Abstract
This study sought to assess the impact of body mass index (BMI) on the risk of left atrial (LA)/left atrial appendage (LAA) thrombus in patients with atrial fibrillation (AF) before catheter ablation. From January 2007 to March 2008, 433 consecutive patients with nonvalvular AF were enrolled. Patients with valvular heart disease, deep vein thrombosis, or pulmonary embolism were excluded. All patients underwent transesophageal echocardiography. Twenty-six of 433 patients (6.0%) had LA/LAA thrombus and the patients with thrombus had a significantly higher BMI (27.9 ± 3.1 vs 26.0 ± 3.3 kg/m2, p = 0.005). The area under the receiver operating characteristic curve of BMI predicting thrombus was 0.662. With a cut-off point of 27.0 kg/m2, the sensitivity and specificity of BMI for the diagnosis of thrombus were 69.2% and 83.1%, respectively. The incidence of LA/LAA thrombus was 10.6% in patients with BMI >27.0 kg/m2 versus only 3.0% for patients with BMI <27.0 kg/m2 (p = 0.001). In multivariable analysis, BMI ≥27.0 kg/m2 (odds ratio 4.02, 95% confidence interval 1.19 to 13.55, p = 0.025), Cardiac Failure, Hypertension, Age, Diabetes, Stroke Doubled score ≥2, and nonparoxysmal AF were independent risk factors of LA/LAA thrombus. In conclusion, BMI ≥27.0 kg/m2 is an independent risk factor of LA/LAA thrombus in patients with AF.
Atrial fibrillation (AF) is the most common sustained arrhythmia in adults, affecting >2 million patients in United States alone.1 Although obesity is a growing epidemic and the cause of various negative cardiovascular outcomes,2-4 its association with AF has been recently reported by several groups.5,6 In particular, an increasing body of evidence has shown that obesity is associated with a poor prognosis in patients with AF.2 At the pathophysiologic level, obesity was found to set the stage for hypofibrinolysis, inflammation, and prothrombosis, suggesting that obese patients with AF could be a population at high risk of thromboembolic complications.7-9 Thus, we prospectively investigated patients admitted for catheter ablation of AF and hypothesized that obesity was associated with a larger risk of left atrial (LA)/left atrial appendage (LAA) thrombus.
Methods
From January 2007 to March 2008, 464 consecutive patients with refractory AF who underwent transesophageal echocardiography (TEE) before AF ablation were included. We excluded patients with valvular heart disease, deep venous thrombosis, or pulmonary embolism. In total, 433 patients were enrolled and 31 patients were excluded. Height (meters) and weight (kilograms) and body mass index (BMI) calculated were recorded at the time of admission. Body surface area was calculated according to a simplified formula10 as the square root of (height [centimeters] × weight [kilo-grams]) × 0.015925. Since admission, study patients received subcutaneous low-molecular-weight heparin (enoxaparin, Sanofi Aventis, Paris, France) 1 mg/kg 2 times/day, rather than warfarin and antiplatelet medications. Stroke risk was then evaluated by the Cardiac Failure, Hypertension, Age, Diabetes, Stroke Doubled (CHADS2) score. As previously reported,11 the CHADS2 score (range 0 to 6) is calculated as follows: 2 points are assigned for a history of stroke, transient ischemic attack, or systemic embolism and 1 point is assigned for age >75 years, a history of hyper-tension, diabetes, or recent heart failure. A score ranging from 0 to 6 was determined for each patient at the time of TEE. To simplify the analysis, we combined all patients with a CHADS2 score of 2 to 6 in a category CHADS2 ≥2. Also, we defined paroxysmal AF as lasting ≤7 days with spontaneous termination according to published guidelines,11 and other AF presentations, including persistent and permanent AF, were categorized as nonparoxysmal AF. Metabolic syndrome was defined according to the modified National Cholesterol Education Program-Adult Treatment Panel III criteria and following Chinese ethnic criteria.12,13 Diabetes mellitus was diagnosed according to American Diabetes Association diagnostic criteria (fasting glucose level ≥7.0 mmol/L, insulin or oral hypoglycemic agent at time of admission).14 This study was approved by the institutional review board. All patients gave a written and informed consent.
