Abstract
OBJECTIVES: To compare the clinical presentation, autonomic dysfunction, and pregnancy outcomes in parous and nulliparous women with postural tachycardia syndrome (POTS) and in women with POTS before and after pregnancy.
PATIENTS AND METHODS: This study consists of women who had at least 1 pregnancy during which time they met criteria for POTS between May 1993 and July 2009. All patients underwent standard autonomic testing. POTS was defined as a heart rate (HR) increase of greater than 30 beats/min on head-up tilt (HUT) with symptoms of orthostatic intolerance. Patients' charts were reviewed retrospectively to determine pregnancy outcomes.
RESULTS: Clinical characteristics related to POTS did not differ between parous and nulliparous women except for disease duration (parous, 3.7±2.6; nulliparous, 2.1±2.2; P<.001). Autonomic dysfunction did not differ between groups (change in HR on HUT: parous, 42.6±12.0 beats/min; nulliparous, 41.3±10.6 beats/min; P=.39). Of 116 total pregnancies, adverse pregnancy outcomes were reported in 9% and maternal complications in 1%. No complication was related to POTS. There was a trend toward modest improvement in autonomic dysfunction before and after pregnancy (change in HR on HUT: before pregnancy, 38.1±22.7 beats/min; after pregnancy, 21.9±14.9 beats/min; P=.07).
CONCLUSION: The long-term impact of pregnancy on POTS does not appear to be clinically important. However, there does appear to be a trend toward improvement in the short-term postpartum period. Adverse pregnancy events were similar to those seen in the general public and do not present a barrier to women with POTS who want to have children.
The long-term impact of pregnancy on postural tachycardia syndrome does not appear to be clinically important; however, there does appear to be a trend toward improvement in the short-term postpartum period. Adverse pregnancy events were similar to those seen in the general population and do not present a barrier to woman with postural tachycardia syndrome who want to have children.
CASS = composite autonomic severity score; HR = heart rate; HUT = head-up tilt; OI = orthostatic intolerance; POTS = postural tachycardia syndrome; TST = thermoregulatory sweat test
Postural tachycardia syndrome (POTS) is defined as the development of orthostatic symptoms associated with an increase in heart rate (HR) greater than 30 beats/min. More severe cases are associated with a standing HR increase greater than 120 beats/min.1 Orthostatic symptoms most often include light-headedness, palpitations, presyncope, and exacerbations with heat or exercise.2 Overall, the prognosis of POTS has been reported to be favorable in follow-up studies, with 80% of patients reporting improvement and 60% becoming functionally normal.3
Onset of POTS occurs predominantly between the ages of 15 and 50 years,4 with a female predominance.1,2,5-7 The female-to-male ratio has been reported to be as high as 5:1.8 Given the female predominance and the occurrence of POTS during the childbearing years, the effects of this disorder on pregnancy is often raised in clinical practice. However, until recently published data have been minimal. A recent study in 22 women indicated that, although the effect on symptoms related to POTS during pregnancy varied, the actual pregnancy was not affected.9
The objectives of this study were as follows: (1) to determine whether differences exist in clinical presentation, autonomic dysfunction, and/or laboratory parameters between parous and nulliparous women who meet the criteria for the diagnosis of POTS; (2) to describe pregnancy outcomes in women diagnosed with POTS; (3) to determine whether there are differences in autonomic function testing results and orthostatic intolerance (OI) before and after pregnancy in women diagnosed with POTS; and (4) to describe changes with regard to OI that occurs during the course of pregnancy.
PATIENTS AND METHODS
Participants included a consecutive series of women seen at the Autonomic Disorders Center at Mayo Clinic in Rochester, MN, who have a diagnosis of POTS and had at least 1 pregnancy during the course of this disorder. POTS was defined as a heart rate increase of 30 beats/min on head-up tilt (HUT) and associated symptoms of OI. For inclusion, all 4 predetermined criteria were fulfilled: (1) onset of POTS between the ages of 16 and 50 years; (2) orthostatic HR increment of 30 beats/min within 5 minutes of HUT; (3) symptoms of OI; and (4) at least 1 pregnancy during the course of POTS (parous women only). Patients who developed POTS remote to a prior pregnancy were excluded. In comparing prepregnancy with postpregnancy autonomic function, women were required to have a prepregnancy autonomic evaluation (including standard autonomic testing) and to have met all the aforementioned criteria. In addition, these women were required to have a second autonomic evaluation (including standard autonomic testing) within a maximum of 3 years after giving birth.
