might this affect the preferred forms of social interaction?
SOCIAL SENSITIVITY ALLELES AND CULTURAL NORMS
Given that there is a higher prevalence of these putative social sensitivity alleles in East Asian populations than in Caucasian populations, there may be a relationship between the relative proportion of these alleles and the predominant cultural forms in a population. In collectivistic cultures, relationships are enduring due to social ties that are reified by mutual obligations between members of the family, clan, or religion. These relationships are so salient that the self is defined by them. Thus, the implicit construction of the self in members of these cultures is inherently relational (Fiske et al., 1998). This social construction of the self may function akin to an implicit social support network (Kim et al., 2008) that is likely to buffer individuals with social sensitivity alleles from the adverse consequences of stress and improve life satisfaction.
In contrast, in individualistic cultures, where there is a high degree of focus upon personal autonomy, individual needs often supersede the needs of the group. Thus, relationships can be more transitory (Adams and Plaut, 2003), which can lead to the perception that one is not a part of a social network. This might be particularly challenging for individuals with social sensitivity alleles.
Heightened concern of social rejection also appears to be related to collectivism. Yamaguchi and colleagues (Yamaguchi, 1994; Yamaguchi et al., 1995) found that higher levels of collectivism were correlated with greater sensitivity to social rejection using Mehrabian’s (1976) sensitivity to rejection scale, which is the same scale that was associated with the A118G polymorphism (Way et al., 2009). This greater sensitivity to cues of rejection and greater concern over the consequences of rejection could lead to the subjugation of self-interest for the interest of the in-group, a hallmark of collectivism (Yamaguchi, 1994). High levels of concern over social rejection may also encourage the reification of social ties in order to reduce the risk of losing one’s social network. Thus, collectivism may be more compatible with populations possessing a high proportion of social sensitivity alleles and may lead to higher levels of well-being in such populations.
Frequency of social sensitivity alleles and individualism–collectivism
In order to study the relationship between biological factors and collectivism, Fincher et al. (2008) compiled a comprehensive database of four different measures of individualism–collectivism for each nation in the world. These measures were drawn from global surveys (Hofstede, 1980; Gelfand et al., 2004), expert opinion (Suh et al., 1998), or language (e.g. pronoun) usage (Kashima and Kashima, 1998). Recently, Chiao and Blizinsky (2010) surveyed the literature for studies on the 5-HTTLPR from different countries and correlated the relative proportion of short and long alleles in the population of each country with that country’s individualism–collectivism score. A robust relationship was found such that the 5-HTTLPR short allele, which we have referred to here as a social sensitivity allele, was much more prevalent in collectivistic populations than individualistic populations.
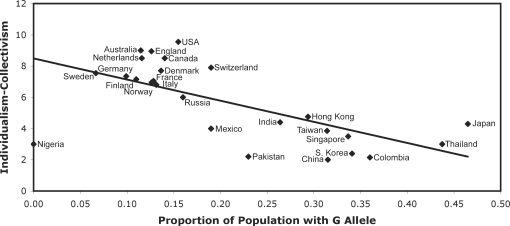
Using a similar approach, we have found a robust correlation (Way, B.M., Hunter, J.F., and Lieberman, M.D. manuscript in preparation; see Supplementary Material) between the A118G polymorphism and individualism–collectivism (Figure 1). Again, the putative social sensitivity allele, the G allele, was more prevalent in populations with greater collectivism. The relationship remained significant when per capita Gross Domestic Product was entered as a covariate as well as when latitude, a measure of historical climate as well as ultraviolet radiation exposure (Hancock et al., 2008), was controlled for. Also, when cultural region (Gupta et al., 2002) rather than nation was used as the unit of analysis, the relationship was significant.
Fig. 1.
Correlation between the proportion of the population with the G allele of the A118G polymorphism and individualism-collectivism [Suh et al., 1998; r(26) = 0.65, P < 0.001]; higher scores represent greater individualism and lower collectivism.
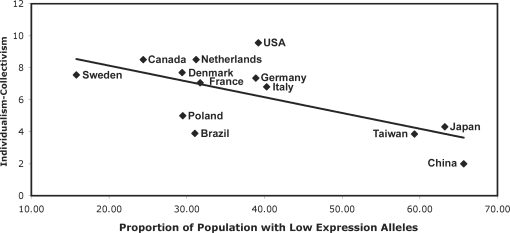
Although fewer countries have data on the allele frequency of the MAOA polymorphism, there was a significant correlation between allele prevalence and individualism–collectivism (Figure 2) as well. Consistent with the other two polymorphisms, the low expression alleles were more prevalent in collectivistic populations. Thus, in all three cases (5-HTTLPR, A118G, MAOA-uVNTR), the alleles hypothesized to influence social sensitivity were more prevalent in collectivistic cultures.