All patients underwent transthoracic echocardiography and TEE before AF catheter ablation (mean 1.6 ± 0.9 days, range 0 to 4, from admission). TEE was performed with a 5-MHz multiplane probe (Sonos 4500/5500, Philips Medical Systems, Andover, Massachusetts) and live images were interpreted by an experienced physician who was blind to BMI. Images of the left atrium including the LAA were evaluated in the horizontal plane (0°) and in contiguous planes obtained by rotation of the imaging sector from 0° to 180° during continuous visualization of the left atrium and LAA. LA/LAA thrombus was defined as a well-circumscribed echogenic mass with a unique echotexture contrasting with the adjacent or underlying myocardium. The presence of spontaneous echocardiographic contrast within the atrial blood pool, seen independently from background artifacts such as reverberation in turbulent blood flow, was also characterized. Because this study dealt with BMI, echocardiographic measurements were indexed to body surface area.
Venous blood samples were obtained by sterile antecubital venous puncture after overnight fasting on the morning of the first hospitalization. Fibrinogen was analyzed by the Clauss method using the hematology autoanalyzer (CA7000, Sysmex, Kobe, Japan).
All analyses were performed with the SPSS 13.0 (SPSS, Inc., Chicago, Illinois). Continuous data are presented as mean ± SD. Univariate analysis was computed using un-paired independent samples t test for continuous variables and chi-square test or Fisher's exact test, if necessary, for categorical variables. Receiver operating characteristic curve (constructed by plotting sensitivity vs 1-specificity) analysis was used to evaluate the efficiency of BMI count for predicting LA/LAA thrombus. The optimal cut-off point was determined by receiver operating characteristic curve, as were sensitivity and specificity of BMI for prediction of LA/LAA thrombus, using TEE as a gold standard. Multivariable logistic regression was employed to examine the risks for LA/LAA thrombus. We entered in the multivariable model the patient CHADS2 score, antithrombotic medication, and all other variables with a p value <0.10 on univariate analysis. All probability values were 2-sided and a p value <0.05 was considered statistically significant.
Results
Patients' clinical characteristics are presented in Table 1. In total, 433 patients (57 ± 11 years old) with AF (315 patients with paroxysmal AF) were enrolled. Twelve patients (2.8%) were on warfarin medication at admission, and mean international normalized ratio was 2.49 ± 0.61 on the first day after admission.
Table 1.
Characteristics of patients with and without left atrial/left atrial appendage (LA/LAA) thrombus
| All (n = 433) |
No Thrombus (n = 407) |
Thrombus (n = 26) |
p Value | |
|---|---|---|---|---|
| Age (years) | 57 ± 11 | 57 ± 11 | 57 ± 10 | 0.924 |
| Men | 318 (73.4%) | 300 (73.7%) | 18 (69.2%) | 0.616 |
| Atrial duration (years) | 6.4 ± 6.1 | 6.4 ± 6.1 | 5.6 ± 6.1 | 0.481 |
| Nonparoxysmal AF | 118 (27.3%) | 102 (25.1%) | 16 (61.5%) | <0.001 |
| Type 2 diabetes mellitus | 46 (10.6%) | 43 (10.6%) | 3 (11.5%) | 0.876 |
| Hypertension | 204 (47.1%) | 187 (45.9%) | 17 (65.4%) | 0.054 |
| Heart failure | 23 (5.3%) | 19 (4.7%) | 4 (15.4%) | 0.018 |
| Previous embolic events | 27 (6.2%) | 22 (5.4%) | 5 (19.2%) | 0.005 |
| Cardiac failure, hypertension, age, diabetes, stroke doubled | 0.003 | |||
| 0 | 189 (43.6%) | 186 (45.7%) | 3 (11.5%) | |
| 1 | 168 (38.8%) | 153 (37.6%) | 15 (57.7%) | |
| ≥2 | 76 (17.6%) | 68 (16.7%) | 8 (30.8%) | |
| Coronary artery disease | 35 (8.1%) | 33 (8.1%) | 2 (7.7%) | 0.940 |
