Abstract
Objective
To confirm that primary intervertebral disc cells cultured in monolayer transduced with adenovirus maintained their phenotype, hence is an appropriate system to test gene therapy agents.
Design
Adult bovine nucleus pulposus and anulus fibrosus cells cultured in monolayer were transduced with adenoviruses expressing human bone morphogenetic proteins (AdBMPs) or Sox9 (AdSox9), or green fluorescence protein (AdGFP, as control). Chondrocyte phenotypic markers (e.g., type II collagen and aggrecan) and the chondrocyte hypertrophy marker (type X collagen) were measured 6 days after viral transduction by reverse-transcription polymerase chain reaction.
Results
Primary nucleus pulposus and anulus fibrosus cells transduced with AdBMPs, AdSox9, or adenovirus-expressing green fluorescence protein only (AdGFP, as control) continue to express healthy chondrocyte phenotypic markers and showed no evidence of the expression of the chondrocyte hypertrophy marker (type X collagen gene). Thus, we have shown that bovine nucleus pulposus and anulus fibrosus cells transduced with adenovirus overexpressing 12 different bone morphogenetic proteins or Sox9 maintain their phenotype in short-term culture.
Conclusions
In this study, primary bovine intervertebral disc cells transduced with adenovirus overexpressing 12 bone morphogenetic proteins or Sox9 preserved their phenotype in short-term culture. These cells did not express the type X collagen gene, an undesirable chondrocyte hypertrophic gene that could lead to ossification. Therefore, low-passage intervertebral disc cells cultured in monolayer is an appropriate culture system to test therapeutic genes. We further suggest that these cells may also be appropriate for engineering tissues or for cell therapy for degenerative disc diseases.
Keywords: Intervertebral Disc, Bone Morphogenetic Proteins, Gene Expression, Phenotype, Extracellular Matrix
Lifetime prevalence of low-back pain in Western populations is ~58 – 84%,1 and the cost to society in direct medical care and loss of productivity is staggering.2 Because existing medical and surgical therapies have not adequately addressed the problem of symptomatic intervertebral disc (IVD) degeneration, many clinicians and researchers are seeking biological therapies. Physiatrists, armed with the skill set to access the IVD under fluoroscopic guidance, should take a leading role in developing and implementing biological treatments. The study of cultured IVD cells in vitro has been a powerful tool to evaluate the effects of biological or physical agents.
The maintenance of the IVD cell phenotype in culture is important because dedifferentiation may alter responses to cytokines or physical stimuli.3 Although a three-dimensional (3D) culture system such as the alginate bead culture system has been shown to support IVD cell phenotype and extracellular matrix production, it is difficult to assess viral transduction efficiency by direct fluorescence microscopy in alginate-encapsulated cells. Also, cells have limited expansion capacity when suspended in alginate, thus limiting the use of this system when more cells are needed. In our previous work, we studied the effects of transducing bovine nucleus pulposus (NP) and anulus fibrosus (AF) cells with a series of recombinant bone morphogenetic proteins (BMPs) and Sox9 on extracellular matrix metabolism.4 The main reason to use monolayer culture in these experiments is that transduction efficiency can be monitored very closely by visualizing green fluorescence protein (GFP). We have found that some of the growth factors tested have consistently stimulated the metabolic output of primary disc cells in monolayer culture. However, the possibility of an inappropriate or undesirable phenotypic response has not been excluded. For example, a potentially undesirable response is type X collagen expression, which is a marker for chondrocyte hypertrophy that might lead to tissue calcification or even ossification. In this study, we describe the phenotype of IVD cell cultured in monolayer, after viral transduction.