Patients were excluded if there was another cause of autonomic failure. In addition, patients who had failure of other organ systems or systemic illness that could affect autonomic function or their ability to cooperate during testing were excluded. These included dementia, pheochromocytoma, heart failure, hypertension, renal or hepatic disease, severe anemia, alcoholism, malignant neoplasms, hypothyroidism, sympathectomy, or strokes. Patients taking medications such as anticholinergic, α-adrenergic, and β-adrenergic antagonists that could not be withheld before autonomic testing were excluded.
Pregnancy Measures
Pregnancy outcome measures included (when available) a complete pregnancy history and a descriptive history of all pregnancies before and during the course of POTS. Data included the following: complications during pregnancy and postpartum (maternal and fetal); whether complications were related to the diagnosis of POTS; birth weight; gestational age; Apgar scores (1 and 5 minutes); health of newborn; and changes in OI related to POTS prepregnancy and postpregnancy as well as per trimester.
Autonomic Testing
Standardized autonomic testing was performed as previously described.10,11 Medications known to interfere with autonomic testing, including anticholinergic, α-adrenergic and β-adrenergic antagonists, were withheld before evaluation. The quantitative sudomotor axon reflex test evaluates the postganglionic sympathetic sudomotor axon. The resulting sweat response was recorded routinely from 4 sites (forearm, proximal leg, distal leg, and foot). Results were compared to normative data derived from studies of 223 healthy people aged 10 to 83 years.12 Cardiovagal function was assessed using HR response to deep breathing and Valsalva ratio. Results were compared to normative data derived from 157 healthy people aged 10 to 83 years.12 Cardiovascular adrenergic function was evaluated by measuring blood pressure and HR responses to Valsalva maneuver and HUT.10,11 The thermoregulatory sweat test (TST) was performed as previously described13; it provides a quantitative measure of total body sweating. The percentage of anhidrosis on the anterior body surface was calculated from the distribution of total body sweating (100% indicates complete anhidrosis). Serum norepinephrine levels (supine and standing), 24 hour urine volume, and 24 hour urine sodium levels were obtained as per standard clinical laboratory protocols (Mayo Clinic, Rochester, MN).
Composite Autonomic Severity Score
The composite autonomic severity score (CASS) is derived from the autonomic reflex screen as previously described.14 CASS provides a measure of the severity and distribution of autonomic failure and has been previously validated to quantify autonomic dysfunction on standard autonomic testing.15 The 11-point CASS is divided into 3 subscores: sudomotor (0-3), cardiovagal (0-3), and adrenergic (0-4). Each score is normalized for the confounding variables of age and sex.12
OI Scale
The OI Scale is 5-point scale, with 0 being normal and 4 the most severe. The scale was developed to describe the severity of clinical symptoms and the effect on activities of daily living related to OI. This scale has been previously described and validated.16
Statistical Analyses
Descriptive statistics are presented as mean ± SD. Data (nonparametric) comparing nulliparous vs parous women were analyzed using the Mann-Whitney test. Data (parametric) comparing women with POTS before and after pregnancy were analyzed using the paired t test. Repeated measures of analysis of variance were used to detect differences in parametric data for one continuous variable (OI) at multiple times (prepregnancy; first, second, and third trimesters; and postpregnancy). All tests were 2-tailed; P<.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software, version 15 for Windows (SPSS Inc, Chicago, IL).
