Abstract
Nodal marginal zone lymphoma (NMZL) represents a rare and heterogeneous group that lacks markers specific for the diagnosis. We evaluated morphologic and immunoarchitectural features of 51 NMZL and the following immunostains were performed: CD20, CD21, CD23, CD5, CD3, CD43, CD10, Ki67, BCL1, BCL2, BCL6, HGAL and LMO2. Four immunoarchitectural patterns were evident: diffuse (75%), well-formed nodular/follicular (10%), interfollicular (14%) and perifollicular (2%). Additional features included a monocytoid component (70%), admixed large cells (40%), plasma cells (47%), compartmentalizing stromal sclerosis (28%), and prominent blood vessels (66%). CD21 highlighted disrupted follicular dendritic cell meshworks in 72% and CD43 co-expression was present in 24% of cases. A panel of germinal center-associated markers was helpful in eliminating cases of diffuse follicle center lymphoma. Our results highlight the histologic and immunoarchitectural spectrum of NMZL and the utility of immunohistochemistry for CD43, CD23, CD21, BCL6, HGAL and LMO2 in the diagnosis of NMZL.
Keywords: Nodal Marginal zone lymphoma, monocytoid B-cells, low-grade B-cell lymphoma, immunoarchitecture, immunohistochemistry
INTRODUCTION
Nodal marginal zone lymphoma (NMZL) was initially described by Sheibani and colleagues in 1986 as ‘monocytoid B-cell lymphoma’ because of the similarity of the neoplastic cells to monocytoid B-cells that are typically seen in toxoplasma lymphadenitis.1 Cousar and colleagues used the term ‘parafollicular lymphoma’ to best illustrate the distribution of the neoplastic cells around hyperplastic follicles.2 Shortly thereafter, Piris and colleagues recognized that lymphomas arising from monocytoid B-cells shared similarities with marginal zone cells based on the presence of distinctive nodal architectural and cytologic features, expression of IgM and electron microscopic observations of short cellular processes and moderately well developed endoplasmic reticula.3 The Revised European American Classification of Lymphoid Neoplasms (REAL) and the World Health Organization (WHO) Classification of Hematological Malignancies (2001 and 2008) recognized and classified this entity as NMZL.4–6 The term NMZL is used to define marginal zone lymphomas with primary presentation in the lymph node in the absence of clinical evidence of prior or concurrent involvement of extranodal sites (other than the bone marrow) or spleen.7 Although the relationship between nodal, splenic and extranodal presentations of marginal zone lymphoma remains a controversial issue, multiple studies report differences in disease distribution and clinical outcome among these entities that argue for consideration of NMZL as a distinct entity,8–12 and it is therefore recognized as such in the current WHO classification.6
NMZL is an uncommon form of lymphoma representing 1.5–1.8% of lymphoid neoplasms,9,10 with only rare reports in the literature that have attempted morphologic or immunophenotypic characterization. Typically, NMZL has been recognized to exhibit two morphologic patterns, one resembling nodal involvement by extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) and the other resembling splenic marginal zone lymphoma. The resemblance of subsets of primary NMZL to extranodal and splenic marginal zone lymphomas has led to the proposal of subdividing NMZL into MALT-type and splenic type NMZL.13 In addition, highly variable plasma cell, monocytoid B-cell and large cell components as well as follicular colonization are also described. The lack of definitive immunophenotypic or molecular markers that are specific to this lymphoma offers a continuing challenge for diagnostic accuracy and reproducibility. We undertook this study to better characterize the morphologic and immunoarchitectural features of NMZL to enable separation of typical as well as variant patterns of NZML from other categories of low-grade B-cell lymphomas with which they overlap.
MATERIALS AND METHODS
Case selection
We conducted a retrospective review of cases between 1995 and 2004 of the Department of Pathology archives at Stanford University Medical Center. Institutional Review Board approval was obtained for these studies. Cases were selected based on the inclusion criteria of marginal zone lymphoma with primary presentation in lymph nodes without either extranodal or splenic involvement prior to or at the time of presentation. Extranodal and splenic marginal zone lymphomas were excluded by these clinical features. Although separating NMZL with clonal plasma cells from lymphoplasmacytic lymphoma (LPL) involving lymph nodes is a problem area and there are no unambiguous criteria for this distinction, cases with significant monoclonal plasma cell component, a high degree of bone marrow involvement by a lymphoplasmacytic plasmacytic component or clinical findings suggestive of LPL or plama cell myeloma were excluded from the study.
Fifty-three cases with primary nodal presentation were identified. Two of these cases were eliminated after additional germinal center-associated markers were performed and found to be positive. These two cases represented follicular lymphomas and were excluded on the basis of immunophenotypic criteria. The remaining 51 cases comprise the study population. The patients ranged in age from 19 to 87 years with a male to female ratio of 2:3. One patient had progressed to diffuse large B-cell lymphoma on a repeat biopsy obtained 14 months after the initial diagnosis. The vast majority of the cases were seen in consultation and further clinical follow-up was not pursued, as clinicopathologic correlation was not the main intent of this study.
