Abstract
Murine intestinal intraepithelial lymphocytes (IELs) can be classified according to expression of a CD43 glycoform recognized by the S7 monoclonal antibody. In this study, we examined the response of S7+ and S7- IELs in mice during acute reovirus serotype 3 (Dearing strain) infection, which was confirmed by virus-specific real-time PCR. In vivo proliferation increased significantly for both S7- and S7+ IELs on day 4 post-infection as determined by BrdU incorporation; however, expression of the inducible costimulatory (ICOS) molecule, which peaked on day 7 post-infection, was upregulated on S7+ CD4+ T cells, most of which were CD4+8- IELs. In vitro ICOS stimulation by syngeneic peritoneal macrophages induced IFN-γ secretion from IELs from day 7 infected mice, and was suppressed by treatment with anti-ICOS mAb. Additionally, IFN-γ mRNA increased in CD4+ IELs on day 6 post-infection. These findings indicate that S7- and S7+ IELs are differentially mobilized during the immune response to reovirus infection; that the regulated expression of ICOS is associated with S7+ IELs; and that stimulation of IELs through ICOS enhances IFN-γ synthesis during infection.
Keywords: Cell activation, Cell surface molecule, Intestinal, T cell, Rodent, Viral
Introduction
CD43 is expressed at high density on the surface of bone marrow cells, thymocytes, and peripheral T cells and some B cells [1-3]. Functionally, the CD43 molecule contributes to T cell recognition and activation [4-7] and has costimulatory activity with TCR/CD3 stimulation [8-12]. As part of our studies aimed at defining effector and regulatory populations of T cells in the gut epithelium, we recently determined that small intestinal intraepithelial lymphocytes (IELs) can be divided according to whether they express or lack a CD43 glycoform recognized by the S7 mAb to mouse CD43 [13]. That reagent demarcates two functional groups of IELs such that S7+ cells produce significantly more Th1 and Th2 cytokines, are more cytotoxic, and undergo more homeostatic proliferation than CD43 S7- IELs [13]. Additionally, CD43 S7+ IELs from IL-10-/- mice with intestinal inflammation spontaneously produce IFN-γ in the terminal ileum [13]. Consistent with those findings, gene array profiles of CD43 S7+ and CD43 S7- IELs revealed that CD43 S7+ IELs express genes of activated T cells (cd6, cd44, cd97) and genes of T cell-associated chemotactic and/or inflammatory mediators (CCR2, CXCR3, MIP-1β) [13]. In contrast, CD43 S7- IELs preferentially express NK activating/inhibitory receptor genes (Ly49E-GE, Ly49G.2, CD94/NKG2, Ly49H, Ly49C). Thus, CD43 S7+ IELs exhibit properties typically associated with adaptive immunity, whereas S7- IELs exhibit properties of innate immunity [13].
Respiratory enteric orphan virus (reovirus) is a non-enveloped RNA virus. Both serotype 1 (Lang strain) and serotype 3 (Dearing strain) have been extensively used for murine studies of enteric virus infection given that both elicit strong anti-viral immune responses [14-16]. Oral infection of adult mice with reovirus serotype 1 or 3 generally results in a self-limiting infection [17]; however, a role for the immune response in curtailing the infectious process is evident from studies in severe-combined immunodeficient (SCID) mice, which develop systemic reovirus infections leading to death of the host [17]. Anti-reovirus IEL cytotoxic T cell responses mediated principally by TCRαβ IELs develop in mice following oral infection with reovirus type 1 [18], and antigen-specific T cells and CTL have been shown to develop in response to reovirus type 1 and 3 infection [19-21]. Peyer’s patch dendritic cells appear to play a role in antigen presentation to CD4+ T cells [22]. The participation of gut γδ T cells in immunity to reovirus remains unclear and natural killer cells, although present in significant numbers in the mouse intestinal mucosa, do not appear to contribute to the protective immune response to reovirus as witnessed by susceptibility of SCID mice [17], which have high levels of natural killer cells.
The goal of the present study was to determine the extent to which CD43 S7+ and S7- IELs are involved in the intestinal immune response to reovirus infection. In so doing, we have evaluated the in vivo proliferative changes of S7+ and S7- IELs during infection, and have examined the functional contribution of the ICOS costimulatory molecule in the local immune response to infection. As reported here, ICOS expression was upregulated on S7+ CD4+ IELs at day 7 post-infection. Moreover, stimulation of IELs through ICOS at day 7 post-infection enhanced IFN-γ synthesis. These findings indicate that S7 is an effective marker for differentiating murine IELs according to functional activity during a natural intestinal immune response in ways that have not been approached hitherto.
Materials and methods
Mice and reagents
Adult female C57BL/6 mice, 6-8 weeks of age, were purchased from Harlan Spargue-Dawley; Indiana, IN. Mice were housed under specific pathogen free conditions. Animals were used according to protocols approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Houston. Cell culture medium consisted of RPMI-1640 supplemented with FCS (10% v/v) (Invitrogen; Carlsbad, CA), 100 U/ml penicillin-streptomycin, 2 mM L-glutamine, and 5 × 10-5 M 2-ME (all reagents; Sigma-Aldrich, St. Louis, MO).
IEL isolation and sorting
Isolation of small intestine IELs was done in a manner similar to that described previously [13, 23] with some modifications. Briefly, small intestine tissue pieces were stirred gently at 37°C in 50 ml of Ca++/Mg++ free PBS containing 2 mM DTT and 5 mM EDTA for 30 min. The tissue slurry was separated into two 25 ml volumes and each was passed through two 10 cc syringe barrels partially filled with wetted nylon wool. Each cell suspension was collected by centrifugation, mixed in 3 ml of 40% isotonic Percoll (Amersham Biosciences; Uppsala, Sweden), layered onto 70% isotonic Percoll, and centrifuged at 600 x g for 30 min. IELs were collected from the 40/70% interfaces, washed with cell culture medium, resuspended in 40% Percoll overlayed onto 70% Percoll, and centrifuged again at 600 x g for 30 min. IELs were collected from the 40/70% Percoll interface; this cell preparation was consistently 75-85% pure for IELs.