RESULTS
Parous vs Nulliparous Women With POTS
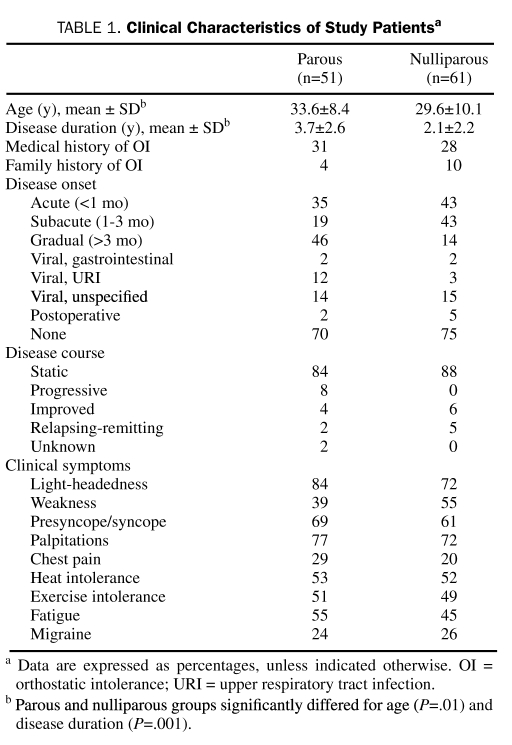
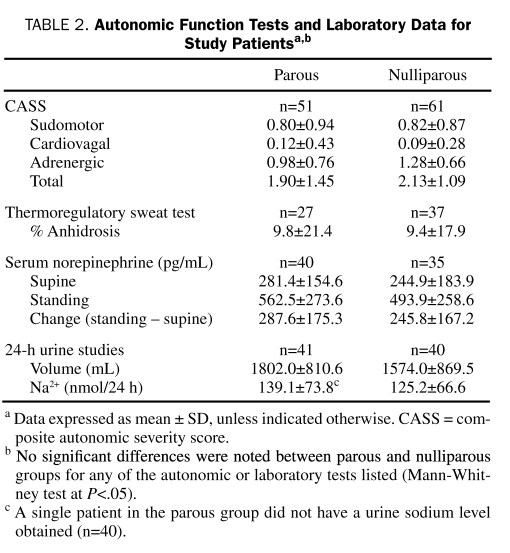
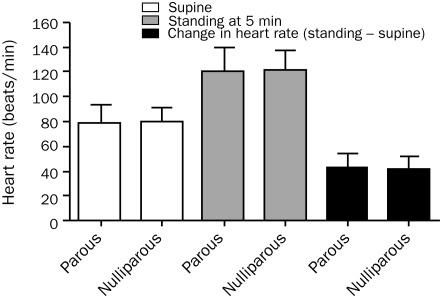
Age (parous, 33.6±8.4 years; nulliparous, 29.6±10.1 years; P=.01 [n=51]) and disease duration (parous, 3.7±2.6 years; nulliparous, 2.1±2.2 years; P<.001 [n=61]) differed significantly between parous and nulliparous women. The frequency of a more rapid onset (<3 month to peak onset) was higher in nulliparous vs parous women (parous, 86%; nulliparous, 54%). However, the rate of clinical symptoms, disease course, and preceding illness did not differ between groups by more than 10% (Table 1). Autonomic dysfunction did not differ between groups, including CASS (totals and all subdomains) and percentage of anhidrosis on TST (Table 2). Indices of HR did not differ significantly between parous and nulliparous women on HUT (change in HR: parous, 42.6±12.0 beats/min; nulliparous, 41.3±10.6 beats/min; P=.39; Figure 1).
TABLE 1.
Clinical Characteristics of Study Patientsa
TABLE 2.
Autonomic Function Tests and laboratory data for Study Patientsa,b
FIGURE 1.
Heart rate changes in response to head-up tilt in parous vs nulliparous women. No significant difference in supine or standing (at 5 minutes) heart rate was noted between parous and nulliparous women (Mann-Whitney test, P=.57; P=.94, respectively). Heart rate increment on head-up tilt (standing – supine) also did not differ significantly (Mann-Whitney test, P=.39). Error bars show mean ± SD.