A major difficulty in describing disc cell phenotype is the lack of well-defined markers. Previous studies of disc cells indicated that their phenotypic profile is similar to those of articular chondrocytes.5-8 In hyaline cartilage, type II collagen is the major collagen species present; it is widely accepted as a marker of the “chondrocytic” phenotype.9 Similarly, IVD tissues contain collagen (types II, I, VI, III, IX, etc.)10 and proteoglycans (PG).11-14 However, the IVD has a much more complex structure than articular cartilage; there are clear morphologic and metabolic differences among cells from the NP, inner AF, and outer AF.15,16
In this study, we have selected aggrecan and type II collagen gene expression to monitor IVD cell phenotype. Proteoglycan synthesis has long been used as a chondrocyte-like phenotype marker because these macromolecules are responsible for many of the physicochemical properties of the disc and other proteoglycan-rich cartilaginous tissues.11,12,15,17 Aggrecan, the major aggregating proteoglycans in the human IVD, is a well-known marker of the IVD phenotype.18,19 The human NP and inner AF have been reported to contain predominantly type II collagen, whereas the outer AF contains significant amount of type I collagen.10,20,21 In the absence of clearly defined markers of IVD phenotype, the combination of type II collagen and aggrecan gene expression has often been used to show that cells maintain at least some of the important properties of the IVD-like phenotype.22
We studied the expression of the type X collagen gene to address the issue of potential ossification or calcification in the cells cultured in monolayer and transduced with adenovirus. Chondrocyte hypertrophy or collagen X expression or both could potentially lead to calcification within the IVD, which would prevent the desired restoration of the biomechanical characteristics of the organ. Therefore, a gene transfer approach that would stimulate IVD tissue calcification is not suited for disc repair purpose. The rationale for this study is again based on the similarities between articular chondrocytes and IVD cells. In the case of hypertrophic chondrocytes, the most widely used marker has been type X collagen.23 During endochondral bone formation, type X collagen is expressed in hypertrophic (enlarged) chondrocytes24 and plays a key role in regulating matrix mineralization.25 Furthermore, type X collagen gene expression was detected in chondrocytes present in osteoarthritic tissue in areas where there appeared to be a reinitiation of endochondral bone formation.26 In the human IVD, type X collagen has been described in the extracellular matrix of immature discs (<2 yrs of age).27 In adult IVDs, on the other hand, type X collagen is seen only in the aged nucleus and inner anulus; this appearance of type X collagen is likely to be related to advanced disc degeneration.27,28 Although it is unknown whether the reappearance of type X collagen is related to disc calcification, there is a theoretical risk for ossification when the gene expression of this collagen is reactivated.
The main goal of the present work is to confirm that low-passage IVD cells cultured in monolayer transduced with AdBMPs or AdSox9 would express only the desirable chondrocytic phenotype marker genes (i.e., type II collagen and aggrecan). We aim to show that type X collagen gene expression, a chondrocyte hypertrophy marker associated with subsequent mineralization, did not occur. The cells in the inner AF seem to produce a matrix that shares properties with both the outer AF and NP.15,16 Data on the inner AF were not shown because it seems to be a blend of NP and AF. The long-term goal of this work is to provide basic information on the response of IVD cells to growth factor treatment and gene transfer, thus providing essential building blocks for future clinical applications of this technology.
METHODS
Bovine Tissue Collection
Bovine IVDs were dissected, as previously described,16 from young adult bovine tails (15–18 mos old) obtained from a local slaughterhouse. It is worth noting that, by 15 mos of age, bovine discs no longer contain a significant proportion of notochordal cells; the matrix is populated predominantly by adult cells.29 The NP tissue was carefully separated from the AF tissue with a scalpel. The AF tissue was further separated into inner AF (inner 1/3 of the AF tissue) and outer AF tissue (outer 2/3 of the AF tissue); only the outer AF tissue was used, the inner AF tissue was discarded. These tissues were used for cell culture and RNA extraction.
As a positive control tissue, a bovine fetus was also obtained from the slaughterhouse; the precise term of gestation was unknown. The femurs of the fetus were isolated, and the growth plates (GPs) on each end of the femurs were isolated and subjected to RNA extraction.
