Abstract
Intraoperative (IO) transesophageal echocardiography (TEE) is widely used for assessing the results of valvular heart disease (VHD) surgery. Epiaortic ultrasonography (EAU) has been recommended for prevention of perioperative strokes. To what extent does high-quality evidence justify the widespread use of these imaging modalities? In March 2009, we searched MEDLINE (PubMed and OVID interfaces) and EMBASE for studies published in English using database-specific controlled vocabulary describing the concepts of IOTEE, cardiac surgery, VHD, and EAU. We found no randomized trials or studies with control groups assessing the impact of IOTEE in VHD surgery. Pooled analysis of 8 observational studies including 15,540 patients showed an average incidence of 11% for prebypass surgical changes and 4% for second pump runs, suggesting that patients undergoing VHD surgery may benefit significantly from IOTEE, particularly from postcardiopulmonary bypass IOTEE in aortic repair and mitral repair and replacement, but less so in isolated aortic replacement. Further available indirect evidence was satisfactory in the test accuracy and surgical quality control aspects, with low complication rates for IOTEE. The data supporting EAU included 12,687 patients in 2 prospective randomized studies and 4 nonrandomized, controlled studies, producing inconsistent outcome-related results. Despite low-quality scientific evidence supporting IOTEE in VHD surgery, we conclude that indirect evidence supporting its use is satisfactory and suggests that IOTEE may offer considerable benefit in valvular repairs and mitral replacements. The value of IOTEE in isolated aortic valve replacement remains less clear. Evidence supporting EAU is scientifically more robust but conflicting. These findings have important clinical policy and research implications.
AR = aortic regurgitation; AVR = aortic valve replacement; CPB = cardiopulmonary bypass; EAU = epiaortic ultrasonography; IO = intraoperative; LVOT = left ventricular outflow tract; MR = mitral regurgitation; OR = operating room; PFO = patent foramen ovale; TEE = transesophageal echocardiography; TR = tricuspid regurgitation; TTE = transthoracic echocardiography; VHD = valvular heart disease
Significant valvular heart disease (VHD) affects 13% of patients aged 75 years or older, has a national prevalence of 2.5%,1 and is primarily due to mitral regurgitation (MR).
Intraoperative (IO) transesophageal echocardiography (TEE) is widely used to monitor patients undergoing VHD surgery. Landmark practice guidelines for the implementation of IOTEE2-4 recommend its use in VHD surgery.
The incidence of perioperative stroke in cardiac surgery has been estimated to range between 1% to 2% (Society of Thoracic Surgeons, 2007) and 4% to 5%.5 Intraoperative TEE is superior to surgical palpation in detecting atheromas6,7; however, epiaortic ultrasonography (EAU) is more sensitive than IOTEE and has been recommended recently for use in patients at risk for stroke.8
Given the expansion in the use of these technologies, we sought to review the literature to determine the extent to which high-quality scientific evidence justifies their use.
METHODS
Review Question
To what extent do IOTEE and EAU improve outcomes in patients undergoing VHD surgery?
Eligibility Criteria
Randomized trials of test management using IOTEE or EAU and consecutive case series of IOTEE or EAU reporting patient-important outcomes were eligible. In their absence, observational, nonconsecutive series and case reports became eligible. To ensure that observational studies reflected mature learning curves associated with IOTEE, we chose arbitrarily to limit studies to those describing experience with more than 100 patients.
Search Strategy
In March 2009, we searched MEDLINE (PubMed and OVID interfaces) and EMBASE for studies published in English using database-specific controlled vocabulary describing the concepts of IOTEE, cardiac surgery, VHD, and EAU (Table 1).
TABLE 1.
Literature Search Strategy
Data Analysis
For each study, we noted methodological quality, surgical indications, change in the surgery planned, rate of second pump run, morbidity and mortality, and complications from imaging. We contacted the authors of one study to clarify its methodological characteristics.9
For pooled analysis of IOTEE impact in VHD surgery, we chose studies reporting actual alterations in surgical (not medical) management, in which a single valve was addressed or in which a valve-by-valve breakdown of results was provided. For EAU in stroke prevention, we chose available randomized trials and nonrandomized controlled studies.