Enrichment of CD4+ IELs was done by autoMACS sorting (Miltenyi Biotec; Auburn, CA) by first conducting a negative sort to remove epithelial cells using the G8.8 mAb [24] as previously reported by our laboratory [13]. This yielded a cell preparation with 98-99% IEL purity. Positive autoMACS cell sorting was done by labeling cells with biotin-anti-CD4 mAb followed by streptavidin-beads (Miltenyi Biotec) to enrich for a population of CD4+ IELs, from which RNA was isolated for real-time PCR analysis of IFN-γ gene expression.
Virus preparation and animal infection
Reovirus type 3 (Dearing strain) was purchased from the American Type Culture Collection (Manassas, VA). Virus stocks were grown in L929 (American Type Culture Collection; cat. No. CCL-1) cell monolayers in RPMI-1640 supplemented as described above. Tissue supernatants were collected from monolayers showing 50-60% cytopathic effect; supernatants were clarified by centrifugation and virus titers were calculated to be 107.5 pfu using a virus plaque assay with L929 cells. Mice were anesthetized by IsoFlo (Abbot Laboratories; Chicago, IL) inhalation and infected by oral gavage with 107.5 pfu reovirus type 3 Dearing strain.
Antibodies and flow cytometry
Antibodies used in this study were: FITC-anti-TCRβ (H57.597), PE-anti-TCRδ (GL3), biotin- and PE-anti-CD43 (S7), FITC-anti-BrdU, FITC-anti-CD8α (53-6.7), FITC-anti-CD8β (53-5.8), anti-CD16/32 (2.4G2), strepavidin-APC (all reagents, BD-PharMingen; San Diego, CA), FITC-anti-CD4 (BVD6-24G2), PE- and purified functional grade anti-ICOS (7E.17G9); biotin-anti-B7RP-1 (HK5.3), biotin-anti-CD4 (L3T4) (all reagents, e-Bioscience; San Diego, CA). Flow cytometric analysis was done on a FACSCalibur flow cytometer using CellQuest software (BD Bioscience; San Jose, CA).
In vivo BrdU labeling
Mice were injected i.p. twice at twenty four hour intervals with 1 mg of 5-bromo-2′deoxyuridine (BrdU) (Sigma-Aldrich) suspended in PBS. Forty-eight hours after the last injection, IELs were isolated and 1×106 IELs were reacted with anti-CD16/32 mAb for 15 min at 4°C followed by PE-anti-CD43 S7 for 20 min at 4°C. Cells were washed, and stained for BrdU incorporation using a BrdU staining kit (BD-PharMingen #559619). Cells were suspended in Cytofix/Cytoperm for 15 min at room temperature in the dark, washed, reacted with Cytoperm Plus for 10 on ice in the dark. Cells were washed with Perm/Wash and reacted with FITC-anti-BrdU for 20 min at room temperature in the dark. Cells were washed in Perm/Wash, resuspended in staining buffer, and analyzed for CD43 S7 expression and BrdU incorporation by flow cytometry.
IFN-γ assay and ICOS blockade experiments
IFN-γ synthesis was measured using IELs from day 4 and 7 C57BL/6 reovirus-infected mice. Cells were isolated from the small intestine and cultured with a transformed peritoneal macrophage line (PM) of C57BL/6 origin (ATCC number CRL-2458). PM cells were irradiated with 3000 rad, washed, and added to cultures at an IEL:PM cell ratio of 10:1 using 1.5×106 IELs in 2 ml of supplemented medium in a 24 well tissue culture plate. Interactions between IELs and PM cells mediated by ICOS were disrupted by the addition of 5 μg/ml anti-ICOS mAb to IELs for 10 min prior to culture with PM cells. Cell-free supernatants were harvested after 24 hrs and IFN-γ levels were measured by ELISA (e-Bioscience).
Q-RT-PCR
Intestinal tissues were collected from the mid-duodenum, mid-jejunum, and mid-ileum. Disruption and homogenization of tissues were performed with an Omni Tissue Master 240 (Omni International; Marietta, GA) for 30 sec in RLT Buffer containing β-ME (Qiagen; Valencia, CA). Total RNA was isolated from 7 mm3 (≤30 mg) of intestinal tissue using an RNAeasy Protect Mini Kit-50; samples were treated with DNase using the RNase-Free DNase Set-50 (Qiagen) according to the manufacturer’s protocol. RNA concentration was estimated spectrophotometrically at A260. Forward and reverse primers (Integrated DNA Technologies; Coralville, IA) for reovirus type 3 were based on the design by Besselsen et al. [25], with a target amplicon size of 72 bp using a forward primer sequence (5′-TGATTTCCATTACTTCTGCTGCTT-3′) in nucleotide positions 1085-1108, and a reverse primer sequence (5′-TCCTGTTCACGATTCCATCAGAT-3′) in nucleotide positions 1156-1134 on the reovirus 3 (Dearing) Sequence (GenBank Accession no. M27262). The validity of those to amplify a reovirus product was confirmed using ten-fold dilutions of RNA from reovirus-infected 3T3 cells, from which a standard curve was generated. IFN-γ primers (GenBank Accession no. NM_008337) were purchased from SuperArray Biosciences (Frederick, MD), cat. no. PPM03121A, for a 95 bp fragment with reference position 310-331 of the IFN-γ mRNA.