| Metabolic syndrome | 220 (50.8%) | 202 (49.6%) | 18 (69.2%) | 0.053 |
| Body mass index (kg/m2) | 26.1 ± 3.3 | 26.0 ± 3.3 | 27.9 ± 3.1 | 0.005 |
| Body mass index | 0.074 | |||
| <25 kg/m2 | 163 (37.6) | 158 (38.8%) | 5 (19.2%) | |
| 25–30 kg/m2 | 215 (49.7%) | 200 (49.1%) | 15 (57.7%) | |
| >30 kg/m2 | 55 (12.7%) | 49 (12.0%) | 6 (23.1%) | |
| Left atrial size (mm) | 38.4 ± 6.3 | 38.0 ± 6.0 | 44.1 ± 7.7 | <0.001 |
| Left atrial size/body surface area (mm/m2) | 21.5 ± 3.5 | 21.3 ± 3.3 | 23.8 ± 5.4 | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 48.5 ± 5.7 | 48.3 ± 5.6 | 51.3 ± 5.9 | 0.010 |
| Left ventricular end-diastolic diameter/body surface area (mm/m2) | 27.2 ± 3.4 | 27.2 ± 3.4 | 27.6 ± 3.6 | 0.562 |
| Left ventricular end-systolic diameter (mm) | 31.6 ± 5.7 | 31.3 ± 5.5 | 35.9 ± 7.0 | <0.001 |
| Left ventricular end-systolic diameter/body surface area (mm/m2) | 17.7 ± 3.1 | 17.6 ± 3.0 | 19.3 ± 4.0 | 0.005 |
| Ejection fraction (%) | 63.5 ± 8.5 | 63.9 ± 8.1 | 56.9 ±11.5 | <0.001 |
| Creatinine (μmol/L) | 76.5 ± 19.2 | 76.4 ± 19.4 | 78.0 ± 15.1 | 0.498 |
| Fibrinogen (g/L) | 2.64 ± 0.58 | 2.63 ± 0.58 | 2.93 ± 0.68 | 0.026 |
| Warfarin | 12 (2.8%) | 12 (2.9%) | 0 | 1.000 |
| Aspirin | 65 (15.0%) | 60 (14.7%) | 5 (19.2%) | 0.534 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 123 (28.4%) | 109 (26.8%) | 14 (53.8%) | 0.003 |
| Statins | 28 (6.5%) | 28 (6.9%) | 0 | 0.398 |
In total, 26 of 433 patients (6.0%) had LA/LAA thrombus detected by TEE as follows: thrombus without spontaneous echocardiographic contrast (n = 15), thrombus with spontaneous echocardiographic contrast (n = 11), and 26 additional patients had only spontaneous echocardiographic contrast. Characteristics of patients with and without LA/LAA thrombus are presented in Table 1. To be noted, patients with LA/LAA thrombus had a higher CHADS2 score, a higher BMI, and higher levels of fibrinogen. Notably, patients with LA/LAA thrombus had an increased prevalence of nonparoxysmal AF (61.5% vs 25.1%, p <0.001). There was a tendency of a higher prevalence of metabolic syndrome between patients with and without LA/LAA thrombus, albeit not significant (69.2% vs 49.6%, p = 0.053).
The BMI of patients with thrombus was significantly higher than that of patients without thrombus (27.9 ± 3.1 vs 26.0 ± 3.3 kg/m2, p = 0.005). There was no significant difference in the prevalence of overweight (BMI 25.0 to 30.0 kg/m2) and obesity (BMI ≥30.0 kg/m2) between patients with LA/LAA thrombus and those without. Incidences of thrombus were 3.1%, 7.0%, and 10.9% in normal, overweight, and obese groups (p = 0.073, p for trend = 0.023), respectively. The area under the receiver operating characteristic curve of BMI for predicting the presence of a LA/LAA thrombus was 0.662 (95% confidence interval [CI] 0.559 to 0.765) and the optimal cut-off point for BMI for predicting the presence of thrombus was 27.0 kg/m2 (Figure 1). With a cut-off value of 27.0 kg/m2, the sensitivity and specificity of BMI for the diagnosis of thrombus were 69.2% and 83.1%, respectively. Also, patients with a BMI ≥27.0 kg/m2 had a larger left atrium, larger left ventricular end-diastolic and end-systolic diameters, and higher levels of fibrinogen; in addition, they had a significantly higher prevalence of nonparoxysmal AF (Table 2). Importantly, the incidence of LA/LAA thrombus was 10.6% in patients with BMI ≥27.0 kg/m2 versus only 3.0% in patients with BMI <27.0 kg/m2 (p = 0.001). As a further example of the good predictability value of a BMI ≥27.0 kg/m2, all 3 patients (nonparoxysmal AF) with a CHADS2 score equal to 0 who had LA/LAA thrombus had a BMI ≥27.0 kg/m2.