Cell Cultures
The bovine NP and AF tissues were dissected as described earlier and minced to ~1–2 mm3 in size. The cells were released using serial enzymatic digestion, as previously described,16 and subsequently cultured in monolayer at 4 × 104 cells/cm2 overnight in complete medium (Dulbecco’s modified Eagle’s medium and Ham’s F-12 medium [Mediatech, Herndon, VA] with 360 μg/ml of l-glutamate (Mediatech), 50 μg/ml of gentamicin (Gibco BRL, Grand Island, NY), 25 μg/ml of ascorbic acid (Sigma-Aldrich, St. Louis, MO), and fetal bovine serum at 20% (Hyclone, Logan, UT]). The next day, the NP and AF cell cultures were assigned to 1 of 16 groups: the no-treatment group, the rhBMP-7 (positive control), the AdGFP group (negative control), and the AdBMP-2, -3, -4, -5, -7, -8, -10, -11, -12, -13, -14, -15, and Sox9 groups. In the rhBMP-7 group, cells were cultured without any exogenous gene in the presence of recombinant human BMP-7 (also referred to as osteogenic protein-1, a gift from Stryker Biotech, Hopkinton, MA) at 100 ng/ml during the first day of culture. In the AdGFP, AdBMP-2, -3, -4, -5, -7, -8, -10, -11, -12, -13, -14, -15, and Sox9 groups, adenovirus-expressing GFP only, or both GFP and BMPs (AdBMPs) or both GFP and Sox 9 (AdSox9) were included in the cultures at the time of plating for 16 hrs at an optimized multiplicity of infection (at about 1:100). In each case, by visualizing GFP-positive cells under a fluorescence microscope, the transduction efficiency was confirmed to be about 90%. The cultures were maintained for 6 days after transduction at 37°C with daily changes of medium. At day 6 of culture, cells grow to confluence and were harvested for analysis by reverse-transcription polymerase chain reaction (RT-PCR) (described here) or extracellular matrix accumulation (published in two manuscripts previously).4,30 Briefly, at the end of the culture period, the cells and extracellular matrix were digested with papain within each culture well.31 The papain digests were analyzed for contents of total sulfated PG by the dimethylmethylene blue (Polysciences, Inc., Warrington, PA) dye-binding method.12 Hydroxyproline, as a measure of collagen, was quantified by reverse-phase high performance liquid chromatography after hydrolysis with 6 M hydrochloric acid for 16 hrs at 120°C and derivatization with phenylisothiocyanate.32 The papain digests of cells were analyzed for DNA content using the Hoechst dye method (Hoechst 33,258: Polysciences, Inc.).31 One hundred microliters of each papain-digested sample was mixed with 1 μg/ml of Hoechst dye solution. The dye/sample complex was excited with ultraviolet light (wavelength, 360 nm), and the emission at 460 nm was measured. Calf thymus DNA type I (Sigma Chemical) was used as a standard.
RNA Extraction
Total RNA was isolated from bovine cells cultured in monolayers, using the RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions, on day 6 postviral infection. Total cellular RNA was also extracted from the bovine IVD tissues and GP tissue. Briefly, ~200 mg of tissues were subjected to homogenization using a rotor-stator device (Omni International, Marietta, GA), and RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by further purification using the RNeasy kit.
Measurement of mRNA Levels with the RT-PCR
To compare the relative levels of chondrocyte-specific gene expression in transduced NP and AF cells, mRNA levels were assessed using the RT-PCR method. The first-strand cDNA was synthesized using Superscript III reverse transcriptase (Invitrogen) and oligo(dT) primers from 500 ng of total RNA. PCR amplification was performed with the platinum Taq DNA polymerase (Invitrogen), using primers paired for each gene of interest. Preliminary experiments were carried out for each gene to select the optimal number of cycles to enable the amplification reaction to proceed in a linear range for semiquantitative analysis. PCR amplification of a constitutively expressed gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was used as a control for the amount of input RNA. The sequences of the primers and the GenBank or Ensembl accession numbers of the corresponding genes are shown in Table 1. PCR products were separated on 2% agarose gels in the presence of ethidium bromide and visualized using a Bio-Rad Gel Doc EQ imaging system (Bio-Rad, Hercules, CA).
TABLE 1.
PCR primer sets
| Gene | Primer Sequence: Sense/Antisense | Product Size | GenBank Accession |
|---|---|---|---|
| Col Iα2 | 5′-TCAGAGCATTGTGCAATAC-3′ 5′-GTGGATCACACTCACAGG-3′ |
268 bp | NM_174520 |
| Col IIα1 | 5′-TCTAAGAGACCTGAACTGGG-3′ 5′-CAGAGGTGTTTGACACAG-3′ |
230 bp | X02420 |
| Aggrecan | 5′-GCTACACAGGTGAAGACTTTGTGG-3′ 5′-TTCACCCTCAGTGATATTTCGGG-3′ |
134 bp | U76615 |
| Col Xα1 | 5′-CTAAGTGGCCCCTTTTGTC-3′ 5′-GCTTTCAGCAATCCACAG-3′ |
161 bp | NM_174634 |
| GAPDH | 5′-CAACGTGTCTGTTGTGGA-3′ 5′-GCTGTAGCCAAATTCATTG-3′ |
250 bp | U85042 |
RESULTS
Native Bovine IVD Tissue Gene Expression by RT-PCR
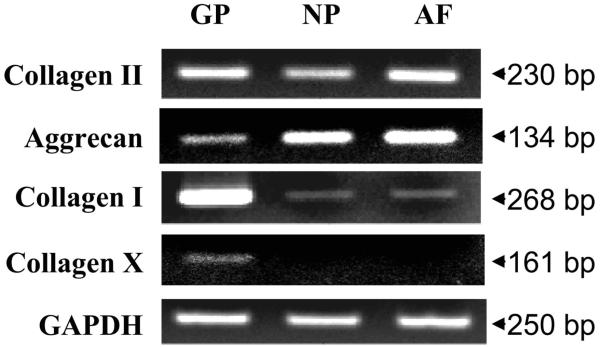
Fetal bovine GP tissue was included in this study as a positive control. The GP expressed significant levels of type I collagen, type II collagen, aggrecan, and type X collagen mRNA. In contrast, tissues from young adult bovine NP and outer AF expressed types I and II collagen, as well as aggrecan mRNA, but not significant type X collagen gene expression (Fig. 1). Interestingly, when type X collagen gene is amplified for 35 cycles, we have noted a faint band of type X collagen PCR product in the NP and AF tissues (data not shown). This may indicate that the type X collagen gene is expressed, but at a very low level, in young, normal bovine IVD tissues.