RESULTS
Search Findings
Duplicates, reviews, commentaries, congenital and pediatric heart disease articles, and studies related to commercial valve brands or specific surgical techniques were eliminated. Of the remaining articles, 8 were appropriate for pooled analysis of the surgical impact of IOTEE in VHD9-16 (Table 2) and 6 for analysis of EAU impact on stroke prevention17-22 (Table 3). Five additional observational studies of the impact of IOTEE 23-27 and 1 additional EAU study28 were eligible. The remaining studies related to test accuracy,29-43 IOTEE-related outcomes,44-47 surgical quality control by IOTEE,48-54 IOTEE safety reports,55,56 and case reports.57-60
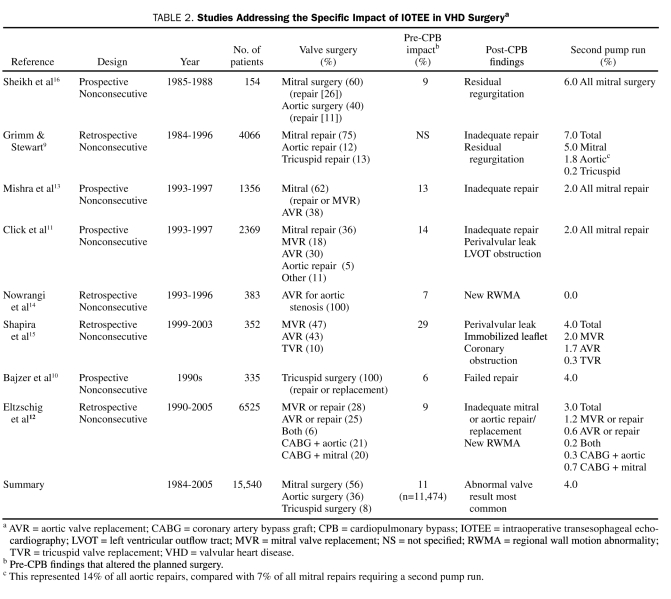
TABLE 2.
Studies Addressing the Specific Impact of IOTEE in VHD Surgerya
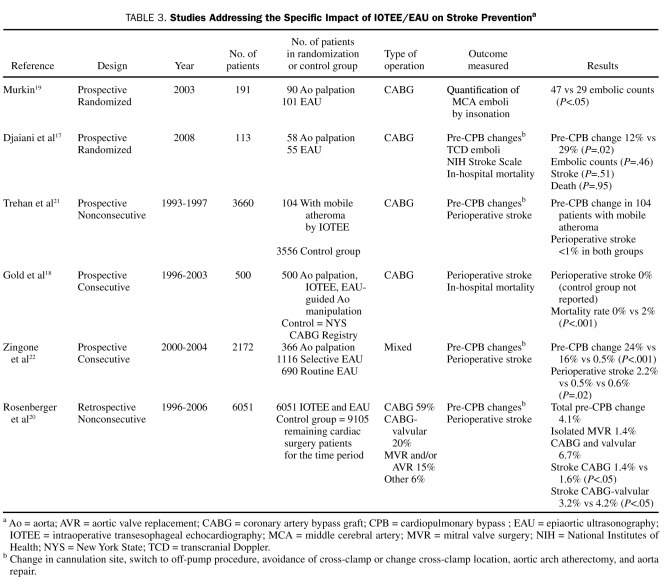
TABLE 3.
