Abstract
Microsporidiosis poses a problem for immunocompromised individuals including patients with HIV infection as well as those with organ transplantation. Recent reports from Africa have suggested that microsporidiosis with diarrhea is an independent risk factor for malnutrition in children. Previous studies from our laboratory have demonstrated that CD8+ T cells are an essential component of protective immunity against the microsporidium Encephalitozoon cuniculi. Mutant mice lacking this T cell subset or cytotoxic function are unable to clear the infection and ultimately succumb to the disease. However, information regarding the antigens involved in the elicitation of CD8+ T cell response is not available. In this study, we report that immunization of animals with Encephalitozoon hellem polar tube protein 1 (rEhPTP1) induces a strong T cell response in vaccinated animals. Splenic dendritic cells pulsed with rEhPTP1 are able to induce E. cuniculi specific CD8+ T cell response with no effect on the CD4+ T cell subset. This is the first report identifying a protein capable of inducing CD8+ T cell immunity, which is conserved in other microsporidial species of human importance.
Keywords: Microsporidia, T cells, Immunization
1. Introduction
Microsporidia are obligate intracellular parasites that infect a wide range of hosts, vertebrates and invertebrates [1]. With the onset of the AIDS pandemic, more attention has been paid to several microsporidia species including Encephalitozoon, due to their ability to cause disease in humans [2–6]. Because of its ability to grow in tissue culture, most of what is known about the biology of microsporidia is based on E. cuniculi [7]. Protective immunity against E. cuniculi infection is primarily dependent on the cellular immune response [8]. In the absence of immune T cells, animals are unable to withstand intraperitoneal (i.p.) infection with the parasite [7]. T cells are the first line of defense against the pathogen, and to a large extent play an important role in preventing the dissemination of organisms to peripheral organs [9]. Although T cells are known to play an important role against E. cuniculi infection, the antigens involved in the elicitation of this response are unknown. In the present study, we evaluated the role of polar tube protein 1, a highly conserved major polar protein of microsporidia.
2. Materials and methods
2.1. Animals and infection
Six to 7 week-old female C57BL/6 mice were obtained from the National Cancer Institute (Frederick, MD), sex and age matched CD8−/− animals were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed under approved conditions at the Animal Research Facility at Louisiana State University (New Orleans, LA) and George Washington University (Washington, DC). Mice were infected with 107 E. cuniculi spores i.p. A rabbit isolate of E. cuniculi (genotype II) was used throughout the study. The parasites were maintained by continuous passage in rabbit kidney (RK-13-CCL37) cells, obtained from American Type Culture Collection (Manassas, VA).
2.2. Production of recombinant PTP1
As previously published [10], PCR employing Pfu, a proof-reading polymerase (Stratagene, La Jolla, CA), was used to clone the E. hellem PTP1 into the EcoRI and XhoI sites of pGEX-4T1, a glutathione S-transferase (GST) expression vector (Pharmacia Biotech, Piscataway, NJ) creating pGEX-EhPTP1. The PCR primers used were designed to provide an EcoRI site at the 5′ end (EhPTPStart4T1-EcoF: 5′CGGAATTCGCAGTTCCGCTTTGCAGT3′) and a XhoI site at the 3′ end (EhPTPEnd4T1-XhoR: 5′CCGCTCGAGCTAACATTGA CAGCAGGAG3′). Recombinant protein was then purified from IPTG induced E.coli containing pGEX-EhPTP1 using glutathione Sepharose 4B (Pharmacia Biotech, Piscataway, NJ) and analyzed by immunoblotting. Sequencing of the pGEX-EhPTP1 insert confirmed that no misincorporations occurred in the cloning process.
2.3. Solubilized Polar Tube Protein Preparation (DTT-PTPs)
Rabbit kidney cells (RK13-CCL37) infected with E. hellem were maintained in continuous culture in minimum essential medium supplemented with 10% heat-inactivated fetal calf serum and 1% penicillin/streptomycin as previously published [11, 12]. Spores (7 × 108 to 1 × 109) were glass bead-disrupted and sequentially extracted with 1% SDS and 9M urea, and solubilized in 2% DTT as previously described [11, 12].