SYBR Green I Q-RT-PCR was performed using an iCycler IQ (Bio-Rad Laboratories; Hercules, CA). The amplicon generation rate was determined by monitoring SYBR Green I fluorescence on a continuous basis. Fluorescence data were collected at a melting temperature greater than that of non-specific PCR products. Q-RT-PCR on the iCycler IQ was performed on 100 ng total RNA in duplicate for all samples using the iScript One-Step RT-PCR Kit with SYBR Green (Bio-RAD) according to the manufacturer’s protocol. RNA from small intestinal tissues of non-infected mice was used as a negative control. A blank sample was included with RNase-free water in place of template. Amplification was done in a 96-well thin-wall plate (Bio-Rad) sealed with optical quality film (Bio-Rad) using a protocol of: 10 min at 50°C for cDNA synthesis, 5 min at 95°C for iScript reverse transcriptase inactivation, followed by 45 cycles of 95°C for 10 sec, and 60°C for 30 sec for the data collection step. Melt curve analysis was performed using a protocol of: 1 min at 95°C, 1 min at 55°C, and increasing the temperature in 0.5°C increments starting at 55°C for 80 cycles of 10 sec each. The presence of a single PCR product was verified by a single melting temperature peak signifying a unique product. Data for reovirus- and 18s-amplified RNAs for each sample were converted into arbitrary units based on reovirus and 18s standard curves using iCycler IQ analysis software (Bio-Rad, ver. 3.1), and were expressed as normalized values based on 18s house-keeping values for the respective sample using a method similar to that reported by others [26] . IFN-γ real-time PCR data were quantified using the 2-ΔΔCt method [27], based on CT values for IFN-γ and 18s genes in order to calculate the fold increase of IELs from infected mice compared to IELs from non-infected mice.
Statistical analysis
Statistical analysis of data was done using Student’s t-test for unpaired observations.
Results
During early phase of reovirus infection, actively proliferating IELs include both S7- and S7+ cells
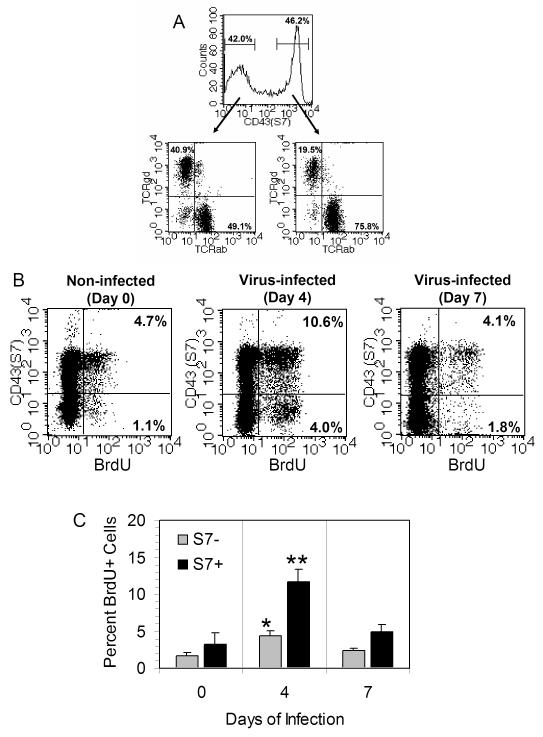
Recent studies from our laboratory demonstrated the CD43 S7 isoform is differentially expressed on small intestinal IELs [13]. That property is also shown in the present study in Fig. 1A, which indicates that roughly equal proportions of IELs were S7- and S7+, and that each population included both TCRαβ+ and TCRγδ+ cells.
FIG. 1.
(A) CD43 S7 expression on small intestinal IELs. The S7 antigen is expressed at roughly equivalent levels on murine IELs. Within each S7 group, IELs consist of both TCRαβ+ and TCRγδ+ cells, although there is a preference for TCRαβ+ cells among the S7+ cells. This is a typical finding in over 20 isolations done in our laboratory. (B) In vivo IEL proliferation of IELs at days 0 (non-infected), 4, and 7 post-infection based on BrdU incorporation and surface S7 staining. Note the increase in BrdU uptake among both S7+ and S7- cells on day 4 post-infection compared to day 0 mice, and the return to normal proliferation levels by day 7 of infection. (C) Graphic representation of BrdU data from 5 mice showing mean values ± SEM of S7+ and S7- cells. *Statistically-significant difference (p < 0.05) compared to day 0 value for S7- cells; **Statistically-significant difference (p < 0.01) compared to day 0 value for S7+ cells.
To understand the relationship between CD43 S7 expression during enteric virus infection, mice were infected with 107.5 pfu of reovirus 3 by oral gavage. Forty-eight hrs prior to IEL isolation, mice were injected twice i.p. with 1 mg of BrdU. IELs were harvested on day 0 (non-infected), day 4, and day 7 post-infection; cells were stained for surface CD43 S7 expression and for incorporated BrdU. In the absence of infection, the S7+ IEL population was the predominant proliferating cell population (Fig. 1B). By day 4 of reovirus infection, however, there was an increase in BrdU uptake in both the S7- and S7+ IEL population (Fig. 1B and C). Proportionally, the greatest increase occurred in the S7- population (3.6 fold increase) on day 4 compared to the S7+ population (2.2 fold increase); however, S7+ cells represented the largest percentage (10.6%) of BrdU+ cells among the total IELs at that time (Fig. 1B and C). Those findings indicate that both S7- and S7+ cells were mobilized during the early phase of enteric reovirus infection. By day 7 post-infection, the levels of BrdU+ cells had returned to normal for both S7- and S7+ IELs (Fig. 1B and C).
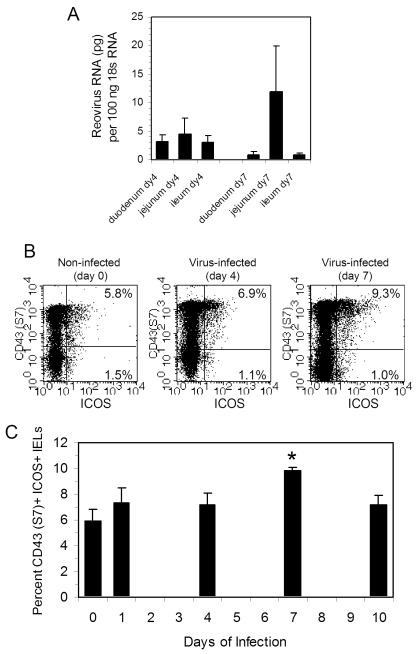
To confirm that the small intestine was infected during this period, intestinal tissue pieces were recovered from the duodenum, jejunum, and ileum on day 0 (non-infected), day 4, and day 7 post-infection, and real-time PCR was done using reovirus-specific primers [25]. Reovirus RNA levels were normalized to 18s housekeeping gene for each tissue sample as described in the Materials and methods. As shown in Fig. 2A, amplified reovirus RNA was detected in all three tissue regions at days 4 and 7 post-infection; no viral RNA was detected in non-infected tissues (data not shown).