Morphologic Evaluation
Morphologic evaluation was performed on formalin-fixed, paraffin-embedded tissue sections from the original diagnostic biopsies of each patient. The overall architecture together with the presence or absence of monocytoid and plasmacytoid cells and a large cell component, and characteristics of stromal elements, sclerosis and vasculature were evaluated. Follicular colonization was defined as reactive follicles that are wholly or partially over-run and virtually replaced by the neoplastic infiltrate, resulting in poorly defined follicles within which fragmented residual germinal center cells and small, darkly-stained mantle zone cells are dispersed. In some cases, follicles are expanded by the infiltrating cells with loss of the characteristic zoning pattern of reactive follicles.14 Cells were defined as large cells if they demonstrated the following features that are characteristic of transformed B-cells: enlarged nuclei (at least two to three times that of small lymphocytes), open chromatin, prominent 1–2 nucleoli and abundant pale cytoplasm. The presence of a large cell component was assessed using a semiquantitative scale based on the percentage of large cells in 10 high-power fields and was placed in the following groups: 0–20%, 21–50%, and >50%. In the evaluation of large cells, colonized or over-run follicles were carefully avoided with the use of antibody stains.
Immunohistologic Studies
Four-micron sections from representative paraffin blocks were deparaffinized in xylene and absolute ethanol and then rehydrated by successive immersions in 95% ethanol, 70% ethanol, and distilled water. All immunohistologic staining was performed on a Ventana ES instrument (Ventana Medical Systems, Tucson, AZ) using an indirect biotin-streptavidin method according to manufacturer recommendations with appropriately staining positive and negative control samples. Primary antibodies to lymphocyte markers including CD20, CD3, CD5, CD43, BCL2, BCL1, CD21, CD23, Ki67, CD10, BCL6, HGAL,15 LMO2,16 CD138, and Kappa and lambda light chains were applied, and a detection system using 3,3′-diaminobenzidine was utilized. Slides were counterstained with hematoxylin. Conditions used for immunohistochemistry, dilutions and sources of all primary antibodies used in this study are shown in Table 1.
Table 1.
Antibodies, Conditions and Sources Used for Immunohistologic Studies
| Antibody | Clone | Pretreatment | Dilution | Manufacturer |
|---|---|---|---|---|
| CD20 | L26 | Ventana; Standard retrieval | 1:1000 | Dako Corporation, Capinteria, CA |
| CD3 | Rabbit polyclonal | Microwave, Tris 0.5 M, pH 10 | 1:200 | Cell Marque, Hot Springs, AZ |
| CD5 | 4C7 | Ventana; Standard retrieval | 1:200 | Novocastra, Newcastle Upon Tyne, UK |
| CD43 | L60 | Ventana; Standard retrieval | 1:500 | BD Biosciences, San Jose CA |
| BCL2 | 124 | Dako; Citrate retrieval | 1:20 | Dako Corporation, Capinteria, CA |
| BCL1 | SP4 | Dako; Citrate retrieval | 1:100 | Thermo Scientific, Fremont, CA |
| IgD | Rabbit polyclonal | Ventana; Mild retreival | 1:500 | Dako Corporation, Carpinteria, CA |
| CD21 | IF8 | Ventana; Protease retrieval | 1:20 | Dako Corporation, Capinteria, CA |
| CD23 | 1B12 | Ventana; Mild retrieval | 1:50 | Novocastra, Newcastle Upon Tyne, UK |
| Ki67 | Polyclonal | Microwave, Tris 0.5 M, pH 10 | 1:1000 | Dako Corporation, Capinteria, CA |
| CD10 | 56C6 | Ventana; Standard retrieval | 1:20 | Novocastra, Newcastle Upon Tyne, UK |
| BCL6 | G/191E/A8 | Ventana; Mild retrieval | Neat | Dako Corporation, Capinteria, CA |
| HGAL | 1H1/A7 | Dako; pH 9.0, Pressure cooker | 1:20 | Reference # 13 |
| LMO2 | 1A1-9 | Ventana; Mild retrieval | 1:150 | Reference # 14 |
| CD138 | B-A38 | Ventana; Standard retrieval | 1:300 | Serotec, Oxford, UK |
| Kappa | Rabbit polyclonal | Ventana; Protease retrieval | 1:2000 | Dako Corporation, Capinteria, CA |
| Lambda | Rabbit polyclonal | Ventana; Protease retrieval | 1:4000 | Dako Corporation, Capinteria, CA |
RESULTS
Architectural patterns
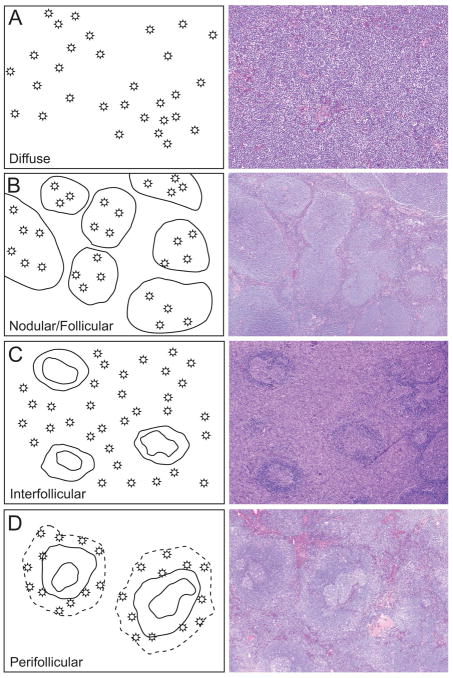
Four architectural patterns were apparent on histologic sections and have been designated diffuse, nodular/follicular, interfollicular, and perifollicular (Figure 1). The frequency of these patterns and associated cytologic features are shown in Table 2.