Studies Addressing the Specific Impact of IOTEE/EAU on Stroke Preventiona
One impact-related study61 not identified by the search was reported for its academic value. To enhance comprehensiveness, 9 additional studies were reported for test accuracy,62-70 2 for surgical quality control,71,72 and 1 for IOTEE safety.73
Methodological Quality of Included Studies
The 8 IOTEE surgical impact studies included for pooled analysis were observational without control groups or consecutive patients (Table 2). Patients were chosen on the basis of probe availability13; presence of images both before and after cardiopulmonary bypass (CPB),15,16 as indicated by the attending anesthesiologist and surgeon9,11,12,14; and availability of preoperative and follow-up echocardiograms.10
Studies of the IOTEE/EAU impact on stroke prevention included 2 available prospective randomized trials,17,19 2 observational retrospective studies of nonconsecutive patients,18,20 and 2 observational prospective studies of consecutive patients21,22 (Table 3). Studies of IOTEE-related outcomes included consecutive patients and control groups.44-46
Surgical Alterations Prompted by IOTEE in VHD Surgery
The surgical impact of IOTEE in mixed cardiac surgical populations has been evaluated in more than 35,000 patients.9-16,23-27,61 Of these patients, the impact of IOTEE in VHD surgery can be analyzed in 15,540 patients from 8 major observational reports.9-16 Changes in the planned surgical procedure related to pre-CPB IOTEE evaluation ranged from 6% to 29%, with a pooled average of 11% (Table 2). Second pump runs prompted by post-CPB IOTEE ranged from 0% to 7%, with a pooled average of 4% (Table 2). The most common pre-CPB finding that resulted in alterations to the surgical procedure was the presence of a patent foramen ovale (PFO). Other pre-CPB IOTEE surgery-altering findings were unsuspected MR, aortic atheroma, intracardiac thrombus, endocarditis complications, left ventricular outflow tract (LVOT) obstruction, and aortic homograft sizing.11,14 Post-CPB alterations (second pump runs) were related to residual valvular abnormalities (Table 2).
The 1996 American Society of Anesthesiologists IOTEE guidelines were prospectively tested in 851 patients undergoing cardiac surgery,61 and surgical alterations were necessary in 17% of patients undergoing class I surgical procedures (includes valve repairs) and in 4% of patients undergoing class II surgical procedures (includes valve replacements) (P<.001).
In a mixed cardiac surgery population of 203 consecutive patients, the incidence of second pump runs was 2.5%24 (vs 4.0% in our VHD pooled analysis). Similarly, the most recent report in a mixed cardiac surgery population showed a second pump run incidence of 2.2% (vs 4.0% in our VHD pooled analysis) in 12,566 nonconsecutive patients.12 This report was also consistent with others showing an IOTEE-prompted incidence of graft revisions of 0.8% to 1.0% in isolated revascularization surgery.12,13,27
Diagnostic Accuracy of IOTEE in VHD and Related Outcomes
Mitral Valve. The evaluation of MR severity with pre-CPB IOTEE was 95% to 97% accurate compared with findings on preoperative trans thoracic echocardiography (TTE) for flail leaflets.30 In a large study of patients with a predominantly degenerative MR mechanism, the exact severity agreement between preoperative TTE and pre-CPB IOTEE was 64%.64 However, when mixed MR mechanisms were studied, the correlation between preoperative TTE and pre-CPB IOTEE was weaker (r=0.40), with an exact agreement of 54%.41 The post-CPB severity of residual MR by IOTEE after mitral repair for degenerative MR correlates fairly well (r=0.66-0.71) with early and late postoperative TTE evaluations.42 Agreement between a pre-CPB, IOTEE-identified dysfunction mechanism and actual operative findings is excellent for both mitral and aortic valve disease.31,63,64
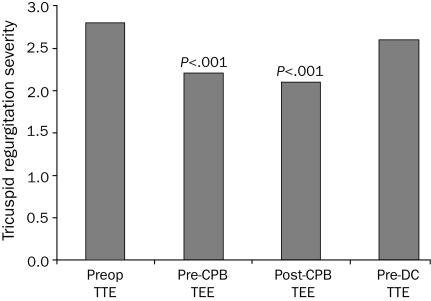
Surprisingly, one study showed that a TTE-defined MR mechanism in 279 patients had a 97% accuracy in predicting mitral valve repair and a 91% agreement with surgical visualization of specific prolapsed segments (no different than pre-CPB IOTEE).39 Importantly, quantification methods by TTE have been validated for TEE,43,65,70 and additional TEE severity-estimation parameters have emerged (ie, pulmonary vein systolic flow reversal in MR).38 Mitral regurgitation may be sensitive to anesthesia-induced hemodynamic conditions, leading to underestimation of severity,30,36 particularly in patients with ischemic MR29; however, administration of intravenous phenylephrine67 and volume to restore physiologic conditions may allow appropriate evaluation of severity.35 In patients undergoing mitral valve repair with concomitant tricuspid regurgitation (TR), we have found that the severity of TR is also significantly underestimated under anesthesia conditions (Figure, submitted data).