2.4. Immunoblot
For whole spore antigen, the E. cuniculi spores were disrupted with a glass bead homogenizer (Minibead-beater, BioSpec, OK) at a concentration of 108 per ml PBS. Disrupted spores were mixed 1:1 with 2X gel sample buffer and DTT-PTPs were mixed 1:1 with 2X gel sample buffer as well. SDS-PAGE electrophoresis was performed using a 10% acrylamide gel and transferred to nitrocellulose using standard techniques. The immunoblot was blocked with 5% Non fat dry milk in PBS, washed and incubated with a 1:1000 dilution of murine sera in PBS for 1 hour. Murine sera were evaluated individually and as a pooled serum. The reaction was visualized using the Western light CSPD system (Applied Biosystems, Foster City, CA) employing a secondary anti-mouse alkaline phosphatase antibody at a 1:2500 dilution following the manufacturers instructions.
2.5. PTP immunization
CD8−/− mice were immunized with 4μg of rEhPTP1 protein or GST in complete Freund’s adjuvant and control animals received same amount of PBS. At day 7 and 14 post-prime immunization, mice were boosted i.p. with 4 μg of rEhPTP1 or GST in incomplete Freund’s adjuvant and subsequently challenged at day 21 post immunization with 1×107 spores of E. cuniculi.
2.6. T cell proliferation
Antigen-specific proliferation of T cell population was determined by thymidine incorporation assay according to a standard protocol in our laboratory [13]. Briefly, splenocytes were isolated and cultured in 96-well flat-bottom plates in RPMI-1640 at a concentration of 2.5×105 cells/wells. The cells were stimulated with rEhPTP1 (20μg/ml), GST (20μg/ml) or E. cuniculi spores (5×103 spores/well). After 72 hours incubation at 37° C in 5% CO2, 3H-thymidine (0.5μCi/well; Amersham, Arlington Heights, IL) was added to the wells. Cells were harvested on a glass filter using an automated multiple sample harvester (Brandel M12, Gaithersburg, MD), dried, and incorporation of radioactive thymidine was determined by liquid scintillation (Beckman Coulter, Fullerton, CA).
2.7. In vitro T cell priming by DC
Splenic DC were isolated from naïve C57BL/6 mice according to the protocol used in our laboratory [14, 15]. Briefly, the tissues were harvested followed by chemical (collagenase D and DNase 1) and mechanical disruption, allowing for the isolation of DC. They were then labeled with anti-CD11c biotin-conjugated antibodies (eBioscience) and positively selected via magnetic purification according to the manufacturer’s protocol (Stem Cell Technology, Vancouver BC). Positively selected cells were then labeled with streptavidin-conjugated PE-Cy5.5, anti-CD19 and anti-NK1.1 antibodies (eBioscience) and CD11c+CD19−NK1.1− DC were further purified on a cell sorter (FACSAria, BD Biosciences). Isolated DC were plated (5×104 cells/wells) and pulsed overnight with various concentrations of rEhPTP1. The next day, T cells from naïve mice were isolated using magnetic sorting as previously described [13] and then added to splenic DC cultures (5×105 cells/well). After a 72-hour incubation, monensin was added to the culture according to the manufacturer’s protocol (BD Biosciences). The next day, surface staining for CD8, CD4 and CD69 as well as intracellular staining for IFNγ were performed using a standard protocol of our laboratory [13]. Samples were acquired on Facscalibur (BD Biosciences) and data were analyzed using Flowjo (Tree Star Inc, Ashland OR).
2.8 CD8+ T cell response in rEhPTP1 immunized mice
C57BL/6 mice were immunized as described above. Day 28 post-immunization, animals were challenged with 107 E. cuniculi spores i.p. Three days later, mice were sacrificed and splenocytes isolated. Cells were restimulated for 12 hours with E. cuniculi spores (5 spores/cell) in presence of monensin and brefeldin according to manufacturer instructions. Cells were labeled for CD8 expression and intracellular staining for IFNγ and granzyme B performed as described above. Samples were acquired on Facscalibur (BD Biosciences) and data were analyzed using Flowjo (Tree Star Inc, Ashland OR).