Adverse pregnancy outcomes were reported in 10 (9%) of 116 total pregnancies. Of these adverse outcomes, 8 (7%) were a result of miscarriage at less than 25 weeks (Table 3). Maternal complications were reported in 1 (1%) of 116 total pregnancies. No fetal or maternal complications could be directly contributed to the underlying diagnosis of POTS. All adverse outcomes are described in Table 3.
TABLE 3.
Pregnancy Outcomes in Women With POTSa
Comparison of Autonomic Dysfunction Before and After Pregnancy
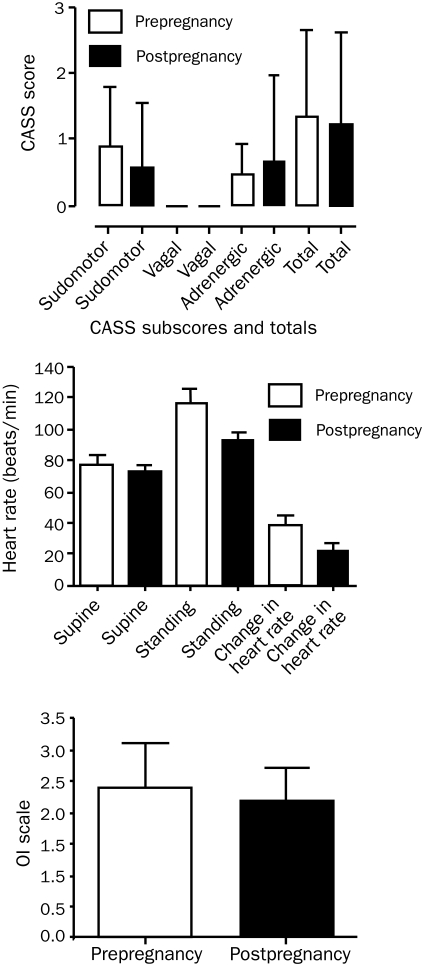
There was no significant difference between autonomic dysfunction before and after pregnancy as measured by standard autonomic testing and by standard measures of OI (Figure 2). However, there appeared to be a slight trend toward modest improvement in autonomic dysfunction in women studied before and after pregnancy (Figure 2). Similarly, HR change after HUT was not significantly different but showed the same trend (change in HR on HUT: prepregnancy, 38.1±22.7 beats/min; postpregnancy, 21.9±14.9 beats/min; P=.07 [n=10]).
FIGURE 2.
Autonomic dysfunction, heart rate changes, and orthostatic intolerance (OI) prepregnancy and postpregnancy. Composite autonomic severity scores (CASS), including sudomotor subscores, adrenergic subscores, and totals, did not differ significantly prepregnancy and postpregnancy (paired t test, P=.48; P=.59; P=.81, respectively [n=9]). There was no significant difference in supine heart rate, standing heart rate (at 5 minutes), or heart rate increment on head-up tilt pre- and postpartum (paired t test, P=.29; P=.06; P=.07; respectively [n=10]). In addition, OI (as per the OI scale, see Patients and Methods section) did not differ significantly between groups (paired t test, P=.51 [n=10]). Error bars show mean ± SD.
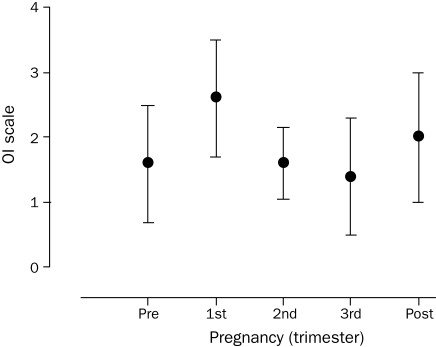
OI per Trimester of Pregnancy
POTS symptoms as measured by the OI scale did not differ statistically during any of the 3 trimesters (F=1.564; P=.20; [n=5]). However, the general trend observed was worsening of OI in the first trimester and improvement in the second and third trimesters (Figure 3). Interestingly, this trend also indicated worsening of POTS symptoms in the first trimester compared to prepregnancy.
FIGURE 3.
Orthostatic intolerance (OI) improved in the later stages of pregnancy. A nonsignificant trend toward a worsening of OI (as per the OI scale, see Patients and Methods section) was noted in the first trimester with subsequent improvement in the second and third trimesters (F=1.564; P=.20 [n=5]). Error bars show mean ± SD.