Figure 1.
Four immunoarchitectural patterns of nodal marginal zone lymphoma
(A) Diffuse pattern characterized by diffuse sheets of neoplastic cells with effacement of nodal architecture; (B) Nodular/follicular pattern characterized by distinct nodules that are well demarcated from the uninvolved interfollicular areas; (C) Interfollicular pattern characterized by neoplastic cells in the interfollicular areas that surround residual germinal centers; (D) Perifollicular pattern characterized by annular distribution of the neoplastic cells around uninvolved normal secondary follicles.
Table 2.
Architectural Patterns and Associated Cytological Features
| Pattern | N | Monocytoid B-cells | Plasma Cell Component | Large Cell Component % |
Prominent Vessels | Compartmentalizing Sclerosis | |||
|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 11–20 | 21–40 | 41–50 | ||||||
| Diffuse | 38 | 27 (71%) | 15 (10 clonal, 4K, 6L) | 25 | 8 | 5 | 0 | 6 | 12 |
| Nodular/follicular | 5 | 4 (80%) | 3 (2 clonal, 1K, 1L) | 5 | 0 | 0 | 0 | 3 | 1 |
| Interfollicular | 7 | 5 (71%) | 5 (3 clonal, 2K, 1L) | 1 | 4 | 0 | 2 | 1 | 0 |
| Perifollicular | 1 | 0 | 1 (1 clonal, L) | 1 | 0 | 0 | 1 | 0 | 0 |
The diffuse pattern, characterized by sheets of neoplastic cells with effacement of nodal architecture, represented the most common pattern and was evident in 38 of 51 cases. Eighteen of the cases with diffuse patterns also exhibited a vague nodular pattern as a minor component (which ranged from 10–40% of the total area of the node evaluated). The diffuse pattern was intermixed with the nodular pattern in one case and the perifollicular pattern in another case; both patterns occupied approximately 50% of the node.
The nodular/follicular pattern was noted in 5 cases and was characterized by well-formed nodules that were well demarcated from the uninvolved interfollicular areas and replaced the normal nodal parenchyma. These nodules were crowded, monotonous in size and shape and were comprised mostly of small neoplastic cells without evidence of germinal centers or a biphasic pattern (i.e. inner darker and outer lighter areas such as seen in the reverse biphasic pattern in splenic MZL).
The neoplastic cells in cases with the interfollicular pattern were limited to the interfollicular areas and spared the normal secondary follicles containing germinal centers. Among the seven cases with the interfollicular pattern, 4 cases showed prominent perivascular/perisinusoidal involvement.
The perifollicular pattern was characterized by annular distribution of the neoplastic cells around uninvolved normal secondary follicles and was noted only in one case. This pattern was noted in a second case as a minor component intermixed with a diffuse pattern.
Increased numbers of blood vessels were evident in 37 cases (70%) with vascular wall sclerosis in 11 cases (22%). Blood vessel sclerosis (thickening of small vessel walls with sclerotic material) was closely associated with the distribution of the neoplastic cells; this was most evident in cases with a nodular/follicular pattern where sclerosis was limited to areas with follicular colonization. Interstitial sclerosis, ranging from fine to coarse sclerotic bands, was seen in 15 cases (28%). These sclerotic bands compartmentalized small groups of neoplastic cells and was most evident in cases with a diffuse pattern: 12 cases with the diffuse pattern and one with the nodular/follicular pattern showed interstitial sclerotic bands. One case showed associated partial involvement by Castleman lymphadenopathy-like features along the periphery of the lymph node.
Cytological features
There was variation in the size of neoplastic cells as well as in the shape and size of nuclei (cellular pleomorphism). Typically, a range from small to large cells was present within the neoplastic infiltrate. Cellular polymorphism was evident in 38 of 51 cases (75%), whereas the remaining 13 cases showed predominantly small cells with occasional, scattered, intermediate-sized cells. A large cell component was identified in the following proportion of cases: 44 cases had 0–20% large cells and eight cases had 21–50% large cells. None of the cases showed greater than 50% large cells. A higher content of larger cells (40–50% large cells) was evident in cases with interfollicular (2 cases) and perifollicular (one case) patterns. In addition, none of the cases with the nodular/follicular pattern showed cellular pleomorphism or a large cell component. A monocytoid B-cell component was present in 36 of 51 cases (71%), with the following distribution: 27 diffuse, four nodular/follicular and five interfollicular patterns. Increased plasma cells, both scattered singly and in small clusters, were present in 24 cases (47%). Overt increase in eosinophils was noted in two cases and increase in neutrophils in association with the tumor cells was noted in another case. One case disclosed increased numbers of histiocytes, and its corresponding involvement of the bone marrow also showed an infiltrate with increased histiocytes. Examples of typical cytologic features are shown in Figure 2.
Figure 2.