Figure 1.
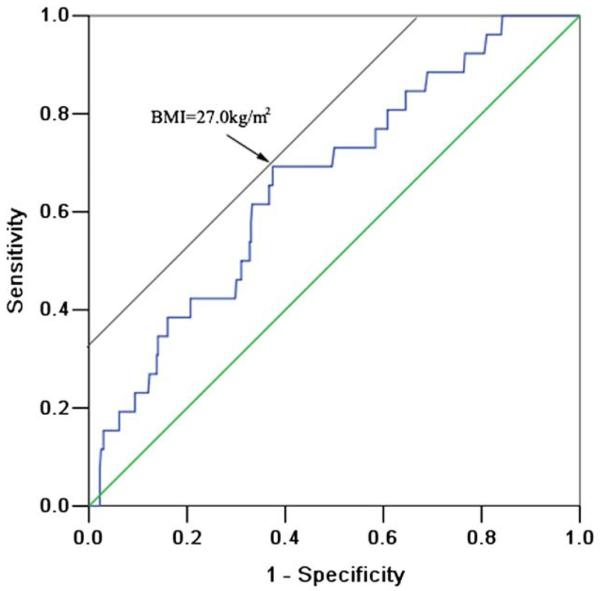
The optimal cut-off value for BMI for predicting the presence of thrombus determined by receiver operating characteristic curve analysis was 27.0 kg/m2. The area under the receiver operating characteristic curve was 0.662 (95% CI 0.559 to 0.765).
Table 2.
Characteristics of patients with body mass index ≥27 or <27 kg/m2
| BMI ≥27 kg/m2 (n = 170) |
BMI <27 kg/m2 (n = 263) |
p Value |
|
|---|---|---|---|
| Age (years) | 56 ± 10 | 57 ± 11 | 0.381 |
| Men | 119 (70.0%) | 199 (75.7%) | 0.192 |
| AF duration (years) | 6.2 ± 6.2 | 6.5 ± 6.0 | 0.563 |
| Nonparoxysmal AF | 63 (37.1%) | 55 (20.9%) | <0.001 |
| Type 2 diabetes mellitus | 20 (11.8%) | 26 (9.9%) | 0.536 |
| Hypertension | 101 (59.4%) | 103 (39.2%) | <0.001 |
| Heart failure | 11 (6.5%) | 12 (4.6%) | 0.387 |
| Previous embolic events | 9 (5.3%) | 18 (6.8%) | 0.515 |
| CHADS2 | <0.001 | ||
| 0 | 54 (31.8%) | 135 (51.3%) | |
| 1 | 85 (50.0%) | 83 (31.6%) | |
| ≥2 | 31 (18.2%) | 45 (17.1%) | |
| Coronary artery disease | 17 (10.0%) | 18 (6.8%) | 0.239 |
| LA size (mm) | 40.9 ± 5.9 | 36.9 ± 6.1 | <0.001 |
| LA size/body surface area (mm/m2) | 21.6 ± 3.3 | 21.4 ± 3.6 | 0.536 |
| Left ventricular end-diastolic diameter (mm) | 49.5 ± 6.3 | 47.9 ± 5.2 | 0.040 |
| Left ventricular end-diastolic diameter/body surface area (mm/m2) | 26.2 ± 3.5 | 27.8 ± 3.2 | <0.001 |
| Left ventricular end-systolic diameter (mm) | 32.6 ±6.1 | 31.0 ± 5.3 | 0.040 |
| Left ventricular end-systolic diameter/body surface area (mm/m2) | 17.3 ± 3.3 | 18.0 ± 3.0 | 0.027 |
| Ejection fraction (%) | 63.2 ± 8.8 | 63.7 ± 8.4 | 0.547 |
| BMI (kg/m2) | 29.4 ± 2.0 | 24.0 ± 2.0 | <0.001 |
| Creatinine (μmol/L) | 77.4 ± 18.9 | 75.2 ± 19.7 | 0.247 |
| Fibrinogen (g/L) | 2.74 ± 0.67 | 2.58 ± 0.51 | 0.008 |
| Warfarin | 5 (2.9%) | 7 (2.7%) | 0.863 |
| Aspirin | 30 (17.6%) | 35 (13.3%) | 0.217 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 61 (35.9%) | 62 (23.6%) | 0.006 |
| Statins | 9 (5.3%) | 19 (7.2%) | 0.549 |
| Thrombus | 18 (10.6%) | 8 (3.0%) | 0.001 |
The prevalence of the 3 CHADS2 score categories was significant different between patients with BMI <27.0 kg/m2 and those with BMI ≥27.0 kg/m2. There were significant differences in LA diameter (37.2 ± 5.6, 39.6 ± 6.8, 38.8 ± 6.3 mm, p = 0.003) and BMI (25.6 ± 2.8, 26.7 ± 3.6, 26.3 ± 3.4 mm, p = 0.003) among the 3 CHADS2 categories. In the 3 CHADS2 categories (CHADS2 0, 1, ≥2), 1.6%, 8.9%, and 10.5% of patients had thrombus (p = 0.003), respectively. Prevalence of nonparoxysmal AF were 23.8%, 33.3%, and 22.4% among the 3 CHADS2 categories, respectively (p = 0.075).