Fig. 2.
Correlation between the proportion of the population with low expression alleles of the MAOA-uVNTR polymorphism and individualism-collectivism [Suh et al., 1998; r(13) = 0.67, P < 0.05]; higher scores represent greater individualism and lower collectivism.
Across multiple genes then it appears that there is a relationship between allele frequency and cultural orientation. As these alleles have been associated with differences in psychological functioning, it suggests that incorporation of genetic variability into models of cross-cultural psychological differences may help elucidate the mechanisms underlying these differences. Unfortunately, African countries were under-represented in these analyses, making it difficult to determine if the relationship between genotype and cultural orientation exists only among non-African populations or across all populations.
National differences in the lifetime prevalence of major depression
These data also raise a fundamental question concerning the nature of the relationship between allele frequency and cultural orientation. In line with the social sensitivity hypothesis, a potential explanation for this relationship is that collectivism improves emotional well-being in populations with a high prevalence of social sensitivity alleles. One measure of well-being that has been studied in many of the countries with genetic data as well as individualism–collectivism data is depression. Therefore, national differences in the lifetime prevalence of depression may serve as one means of addressing the inter-relationship between social orientation, genotype and psychological state. In addition, at the genetic level, there is good reason to suspect that the polymorphisms discussed here may be associated with depression. The serotonin transporter and monoamine oxidase are targets of the two most commonly prescribed classes of antidepressants, selective serotonin reuptake inhibitors (e.g. Prozac) and monoamine oxidase inhibitors (e.g. Nardil), respectively. Agonists of the µ-opioid receptor also have antidepressant effects (Berrocoso et al., 2009).
In order to systematically compare levels of major depressive disorder across different countries, data were drawn from studies in the literature using diagnostic criteria from the third (American Psychiatric Association, 1980) or fourth edition (American Psychiatric Association, 1994) of the diagnostic and statistical manual for mental disorders (DSM). Although DSM criteria were derived according to Western cultural definitions of depression, and may not be sensitive to culturally specific symptoms (e.g. Kleinman, 1982), they offer good reliability across samples (Kessler et al., 2007). Only data from studies with large (e.g. n > 2000), nationally representative samples using interview-based diagnoses were included, which slightly reduced the size of the sample for analysis. The focus was on lifetime prevalence of major depression rather than the number of major depressive episodes in the last year due to variability associated with potential adverse events in this latter measure.
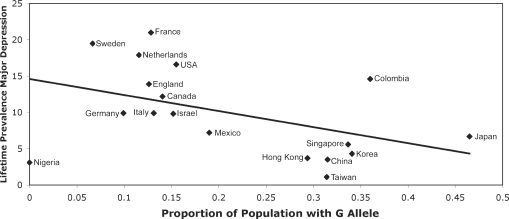
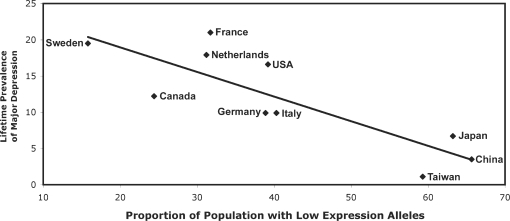
There was a negative relationship between the national prevalence of the G allele and depression (Figure 3) such that countries with a higher prevalence of the G allele in the population had lower levels of depression. A similar relationship was found for both the MAOA-uVNTR (Figure 4) and the 5-HTTLPR (Chiao and Blizinsky, 2010). Because individualism–collectivism was significantly correlated with the lifetime prevalence of depression, a mediation analysis was performed. For the A118G polymorphism, individualism–collectivism partially mediated the relationship between G allele frequency and depression. The mediation analysis for the MAOA-uVNTR was not significant, potentially due to a reduced sample size. This suggests that the reduced lifetime prevalence of depression in populations with a high prevalence of social sensitivity alleles may be due to the increased levels of collectivism in those populations.
Fig. 3.
Relationship between the proportion of the population with G allele of the A118G polymorphism and lifetime prevalence of major depression in each country [r(18) = 0.45, P = 0.05].
Fig. 4.
Relationship between the proportion of the population with low expression alleles of the MAOA-uVNTR polymorphism and lifetime prevalence of major depression in each country [r(11) = 0.83, P < 0.01].