FIGURE.
Tricuspid regurgitation severity (1+ to 4+) by preoperative (Preop) transthoracic echocardiography (TTE), by intraoperative transesophageal echocardiography (TEE) before and after cardiopulmonary bypass (CPB), and predischarge (pre-DC) TTE in 115 patients with more than mild tricuspid regurgitation undergoing mitral valve repair for mitral regurgitation. Severity under general anesthesia (both pre-CPB and post-CPB) was significantly less than both preoperative and pre-DC levels (P<.001 in all).
Aortic Valve. In aortic stenosis, TTE is the criterion standard for severity quantification by virtue of the multiple continuous-wave Doppler interrogation sites that prevent underestimation of severity. Planimetry of aortic valve area by 2-dimensional TEE has produced conflicting accuracy results.62,66 The correlation for aortic regurgitation (AR) severity is fair (r=0.70), with 59% exact agreement with TTE.41 Assessment of cusp mobility and jet direction during pre-CPB IOTEE is highly accurate in defining the AR mechanism,63 and aortic valve repair is highly dependent on post-CPB IOTEE for surgical success.9,12
Perivalvular Leaks. The correlation between periprosthetic leaks described by post-CPB IOTEE and direct surgical observation is excellent.69 Nevertheless, post-CPB perivalvular leaks tend to be mild in the aortic and mitral positions40 and resolve over time in more than 50% of cases, with a benign clinical course at late follow-up for most.45
Outcomes. Observational data in patients with moderate residual valve dysfunction determined by post-CPB IOTEE (aortic or mitral valve replacement) suggest that these patients have more postoperative complications and a higher mortality rate compared with those with a satisfactory valve surgery result.16 In patients with ischemic heart disease who are undergoing surgical revascularization (mitral valve surgery patients excluded), identification of even mild residual MR by IOTEE is associated with a worse clinical prognosis.46 In patients with degenerative MR, postrepair residual 1+ or 2+ MR as determined by post-CPB IOTEE is related to an increased rate of reoperations,44 and the mere use of post-CPB IOTEE decreases the rate of reoperations.47 Intraoperative TEE has been touted as cost-effective because it avoids the high costs of reoperation at follow-up.33,37
Surgical Quality Control by IOTEE in VHD Surgery
In cardiac surgery requiring CPB, more than mild AR may preclude antegrade cardioplegia administration and require further strategies for myocardial protection.52
Aortic Valve. Intraoperative TEE is highly accurate in predicting annular size,53 potentially reducing CPB time by 10 to 30 minutes of thaw time when homografts are used. Recently, attention has been focused on the impact of patient-prosthesis mismatch in the aortic position,74 significant mismatch being an independent predictor of long-term mortality. Small calcified annuli (<2 cm) may require annular debridement and/or pericardial patch aortic enlargement to insert an adequately sized prosthesis. After CPB, LVOT subvalvular obstruction may increase the prosthetic aortic mean gradient and may decrease blood pressure,49 requiring concomitant myectomy with aortic valve replacement (AVR). Calcific aortic stenosis is related to ascending aorta atheroma, especially if concomitant coronary disease is present,54,75 necessitating imaging to potentially guide aorta manipulation.