3. Results
3.1. PTP1 immunization protects the susceptible CD8−/− mice
Earlier studies from our laboratory demonstrated that mice lacking CD8+ T cells are unable to withstand i.p. E. cuniculi infection [16]. These findings suggested that this subset plays a predominant role in protection against the pathogen. To determine if other components of the adaptive immune response could potentially play a role in protective immunity against E. cuniculi, we immunized CD8−/− mice with rEhPTP1 or control GST. The vaccinated animals were subsequently challenged at day 21 post-immunization with 1×107 spores of E. cuniculi. As shown in the table 1, only 2/12 PTP immunized mice became ill and developed a small degree of ascitis while the remaining 10 mice exhibited no sign of illness. Conversely, all of the control GST or saline treated mice showed signs of illness and developed large amount of ascitis. However, as observed earlier [9, 16], saline injected animals succumbed to infection and died at day 15–20 post infection, while GST treated animals did eventually recover from infection. These studies suggest that although GST treatment can induce protection by evoking a non-specific immunity, the more complete protection seen with rEhPTP1 is probably dependent on the elicitation of antigen specific response.
Table 1.
rEhPTP1 immunized CD8−/− animals are protected against lethal challenge with E. cuniculi.
ascites severity was assessed by at least two persons in a blind test. +++ represents the largest ascites.
number in parentheses represents number of animals with ascites over total number of mice in group
3.2. PTP immunization induces antigen-specific immunity
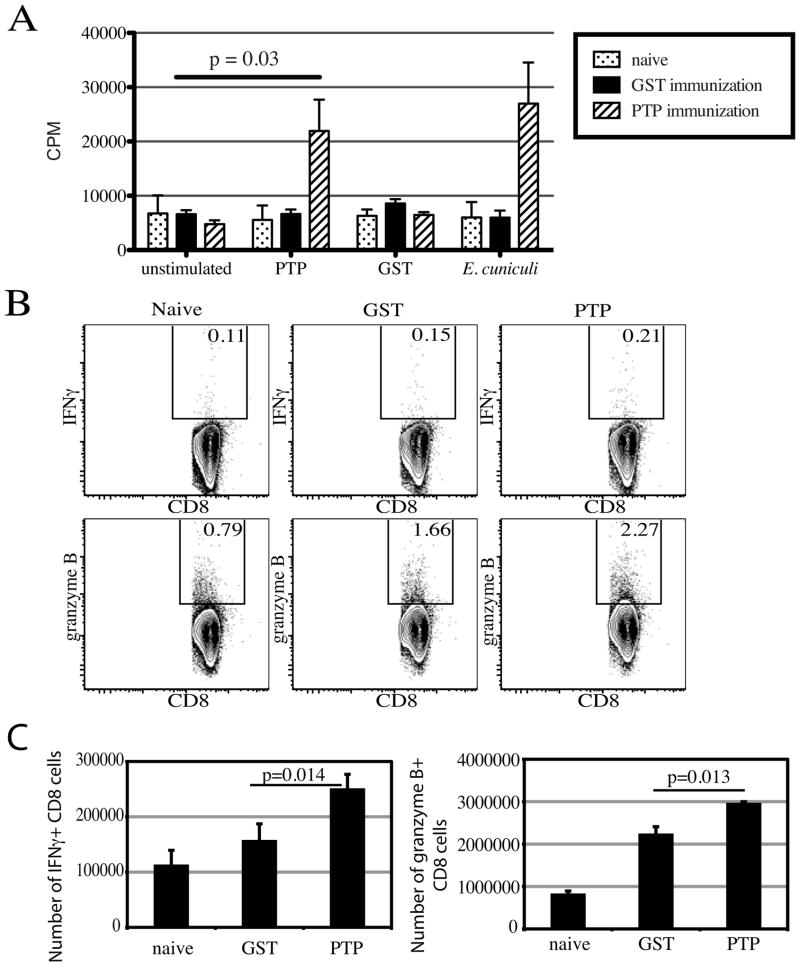
To determine that PTP1 immunization leads to the development of antigen-specific immune response, proliferation assay was performed. Wild type C57BL/6 mice were immunized with rEhPTP1 protein as mentioned above and at day 21-post vaccination the animals were sacrificed, spleens pooled and antigen-specific proliferation was measured by 3H-thymidine incorporation assay. As shown in figure 1A, splenocytes isolated from rEhPTP1 immunized mice showed significant proliferation in response to rEhPTP1 stimulation (p= 0.03). The proliferation of the