FIG. 2.
(A) Reovirus serotype 3 virus transcripts in the duodenum, jejunum, and ileum of mice on days 4 and 7 post-infection as determined by real-time PCR. Data are the mean and ranges of values for two mice for each tissue. (B) ICOS expression increases on S7+ IELs on day 4 and 7 post-reovirus infection, indicating that the S7+ cell population is the primary IEL subset linking ICOS expression to virus infection. (C) Percent of S7+ ICOS+ cells in IELs on the selected days through day 10 post-infection, indicating that the greatest increase in ICOS expression occurs at day 7 post-infection. Analysis was not done on days 2, 3, 5, 6, 8, or 9. Data are mean values ± SEM of 3-7 mice per time point. * Statistically-significant difference (p < 0.05) compared to day 0 value.
As the infection progresses, ICOS expression is upregulated on S7+ CD4+ IELs
To identify factors that promote the activation of an anti-reovirus IEL response, the expression of three T cell costimulatory molecules (OX40, Ly-6C, and ICOS) was examined on IELs. OX40 and Ly-6C showed minimal change in expression on S7- or S7+ IELs when examined on days 4 or 7 post-infection (data not shown). In contrast, ICOS expression increased on IELs on days 4 and 7 post-infection compared to that of IELs from non-infected mice as shown by flow cytometry (Fig. 2B) and in data from multiple isolates (Fig. 2C). Of interest, the increase in ICOS expression was exclusively associated with the S7+ IEL population (Fig. 2B).
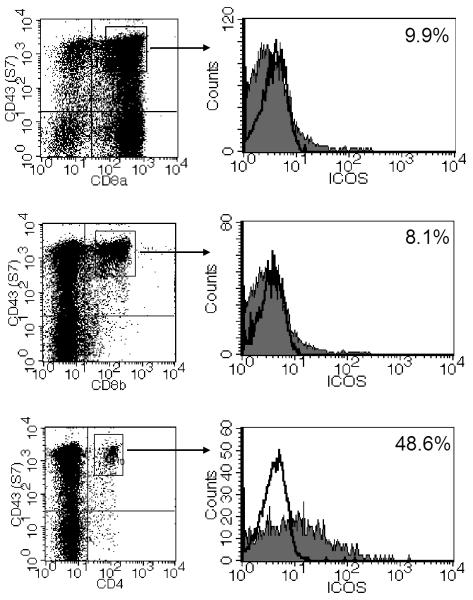
Experiments were done to determine which population of the S7+ IELs expressed ICOS using three-color staining for S7 and CD8α, CD8β, or CD4 with ICOS staining. As shown in Fig. 3, only a small percentage of S7+ cells expressing either CD8α or CD8β expressed ICOS, whereas approximately half of the S7+ CD4+ IELs were ICOS+ cells, thus indicating that the majority of ICOS expression was linked to S7+ CD4+ IELs.
FIG. 3.
Three-color staining of IELs from a day 7 reovirus-infected mouse for ICOS with staining for CD43 S7 and CD8α, CD8β, or CD4. ICOS expression is primarily associated with S7+, CD4+ IELs. Open histograms indicate level of control staining; filled histograms indicate ICOS staining.
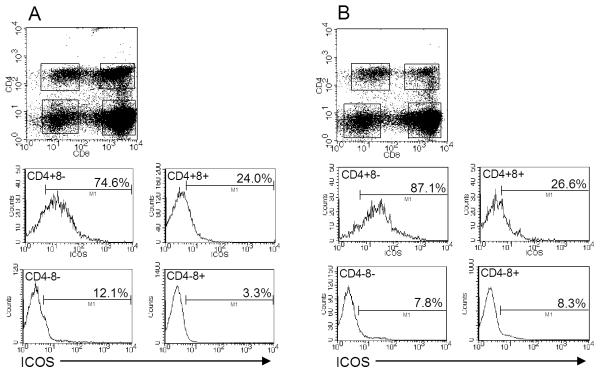
Small intestinal IELs are known to consist of CD4+8- and CD4+8+ populations [28]. To determine which of those IELs expressed ICOS, three-color staining was done for ICOS with CD4 and CD8 staining. As shown in Fig. 4, ICOS expression was primarily associated with the CD4+8- IEL population, although some ICOS+ cells were present among the CD4+8+ population. Few CD4-8- and CD4-8+ cells were ICOS+ (Fig. 4A and B). Notably, the number of ICOS+ cells in the CD4+8- population increased at day 7 post-infection (87.1%) (Fig. 4B) compared to non-infected mice (74.6%) (Fig. 4A), a finding that is consistent with the elevated levels of ICOS at day 7 as shown in Fig. 2B and C. In as much as ICOS has been shown to be primarily expressed on Th2 cells and not on Th1 cells [29], the expression of ICOS on roughly half of the CD4+ IELs may serve as a marker to discriminate intestinal Th2 and Th1 cells.
Fig. 4.
Three-color flow cytometric analysis of small intestinal IELs from (A) non-infected and (B) day 7 reovirus-infected mice. Note that ICOS expression is primarily associated with the CD4+8- IEL population, and that there is an increase in expression on that population in day 7 infected mice compared to non-infected animals. Similar findings to these were observed from mice at day 6 post infection (data not shown).