Such an interpretation is consistent with individuals of East Asian descent living in the USA suffering higher levels of major depression than Asians living in Asia when using the same diagnostic criteria (Chang, 2002). Greater acculturation of Chinese immigrants to the USA is also associated with greater vulnerability to the depressogenic effects of stress than less acculturated immigrants (Hwang and Myers, 2007). Similarly, foreign-born Latino’s, another collectivist group, experience lower rates of depression than do Latino’s born in the USA (Escobar, 1998). This increase in psychopathology following relocating to an individualistic culture among members of ethnic groups with a high proportion of social sensitivity alleles extends to other phenotypes such as social anxiety (Okazaki, 1997) and subclinical depression (Tafarodi and Smith, 2001). This greater risk of psychopathology is also likely to extend to other phenotypes associated with the opioid and serotonin systems for which there is less available data, such as antisocial behavior and substance abuse. Taken together, these studies support the notion that collectivism can protect against psychopathology in populations with a high proportion of social sensitivity alleles.
SOCIAL SENSITIVITY ALLELES AND INDIVIDUALISM–COLLECTIVISM
A weakness of the correlational approach used here is the degree to which this pattern of results could occur as a result of random processes such as genetic drift (fluctuations in allele frequency due to chance; Keinan et al., 2007). At the present time, an insufficient number of the countries from this correlation are represented in the genetic databases [e.g. HapMap (International HapMap Consortium, 2005) and Human Genome Diversity Panel (Li et al., 2008)] to determine if the present results are significantly different from the frequency distribution of randomly selected alleles from representatives of each of these countries. If the relationship between social orientation and genotype remains significant once underlying random genetic variation due to demography is controlled, it would suggest that there were selective pressures whereby the presence of a particular culture increased the frequency of alleles that ‘fit’ this culture. Thus, individuals or groups with a greater innate proclivity to collectivist organization would have had greater fitness, causing these alleles to increase in frequency in collectivist cultures. Conversely, in individualistic cultures there may have been selective pressures decreasing the prevalence of social sensitivity alleles.
Alternatively, if the correlation is not demonstrated to be significantly different from background genetic variation, it would suggest that genetic selection at these loci is not the explanation for the correlation. Rather, it would suggest that collectivism was ‘stickier’, representing a better fit in populations with a high proportion of putative social sensitivity alleles (Lieberman, 2009). In other words, the psychological and behavioral tendencies associated with collectivism may have been more likely to have been adopted and transmitted in populations with a higher prevalence of such social sensitivity alleles. Psychologically, the more integrated social network characteristic of collectivistic cultures may have reduced the risk for psychopathology in these populations due to the high prevalence of sensitivity alleles. Similarly, individualism may have represented a better fit for populations with a low proportion of social sensitivity alleles where less reactivity to social rejection or exclusion would have been beneficial. This would be consistent with theories of gene–culture interaction that posit there are genetic influences creating psychological predispositions that favor the adoption of particular cultural content in a process of biased transmission (Richerson and Boyd, 2005; Henrich and McElreath, 2007). Thus in the first of these two accounts, culture would provide a pressure on genetic selection and in the second, preexisting gene distributions would provide a pressure on the kinds of cultures likely to emerge.
Ultimately, other methodologies will be necessary for clarifying the relationship between allele frequency, culture and measures of well-being like depressive symptomatology. One approach would be to incorporate genotyping into epidemiological studies of immigrants to each respective culture. For example, is greater cultural assimilation among East Asian immigrants to North America associated with higher risk for psychopathology in individuals with social sensitivity alleles? Conversely, does greater acculturation of Caucasian immigrants to East Asian cultures confer greater protection against psychopathology in individuals with social sensitivity alleles? Such studies would extend the cultural fit hypothesis (Ward and Chang, 1997; Caldwell-Harris and Aycicegi, 2006) from the psychological level to the genetic level. According to this hypothesis, psychological adjustment is highest when an individual’s personality profile and cultural orientation are similar to their cultural milieu. In other words, if this hypothesis were evaluated at the genetic level, individuals with a high proportion of social sensitivity alleles would be expected to have higher well-being in collectivistic cultural environments, while individuals with a low proportion of social sensitivity alleles would be expected to have higher well-being in individualistic cultural environments. Unfortunately, in most prior studies of acculturation cultural background and genotype have been confounded. The data presented here suggest that genotyping participants in such studies may help to clarify the nature of the interaction between culture, genetics and psychopathology.
In conclusion, there exists a robust relationship between the cultural construct individualism–collectivism and the prevalence of alleles at several polymorphisms with apparent psychological effects. As the knowledge base of the relationship between genetics and social cognition grows, there will be great refinement in our understanding of the relationship between psychological processes and genetic variation, including the variants discussed here. We hope that the this data will stimulate further theoretical developments as well as experimental studies that will ultimately shed new light onto processes that lie at the heart of cultural psychology.