In multivariable analysis (Table 3), after adjustment for warfarin, aspirin, and angiotensin-converting enzyme inhibitors/angiotensin receptor blocker medications, BMI ≥27.0 kg/m2, CHADS2 ≥2, and nonparoxysmal AF were found to be independent risk factors of LA/LAA thrombus. It should be noted, however, that BMI as a continuous variable was no longer an independent risk factor for thrombus (odds ratio [OR] 1.08, 95% CI 0.92 to 1.27, p = 0.317). After adjustment for the same confounders as in Table 3 (BMI ≥27.0 kg/m2 was not included), overweight (OR 2.44, 95% CI 0.55 to 10.82, p = 0.241) and obesity (OR 2.63, 95% CI 0.44 to 15.70, p = 0.290) were not found to be independent risk factors of LA/LAA thrombus. However, CHADS2 ≥2 (OR 5.33, 95% CI 1.10 to 25.70, p = 0.037) and nonparoxysmal AF (OR 4.02, 95% CI 1.34 to 12.03, p = 0.013) remained independent risk factors of LA/LAA thrombus.
Table 3.
Risk of left atrial/left atrial appendage thrombus in multivariable analysis
| OR | 95% CI | p Value | |
|---|---|---|---|
| Nonparoxysmal atrial fibrillation | 4.06 | 1.32–12.51 | 0.015 |
| CHADS2 | |||
| 0 | reference | ||
| 1 | 1.93 | 0.44–8.59 | 0.386 |
| ≥2 | 5.06 | 1.04–24.76 | 0.045 |
| Left atrial size | 1.04 | 0.95–1.13 | 0.395 |
| Left ventricular end-diastolic diameter | 0.98 | 0.78–1.24 | 0.884 |
| Left ventricular end-systolic diameter | 0.97 | 0.73–1.28 | 0.826 |
| Ejection fraction | 0.91 | 0.81–1.02 | 0.108 |
| Fibrinogen | 1.54 | 0.75–3.18 | 0.241 |
| Body mass index ≥27.0 kg/m2 | 4.02 | 1.19–13.55 | 0.025 |
CI = confidence interval; OR = odds ratio.
Discussion
Although the risk of thromboembolic complications is widely accepted as one of the negative outcomes of patients with AF,15 it is unknown whether patients with an increased BMI have an increased thromboembolic risk. In 433 consecutive patients with nonvalvular AF, including 315 patients with paroxysmal AF, investigated with transesophageal echocardiographic examination before ablation, we prospectively examined the risk factors of thrombus formation. Our results are show that (1) an increased BMI is potentially linked to a higher risk of thrombus formation and (2) a cut-off value of 27.0 kg/m2 stands as a clear limit above which the risk of LA/LAA thrombus in patients with AF is substantial.