FIGURE 1.
Chondrocyte-phenotype marker and hypertrophy marker gene expression in fetal bovine growth plate (GP), adult bovine nucleus pulposus (NP), and anulus fibrosus (AF) tissues.
Cultured Bovine NP Cells Expressed Chondrocyte-Phenotype Marker Genes
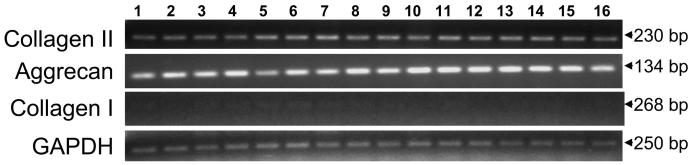
Primary NP cells derived from a young adult bovine animal cultured in monolayer were passaged once (P1) and either stimulated with rhBMP-7 or transduced with AdBMPs or AdSox9. Cultured cells were confluent on day 6. Total cellular RNA was extracted and subjected to RT-PCR studies. Cultured NP cells continue to express type II collagen and aggrecan genes, but only minimal expression of type I collagen gene was detected (Fig. 2). Our results suggest that primary bovine NP cells can preserve their chondrocytic phenotype when transduced with a series of AdBMPs or AdSox9.
FIGURE 2.
Bovine nucleus pulposus (NP) cells transduced with AdBMPs and AdSox9 continue to express chondrocyte-phenotype marker genes. Lanes 1, No treatment; 2, rhBMP-7; 3, AdGFP; 4, AdBMP-2; 5, AdBMP-3; 6, AdBMP-4; 7, AdBMP-5; 8, AdBMP-7; 9, AdBMP-8; 10, AdBMP-10; 11, AdBMP-11; 12, AdBMP-12; 13, AdBMP-13; 14, AdBMP-14; 15, AdBMP-15; 16, AdSox9.
Cultured Bovine AF Cells Expressed Chondrocyte-Phenotype Marker Genes
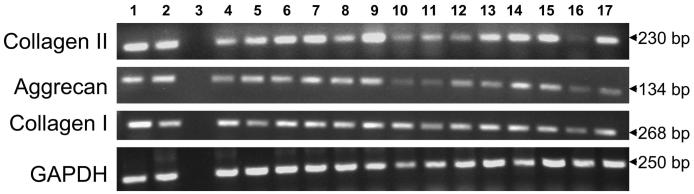
Primary bovine AF cells (P1) cultured in monolayer were stimulated with rhBMP-7 or transduced with AdBMPs or AdSox9. Figure 3 shows chondrocyte phenotypic marker gene expression examined by RT-PCR on day 6 after transduction. Not surprisingly, we have detected significant levels of not only aggrecan and type II collagen but also type I collagen gene expression, both with and without the overexpression of various BMPs or Sox9. Similar to NP cells, primary AF cells in culture did not express type X collagen gene (Fig. 4B). These results suggest that bovine AF cells cultured in monolayer for a short-term preserved their chondrocyte-like phenotype. In addition to the chondrocyte phenotype-marker genes, type I collagen gene is also expressed; this observation underlines the phenotype difference between the NP and AF cells.
FIGURE 3.
Bovine anulus fibrosus (AF) cells transduced with AdBMPs and AdSox9 continue to express chondrocyte-phenotype marker genes and the type I collagen gene. Lanes 1, No treatment; 2, rhBMP-7; 3, empty lane; 4, AdGFP; 5, AdBMP-2; 6, AdBMP-3; 7, AdBMP-4; 8, AdBMP-5; 9, AdBMP-7; 10, AdBMP-8; 11, AdBMP-10; 12, AdBMP-11; 13, AdBMP-12; 14, AdBMP-13; 15, AdBMP-14; 16, AdBMP-15; 17, AdSox9.