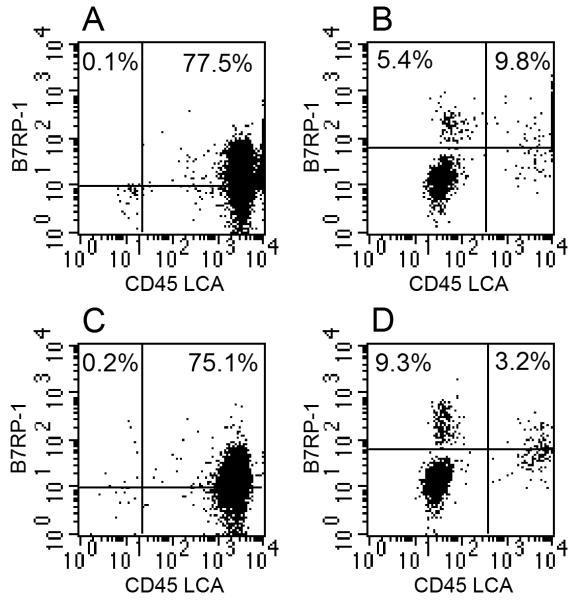
The ICOS ligand, B7RP-1, is expressed on macrophages, dendritic cells, and possibly intestinal epithelial cells. To evaluate changes in B7RP-1 expression in the intestine during reovirus infection, IELs were isolated using the technique described in the Materials and methods, which resulted in an IEL:epithelial ratio of ~4:1. Cells were stained with anti-B7RP-1 mAb in conjunction with mAb to the CD45 leukocyte common antigen (LCA), the latter being a marker that discriminates leukocytes from non-hematopoietic cells. The findings are shown in Fig. 5 for IEL-gated cells (Fig. 5A and C) and epithelial-gated cells (Fig. 5B and D). Two important points emerged from these studies. First, B7RP-1 was expressed on some but not all IELs or epithelial cells. Among the IELs, those cells likely consist of macrophages and dendritic cells, although additional studies will be needed to confirm this. These are shown as ICOS+ CD45 LCA+ cells in Fig. 5A and C. Second, during infection the percent of B7RP-1+ epithelial cells increased from 5.4% on epithelial cells from non-infected mice to 9.3% on epithelial cells from day 7 reovirus-infected animals. These are shown as ICOS+ CD45 LCA- cells in Fig. 5B and D. Similar findings for IELs and epithelial cells were observed at day 6 post-infection (data not shown). These findings collectively suggest that in addition to ICOS binding to B7RP-1 on intestinal leukocytes, some ICOS stimulation may come via B7RP-1+ epithelial cells, and they indicate that there is an increase in B7RP-1 expression on epithelial cells during the infectious cycle.
Fig.5.
B7RP-1 and CD45 LCA expression on IELs (A and C) and epithelial cells (B and D) from non-infected (A and B) and day 7 reovirus-infected (C and D) mice. Note the increase in B7RP-1 expression on the CD45 LCA- population of epithelial cells from mice at (D) day 7 post-infection compared to epithelial cells from (B) non-infected mice. Similar findings to these were observed from mice at day 6 post infection (data not shown).
ICOS ligation induces IFN-γ production by IELs
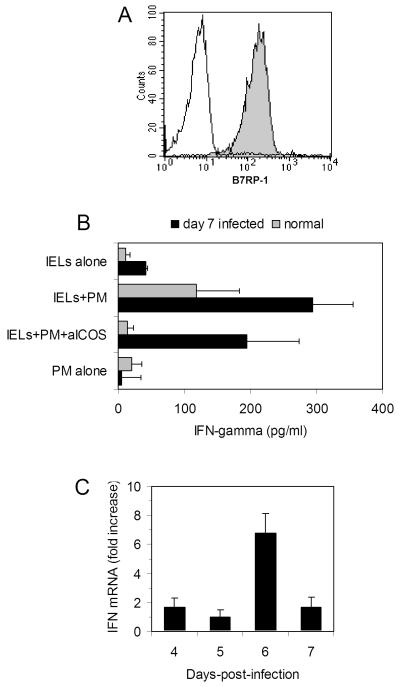
The increase in expression of ICOS on IELs on day 7 post-reovirus infection suggested that ICOS-mediated interactions may contribute to the anti-reovirus immune response mediated by S7+ IELs. To evaluate this, IELs were cultured with PM cells (a syngeneic peritoneal macrophage line) since macrophages express the ICOS ligand, B7RP-1. To formally determine the latter, PM cells were stained with anti-B7RP-1 mAb and found to express high levels of the B7RP-1 antigen (Fig. 6A). IELs were isolated from normal non-infected mice and from mice 7 days post-reovirus infection, a time of maximum ICOS expression. Cells were cultured alone, with PM cells (a syngeneic peritoneal macrophage cell line), or with PM cells with anti-ICOS mAb. Neither IELs nor PM cells from normal or infected mice cultured alone produced significant levels of IFN-γ (Fig. 6B) However, IELs from day 7 infected mice cultured with PM cells produced high levels of IFN-γ (Fig. 6B). Stimulation of IELs from uninfected mice with PM cells also resulted in IFN-γ synthesis; however, this was lower than that of virus-infected animals. It is likely, therefore, that the elevated level of IFN-γ synthesis by IELs from infected mice reflected the increase in ICOS expression on day 7 post-infection as shown in Fig. 2B and C. That stimulation by PM cells was primarily due to activation of IELs through the ICOS receptor was demonstrated by suppressed IFN-γ secretion when IELs were cultured with PM cells in the presence of anti-ICOS mAb (Fig. 6B), an antibody that has previously been shown to disrupt ICOS-driven function [30]. These findings imply that ICOS+ intestinal IELs play a role in the anti-viral immune response through increased synthesis of IFN-γ.
FIG. 6.