Conflict of Interest
None declared.
Acknowledgments
This work was generously supported by grants from the Center for Culture, Brain, and Development and the UCLA Faculty Senate.
REFERENCES
- Adams G, Plaut V. The cultural grounding of personal relationship: friendship in North American and West African worlds. Personal Relationships. 2003;10(3):333–47. doi: 10.1037/0022-3514.88.6.948. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. DSM-III: Diagnostic and Statistical Manual of Mental Disorders. 3rd edn. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–54. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocoso E, Sanchez-Blazquez P, Garzon J, Mico J. Opiates as antidepressants. Current Pharmaceutical Design. 2009;15(14):1612–22. doi: 10.2174/138161209788168100. [DOI] [PubMed] [Google Scholar]
- Brody G, Beach S, Philibert R, et al. Parenting moderates a genetic vulnerability factor in longitudinal increases in youths' substance use. Journal of Consulting and Clinical Psychology. 2009a;77(1):1–11. doi: 10.1037/a0012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody G, Beach S, Philibert R, Chen Y, Murry V. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: gene x environment hypotheses tested via a randomized prevention design. Child Development. 2009b;80(3):645–61. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Krystal AD, Ashley-Koch A, et al. Sleep quality varies as a function of 5-HTTLPR genotype and stress. Psychosomatic Medicine. 2007;69(7):621–4. doi: 10.1097/PSY.0b013e31814b8de6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell-Harris C, Aycicegi A. When personality and culture clash: the psychological distress of allocentrics in an individualist culture and idiocentrics in a collectivist culture. Transcultural psychiatry. 2006;43(3):331–61. doi: 10.1177/1363461506066982. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–4. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chang DF. Understanding the rates and distribution of mental disorders. In: Kurasaki KS, Okazaki S, Sue S, editors. Asian American Mental Health: Assessments Theories and Methods. New York: Kluwer Academic; 2002. pp. 9–27. [Google Scholar]
- Chiao J, Ambady N. Cultural neuroscience: parsing universality and diversity across levels of analysis. Handbook of Cultural Psychology. 2007:237–54. [Google Scholar]
- Chiao J, Blizinsky K. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proceedings of the Royal Society B. 2010;277(1681):529–37. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Molecular Psychiatry. 2007;13(3):334–47. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain's response to social exclusion. Biological Psychiatry. 2007;61(9):1100–8. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Escobar J. Immigration and mental health: why are immigrants better off? Archives of General Psychiatry. 1998;55(9):781–2. doi: 10.1001/archpsyc.55.9.781. [DOI] [PubMed] [Google Scholar]
- Fincher CL, Thornhill R, Murray DR, Schaller M. Pathogen prevalence predicts human cross-cultural variability in individualism/collectivism. Proceedings of the Royal Society B: Biological Sciences. 2008;275(1640):1279–85. doi: 10.1098/rspb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske A, Kitayama S, Markus H, Nisbett R. The cultural matrix of social psychology. The Handbook of Social Psychology. 1998;2:915–81. [Google Scholar]
- Gelfand M, Bhawuk D, Nishii L, Bechtold D. Individualism and collectivism. In: House R, Hanges P, Javidan M, Dorfman P, Gupta V, editors. Culture, Leadership, and Organizations: The GLOBE Study of 62 Societies. Thousand Oaks, CA: Sage Publications; 2004. pp. 437–512. [Google Scholar]
- Ginovart N, Meyer JH, Boovariwala A, et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. Journal of Cerebral Blood Flow and Metababolism. 2006;26(3):330–44. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- Gupta V, Hanges P, Dorfman P. Cultural clusters: methodology and findings. Journal of World Business. 2002;37(1):11–5. [Google Scholar]
- Hancock A, Witonsky D, Gordon A, et al. Adaptations to climate in candidate genes for common metabolic disorders. PLoS Genetics. 2008;4(2):e32. doi: 10.1371/journal.pgen.0040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, McElreath R. Dual inheritance theory: the evolution of human cultural capacities and cultural evolution. In: Dunbar R, Barrett L, editors. Oxford Handbook of Evolutionary Psychology. Oxford University Press; 2007. pp. 555–70. [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacology Biochemistry and Behavior. 1978;9(2):213–20. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Hofstede G. Culture's Consequences: International Differences in Work-Related Values. Newbury Park, CA: Sage; 1980. [Google Scholar]