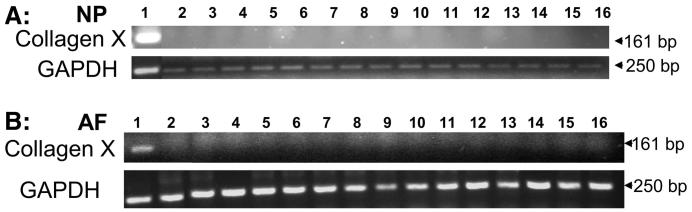
FIGURE 4.
Bovine IVD cells transduced with AdBMPs and AdSox9 did not express the type X collagen gene, a chondrocyte hypertrophy marker gene. Panel A, fetal bovine growth plate (as positive control) and nucleus pulposus (NP) cells; Panel B, fetal bovine growth plate and anulus fibrosus (AF) cells. Lanes 1, fetal bovine growth plate; 2, no treatment; 3, AdGFP; 4, AdBMP-2; 5, AdBMP-3; 6, AdBMP-4; 7, AdBMP-5; 8, AdBMP-7; 9, AdBMP-8; 10, AdBMP-10; 11, AdBMP-11; 12, AdBMP-12; 13, AdBMP-13; 14, AdBMP-14; 15, AdBMP-15; 16, AdSox9.
No Chondrocyte Hypertrophy Marker Gene Expression in Cultured NP or AF Cells
Primary NP cells cultured in monolayer transduced with any of the AdBMPs or AdSox9 did not express significant levels of type X collagen gene, a marker of chondrocyte hypertrophy (Fig. 4A). Similar results were observed when primary AF cells were cultured in monolayer transduced with any of the AdBMPs or AdSox9 (Fig. 4B). Because the GP expressed the type X collagen gene, the total RNA sample extracted from fetal bovine GP tissue was used as a positive control (lane 1). Cells cultured without any treatment (lane 2) also were included as controls.
In summary, primary NP and AF cells cultured in monolayer continued to express chondrocyte marker genes (e.g., type II collagen and aggrecan) but did not express the hypertrophy marker (type X collagen) gene.
DISCUSSION
IVD cell phenotypic profiles are similar to those of chondrocytes.5,6 The gene expression of type II collagen and aggrecan was used in this study because their production is generally considered to be a marker of the “chondrocytic” phenotype.9 Recently, other phenotypic characteristics of the IVD tissue, such as hypoxia-inducing factor-1, glucose transporter-1, and metalloproteinase-2, have been described as potential molecular markers for NP cells.33,34 In articular chondrocytes, (V + C)– fibronectin is a unique characteristic for the chondrocytic phenotype, although this splice form has not been studied in the human IVD yet.35 These valuable markers were not studied here; future studies are indicated for selected, promising growth factors in IVD repair. Specifically, studies implementing powerful techniques such as PCR array, which allow simultaneous study of a large number of genes, are warranted to describe further the molecular markers of NP and AF cell phenotype in their native state and in response to biological stimuli. In addition, collagen II and aggrecan gene expression varied, sometimes markedly, among different interventions. This was particularly true in the case of AF cells where there seemed to be significant suppression of gene expression in response to certain growth factors (e.g., AdBMP-15). These results, if confirmed, may indicate that selected growth factors are not suitable candidates for stimulating IVD repair.
Collagen types vary across the disc. In human IVDs, type II collagen (protein) is found in the NP and AF, whereas type I collagen (protein) is found mainly in the AF.20,36 To our surprise, we found that the gene expression profile was similar in bovine NP and AF tissues (Fig. 1). This finding shows that the mRNA level may not correspond to the amount of protein synthesized. The mechanism regulating protein production might be at posttranscriptional, translational, or even at protein stability levels, resulting in a difference in net protein content in different regions of the IVD. Another observation is that type I collagen gene expression is minimal in cultured bovine NP cells; in contrast, its expression is significant in the AF cells. This observation further indicates the complexities in the regulation of collagen protein production in different regions of the bovine IVD and underscores the limitation of this line of study.