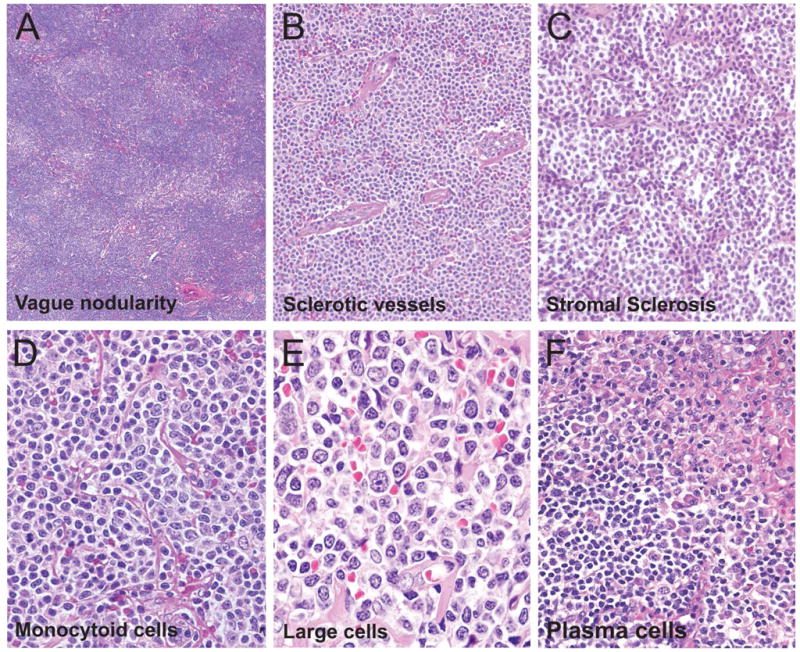
Cytomorphologic features associated with nodal marginal zone lymphoma
The cytomorphologic features associated with NMZL include: (A) vague nodularity, often associated with the diffuse pattern; (B) sclerosed blood vessels closely associated with neoplastic cells; and (C) fine stromal sclerosis that appeared to compartmentalize small groups of neoplastic cells. The typical cell milieu in NMZL showed: (D) a range from small to medium sized cells with clear cytoplasm (monocytoid cells) with occasional admixed large cells; (E) increased large cells with nuclear pleomorphism; and (F) plasmacytoid cells scattered singly and in clusters.
Immunohistologic and immunoarchitectural features
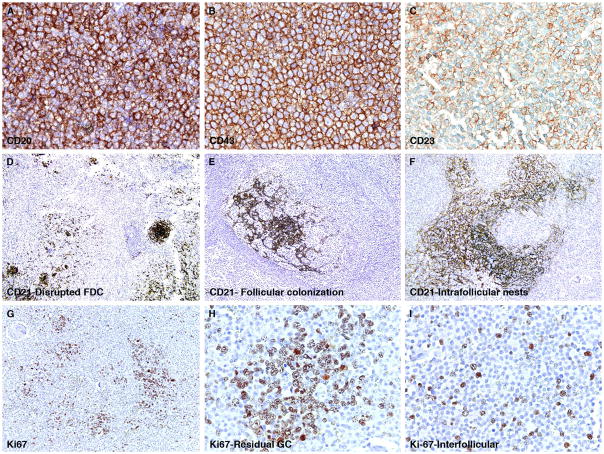
The immunohistologic characteristics are summarized in Table 3. In all cases, the neoplastic cells were positive for CD20. The stain for CD138 highlighted an increase in plasma cells in 24 of 51 cases (47%), and monoclonality (7 kappa-, 9 lambda-light chain restriction) was confirmed by either immunohistochemistry or in-situ hybridization in 16 of 51 cases (31%). CD43 co-expression was observed in 10 of 42 cases (24%) and BCL2 was detected in 12 of 28 cases (43%). The expression of CD43 and did not correlate with any particular immunoarchitectural pattern. The stains for CD3, CD5, cyclin D1 and IgD were negative in all cases tested. CD23 was found to highlight neoplastic cells in one case where the staining was diffuse but weakly positive (Figure 3, row 1).
Table 3.
Summery of Immunohistologic Findings
| Antibody | Total Positive |
|---|---|
| CD20 | 51/51 (100%) |
| CD3 or CD5 | 0/51 (0%) |
| CD43 | 10/42 (24%) |
| BCL2 | 12/28 (43%) |
| BCL1 | 0/13 (0%) |
| IgD | 0/26 (0%) |
| CD21 | 0/49 (0%, lymphoma cells) |
| Follicular colonization | 13/49 (27%, FDC) |
| Disrupted FDC meshwork | 35/49 (72%, FDC) |
| CD23 | 1/20 (5%, lymphoma cells) |
| CD10 | 0/21 (0%) |
| BCL6 | 0/40 (0%) |
| HGAL | 0/34 (0%) |
| LMO2 | 0/43 (0%) |
| CD138 | |
| Increased polyclonal plasma cells | 4/51 (8%) |
| Monoclonal plasma cells | 16/51 (31%) |
| Kappa | 7/16 (44%) |
| Lambda | 9/16 (56%) |
Figure 3.
Immunohistologic features of nodal marginal zone lymphoma
The first row depicts a NMZL case example in which (A) the neoplastic CD20-positive B-cells co-express CD43 (strong and diffuse, B) and CD23 (weak and focal, C). The second row shows different patterns of follicular dendritic cell meshworks highlighted by the stain for CD21 (D), with follicular colonization (E), and intrafollicular nests of FDC cells. The third row shows the variable staining pattern for Ki-67 (G), increased labeling of Ki-67 in found within residual germinal centers (H), whereas low to moderate numbers of Ki-67-postive cells are highlighted in the interfollicular areas where the Ki-67 stain appears to preferentially highlight admixed large transformed cells (I).