cultures stimulated with rEhPTP1 was not significantly different from those treated with whole spores (Figure 1A). Interestingly, cells isolated from GST treated animals failed to proliferate in response to rEhPTP1 or whole spores suggesting the failure of these animals to develop an antigen-specific immune response. As protection against E. cuniculi infection has been reported to be primarily mediated by CD8+ T cell [9, 16], response in immunized mice was assessed by their IFNγ and granzyme B expression (Figure 1B–C). Three days post-challenge, when compared to control mice, immunized animals exhibited a significant increase in both frequency and number of IFNγ+ as well as granzyme B+ CD8 T cells (Figure 1B–C). Immunoblot of murine sera from rEhPTP1 immunized mice demonstrated that immunized mice had a humoral immune response that recognized rEhPTP1 (Figure 2). We have previously demonstrated that antibodies to EhPTP1 can also recognize the PTP1 from Encephalitozoon cuniculi and Encephalitozoon intestinalis [17].
Figure 1. Splenocytes from rEhPTP1 immunized animals demonstrate antigen specific proliferation in vitro.
Animals (3 mice/group) were immunized with rEhPTP1 or GST. At day 21 pi, mice were sacrificed and splenocytes prepared. Cells were restimulated with rEhPTP1 (20μg/ml), GST (20μg/ml) or E. cuniculi spores. After 72h incubation, proliferation was measured by thymidine 3H incorporation (A). In another set of experiments, splenocytes were restimulated overnight with E. cuniculi spores in presence of monensin and brefeldin. Cells were labeled for CD8, IFNγ and granzyme B. Total cells were gated for CD8+ T cells prior to IFNγ or granzyme B analysis. Data are presented as dot plots (B) and histograms of total number of positive cells (C). Experiments were performed twice and data are representative of one experiment.
Figure 2. Immunoblot using whole spore and solublized polar tube proteins.
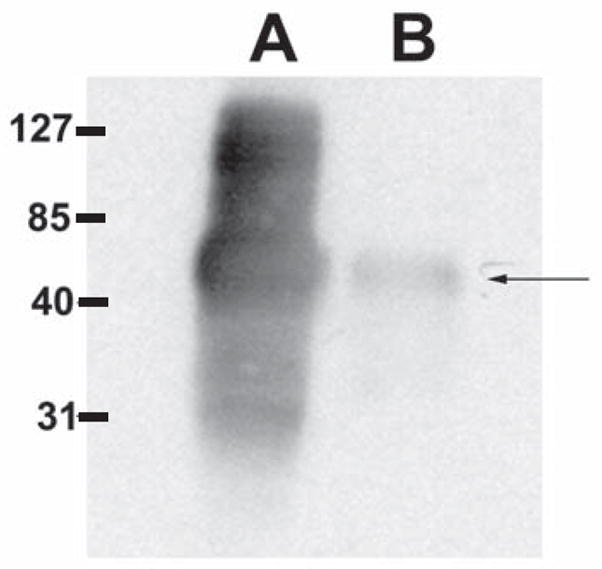
Lane A. DTT solubilized polar tube preparation. Lane B. Disrupted spore lysate. Pooled murine serum from the immunized mice recognized a band consistent with PTP1. Similar results were obtained with serum from the individual mice prior to pooling. Murine sera also recognized rEhPTP1 (data not shown).