- Hwang W, Myers H. Major depression in Chinese Americans. Social Psychiatry and Psychiatric Epidemiology. 2007;42(3):189–97. doi: 10.1007/s00127-006-0152-1. [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437(7063):1299–320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima E, Kashima Y. Culture and language: the case of cultural dimensions and personal pronoun use. Journal of Cross Cultural Psychology. 1998;29:461–86. [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101(49):17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinan A, Mullikin J, Patterson N, Reich D. Measurement of the human allele frequency spectrum demonstrates greater genetic drift in East Asians than in Europeans. Nature genetics. 2007;39(10):1251–5. doi: 10.1038/ng2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Angermeyer M, Anthony J, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6(3):168–76. [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164(11):1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kim H, Sherman D, Taylor S. Culture and social support. American Psychologist. 2008;63(6):518–26. doi: 10.1037/0003-066X. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, et al. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11(10):903–13. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Huang YY, Haverly R, et al. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatric Genetics. 2009;19(3):126–33. doi: 10.1097/YPG.0b013e32832a50a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman A. Neurasthenia and depression: a study of somatization and culture in China. Culture, Medicine and Psychiatry. 1982;6(2):117–90. doi: 10.1007/BF00051427. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Balling U, Gross J, et al. Organization of the human serotonin transporter gene. Journal of Neural Transmission. 1994;95(2):157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. The apportionment of human diversity. Evolutionary biology. 1972;6(38):381–98. [Google Scholar]
- Li J, Lee S. Latent class analysis of antisocial behavior: interaction of serotonin transporter genotype and maltreatment. Journal of Abnormal Child Psychology. in press doi: 10.1007/s10802-010-9409-y. doi:10.1007/s10802-010-9409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Absher DM, Tang H, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319(5866):1100–4. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. What makes big ideas sticky? In: Brockman M, editor. What's Next? Dispatches on the future of science. New York: Vintage Books; 2009. [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29(5):651–68. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Markus H, Kitayama S. Culture and the self: implications for cognition, emotion, and motivation. Psychological review. 1991;98(2):224–53. [Google Scholar]
- Mehrabian A. Questionnaire measures of affiliative tendency and sensitivity to rejection. Psychological Reports. 1976;38:199–209. [Google Scholar]
- Nilsson KW, Sjöberg RL, Damberg M, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biological Psychiatry. 2006;59(2):121–7. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Okazaki S. Sources of ethnic differences between Asian American and White American college students on measures of depression and social anxiety. Journal of Abnormal Psychology. 1997;106:52–60. doi: 10.1037//0021-843x.106.1.52. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience : the foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biological Psychiatry. 1978a;13(5):607–18. [PubMed] [Google Scholar]
- Panksepp J, Vilberg T, Bean NJ, Coy DH, Kastin AJ. Reduction of distress vocalization in chicks by opiate-like peptides. Brain Research Bulletin. 1978b;3(6):663–7. doi: 10.1016/0361-9230(78)90014-x. [DOI] [PubMed] [Google Scholar]
- Richerson P, Boyd R. Not by genes alone: how culture transformed human evolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Journal of the American Medical Association. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32(9):2046–52. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Human Genetics. 1998;103(3):273–9. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annual Review of Neuroscience. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2007;33(2):312–19. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- Suh E, Diener E, Oishi S, Triandis H. The shifting basis of life satisfaction judgments across cultures: Emotions versus norms. Journal of Personality and Social Psychology. 1998;74:482–93. [Google Scholar]
- Tafarodi R, Smith A. Individualismñcollectivism and depressive sensitivity to life events: the case of Malaysian sojourners. International Journal of Intercultural Relations. 2001;25(1):73–88. [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60(7):671–6. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Ward C, Chang W. Cultural fit: a new perspective on personality and sojourner adjustment. International Journal of Intercultural Relations. 1997;21(4):525–33. [Google Scholar]
- Way BM, Gurbaxani BM. A genetics primer for social health research. Social and Personality Psychology Compass. 2008;2(2):785–816. [Google Scholar]
- Way BM, Taylor SE. Social influences on health: Is serotonin a critical mediator? Psychosomatic Medicine. 2010;72(2):107–12. doi: 10.1097/PSY.0b013e3181ce6a7d. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences. 2009;106(35):15079–84. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” Childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biological Psychiatry. 2006;60(7):684–9. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, Lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Science. 1995;57(5):411–41. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler H, Poling J, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry. 2009;66(11):1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. Collectivism among the Japanese: a perspective from the self. Cross-cultural Research and Methodology Series. 1994;18:175. [Google Scholar]
- Yamaguchi S, Kuhlman D, Sugimori S. Personality correlates of allocentric tendencies in individualist and collectivist cultures. Journal of Cross-Cultural Psychology. 1995;26(6):658–72. [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Archives of General Psychiatry. 2003;60(11):1145–53. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]