The stain for CD21 was performed in 49 cases. This stain highlighted disrupted follicular dendritic cell (FDC) meshworks in 35 cases (71%), while prominent intra-follicular nests of neoplastic cells were highlighted in 13 (27%) cases (Figure 3, row 2). The intrafollicular nests of neoplastic cells were found in seven cases with a diffuse pattern, four with the nodular/follicular pattern and in one with the perifollicular pattern. Intact or disrupted FDC meshworks associated with residual germinal centers were absent in eight cases exhibiting a diffuse pattern and in one with the nodular/follicular pattern. The stain for CD23 highlighted residual and disrupted FDC meshworks 8 of 20 cases (40%), all of which corresponded to those that were also highlighted by CD21 staining. The proliferation marker, Ki67 was evaluated in 13 cases: a high growth fraction was present in residual germinal centers; the neoplastic proliferation showed a range of staining from 5–40%, which was primarily localized to scattered, transformed large cells (Figure 3, row 3).
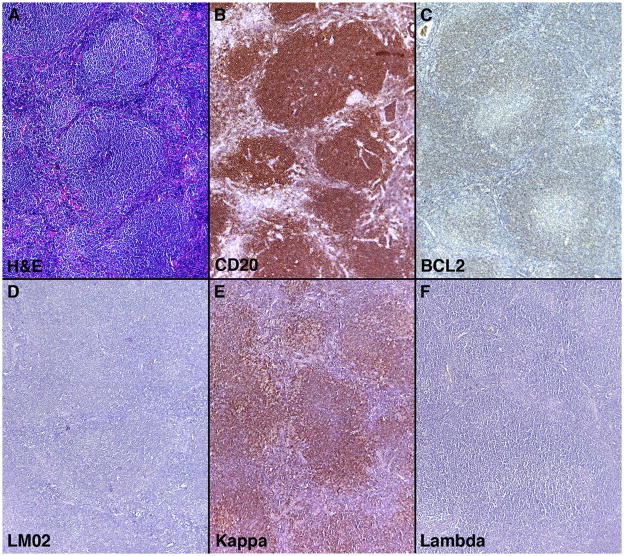
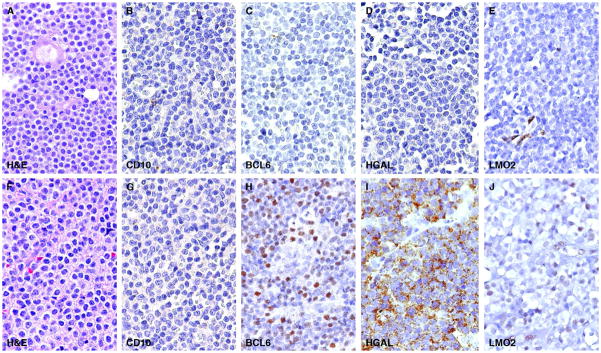
Separation of the nodular/follicular pattern of NMZL from follicular lymphoma, particularly in cases of follicular lymphoma with a diffuse component, can be problematic. The pattern of staining for CD20, BCL2 and germinal center-associated markers can aid in this separation as shown in a typical case example of NMZL with a nodular/follicular pattern and associated immunohistologic stains (Figure 4). A panel of four germinal center-associated markers CD10, BCL6, HGAL and LMO2 were carried out to eliminate cases of diffuse follicle center lymphoma that show morphologic overlap with NMZL. Fifty-three cases were initially retrieved from our files with the diagnosis of NMZL that fulfilled criteria for this study. Staining for CD10 was uniformly absent in all 53 cases; however, two cases showed staining for BCL6, HGAL and LMO2 (both cases showed diffuse staining for BCL6 and LMO2; one case showed weak focal staining for HGAL). This staining was convincingly localized to the neoplastic cells and was not related to residual germinal center cells in colonized or over-run follicles (Figure 5). Both cases exhibited a diffuse growth pattern and were reclassified as follicle center lymphomas (diffuse pattern, low grade) and were not included in the analysis of NMZL immunoarchitectural patterns.
Figure 4.
Nodular/follicular pattern of nodal marginal zone lymphoma and associated Immunohistologic features
(A) Typical case example of the nodular/follicular pattern of NMZL showing distinct and well demarcated nodules; (B) Staining for CD20 found primarily in a nodular pattern, although staining is also seen in interfollicular areas; (C) BCL2 staining highlights the neoplastic infiltrate but does not show staining within follicles as would be typical in cases of follicular lymphoma; (D) Staining for LMO2, a germinal center-associated marker, is absent; (D and E) Staining for kappa and lambda light chains show kappa light chain restriction confirming the clonal nature of the neoplastic lymphoid infitrate.
Figure 5.
Immunohistochemistry for germinal center-associated markers
The first row shows a typical case example of NMZL in which all four germinal center-associated markers CD10, BCL6, HGAL and LMO2 are lacking. The second row shows a diffuse follicle center lymphoma that lacks CD10 but shows diffuse staining for BCL6, HGAL and LMO2.