3.3. DC population is able to present PTP antigen
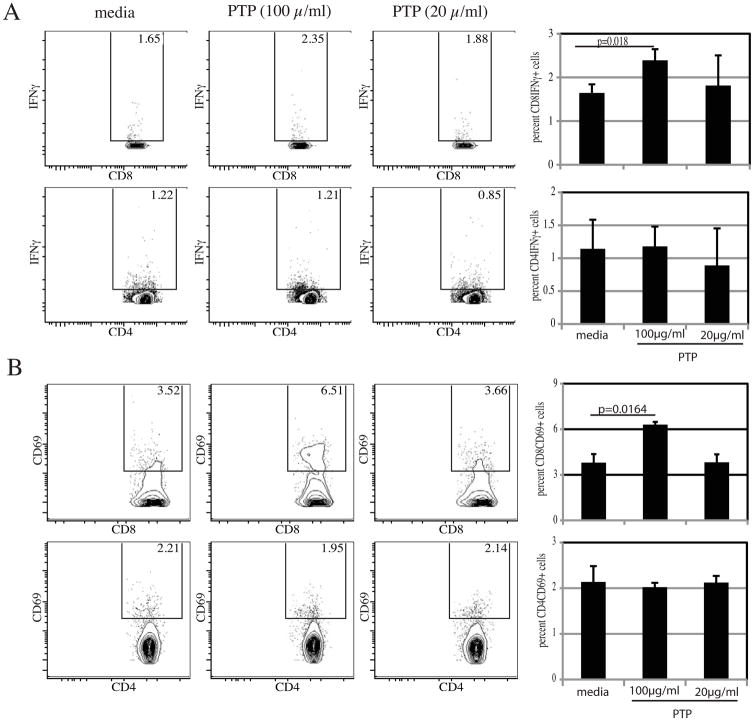
Priming a T cell response by efficient antigen presentation is an important characteristic of DC population and earlier studies from our laboratory have reported the importance of these cells in the induction of adaptive immunity in E. cuniculi infected animals [15]. Next, we determined if DC pulsed with rEhPTP1 could elicit a T cell response against the antigen. As shown in figure 3A, a significant rise in percent of IFNγ producing cells within CD8+ T cell subset was observed when DC were pulsed with the same concentration rEhPTP1 antigen. Similarly, at the concentration of 100 μg/ml, primed DC induce a significant activation of CD8+ T cell subset as measured by CD69 expression, an early activation marker for T cells. (figure 3B). However, no significant increase in the activation of CD4+ T cells in response to rEhPTP1 stimulation as measured by IFNγ production (figure 3A) or CD69 expression (figure 3B) was noted.
Figure 3. rEhPTP1 pulsed DC can prime CD8+ T cells in vitro.
DC were purified from naïve animals (n= 4), plated and pulsed overnight with various concentration of rEhPTP1 (100 or 20 μg/ml). The next day, TCRβ+ T cells were isolated from naïve mice (n= 3) and added to the culture. After 72 h incubation, monensin was added and cells were labeled for CD4, CD8, CD69 and IFNγ. Total cells were gated for CD8 or CD4+ T cells prior to IFNγ (A) or CD69 (B) analysis. Experiment was performed twice and data are representative of one experiment.
4. Discussion
The role of CD8+ T cells in the protective immunity against E. cuniculi infection is well demonstrated by previously published studies conducted in our laboratory [9, 13, 16]. CD8+ T cells in the infected animals exhibit a strong cytotoxic activity and mutant animals lacking perforin gene are highly susceptible to infection [16]. Conversely, CD4+ T cells play a minimal role during E. cuniculi infection, and the knock out animals lacking this T cell subset are able to clear infection [9, 16]. Moreover, the absence of CD4+ T cell has no effect in the generation of CD8+ T cell response against the pathogen [9]. The importance of CD8+ T cells is not restricted to i.p. infection, as these cells are an important component of gut immune response [18].