(A) The B7RP-1 molecule, the ICOS ligand, is expressed (shaded area) at high density on PM cells; control staining (unshaded area). (B) IFN-γ secretion by IELs from normal mice (grey bars) or mice 7 days post-infection (black bars) following 24 culture either alone, with the PM cells, or with PM cells in the presence of anti-ICOS mAb. Although culture of IELs from normal and virus-infected mice resulted in an increase in IFN-γ secretion, IFN-γ was consistently higher from infected mice. Treatment of IELs with anti-ICOS mAb inhibited IFN-γ responses of both normal and virus-infected mice, indicating that stimulation of IELs by PM cells occurs through an ICOS-mediated signal. Data are mean values ± SEM of 5 normal mice and 4 virus-infected mice. (C) Real-time PCR analysis of IFN-γ gene expression in CD4+ cell-sorted IELs from days 4, 5, 6, and 7 post-reovirus infection. Data are mean values of two samples expressed as fold increase over non-infected mice using the 2-ΔΔCt method [27] as described in the Materials and methods.
To determine how these findings may reflect what occurs in vivo during virus infection, CD4+ IELs from reovirus-infected mice were studied for IFN-γ gene expression by real-time PCR. Expression was compared to that of CD4+ cells from non-infected mice. CD4+ cells were chosen for these experiments since they contain the majority of the ICOS+ IELs, as documented in the studies in Fig. 3 and 4. Enrichment of cells was done by autoMACS sorting as described in the Materials and methods, which yielded a highly pure preparation of cells. RNAs from IELs from day 0 (non-infected) mice and from mice each at days 4, 5, 6, and 7 post-infection were assayed for IFN-γ and 18s gene expression. Using the 2-ΔΔCt method [27] for comparing relative gene expression, there was a 6.75 fold increase in IFN-γ gene expression in CD4+ IELs on day 6 post-reovirus infection compared to that of CD4+ IELs from non-infected mice, thus making a direct connection between IFN-γ activity of IELs during in vivo virus infection. These findings, coupled with the rise in ICOS expression by day 7 post-infection (Fig. 2C), the association between ICOS expression and S7+ CD4+ IELs (Fig. 3), and the higher levels of IFN-γ secretion by IELs taken from mice on day 7 post-infection when stimulated with PM cells (Fig. 6B), make a strong case that S7+ IELs contribute significantly to the anti-reovirus adaptive immune response that occurs at about day 7 post-infection.
Discussion
Owing to their phenotypic and functional complexity [13, 31-39], understanding the contribution of intestinal IELs to the natural immune response during local antigen challenge has been difficult to fully assess. In a recent series of studies aimed at better understanding functional populations of IELs, we observed that murine IELs can be classified based on whether they express a CD43 glycoform recognized by mAb S7. Accordingly, S7+ IELs were found to display properties typical of adaptive immunity whereas S7- IELs displayed properties commonly associated with innate immunity [13]. An unexpected finding from those studies, however, was that S7+ and S7- IELs each included TCRαβ and TCRγδ IELs [13] (Fig. 1A), indicating that S7 expression differentiates IELs independent of the class of TCR expression, and suggesting that S7 expression may serve as a useful marker for defining functional subsets of IELs during the course of a natural intestinal immune response.
The present study now extends those observations in a model of reovirus infection by demonstrating that differences in IEL populations defined by S7 expression bear different temporal and functional responses to infection. Although in vivo proliferation studies revealed an increase in IEL expansion following virus infection among both S7+ and S7- IELs, with peak activity occurring at day 4 post-infection, the S7+ IELs constituted the largest percent of proliferating cells (10.6%), whereas the S7- population had the greatest proportional increase in in vivo cell proliferation (3.6 fold increase for the S7- cells vs. 2.25 fold increase for the S7+ IELs) (Fig. 1B). This occurred for both populations at day 4 post-infection. Hence, although both S7- and S7+ IELs respond during the early phase of viral infection, there may be quantitative differences in the numbers of S7- and S7+ cells recruited at that time.
ICOS expression is upregulated on human, rat, and mouse large intestinal leukocytes during induced or naturally-occurring colonic inflammation [40-42]; may play a role in disease pathogenesis during gastrointestinal Trichinella spiralis infection [43]; and contributes to the protective immune response of mice against Listeria monocytogenes in the small intestine [44]. The role of ICOS during enteric reovirus infection has yet to be explored, however. Thus, the finding that ICOS expression is upregulated on IELs during reovirus infection is of interest for a number of reasons. ICOS expression on IELs peaked at day 7 post-infection, a time when high virus titers were still evident in intestinal tissues but after the time of maximum in vivo IEL proliferation following infection. Given that ICOS+ cells were primarily located in the S7+ CD4+ IEL population (Fig. 3), and because S7+ IELs have been linked to activated effector T cells [13], it is likely that the ICOS+ population reflects the generation of cells with properties of adaptive immunity that are responding to virus infection. This is further supported by the fact that ICOS expression peaked relatively late in the infectious cycle (at day 7 post-infection), at a time when anti-viral CTL, activated B cells, and T helper cells would be fully mobilized.
Although ICOS expression was upregulated on IELs following virus infection, it is interesting that 5-6% of the IELs from normal mice also expressed ICOS. This was surprising given that naïve T cells generally do not express ICOS [29, 45-47], a finding that we have found to be true for splenic T cells (data not shown). Although the specificities of those ICOS+ cells in the intestine of normal mice cannot be determined, the possibility must be entertained that they represent recently activated IELs or possibly memory T cells. The potential for this is consistent with the observation in the present study that IELs could be induced to secrete IFN-γ following stimulation through the ICOS ligand (such as with PM cells) without direct TCR engagement. A population of partially-activated T cells could account for this. That some IELs even from normal mice may exist in a state of partial activation has been proposed by ourselves and others [13, 34]. This would account for the ability of IELs from uninfected mice to synthesize IFN-γ upon exposure to the ICOS ligand (Fig. 4B). From the point of view of immune defense during virus infection, this would have considerable potential for curtailing the infectious process by utilizing bystander IELs that have previously acquired ICOS expression upon engagement with the ICOS ligand on macrophages, dendritic cells, and/or epithelial cells. That possibility coupled with the upregulation of ICOS expression on IELs as a direct consequence of virus infection, as seen at day 7 of infection, would offer mechanisms for containing viral spread more effectively through the release of IFN-γ.