We have shown that primary bovine NP and AF cells cultured in monolayer for a short term at a moderately high density (4 × 104 cells per cm2) preserved their chondrocytic phenotype in short-term cultures. Similarly, Horner et al.15 found that bovine NP cells passaged twice (P2) in monolayer at a density slightly lower than what we have used (1.3 × 104 cells per cm2) still preserve their chondrocytic phenotype. Not surprisingly, NP and AF cells cultured in monolayer at very low density (5 × 103 cells per cm2) undergo severe dedifferentiation and eventually cell death.37 The earlier findings suggest that culturing IVD cells at high density is necessary for cell transplantation or tissue-engineering purposes. Although we did not study cells cultured for longer than 2 wks, we expect that in longer term cultures (6 wks or more), collagen gene expression profile may change, as in cultured articular chondrocytes.38
One of the concerns associated with the delivery of growth factors for disc regeneration is that some of these growth factors (e.g., BMP-2 or -7) are known to promote osteogenesis under certain conditions. Several of the BMPs have even been used clinically to promote bone repair.39,40 Furthermore, intradiscal calcification has been observed in degenerative human discs. Although no intradiscal calcification has been associated with treatment with recombinant BMP-7 in the rabbit disc injury model,41 the theoretical possibility of intradiscal bone formation with growth factor treatments persists. Type X collagen gene expression has been detected in osteoarthritic tissue reinitiating endochondral bone formation.26 Neither type X collagen protein10,28 nor type X collagen gene expression was detected in normal human disc.28 However, type X collagen has also been described in aging28 and degenerative27 human IVDs, likely correlating with intradiscal calcification. In this study, we have selected type X collagen gene expression as a marker likely to precede calcification.
In native bovine IVD tissue, when the PCR is carried out in 35 cycles, we did notice a faint band in the position consistent with the molecular weight of type X collagen gene (Fig. 1). This finding may suggest that a very low level of type X collagen gene expression exists in the native bovine tissue. In cultured primary NP or AF cells, after a 6-day treatment with adenovirus overexpressing a panel of growth factors or Sox9, reactivating expression of this gene was not observed.
It is worth noting that in this study, the expression of collagen type II and aggrecan genes seems to be regulated by transducing the cells with adenovirus overexpressing various growth factors or Sox9. However, the level of changes cannot be measured and compared reliably by routine RT-PCR, which requires a 2-fold or more change to be considered significant. Instead, these changes may be assessed by the real-time PCR method, which is a more sensitive method to quantify changes in gene expression. In future studies, the relative changes in gene expression may be measured by real-time PCR after treatment with growth factor(s) of interest. This may serve as an indicator of efficacy of the particular growth factor(s) treatment. Furthermore, exposure to most of the selected growth factors resulted in an increase in total PG accumulation by both NP and AF cells.4,30 Additional studies are thus indicated to determine whether this PG accumulation is the result of increased PG production or decreased PG degradation.
It is known that monolayer culture of articular chondrocytes can lead to a loss of the chondrogenic phenotype with multiple passages of the cells. For instance, Kuettner et al.9,42 showed that bovine adult articular chondrocytes would retain their chondrocytic phenotype for up to 3 wks when cultured as high-density monolayers, but would dedifferentiate after this point. For this reason, alternative culture systems have been sought that would allow a 3D growth of the cells that is more similar to the native environment where articular chondrocytes have been shown to maintain their phenotype for months.43 Similar findings have been made for IVD cells.15,44 A body of literature has demonstrated the superiority of the 3D culture system in preserving chondrocyte-like phenotype. Unfortunately, 3D culture systems have certain disadvantages. First, it is not possible to assess transduction efficiency by fluoroscopy without dissolving the culture sphere. Second, the ability of cells in 3D culture systems to expand markedly in cell number is generally low because the rate of cell division in these systems is typically low. Thus, the use of a monolayer culture system is desirable for certain applications in cell-based tissue engineering for the degenerative disc.
Data derived from this study suggest that the primary IVD cells from the NP and outer AF cultured in monolayer continue to express genes appropriate for their tissue of origin. When these cells were transduced with adenovirus overexpressing growth factors or Sox9, they preserved their native phenotype. In addition, these cells did not express the type X collagen gene, suggesting that these cells show no tendency to form an altered phenotype similar to that of hypertrophic chondrocytes. This information is important to those seeking a cell-based tissue-engineering strategy for symptomatic disc degeneration.
In summary, physiatrists have the skill set to access the IVD under fluoroscopic guidance, without open surgery. Therefore, physiatrists should collaborate closely with surgeons and researchers in basic sciences in developing and implementing biological treatments. In our previous studies, we investigated the role of gene transfer in restoring IVD tissue structure. Our current data suggest that a monolayer culture system can be used successfully without significant change in cellular phenotype, even with growth factor stimulation, as long as cells are plated at high density for a short-term culture. On the basis of these findings, we conclude that low-passage IVD cells cultured in monolayer is an appropriate culture system to test therapeutic genes. We further suggest that these cells may also be appropriate for engineering tissues or for cell therapy for degenerative disc diseases.