DISCUSSION
Currently, the WHO guidelines for the diagnosis of lymphoid malignancies require the integration of clinical, morphologic, immunophenotypic and genetic information for a definitive diagnosis. To date, no immunophenotypic or genetic marker specific to NMZL has been identified. Therefore, the diagnosis of this entity relies on the recognition and development of diagnostic criteria based solely on morphologic and immunoarchitectural patterns and the integration of clinical data at the time of presentation to render a diagnosis. Because of the rarity of NMZL, large series that thoroughly characterize their pathologic features are limited. In this study, we have morphologically and immunphenotypically characterized, to the best of our knowledge, the largest series of primary NMZL that meet the current WHO creteria for this entity. We define four distinct immunoarchitectural patterns of NMZL that we have designated diffuse, nodular/follicular, interfollicular and perifollicular, and describe their histologic and immunoarchitectural features.
Peripheral lymph node involvement can be seen in both extranodal and splenic MZL.9,17 Thus in our cohort, we excluded any case with extranodal disease, splenic or splenic hilar lymph node involvement. The distinction between of NMZL with clonal plasma cells and LPL involving lymph nodes is a problematic one and currently there are no unequivocal criteria to separate these entities. As such the morphologic and immunoarchitectural features need to be assessed in light of the clinical presentation. In the current study, we excluded cases with an increased monoclonal plasma cell component in the lymph node, extensive marrow involvement and clinical or radiologic features suggestive of LPL or plasma cell myeloma. Although plasma cells were noted in a large proportion of our cases (47%), they represented only a minor component f the neoplastic infiltrate in the lymph node. All cases presented in this report met strict diagnostic criteria for NMZL with primary lymph node presentation and absence of extranodal or splenic disease at the time of this study. It is however important to consider that some cases of NMZL may manifest as extranodal disease on follow-up and represent secondary involvement of lymph nodes.13
In accord with previous studies, a diffuse effacement of architecture (the diffuse pattern) was most commonly encountered with an associated vague nodular pattern in 45% of cases. In cases with both patterns, the diffuse pattern usually represented the major component and nodular pattern was a minor component occupying approximately 10–40% of the lesion. Traverse-Glehen and colleagues made a similar observation that cases with the diffuse pattern are closely associated with a nodular pattern.18 In contrast to previous studies,10,18 we identified well-formed follicles in 9% of cases, but did not observe any with the so-called “inverse follicular pattern”. This inverse follicular pattern, which has been described as a follicle with a dark-staining inner zone surrounded by a light-staining outer zone on H&E sections, is typically seen in a subset of splenic MZL and a small number of NMZL (2 of 21 cases) in one prior study.18
A problematic area in the diagnosis of NMZL with a well-defined follicular/nodular or diffuse pattern is its separation from low-grade follicular lymphoma. Germinal center-associated markers that highlight follicular lymphoma cells are typically absent in NMZL. However, subsets of follicular lymphoma may also lack germinal center-associated markers, making the distinction between these entities a diagnostic challenge. We encountered two such cases with a diffuse pattern that were originally diagnosed as NMZL based on the lack of CD10 staining. However, with the use of additional germinal center-associated markers, these cases were reclassified as diffuse follicle center lymphomas. In this context, BCL6, HGAL and LMO2 performed better than CD10 and showed more sensitivity and specificity for follicular lymphoma cells. In these two cases, CD21 immunostaining highlighted disrupted FDC meshworks, a finding that further confounded their separation from NMZL. Overall, CD21 highlighted disrupted FDC meshworks with follicular colonization in the majority (71%) of cases in our cohort regardless of the morphologic pattern, although it was more prominent in cases with a well-defined follicular/nodular pattern (4 of 5 cases; 80%). In a recent study of 15 NMZL with prominent follicular colonization, the difficulty in distinguishing NMZL from other low-grade lymphomas with a follicular pattern, are well illustrated.19 Our findings are in agreement with that study and further highlight the need for recognition of the extensive disruption of FDC meshworks and follicular colonization that is often seen in NMZL. Moreover, the presence of follicular colonization and disrupted FDCs in some cases and the complete absence of FDCs in other cases suggest a possible evolution of tumor pattern in NMZL. The cases that showed a complete absence of FDCs likely represent more advanced tumors that replace the lymph node architecture. We were unable to verify whether the attrition and eventual absence of FDC meshworks correlate with progression of disease in our cohort of cases as clinical follow-up information was limited. However, this concept warrants further examination on clinically well-characterized cases.
Immunohistologic staining for CD43 and BCL2 can be helpful in the diagnosis of NMZL, but shows significant variation among cases and in rates of positivity reported in previously published studies. Staining for CD43 is reported to range from 20–75% whereas the staining for BCL2 has been reported to range from 62–100%.12,13,18–22 Lai and colleagues previously showed that CD43 staining was positive in 20–40% of nodal and extranodal marginal zone lymphomas and staining for CD43 in our cohort was in the same range. However, BCL2 positivity was significantly lower in our cohort. A number of factors may be responsible for this difference including differences in methodology and antigen retrieval among cases sent in consultation. In addition, the inclusion criteria used in some studies whereby cases were selected based on their positivity of CD43 and/or BCL2 may also play into the higher number of positive cases reported in published series.12 Nonetheless, it is important to recognize that although immunohistologic stains for CD43 and BCL2 are helpful when present and aid in identifying the neoplastic B-cell infiltrates on which they are co-expressed, their expression is not essential for the diagnosis of NMZL. In addition, other B-cell non-Hodgkin lymphoma such as mantle cell lymphoma (frequently positive for both CD43 and BCL2) and follicular lymphoma (particularly, diffuse follicle center lymphoma) should also be carefully eliminated from the differential diagnosis.