ICOS expression is most frequently though not exclusively linked to Th2 cells [45, 46]. In our study, stimulation of IELs via ICOS resulted in a significant increase in IFN-γ secretion, although that finding cannot be construed to mean the Th2 cytokines are not also produced by ICOS+ IELs. Additional studies are underway to address this. In that vein also, studies using lymphocytic choriomeningitis virus and vesicular stomatitis virus infected mice indicate that ICOS participates in the generation of Th1 and Th2 responses but that its contribution to CTL activation is minimal [48]. This is consistent with the association noted here of ICOS expression CD4+ T cells.
In summary, the findings in the present study provide additional evidence that IELs can be functionally defined based on whether they express the CD43 S7 antigen. It is likely that this marker will continue to be useful as a means of understanding immune regulatory and effector populations of IELs in other infectious disease models, as well as in systems in which inflammation, T cell activation, and intestinal innate immunity are involved.
Acknowledgments
This work was supported by NIH grant DK35566 and by Public Health Service Grant DK56338 to the Texas Gulf Coast Digestive Diseases Center.
References
- [1].Rosenstein Y, Santana A, Pedraza-Alva G. CD43, a molecule with multiple functions. Immunol Res. 1999;20:89–99. doi: 10.1007/BF02786465. [DOI] [PubMed] [Google Scholar]
- [2].Gulley ML, Ogata LC, Thorson JA, Dailey MO, Kemp JD. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988;140:3751–3757. [PubMed] [Google Scholar]
- [3].Moore T, Huang S, Terstappen LW, Bennett M, Kumar V. Expression of CD43 on murine and human pluripotent hematopoietic stem cells. J Immunol. 1994;153:4978–4987. [PubMed] [Google Scholar]
- [4].Woodside DG, Long DA, McIntyre BW. Intracellular analysis of interleukin-2 induction provides direct evidence at the single cell level of differential coactivation requirements for CD45RA+ and CD45RO+ T cell subsets. J Interferon Cytokine Res. 1999;19:769–779. doi: 10.1089/107999099313622. [DOI] [PubMed] [Google Scholar]
- [5].Hare KJ, Pongracz J, Jenkinson EJ, Anderson G. Modeling TCR signaling complex formation in positive selection. J Immunol. 2003;171:2825–2831. doi: 10.4049/jimmunol.171.6.2825. [DOI] [PubMed] [Google Scholar]
- [6].Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- [7].Sanchez-Mateos P, Campanero MR, del Pozo MA, Sanchez-Madrid F. Regulatory role of CD43 leukosialin on integrin-mediated T-cell adhesion to endothelial and extracellular matrix ligands and its polar redistribution to a cellular uropod. Blood. 1995;86:2228–2239. [PubMed] [Google Scholar]
- [8].Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, Thompson CB. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Todeschini AR, Nunes MP, Pires RS, Lopes MF, Previato JO, Mendonca-Previato L, DosReis GA. Costimulation of host T lymphocytes by a trypanosomal trans-sialidase: involvement of CD43 signaling. J Immunol. 2002;168:5192–5198. doi: 10.4049/jimmunol.168.10.5192. [DOI] [PubMed] [Google Scholar]
- [10].Mattioli I, Dittrich-Breiholz O, Livingstone M, Kracht M, Schmitz ML. Comparative analysis of T-cell costimulation and CD43 activation reveals novel signaling pathways and target genes. Blood. 2004;104:3302–3304. doi: 10.1182/blood-2004-04-1536. [DOI] [PubMed] [Google Scholar]
- [11].Bagriacik EU, Tang M, Wang HC, Klein JR. CD43 potentiates CD3-induced proliferation of murine intestinal intraepithelial lymphocytes. Immunol Cell Biol. 2001;79:303–307. doi: 10.1046/j.1440-1711.2001.01007.x. [DOI] [PubMed] [Google Scholar]
- [12].Hamad M, Mosley RL, Wang J, Klein JR. Stimulation via the CD43 coreceptor augments T cell proliferation during the early phase of antigen-induced activation. Dev Comp Immunol. 1996;20:77–82. doi: 10.1016/0145-305x(95)00037-t. [DOI] [PubMed] [Google Scholar]
- [13].Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol. 2002;169:4717–4722. doi: 10.4049/jimmunol.169.9.4717. [DOI] [PubMed] [Google Scholar]
- [14].Fulton JR, Cuff CF. Mucosal and systemic immunity to intestinal reovirus infection in aged mice. Exp Gerontol. 2004;39:1285–1294. doi: 10.1016/j.exger.2004.06.013. [DOI] [PubMed] [Google Scholar]
- [15].Chen D, Rubin DH. Mucosal T cell response to reovirus. Immunol Res. 2001;23:229–234. doi: 10.1385/IR:23:2-3:229. [DOI] [PubMed] [Google Scholar]
- [16].Forrest JC, Dermody TS. Reovirus receptors and pathogenesis. J Virol. 2003;77:9109–9115. doi: 10.1128/JVI.77.17.9109-9115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].George A, Kost SI, Witzleben CL, Cebra JJ, Rubin DH. Reovirus-induced liver disease in severe combined immunodeficient (SCID) mice. A model for the study of viral infection, pathogenesis, and clearance. J Exp Med. 1990;171:929–934. doi: 10.1084/jem.171.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fulton JR, Smith J, Cunningham C, Cuff CF. Influence of the route of infection on development of T-cell receptor β-chain repertoires of reovirus-specific cytotoxic T lymphocytes. J Virol. 2004;78:1582–1590. doi: 10.1128/JVI.78.3.1582-1590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].London SD, Cebra-Thomas JA, Rubin DH, Cebra JJ. CD8 lymphocyte subpopulations in Peyer’s patches induced by reovirus serotype 1 infection. J Immunol. 1990;144:3187–3194. [PubMed] [Google Scholar]