CD8+ T cell immunity plays an important role in immunoprotection against number of intracellular viral, bacterial and parasitic infections [19–22]. Over the years, efforts have been made to identify the antigenic components of the pathogen responsible for the elicitation of this response. This has led to the mapping of CD8+ T cell epitopes, which serve as essential tools for in depth evaluation of the response and helps in the development of appropriate immunotherapeutic agents against the infection. The information regarding the antigens involved in the elicitation of CD8+ T cell immunity against E. cuniculi infection is non-existent. In the present study, we demonstrate that immunization with recombinant PTP antigen leads to the generation of a strong T cell response against the pathogen. In vitro stimulation of DC with this antigen induces antigen-specific CD8+ T cell activation.
Although rEhPTP1 pulsed DC failed to evoke CD4+ T cell response against the antigen, immunization of CD8 deficient mice did enhance their protection against parasite infection. As antigen fails to induce a CD4+ T cell response, it appears that rEhPTP1 immunization may be stimulating other components of immune system, which afford a certain degree of protection in the absence of CD8+ T cells. Although our data demonstrates that rEhPTP1 induces a strong antibody response in infected animals, earlier studies have reported the inability of immune sera to protect infected animals [23]. Adoptive transfer of immune B lymphocytes into athymic BALB/c (nu/nu) or SCID mice does not protect these animals from death following E. cuniculi infection [23]. Moreover, passive transfer of hyper-immune serum into these mice does not prevent lethal infection [7]. As GST immunization leads to a certain degree of protection, it is most likely that innate immune components may be involved in the protection of CD8−/− animals.
The data obtained from these studies has important implications in analyzing the generation of protective immunity against E. cuniculi infection. This information will form the basis for the development of tools necessary for the measurement of immune response in infected versus immunized individuals. As E. cuniculi is acquired via oral route, further studies need to be performed to determine if vaccination is also effective after oral challenge. Further studies on the duration of immunity following rEhPTP1 immunization will help define the components of this protective immune response. Additionally, this data is the first demonstration of cross immune protection in the microsporidia and suggests that immunization strategies can be developed to provide immune protection against several of these organisms. This has implications for veterinary medicine including aquaculture where microsporidiosis is an important problem, as well as for the development of possible control strategies for human infections with these pathogenic protists.
Acknowledgments
We thank Teresa Hawley for her help with flow cytometry. This work was supported by NIH AI 043693 awarded to IAK and AI37188 award to LMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Canning JLE. The microsporidia of vertebrates. Academic Press; New York, NY: 1986. [Google Scholar]
- 2.Fournier S, Liguory O, Sarfati C, David-Ouaknine F, Derouin F, Decazes JM, Molina JM. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med. 2000;1:155–161. doi: 10.1046/j.1468-1293.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 3.Weitzel T, Wolff M, Dabanch J, Levy I, Schmetz C, Visvesvara GS, Sobottka I. Dual microsporidial infection with Encephalitozoon cuniculi and Enterocytozoon bieneusi in an HIV-positive patient. Infection. 2001;29:237–239. doi: 10.1007/s15010-001-1164-0. [DOI] [PubMed] [Google Scholar]
- 4.Botterel F, Minozzi C, Vittecoq D, Bouree P. Pulmonary localization of Enterocytozoon bieneusi in an AIDS patient: case report and review. J Clin Microbiol. 2002;40:4800–4801. doi: 10.1128/JCM.40.12.4800-4801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scaglia M, Sacchi L, Croppo GP, da Silva A, Gatti S, Corona S, Orani A, Bernuzzi AM, Pieniazek NJ, Slemenda SB, Wallace S, Visvesvara GS. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J Infect. 1997;34:119–126. doi: 10.1016/s0163-4453(97)92414-2. [DOI] [PubMed] [Google Scholar]