Another important morphologic consideration in the diagnosis of NMZL is its relationship to diffuse large B-cell lymphoma (DLBCL), which is particularly problematic in NMZL with increased large transformed B-cells. Nathwani and colleagues previously described progression to DLBCL as those cases with >20% large cells.10 In our cohort, however, we found >20% large cells in 23% of cases: many of these cases exhibited increased numbers of scattered large cells, and in a minority of cases, up to 50% of large cells were present. These cases showed no significant correlation with a particular morphologic pattern and did not correspond to the single case that showed transformation to large cell lymphoma in a subsequent biopsy. In addition, none of our cases demonstrated sheets of large cells. Similar to Traverse-Glehen et al,18 our findings suggest that the presence of a large cell component in NMZL is more frequent than previously recognized.3,9,10 This finding of increased scattered large cells should not be confused with sheets of large cells, particularly if the latter is associated with an increase in proliferation (as measured by Ki-67 staining), as sheets of large cells, even if focal should raise concern for progression to large cell lymphoma. Kojima and colleagues recently reported a series of 65 cases of NMZL of which 20 cases had either >50% large cells or sheets of large cells and were classified as “DLBCL + MALT-type NMZL”; these cases were associated with a significantly worse outcome.12 Our cohort did not include cases with >50% large cells or sheets of large cells, and thus meet the current WHO definition of primary NMZL. Nevertheless, the criteria for the diagnosis of progression to large B-cell lymphoma from NMZL is not well established and currently there is no set cut-off for proliferation (as assessed by Ki-67) that is utilized to aid in this distinction.
B-cell lymphomas arise as a result of deregulation and clonal expansion of B-cells at distinct developmental stages, and NMZL are thought to arise from marginal zone B-cells of the lymphoid follicle. Previously, Conconi and colleagues observed various patterns of immunoglobulin heavy chain variable region (VH) gene mutations in NMZL and identified distinct subsets of B-cells including naïve (unmutated V regions) and germinal center and memory B-cells (undergone somatic hypermutation) from which NMZL were derived.23 These findings suggest that NMZL originate from different subsets of marginal zone cells and provide a plausible explanation for the immunoarchitectural heterogeneity of this group. Furthermore, although NMZL is thought to arise from marginal zone B cells of lymphoid follicles, aberrant expression of germinal center-associated markers has also been reported in NMZL. From a diagnostic standpoint, the expression of germinal center-associated markers in rare cases of NMZL and their lack in a subset of follicular lymphoma, make the separation of NMZL from diffuse follicle center lymphoma particularly challenging. In our experience, the use of additional germinal center-associated markers is helpful in ruling out a follicular lymphoma particularly if staining for CD10, a marker that is frequently employed in immunohistologic panels, is absent. In this study, we found that together with BCL6, HGAL and LMO2, two germinal center B-cell-associated markers that we recently characterized, are excellent adjuncts in the immunohistologic work-up of low grade B-cell lymphomas and especially those that exhibit a diffuse small lymphoid proliferation.
In summary, we describe a large series of primary NMZL that highlights the heterogeneity of this disease and exhibits a spectrum of morphologic and immunophenotypic variability. The four immunoarchitectural patterns we describe, together with their frequency and potential overlap with other lymphomas in the differential diagnosis, affords a greater understanding of the inherent heterogeneity of this lymphoma. The presence of large cells in a significant proportion of cases and the inclusion of germinal center-associated markers in their work-up support and extend previous observations. In addition, we found that CD21 staining was particularly useful in highlighting disrupted FDC meshworks and follicular colonization, a feature that lends support for the diagnosis of MZL. Recent gene expression profiling studies of splenic marginal zone lymphoma have pointed to specific abnormalities in that entity,24 which after further testing and validation, can be employed for the diagnosis of splenic MZL. Similarly, genomic and proteomic approaches to interrogate NMZL will be needed and will likely furnish candidate markers specific to the diagnosis. In the interim, awareness of the immunoarchitectural patterns of NMZL is important for rendering an accurate and reproducible diagnosis and to distinguish this entity from other lymphomas in its differential diagnosis.