In the present study, the incidence of LA/LAA thrombus was 6.0%. The incidence and predictors of LA/LAA thrombus have been investigated in several recent studies.16-21 Notably, the incidence of LA/LAA thrombi were found to vary widely depending on the patient population studied (from 0% to 9.0%).16-22 First, Scherr et al19 showed that 1.6% of patients before AF ablation had LA thrombus. In the study by Scherr et al, all patients underwent anticoagulation for ≥4 weeks before the procedure, whereas only 2.8% of patients had warfarin before the ablation procedure. The striking underuse of warfarin reflected current anticoagulant use for prophylaxis in patients with AF in China.23 In contrast, in a study by Mazouz et al,20 thrombi were detected in 30 of 381 patients (7.9%) before a transesophageal echocardiographically guided cardioversion. The fact that, in the work by Mazouz et al, 41% of the population had valvular AF could have led to a higher incidence of thrombus formation. It is indeed well known that valvular AF brings about a higher risk of LA/LAA thrombus than nonvalvular AF.11,24
In our work, the fact that nonparoxysmal AF was found to be an independent risk factor of LA/LAA thrombus confirmed the results obtained by Habara et al,16 which indicated that nonparoxysmal AF imposes a 2.13-fold higher risk of LAA thrombus. In addition, we found that the CHADS2 score was significantly higher in patients with thrombus or with a BMI ≥27.0 kg/m2. Accordingly, it has been previously reported that the CHADS2 score was associated with LA/LAA thrombus19,21 and its predictability value has been recently included in recent AF guidelines.11,25
In the present work, we provide the first evidence that BMI values ≥27.0 kg/m2 stand as a risk factor of LA/LAA thrombus in patients investigated before catheter ablation of AF. Interestingly, our results confirm previous pathophysiologic findings linking obesity and prothrombosis. Patients with BMI ≥27.0 kg/m2 have higher fibrinogen levels, which are known to be associated with the presence of LA/LAA thrombus. Those findings well corroborate previous works that associated obesity with hypofibrinolysis, inflammation, and prothrombosis.7,8 Particularly, it was indicated that obese patients are exposed to a higher risk of venous thrombosis.26 For instance, it was noted that over-weight and obesity are associated with a prothrombotic state and that obese patients have a higher level of fibrinogen,27,28 which promotes thrombosis through increased fibrin formation.
The exact mechanism linking obesity to a higher risk of LA/LAA thrombus remains to be investigated. The hormones, cytokines, and growth factors secreted by adipocytes such as leptin, adiponectin, resistin, or C-reactive protein could be major players and their role will have to be examined in detail. Also, in our study, transesophageal echocardiogram was interpreted by only 1 experienced physician. Inter- and intraobserver variabilities of LA/LAA thrombus assessment have not been determined. Multiple readers of transesophageal echocardiograms would improve the accuracy of transesophageal echocardiographic interpretation. CHADS2 scores have comparable, but only limited, overall ability to predict thromboembolism in patients with AF29; thus patients' classification implementing this score should be considered with caution. It is unknown if BMI ≥27.0 kg/m2 would be a risk factor of stroke in a larger population of patients with AF. The applicability of the data presented in a population with obesity defined as a BMI >30 kg/m2 is not established in our study.
Acknowledgment
We thank Chang Liu for collecting the data.
This work was funded by the National Science Foundation Council of China (Grants 30670843, 30871048, and 30770876) and the Beijing Science Foundation Council (Grant 07G0179), Beijing, China.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madala MC, Franklin BA, Chen AY, Berman AD, Roe MT, Peterson ED, Ohman EM, Smith SC, Jr, Gibler WB, McCullough PA. Obesity and age of first non-ST-segment elevation myocardial infarction. JAm Coll Cardiol. 2008;52:979–985. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 4.Tang RB, Dong JZ, Liu XP, Long DY, Yu RH, Kalifa J, Ma CS. Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circ J. 2009;73:438–443. doi: 10.1253/circj.cj-08-0832. [DOI] [PubMed] [Google Scholar]