Mitral Valve. In degenerative MR, repairing instead of replacing the valve conveys a survival benefit.76 Probability of repair is substantial when leaflet tissue is abundant32 and annular calcification is not severe. Severe annular calcification predisposes to perivalvular leaks after replacement. In patients with extensive Barlow bileaflet mitral valve disease, it is critical to prioritize scallops requiring intervention, a task accomplished by IOTEE. After repair, the left ventricle is insufflated with saline to visually assess mitral valve integrity. In Barlow disease with dilated ventricle and annulus, it is often difficult to fully insufflate the leaflets to assess coaptation in a static and flaccid heart. It is only after CPB under physiologic loading conditions that mitral valve competence is confirmed by IOTEE. Residual commissural jets are very difficult to assess by methods other than IOTEE. A cause of persistent MR and hemodynamic embarrassment is LVOT obstruction due to systolic anterior motion, which has been described in 1% to 9% of mitral valve repairs.34,50,51 Failure to medically eliminate systolic anterior motion of the mitral valve apparatus in the post-CPB period often necessitates a second pump run for correction. Pre-CPB IOTEE may be predictive of systolic anterior motion at the pre-CPB stage,68,77 potentially modifying the surgeon's repair approach.
In minimally invasive mitral valve repair, pre-CPB IOTEE verifies the cardioplegia catheter in the coronary sinus, the venous cannula position across the right atrium, and the arterial cannula in the descending thoracic aorta.48 After mitral valve replacement, mechanical leaflets may be “stuck” to residual subvalvular tissue,58,59 with resultant obstruction or regurgitation. After mitral annuloplasty, severe AR caused by left coronary cusp retraction has been reported.57 Damage to the circumflex artery after mitral valve repair has been described.60 These conditions are recognized by post-CPB IOTEE.
Mitral abnormalities prompting additional mitral surgery are found in 7% of patients26 on pre-CPB IOTEE during septal myectomy for obstructive hypertrophic cardiomyopathy. In up to 7% of patients, post-CPB IOTEE detects new findings, potentially requiring a second pump run.
Tricuspid Valve. Despite repair, recurrence of significant TR is not uncommon and may be predicted by IOTEE. Tricuspid annulus dilatation,71 amount of leaflet tethering,72 and significant residual regurgitation after repair are predictors of recurrence and have correction potential before the patient leaves the operating room (OR); however, IOTEE underestimates TR severity (Figure).
Safety of IOTEE
Studies including more than 25,000 patients undergoing TEE and IOTEE demonstrate a complication rate of 0.2% or less and a mortality rate of less than 0.1%.11,13,23,55,73 However, a recent retrospective study of 859 cardiac surgery patients found an incidence of major gastrointestinal complications of 1.2%, mostly gastric tears and perforations,56 occurring late (after >24 hours). However, inexperienced echocardiographers performed those procedures, limiting the applicability of the results to experienced centers. In 22,179 patients undergoing IOTEE from 1991 to 2007 at Mayo Clinic in Rochester, MN, 7 esophageal perforations (0.032%) occurred, 3 (0.014%; 43% of perforations) of which were fatal (unpublished data). Therefore, the complication risk of IOTEE in experienced hands is very low, but the mortality associated with complications is considerable. Available data suggest that perforations tend to occur in older patients with poor-quality echocardiographic images and tend to involve the lower esophagus, gastroesophageal junction, and cardias. Whether avoiding low-esophageal and gastric imaging prevents perforations remains unknown.