DISCUSSION
Our results answer 4 important questions regarding the association between pregnancy and POTS. First, no clinically important differences were noted between parous and nulliparous women in clinical presentation, autonomic testing, or laboratory evaluation. Second, the adverse event rate for mother and child during pregnancy in patients with POTS was similar to that seen in the general public. Third, autonomic evaluation showed a trend toward improvement but was not significant when comparing data before and after pregnancy. Finally, a nonsignificant trend toward improvement in OI was noted in the second and third trimesters.
Our study has 2 major limitations. The first is the lack of data for method of delivery and is related to the difficulties encountered in retrospective studies. Data on method of delivery (ie, spontaneous vaginal delivery, cesarean section) were not captured in most available records. Therefore, a reliable estimation of frequency per birth for a particular method of delivery was not possible. This was confounded because, when an adverse event occurred, data were consistently present. This would have likely led to a possible association between cesarean section and adverse outcomes, which would be misleading. Of note, one of the women in the parous group opted for a planned cesarean section solely on the basis of her prior POTS diagnosis. Ultimately, the question of method of delivery is important and will likely require future prospective studies to provide an answer.
The second limitation is the lack of standardized data on clinical autonomic testing and symptom profiles during pregnancy. Standard autonomic testing is noninvasive, safe, and performed on pregnant women (with the exception of the TST) at our center (Autonomic Disorders Center, Mayo Clinic) when clinically necessary. However, standardized control values do not exist for pregnant women.10,12 Therefore, our data cannot be compared to those of pregnant women who do not have POTS.
Interestly, a significant difference in age (33.6 vs 29.6 years) and disease duration (3.7 vs 2.1 years) was noted between parous and nulliparous women, respectively. The age of parous women was higher than that previously reported at 30.2 years, but disease duration was similar to that previously reported at 4.1 years.2 However, approximately 20% of the patients in the study by Thieben et al2 were male. The difference in age most likely reflects the fact that pregnancy increases in frequency with age, and it could be argued that this is an expected result given the study design. However, the statistically significant differences we report in age and disease duration are highly unlikely to have any clinical importance in regard to either treatment or prognosis, particularly because the clinical characteristics and autonomic study results did not differ between groups.
The adverse events reported during pregnancy in our POTS cohort (total fetal complications, 9%; miscarriages, 7%) are similar to previously reported miscarriage rates of approximately 8% in the general public.17 Of the other 2 fetal complications, 1 was placenta previa with subsequent miscarriage, which would have only increased the frequency of miscarriage in our study to 8%, still comparable to rates in the general population and to that previously published in patients with POTS.9 The other complication was placental abruption and malpresentation, resulting in a healthy newborn but an obligatory hysterectomy for the mother (the only known maternal complication). We were surprised that maternal complications were minimal. Our prior clinical impression was that some of these women would have difficulty with OI during delivery and immediately postpartum. Even more conservative measures, such as intravenous fluid rehydration, for treating POTS-related symptoms were not found in the available clinical records. This may be related to our data showing a trend toward improvement in several autonomic parameters in the later trimesters and in the short-term postpartum period.
Changes in the cardiovascular system of pregnant women are substantial, reaching a maximum in the middle of the second trimester and continuing through the third trimester. These result in decreased systemic vascular resistance, increased cardiac output, and a large increase in plasma and blood volume.18 Blood and plasma volume can increase as much as 40% and 45% to 55%, respectively, compared with prepregnancy levels.19 These changes may underlie the trend we observed toward improvement in OI during the second and third trimesters. This would be comparable to the mainstays of conservative treatment of POTS (increased fluid and salt intake) aimed at increasing plasma volume and resulting in clinical improvement.20
CONClUSION
To our knowledge, this is the first systematic study of the association between POTS and pregnancy. The long-term impact of pregnancy on POTS does not appear to be clinically important, and the diagnosis of POTS does not present an increased risk for women during pregnancy and childbirth. To arrive at these conclusions, we used standard clinical instruments, such as the OI scale, that are practical, quick, and valid measures easily incorporated into routine clinical practice.16 The standard autonomic testing used in this study, specifically HUT, is commercially available and provides the criterion standard for which POTS is diagnosed.1-3 As such, our analysis of POTS during pregnancy is readily applicable to clinical practice. Our hope is that the data provided in this study will help clinicians make decisions in the management of pregnant women with POTS or those thinking of becoming pregnant who have POTS.