Acknowledgments
Funding: Supported in part by NIH POI CA34233, NIH CA109335, NIH CA122105
References
- 1.Sheibani K, Sohn CC, Burke JS, Winberg CD, Wu AM, Rappaport H. Monocytoid B-cell lymphoma. A novel B-cell neoplasm. Am J Pathol. 1986;124:310–318. [PMC free article] [PubMed] [Google Scholar]
- 2.Cousar JB, McGinn DL, Glick AD, List AF, Collins RD. Report of an unusual lymphoma arising from parafollicular B-lymphocytes (PBLs) or so-called “monocytoid“ lymphocytes. Am J Clin Pathol. 1987;87:121–128. doi: 10.1093/ajcp/87.1.121. [DOI] [PubMed] [Google Scholar]
- 3.Piris MA, Rivas C, Morente M, Cruz MA, Rubio C, Oliva H. Monocytoid B-cell lymphoma, a tumour related to the marginal zone. Histopathology. 1988;12:383–392. doi: 10.1111/j.1365-2559.1988.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 4.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 5.Jaffe ES, Harris NL, Stein H, Vardiman JW. Pathology and genetics of tumors of the hematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. [Google Scholar]
- 7.Mollejo M, Camacho FI, Algara P, Ruiz-Ballesteros E, Garcia JF, Piris MA. Nodal and splenic marginal zone B cell lymphomas. Hematol Oncol. 2005;23:108–118. doi: 10.1002/hon.762. [DOI] [PubMed] [Google Scholar]
- 8.Arcaini L, Paulli M, Boveri E, et al. Splenic and nodal marginal zone lymphomas are indolent disorders at high hepatitis C virus seroprevalence with distinct presenting features but similar morphologic and phenotypic profiles. Cancer. 2004;100:107–115. doi: 10.1002/cncr.11893. [DOI] [PubMed] [Google Scholar]
- 9.Berger F, Felman P, Thieblemont C, et al. Non-MALT marginal zone B-cell lymphomas: a description of clinical presentation and outcome in 124 patients. Blood. 2000;95:1950–1956. [PubMed] [Google Scholar]
- 10.Nathwani BN, Anderson JR, Armitage JO, et al. Marginal zone B-cell lymphoma: A clinical comparison of nodal and mucosa-associated lymphoid tissue types. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1999;17:2486–2492. doi: 10.1200/JCO.1999.17.8.2486. [DOI] [PubMed] [Google Scholar]
- 11.Oh SY, Ryoo BY, Kim WS, et al. Nodal marginal zone B-cell lymphoma: Analysis of 36 cases. Clinical presentation and treatment outcomes of nodal marginal zone B-cell lymphoma. Ann Hematol. 2006;85:781–786. doi: 10.1007/s00277-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 12.Kojima M, Inagaki H, Motoori T, et al. Clinical implications of nodal marginal zone B-cell lymphoma among Japanese: study of 65 cases. Cancer Sci. 2007;98:44–49. doi: 10.1111/j.1349-7006.2006.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campo E, Miquel R, Krenacs L, Sorbara L, Raffeld M, Jaffe ES. Primary nodal marginal zone lymphomas of splenic and MALT type. Am J Surg Pathol. 1999;23:59–68. doi: 10.1097/00000478-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson PG, Norten AJ. Extranodal Lymphomas. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 15.Natkunam Y, Lossos IS, Taidi B, et al. Expression of the human germinal center-associated lymphoma (HGAL) protein, a new marker of germinal center B-cell derivation. Blood. 2005;105:3979–3986. doi: 10.1182/blood-2004-08-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natkunam Y, Zhao S, Mason DY, et al. The oncoprotein LMO2 is expressed in normal germinal-center B cells and in human B-cell lymphomas. Blood. 2007;109:1636–1642. doi: 10.1182/blood-2006-08-039024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thieblemont C, Bastion Y, Berger F, et al. Mucosa-associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624–1630. doi: 10.1200/JCO.1997.15.4.1624. [DOI] [PubMed] [Google Scholar]
- 18.Traverse-Glehen A, Felman P, Callet-Bauchu E, et al. A clinicopathological study of nodal marginal zone B-cell lymphoma. A report on 21 cases. Histopathology. 2006;48:162–173. doi: 10.1111/j.1365-2559.2005.02309.x. [DOI] [PubMed] [Google Scholar]
- 19.Naresh KN. Nodal marginal zone B-cell lymphoma with prominent follicular colonization - difficulties in diagnosis: a study of 15 cases. Histopathology. 2008;52:331–339. doi: 10.1111/j.1365-2559.2007.02951.x. [DOI] [PubMed] [Google Scholar]
- 20.Camacho FI, Algara P, Mollejo M, et al. Nodal marginal zone lymphoma: a heterogeneous tumor: a comprehensive analysis of a series of 27 cases. Am J Surg Pathol. 2003;27:762–771. doi: 10.1097/00000478-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lai R, Arber DA, Chang KL, Wilson CS, Weiss LM. Frequency of bcl-2 expression in non-Hodgkin’s lymphoma: a study of 778 cases with comparison of marginal zone lymphoma and monocytoid B-cell hyperplasia. Mod Pathol. 1998;11:864–869. [PubMed] [Google Scholar]
- 22.Lai R, Weiss LM, Chang KL, Arber DA. Frequency of CD43 expression in non- Hodgkin lymphoma. A survey of 742 cases and further characterization of rare CD43+ follicular lymphomas. Am J Clin Pathol. 1999;111:488–494. doi: 10.1093/ajcp/111.4.488. [DOI] [PubMed] [Google Scholar]
- 23.Conconi A, Bertoni F, Pedrinis E, et al. Nodal marginal zone B-cell lymphomas may arise from different subsets of marginal zone B lymphocytes. Blood. 2001;98:781–786. doi: 10.1182/blood.v98.3.781. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Ballesteros E, Mollejo M, Rodriguez A, et al. Splenic marginal zone lymphoma: proposal of new diagnostic and prognostic markers identified after tissue and cDNA microarray analysis. Blood. 2005;106:1831–1838. doi: 10.1182/blood-2004-10-3898. [DOI] [PubMed] [Google Scholar]