ACKNOWLEDGMENTS
We thank Ms. Mary Ellen Lenz for help in the preparation of the manuscript and Drs. Hong Chen, Chuen-Liu Zhu, Jorge Raman-Blas, and Ms. Yiding Shen for technical assistance. We also thank Drs. Thomas M. Schmid, Irving Shapiro, Masahiro Iwamoto, and Maurizio Pacifici for valuable insights and discussions and Dr. Alvaro Sandroni for statistical advice.
Footnotes
Disclosures:
Supported by the NICHHD (1K08 HD049598-01) (to Y.Z.).
REFERENCES
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Demoor-Fossard M, Boittin M, Redini F, et al. Differential effects of interleukin-1 and transforming growth factor β on the synthesis of small proteoglycans by rabbit articular chondrocytes cultured in alginate beads as compared to monolayers. Mol Cell Biochem. 1999;199:69–80. doi: 10.1023/a:1006947015094. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, An HS, Thonar EJ, et al. Comparative effects of adenovirus-expressing bone morphogenetic proteins and Sox9 overexpression on extracellular matrix metabolism of bovine nucleus pulposus cells. Spine. 2006;31:2173–9. doi: 10.1097/01.brs.0000232792.66632.d8. [DOI] [PubMed] [Google Scholar]
- 5.Boos N, Weissbach S, Rohrbach H, et al. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Poiraudeau S, Monteiro I, Anract P, et al. Phenotypic characteristics of rabbit intervertebral disc cells: Comparison with cartilage cells from the same animals. Spine. 1999;24:837–44. doi: 10.1097/00007632-199905010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Richardson SM, Mobasheri A, Freemont AJ, et al. Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol. 2007;22:1033–41. doi: 10.14670/HH-22.1033. [DOI] [PubMed] [Google Scholar]
- 8.Freemont AJ, Watkins A, Le Maitre C, et al. Current understanding of cellular and molecular events in intervertebral disc degeneration: Implications for therapy. J Pathol. 2002;196:374–9. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 9.Kuettner KE, Pauli BU, Gall G, et al. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–50. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyre DR. Biochemistry of the intervertebral disc. Int Rev Connect Tissue Res. 1979;8:227–91. doi: 10.1016/b978-0-12-363708-6.50012-6. [DOI] [PubMed] [Google Scholar]
- 11.Buckwalter JA, Pedrini-Mille A, Pedrini V, et al. Proteoglycans of human infant intervertebral disc: Electron microscopic and biochemical studies. J Bone Joint Surg Am. 1985;67:284–94. [PubMed] [Google Scholar]
- 12.Johnstone B, Bayliss MT. The large proteoglycans of the human intervertebral disc: Changes in their biosynthesis and structure with age, topography, and pathology. Spine. 1995;20:674–84. doi: 10.1097/00007632-199503150-00008. [DOI] [PubMed] [Google Scholar]
- 13.Roberts S, Caterson B, Evans H, et al. Proteoglycan components of the intervertebral disc and cartilage endplate: An immunolocalization study of animal and human tissues. Histochem J. 1994;26:402–11. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- 14.Sztrolovics R, White RJ, Roughley PJ, et al. The mechanism of aggrecan release from cartilage differs with tissue origin and the agent used to stimulate catabolism. Biochem J. 2002;362:465–72. doi: 10.1042/0264-6021:3620465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horner HA, Roberts S, Bielby RC, et al. Cells from different regions of the intervertebral disc: Effect of culture system on matrix expression and cell phenotype. Spine. 2002;27:1018–28. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, An HS, Song S, et al. Growth factor osteogenic protein-1: Differing effects on cells from three distinct zones in the bovine intervertebral disc. Am J Phys Med Rehabil. 2004;83:515–21. doi: 10.1097/01.phm.0000130031.64343.59. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter JA, Mow VC, Boden S, et al. Intervertebral disk structure, composition, and mechanical function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. , American Academy of Orthopaedic Surgeons; Rosemont, IL: 2000. [Google Scholar]
- 18.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans. 2002;30:869–74. doi: 10.1042/bst0300869. [DOI] [PubMed] [Google Scholar]
- 19.Roughley PJ, Melching LI, Heathfield TF, et al. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(suppl 3):S326–S332. doi: 10.1007/s00586-006-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 21.Nerlich AG, Boos N, Wiest I, et al. Immunolocalization of major interstitial collagen types in human lumbar intervertebral discs of various ages. Virchows Arch. 1998;432:67–76. doi: 10.1007/s004280050136. [DOI] [PubMed] [Google Scholar]