Footnotes
This work was supported in part by the National Institutes of Health (NS32352, NS44233, NS22352, NS43364), Mayo CTSA (UL1 RR24150), and Mayo funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
An earlier version of this article appeared Online First.
REFERENCES
- 1.Low PA, Opfer-Gehrking TL, Textor SC, et al. Comparison of the postural tachycardia syndrome (POTS) with orthostatic hypotension due to autonomic failure. J Auton Nerv Syst. 1994;50(2):181-188 [DOI] [PubMed] [Google Scholar]
- 2.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82(3):308-313 [DOI] [PubMed] [Google Scholar]
- 3.Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin Proc. 1999;74(11):1106-1110 [DOI] [PubMed] [Google Scholar]
- 4.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43(1):132-137 [DOI] [PubMed] [Google Scholar]
- 5.Jacob G, Costa F, Shannon JR, et al. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343(14):1008-1014 [DOI] [PubMed] [Google Scholar]
- 6.Raj SR, Biaggioni I, Yamhure PC, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005;111(13):1574-1582 [DOI] [PubMed] [Google Scholar]
- 7.Rosen SG, Cryer PE. Postural tachycardia syndrome: reversal of sympathetic hyperresponsiveness and clinical improvement during sodium loading. Am J Med. 1982;72(5):847-850 [DOI] [PubMed] [Google Scholar]
- 8.Low PA, Sandroni P, Joyner MJ, Shen WK. Postural tachycardia syndrome. In: Low PA, Benarroch EE, eds. Clinical Autonomic Disorders 3rd ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2008:515-533 [Google Scholar]
- 9.Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Outcomes of pregnancy in patients with preexisting postural tachycardia syndrome. Pacing Clin Electrophysiol. 2009;32(8):1000-1003 [DOI] [PubMed] [Google Scholar]
- 10.Low PA, Opfer-Gehrking TL. The autonomic laboratory. Am J Electroneurodiagnostic Technol. 1999;39(2):65-76 [PubMed] [Google Scholar]
- 11.Low PA. Testing the autonomic nervous system. Semin Neurol. 2003;23(4):407-421 [DOI] [PubMed] [Google Scholar]
- 12.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20(12):1561-1568 [DOI] [PubMed] [Google Scholar]
- 13.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clin Proc. 1989;64(6):617-628 [DOI] [PubMed] [Google Scholar]
- 14.Low PA, Sletten DM. Laboratory evalution of autonomic failure. In: Low PA, Benarroch EE, eds. Clinical Autonomic Disorders 3rd ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2008:130-163 [Google Scholar]
- 15.Low PA. Composite autonomic scoring scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68(8):748-752 [DOI] [PubMed] [Google Scholar]
- 16.Schrezenmaier C, Gehrking JA, Hines SM, Low PA, Benrud-Larson LM, Sandroni P. Evaluation of orthostatic hypotension: relationship of a new self-report instrument to laboratory-based measures. Mayo Clin Proc. 2005;80(3):330-334 [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577-584 [DOI] [PubMed] [Google Scholar]
- 18.Ganzevoort W, Rep A, Bonsel GJ, de Vries JI, Wolf H. Plasma volume and blood pressure regulation in hypertensive pregnancy. J Hypertens. 2004;22(7):1235-1242 [DOI] [PubMed] [Google Scholar]
- 19.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol. 1983;245(5, pt 1): R20-R729 [DOI] [PubMed] [Google Scholar]
- 20.Low PA, Sandroni P, Joyner MJ, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. 2009;20(3):352-358 [DOI] [PMC free article] [PubMed] [Google Scholar]