- [20].Hoffman LM, Hogan KT, Cashdollar LW. The reovirus nonstructural protein sigma1NS is recognized by murine cytotoxic T lymphocytes. J Virol. 1996;70:8160–8164. doi: 10.1128/jvi.70.11.8160-8164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bharhani MS, Grewal JS, Pilgrim MJ, Enocksen C, Peppler R, London L, London SD. Reovirus serotype 1/strain Lang-stimulated activation of antigen-specific T lymphocytes in Peyer’s patches and distal gut-mucosal sites: activation status and cytotoxic mechanisms. J Immunol. 2005;174:3580–3589. doi: 10.4049/jimmunol.174.6.3580. [DOI] [PubMed] [Google Scholar]
- [22].Fleeton MN, Contractor N, Leon F, Wetzel JD, Dermody TS, Kelsall BL. Peyer’s patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J Exp Med. 2004;200:235–245. doi: 10.1084/jem.20041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Montufar-Solis D, Klein JR. An improved method for isolating intraepithelial lymphocytes (IELs) from the murine small intestine with consistently high purity. J Immunol Methods. 2006;308:251–254. doi: 10.1016/j.jim.2005.10.008. [DOI] [PubMed] [Google Scholar]
- [24].Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39:645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- [25].Uchiyama A, Besselsen DG. Detection of Reovirus type 3 by use of fluorogenic nuclease reverse transcriptase polymerase chain reaction. Lab Anim. 2003;37:352–359. doi: 10.1258/002367703103051903. [DOI] [PubMed] [Google Scholar]
- [26].Loke P, Zang X, Hsuan L, Waitz R, Locksley RM, Allen JE, Allison JP. Inducible costimulator is required for type 2 antibody isotype switching but not T helper cell type 2 responses in chronic nematode infection. Proc Natl Acad Sci U S A. 2005;102:9872–9877. doi: 10.1073/pnas.0503961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [28].Mosley RL, Styre D, Klein JR. CD4+CD8+ murine intestinal intraepithelial lymphocytes. Int Immunol. 1990;2:361–365. doi: 10.1093/intimm/2.4.361. [DOI] [PubMed] [Google Scholar]
- [29].Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, Gosselin M, Owen LR, Rudd CE, Gutierrez-Ramos JC. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- [30].Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, Blazar BR. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- [31].Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing γδ T-cell antigen receptors. Proc Natl Acad Sci U S A. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- [33].Cheroutre H. IELs: enforcing law and order in the court of the intestinal epithelium. Immunol Rev. 2005;206:114–131. doi: 10.1111/j.0105-2896.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- [34].Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–434. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- [35].Roccabianca P, Woo JC, Moore PF. Characterization of the diffuse mucosal associated lymphoid tissue of feline small intestine. Vet Immunol Immunopathol. 2000;75:27–42. doi: 10.1016/S0165-2427(00)00181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fujihashi K, Taguchi T, Aicher WK, McGhee JR, Bluestone JA, Eldridge JH, Kiyono H. Immunoregulatory functions for murine intraepithelial lymphocytes: γ/δ T cell receptor-positive (TCR+) T cells abrogate oral tolerance, while α/β TCR+ T cells provide B cell help. J Exp Med. 1992;175:695–707. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- [38].Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien YH. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci U S A. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Onodera T, Ray UR, Melez KA, Suzuki H, Toniolo A, Notkins AL. Virus-induced diabetes mellitus: autoimmunity and polyendocrine disease prevented by immunosuppression. Nature. 1982;297:66–68. doi: 10.1038/297066a0. [DOI] [PubMed] [Google Scholar]
- [40].Totsuka T, Kanai T, Iiyama R, Uraushihara K, Yamazaki M, Okamoto R, Hibi T, Tezuka K, Azuma M, Akiba H, Yagita H, Okumura K, Watanabe M. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124:410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- [41].Sato T, Kanai T, Watanabe M, Sakuraba A, Okamoto S, Nakai T, Okazawa A, Inoue N, Totsuka T, Yamazaki M, Kroczek RA, Fukushima T, Ishii H, Hibi T. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- [42].Ishii K, Kanai T, Totsuka T, Uraushihara K, Ishikura T, Yamazaki M, Okamoto R, Araki A, Miyata T, Tezuka K, Nakamura T, Watanabe M. Hyperexpression of inducible costimulator on lamina propria mononuclear cells in rat dextran sulfate sodium colitis. J Gastroenterol Hepatol. 2004;19:174–181. doi: 10.1111/j.1440-1746.2004.03202.x. [DOI] [PubMed] [Google Scholar]
- [43].Scales HE, Ierna MX, Gutierrez-Ramos JC, Coyle AJ, Garside P, Lawrence CE. Effect of inducible costimulator blockade on the pathological and protective immune responses induced by the gastrointestinal helminth Trichinella spiralis. Eur J Immunol. 2004;34:2854–2862. doi: 10.1002/eji.200324364. [DOI] [PubMed] [Google Scholar]
- [44].Mittrucker HW, Kursar M, Kohler A, Yanagihara D, Yoshinaga SK, Kaufmann SH. Inducible costimulator protein controls the protective T cell response against Listeria monocytogenes. J Immunol. 2002;169:5813–5817. doi: 10.4049/jimmunol.169.10.5813. [DOI] [PubMed] [Google Scholar]
- [45].Dong C, Nurieva RI, Prasad DV. Immune regulation by novel costimulatory molecules. Immunol Res. 2003;28:39–48. doi: 10.1385/IR:28:1:39. [DOI] [PubMed] [Google Scholar]
- [46].Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, Odermatt B, Ho A, Itie A, Horan T, Whoriskey JS, Pawson T, Penninger JM, Ohashi PS, Mak TW. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- [47].Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- [48].Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos JC, Bachmann MF. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J Exp Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]