Intraoperative TEE and EAU in Stroke Prevention During Cardiac Surgery
When compared with direct palpation and IOTEE, EAU has been found to have a better sensitivity and specificity for atheroma detection than IOTEE.28 The incidence of stroke may be reduced with the use of pre-CPB IOTEE18 and IOTEE- and EAU-guided aortic cannulation to minimize handling of the aorta. Randomly compared with surgical palpation alone, EAU is associated with a decrease in perioperative stroke markers19 (Table 3). However, a prospective series of elderly patients undergoing coronary revascularization randomized to EAU-guided aortic cannulation or cannulation guided by IOTEE and aortic palpation showed no differences in stroke incidence between the groups, despite significantly higher alteration of the cannulation procedure prompted by EAU.17 Although EAU has a higher sensitivity for atheromas than IOTEE, these findings suggest that adding EAU to IOTEE may have little clinical impact (Table 3). A recent retrospective study of 6051 nonconsecutive patients undergoing EAU for cardiac surgery without a formal control population revealed an impact of EAU in surgical decision making of 4% and a decreased stroke rate compared with patients not receiving EAU during the same period20 (Table 3). Another study in patients with severe atheroma showed no difference in stroke rates despite substantial IOTEE-prompted changes in aorta manipulation in patients with severe atheroma.21 Zingone et al22 showed that selective and routine use of EAU decreased stroke rates in a large number of patients; however, the comparison was made with aortic palpation and not IOTEE (Table 3).
DISCUSSION
Our review found low-quality evidence supporting the use of IOTEE for VHD surgery. We found no randomized trial of test management that clearly established the value of IOTEE and its impact on patient-important outcomes. However, a body of indirect evidence supports IOTEE in VHD and has been explored in this review. Because aortic atheroma evaluation cannot be excluded from the echocardiographic assessment of patients undergoing valvular surgery, we have also analyzed EAU. Evidence supporting EAU was scientifically more robust but contradictory.
Most recommendations about diagnostic testing rely on an implicit 2-step process to assess how the accuracy of a test indirectly changes patient-important outcomes.78
The first step involves comparing the new test to a reference test to determine accuracy. The accuracy of IOTEE for both severity estimation and mechanism identification must be assessed. Transthoracic echocardiography has been used to validate IOTEE findings, with overall favorable comparative results in regurgitant lesions; however, an important caveat is that MR and TR are underestimated. Mechanistically, IOTEE shows excellent performance compared with operative findings. Furthermore, real-time 3-dimensional IOTEE, which is superior to 2-dimensional TEE for identification of native mitral valve regurgitation mechanisms,79 is currently available and can be performed expeditiously in the OR. Three-dimensional TEE also provides additional information on the anatomy of dehiscence of mitral prosthetic rings and valves.80 However, patient-important outcomes have not been tested for this technology. Interestingly, preoperative TTE is also highly accurate for determination of mechanisms in MR. Also, for aortic stenosis, the most accurate evaluation is achieved by TTE. Thus, a high value must be placed on preoperative TTE in VHD. Furthermore, several of the pre-CPB surgical quality control aspects can readily be evaluated by preoperative TTE; for example, the presence of AR can be established for cardioplegia planning and the prevention of patient-prosthesis mismatch. These advantages of preoperative TTE and the uncertain clinical benefit of correcting mild residual valvular abnormalities with a second pump run raises the following question: Should all patients with VHD undergo IOTEE, a technique that increases costs and whose complications are minimal but potentially life-threatening? The indirect evidence evaluated suggests that both pre-CPB and post-CPB IOTEE are relevant for valvular repairs and that post-CPB IOTEE is relevant for mitral replacements and hypertrophic cardiomyopathy surgery. Isolated AVRs appear to benefit the least from IOTEE (Table 2), except in the case of homograft sizing.
The second step is to assess the relevance of test accuracy as a surrogate to patient-important outcomes (eg, the consequences of being correctly or incorrectly classified as having or not having a disease).78 Judgments must be transparent. In an effort to be transparent, we note that the overall impact of IOTEE in VHD could be significantly lower than our pooled analysis suggests if all consecutive patients had been included in the studies. Furthermore, due to their observational nature, these reports may be biased by false-positive results that may prompt unnecessary second pump runs. The incidence, cost, and morbidity and mortality of unnecessary second pump runs have not been studied and could eliminate any savings prompted by true-positive results or incur additional costs. False-negative results also limit cost-effectiveness and incur future morbidity and mortality. False-negative results seem particularly important in the evaluation of MR, TR, and aortic stenosis severities by IOTEE.