- 6.Svedhem V, Lebbad M, Hedkvist B, Del Aguila C, Hedman P, Larsson R, Navajas R, Aust-Kettis A. Disseminated infection with encephalitozoon intestinalis in AIDS patients: report of 2 cases. Scand J Infect Dis. 2002;34:703–705. doi: 10.1080/00365540210147598. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt EC, Shadduck JA. Murine encephalitozoonosis model for studying the host-parasite relationship of a chronic infection. Infect Immun. 1983;40:936–942. doi: 10.1128/iai.40.3.936-942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt EC, Shadduck JA. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol. 1984;133:2712–2719. [PubMed] [Google Scholar]
- 9.Moretto M, Casciotti L, Durell B, Khan IA. Lack of CD4(+) T cells does not affect induction of CD8(+) T-cell immunity against Encephalitozoon cuniculi infection. Infect Immun. 2000;68:6223–6232. doi: 10.1128/iai.68.11.6223-6232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keohane EM, Orr GA, Zhang HS, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol Biochem Parasitol. 1998;94:227–236. doi: 10.1016/s0166-6851(98)00071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Purification and characterization of human microsporidian polar tube proteins. J Eukaryot Microbiol. 1996;43:100S. doi: 10.1111/j.1550-7408.1996.tb05023.x. [DOI] [PubMed] [Google Scholar]
- 12.Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Purification and characterization of a microsporidian polar tube protein. Mol Biochem Parasitol. 1996;79:255–259. doi: 10.1016/0166-6851(96)02666-7. [DOI] [PubMed] [Google Scholar]
- 13.Moretto M, Durell B, Schwartzman JD, Khan IA. Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection. J Immunol. 2001;166:7389–7397. doi: 10.4049/jimmunol.166.12.7389. [DOI] [PubMed] [Google Scholar]
- 14.Moretto MM, Lawlor EM, Khan IA. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J Immunol. 2008;181:7977–7984. doi: 10.4049/jimmunol.181.11.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretto MM, Weiss LM, Combe CL, Khan IA. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J Immunol. 2007;179:2485–2492. doi: 10.4049/jimmunol.179.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan IA, Schwartzman JD, Kasper LH, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol. 1999;162:6086–6091. [PubMed] [Google Scholar]
- 17.Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Polar tube proteins of microsporidia of the family encephalitozoonidae. J Eukaryot Microbiol. 1999;46:1–5. doi: 10.1111/j.1550-7408.1999.tb04569.x. [DOI] [PubMed] [Google Scholar]
- 18.Moretto M, Weiss LM, Khan IA. Induction of a rapid and strong antigen-specific intraepithelial lymphocyte response during oral Encephalitozoon cuniculi infection. J Immunol. 2004;172:4402–4409. doi: 10.4049/jimmunol.172.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolls JK, Habetz S, Shean MK, Vazquez C, Brown JA, Lei D, Schwarzenberger P, Ye P, Nelson S, Summer WR, Shellito JE. IFN-gamma and CD8+ T cells restore host defenses against Pneumocystis carinii in mice depleted of CD4+ T cells. J Immunol. 1999;162:2890–2894. [PubMed] [Google Scholar]
- 20.Khan IA, Matsuura T, Kasper LH. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leav BA, Yoshida M, Rogers K, Cohen S, Godiwala N, Blumberg RS, Ward H. An early intestinal mucosal source of gamma interferon is associated with resistance to and control of Cryptosporidium parvum infection in mice. Infect Immun. 2005;73:8425–8428. doi: 10.1128/IAI.73.12.8425-8428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Kang H, Kikuchi T, Suzuki Y. Gamma interferon production, but not perforin-mediated cytolytic activity, of T cells is required for prevention of toxoplasmic encephalitis in BALB/c mice genetically resistant to the disease. Infect Immun. 2004;72:4432–4438. doi: 10.1128/IAI.72.8.4432-4438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enriquez E. MIcrosporidia: immunoty and immunodiagnosis. 2nd Worshop Microsporidiosis and Cryptosporidiosis Immunodeficient Patients; Ceske Budejovice, Cezh Republic. 1997. p. 16. [Google Scholar]