- 22.Wang JY, Baer AE, Kraus VB, et al. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001;26:1747–51. doi: 10.1097/00007632-200108150-00003. discussion 1752. [DOI] [PubMed] [Google Scholar]
- 23.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–7. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985;100:598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid TM, Bonen DK, Luchene L, et al. Late events in chondrocyte differentiation: hypertrophy, type X collagen synthesis and matrix calcification. In Vivo. 1991;5:533–40. [PubMed] [Google Scholar]
- 26.Hoyland JA, Thomas JT, Donn R, et al. Distribution of type X collagen mRNA in normal and osteoarthritic human cartilage. Bone Miner. 1991;15:151–63. doi: 10.1016/0169-6009(91)90005-k. [DOI] [PubMed] [Google Scholar]
- 27.Boos N, Nerlich AG, Wiest I, et al. Immunolocalization of type X collagen in human lumbar intervertebral discs during aging and degeneration. Histochem Cell Biol. 1997;108:471–80. doi: 10.1007/s004180050187. [DOI] [PubMed] [Google Scholar]
- 28.Aigner T, Gresk-Otter KR, Fairbank JC, et al. Variation with age in the pattern of type X collagen expression in normal and scoliotic human intervertebral discs. Calcif Tissue Int. 1998;63:263–8. doi: 10.1007/s002239900524. [DOI] [PubMed] [Google Scholar]
- 29.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–37. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Anderson DG, Phillips FM, et al. Comparative effects of bone morphogenetic proteins and Sox9 overexpression on matrix accumulation by bovine anulus fibrosus cells: Implications for anular repair. Spine. 2007;32:2515–20. doi: 10.1097/BRS.0b013e318158cc09. [DOI] [PubMed] [Google Scholar]
- 31.Mok SS, Masuda K, Hauselmann HJ, et al. Aggrecan synthesized by mature bovine chondrocytes suspended in alginate: Identification of two distinct metabolic matrix pools. J Biol Chem. 1994;269:33021–7. [PubMed] [Google Scholar]
- 32.Dunphy MJ, Bhide MV, Smith DJ. Determination of hydroxyproline in tissue collagen hydrolysate by derivatization and isocratic reversed-phase high-performance liquid chromatography. J Chromatogr. 1987;420:394–7. doi: 10.1016/0378-4347(87)80195-0. [DOI] [PubMed] [Google Scholar]
- 33.Rajpurohit R, Risbud MV, Ducheyne P, et al. Phenotypic characteristics of the nucleus pulposus: Expression of hypoxia-inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–7. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 34.Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF-1α under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–9. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 35.Stewart MC, Saunders KM, Burton-Wurster N, et al. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J Bone Miner Res. 2000;15:166–74. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 36.Eyre DR, Matsui Y, Wu JJ. Collagen polymorphisms of the intervertebral disc. Biochem Soc Trans. 2002;30:844–8. doi: 10.1042/bst0300844. [DOI] [PubMed] [Google Scholar]
- 37.Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: Effect of degeneration and culture system on cell phenotype. Spine. 2005;30:2743–8. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 38.Marlovits S, Hombauer M, Truppe M, et al. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J Bone Joint Surg Br. 2004;86:286–95. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton DK, Jones-Quaidoo SM, Sansur C, et al. Outcomes of bone morphogenetic protein-2 in mature adults: Posterolateral non-instrument-assisted lumbar decompression and fusion. Surg Neurol. 2008;69:457–61. doi: 10.1016/j.surneu.2007.09.008. discussion 461–2. [DOI] [PubMed] [Google Scholar]
- 40.White AP, Vaccaro AR, Hall JA, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–41. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. discussion 32. [DOI] [PubMed] [Google Scholar]
- 42.Kuettner KE, Memoli VA, Pauli BU, et al. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. II. Maintenance of collagen and proteoglycan phenotype. J Cell Biol. 1982;93:751–7. doi: 10.1083/jcb.93.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauselmann HJ, Fernandes RJ, Mok SS, et al. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 44.Gruber HE, Fisher EC, Jr, Desai B, et al. Human intervertebral disc cells from the annulus: Three-dimensional culture in agarose or alginate and responsiveness to TGF-β1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]