The available relationships between post-CPB IOTEE findings and outcomes are limited and observational. Regarding the relationship between residual MR and adverse clinical outcomes, the latter are thought to be prevented by IOTEE detection and immediate correction; however, we found no prospective, randomized trial determining whether a second pump run to correct less than moderate MR has any positive clinical impact in ischemic or degenerative MR. Also, immediate and effective correction of post-CPB abnormalities requires appropriate communication between echocardiographer and surgeon regarding the anatomic location of residual valvular abnormalities, an issue that we find recurrent in our practice and that remains unstudied in the literature.
A definitive answer to our research question, akin to one on the effectiveness of a therapy, would require direct evidence (randomized trials) regarding the impact of IOTEE and EAU in VHD surgery. In the absence of direct evidence, the diagnostic advantages afforded by IOTEE and EAU provide only indirect evidence of their effectiveness. Nevertheless, improvement in patient-important outcomes can be inferred from the results of nonrandomized trials of test accuracy, provided that effective treatment for the abnormalities detected by that test is available and that test-related adverse effects (ie, IOTEE-related complications) can be reduced.81 Case reports may offer lower-quality evidence of efficacy that could be considered of higher quality if the effect of IOTEE is considered large (eg, it obviously prevented severe morbidity or obviated the need for emergent reoperation in a patient). These conditions are obviously met by IOTEE in VHD, particularly post-CPB IOTEE. The available literature on the surgical impact of IOTEE suggests that the incidence of IOTEE-prompted second pump runs is greater for VHD surgery than for other cardiac surgeries. Mitral valve repair is the leading cause of second pump runs (Table 2), and aortic valve repair is the most common single-valve procedure requiring a second pump run (Table 2). Mitral replacements are subject to a number of uncommon but real complications that are detectable by post-CPB IOTEE and are immediately correctable. Interestingly, the need for second pump runs has decreased from the earliest series to the most current (Table 2), likely reflecting superior prostheses and improved surgical repair techniques. Even this lower second pump rate continues to be significantly higher than the complication rate of IOTEE.
Evidence is lacking as to whether correction of PFO, the most common surgery-altering finding on pre-CPB, has any impact on prevention of stroke, and a call to completion of randomized trials has been issued.82 Moreover, in a recent retrospective study of 2277 cardiac surgery patients with incidental pre-CPB IOTEE-diagnosed PFO, patients with repaired PFO were surprisingly twice as prone to develop postoperative stroke as patients with unrepaired PFO (P=.04).83
Despite conflicting data on clinical outcomes and acknowledging potential limitations of EAU (possible contamination of the sterile field, need for more expensive equipment and training), recent guidelines advocate the use of EAU8 in patients considered to be at high risk for stroke. Whether EAU adds clinical benefit beyond that afforded by IOTEE alone for prevention of stroke remains as yet unanswered.
LIMITATIONS
Our review is limited by the scope of the search strategy (limited to the English language), the high risk of bias in the available evidence (selective reporting of nonconsecutive patients may have overestimated the usefulness of the procedure), and the lack of randomized trials of diagnostic management. Our appraisal raises more questions than it answers. Nonetheless, these questions have the potential of improving institutional quality-control measures and stimulating further scientific exploration of the issues raised.
Given the lower prevalence of pulmonary valve disease in adults, we have not addressed this valve.
IMPLICATIONS FOR CLINICAL POLICY AND RESEARCH
In VHD surgery, available indirect evidence favors the use of IOTEE, placing a high value on avoiding reoperation and a low value on avoiding second pump runs, the very low rate of complications in experienced hands, and the potential incremental costs. Research efforts must concentrate on improving the accuracy of the technique and preventing its related complications. One strategy must be enforcement of guidelines for training in IOTEE, with advanced training of all operators.84 To prevent false-negative results, preoperative TTE should be performed and reviewed for MR, TR, and aortic stenosis, such that operative indications are secured by TTE before the patient reaches the OR. To prevent false-positive results, all available specific and supportive echocardiographic signs of regurgitation should be explored before deciding on second pump runs.85 The ability of the echocardiographer and the surgeon to communicate effectively regarding the residual defect location should be studied and developed.