- 5.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity—results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 7.Bowles LK, Cooper JA, Howarth DJ, Miller GJ, MacCallum PK. Associations of haemostatic variables with body mass index: a community-based study. Blood Coagul Fibrinolysis. 2003;14:569–573. doi: 10.1097/00001721-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Darvall KA, Sam RC, Silverman SH, Bradbury AW, Adam DJ. Obesity and thrombosis. Eur J Vasc Endovasc Surg. 2007;33:223–233. doi: 10.1016/j.ejvs.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, Keaney JF, Jr, Wang TJ, Vasan RS, Benjamin EJ. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104:92–96. doi: 10.1016/j.amjcard.2009.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu CY, Lo YH, Chiou WK. The 3D scanner for measuring body surface area: a simplified calculation in the Chinese adult. Appl Ergon. 2003;34:273–278. doi: 10.1016/S0003-6870(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 11.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the European Society of Cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(suppl):e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 12.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 13.Lu YH, Lu JM, Wang SY, Li CL, Liu LS, Zheng RP, Tian H, Wang XL, Yang LJ, Zhang YQ, Pan CY. Comparison of the diagnostic criteria of metabolic syndrome by international Diabetes Federation and that by Chinese Medical Association Diabetes Branch. Zhonghua Yi Xue Za Zhi. 2006;86:386–389. [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 15.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 16.Habara S, Dote K, Kato M, Sasaki S, Goto K, Takemoto H, Hasegawa D, Matsuda O. Prediction of left atrial appendage thrombi in nonvalvular atrial fibrillation. Eur Heart J. 2007;28:2217–2222. doi: 10.1093/eurheartj/ehm356. [DOI] [PubMed] [Google Scholar]
- 17.Khan MN, Usmani A, Noor S, Elayi S, Ching CK, Di Biase L, Patel D, Burkhardt JD, Cummings J, Schweikert R, Saliba W, Natale A. Low incidence of left atrial or left atrial appendage thrombus in patients with paroxysmal atrial fibrillation and normal EF who present for pulmonary vein antrum isolation procedure. J Cardiovasc Electro-physiol. 2008;19:356–358. doi: 10.1111/j.1540-8167.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 18.Kleemann T, Becker T, Strauss M, Schneider S, Seidl K. Prevalence and clinical impact of left atrial thrombus and dense spontaneous echo contrast in patients with atrial fibrillation and low CHADS2 score. Eur J Echocardiogr. 2009;10:383–388. doi: 10.1093/ejechocard/jen256. [DOI] [PubMed] [Google Scholar]
- 19.Scherr D, Dalal D, Chilukuri K, Dong J, Spragg D, Henrikson CA, Nazarian S, Cheng A, Berger RD, Abraham TP, Calkins H, Marine JE. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:379–384. doi: 10.1111/j.1540-8167.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 20.Mazouz B, Keren A, Chenzbraun A. Age alone is not a risk factor for left atrial thrombus in atrial fibrillation. Heart. 2008;94:197–199. doi: 10.1136/hrt.2006.110502. [DOI] [PubMed] [Google Scholar]
- 21.Khumri TM, Idupulapati M, Rader VJ, Nayyar S, Stoner CN, Main ML. Clinical and echocardiographic markers of mortality risk in patients with atrial fibrillation. Am J Cardiol. 2007;99:1733–1736. doi: 10.1016/j.amjcard.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 22.Rader VJ, Khumri TM, Idupulapati M, Stoner CN, Magalski A, Main ML. Clinical predictors of left atrial thrombus and spontaneous echo-cardiographic contrast in patients with atrial fibrillation. J Am Soc Echocardiogr. 2007;20:1181–1185. doi: 10.1016/j.echo.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol. 2008;52:865–868. doi: 10.1016/j.jacc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 24.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 25.Estes NAM, Iii, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJW, Waldo AL, Wyse DG, Bonow RO, Estes NAM, Iii, DeLong E, Goff DC, Jr, Grady K, Green LA, Hiniker A, Linderbaum JA, Masoudi FA, Pina IL, Pressler S, Radford MJ, Rumsfeld JS. ACC/AHA/physician consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association task force on performance measures and the physician consortium for performance improvement (writing committee to develop clinical performance measures for atrial fibrillation) developed in collaboration with the Heart Rhythm Society. J Am Coll Cardiol. 2008;51:865–884. doi: 10.1016/j.jacc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Eichinger S, Hron G, Bialonczyk C, Hirschl M, Minar E, Wagner O, Heinze G, Kyrle PA. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch Intern Med. 2008;168:1678–1683. doi: 10.1001/archinte.168.15.1678. [DOI] [PubMed] [Google Scholar]
- 27.Sola E, Vaya A, Corella D, Santaolaria ML, Espana F, Estelles A, Hernandez-Mijares A. Erythrocyte hyperaggregation in obesity: determining factors and weight loss influence. Obesity. 2007;15:2128–2134. doi: 10.1038/oby.2007.253. [DOI] [PubMed] [Google Scholar]
- 28.Rosito GA, D'Agostino RB, Massaro J, Lipinska I, Mittleman MA, Sutherland P, Wilson PW, Levy D, Muller JE, Tofler GH. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91:683–689. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- 29.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]