Randomized studies should be undertaken to address the clinical impact of correcting MR down to a trivial level or abolishing it completely with a second pump run for both ischemic and degenerative scenarios.
Randomized studies addressing the clinical value of IOTEE in isolated AVR could be valuable; however, this would require an alternative technique for exploration of aortic atheroma, such as preoperative computed tomography.
A randomized trial comparing IOTEE with EAU in the prevention of perioperative stroke is critical. Comparison with preoperative computed tomography as an alternative technique seems appropriate.
Studies addressing the sensitivity and specificity of IOTEE for detection of significant TR and maneuvers to improve its accuracy should be conducted. At this juncture, reliance on preoperative TTE is critical.
Closure of incidental PFOs is recommended in patients at high risk of hypoxemia (those with a left ventricular assist device or those undergoing heart transplant)86 but remains controversial in all other situations.
Supplementary Material
Acknowledgments
The authors wish to thank Victor M. Montori, MD, for his critical revision and contributions to the submitted manuscript.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-1011 [DOI] [PubMed] [Google Scholar]
- 2.Task Force on Transesophageal Echocardiography Practice guidelines for perioperative transesophageal echocardiography: a report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists. Anesthesiology 1996;84(4):986-1006 [PubMed] [Google Scholar]
- 3.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation 2003;108:1146-1162 [DOI] [PubMed] [Google Scholar]
- 4.Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. J Am Soc Echocardiogr. 1999;12:884-900 [DOI] [PubMed] [Google Scholar]
- 5.Bucerius J, Gummert JF, Borger MA, et al. Stroke after cardiac surgery: a risk factor analysis of 16,184 consecutive adult patients. Ann Thorac Surg. 2003;75:472-478 [DOI] [PubMed] [Google Scholar]
- 6.Marschall K, Kanchuger M, Kessler K, et al. Superiority of transesophageal echocardiography in detecting aortic arch atheromatous disease: identification of patients at increased risk of stroke during cardiac surgery. J Cardiothorac Vasc Anesth. 1994;8:5-13 [DOI] [PubMed] [Google Scholar]
- 7.Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol. 1992;20:70-77 [DOI] [PubMed] [Google Scholar]
- 8.Glas KE, Swaminathan M, Reeves ST, et al. Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists: endorsed by the Society of Thoracic Surgeons. J Am Soc Echocardiogr. 2007;20:1227-1235 [DOI] [PubMed] [Google Scholar]
- 9.Grimm RA, Stewart WJ. The role of intraoperative echocardiography in valve surgery. Cardiol Clin. 1998;16:477-489, ix [DOI] [PubMed] [Google Scholar]
- 10.Bajzer CT, Stewart WJ, Cosgrove DM, Azzam SJ, Arheart KL, Klein AL. Tricuspid valve surgery and intraoperative echocardiography: factors affecting survival, clinical outcome, and echocardiographic success. J Am Coll Cardiol. 1998;32:1023-1031 [DOI] [PubMed] [Google Scholar]
- 11.Click RL, Abel MD, Schaff HV. Intraoperative transesophageal echocardiography: 5-year prospective review of impact on surgical management. Mayo Clin Proc. 2000;75:241-247 [DOI] [PubMed] [Google Scholar]
- 12.Eltzschig HK, Rosenberger P, Loffler M, Fox JA, Aranki SF, Shernan SK. Impact of intraoperative transesophageal echocardiography on surgical decisions in 12,566 patients undergoing cardiac surgery. Ann Thorac Surg. 2008;85:845-852 [DOI] [PubMed] [Google Scholar]
- 13.Mishra M, Chauhan R, Sharma KK, et al. Real-time intraoperative transesophageal echocardiography–how useful? experience of 5,016 cases. J Cardiothorac Vasc Anesth. 1998;12:625-632 [DOI] [PubMed] [Google Scholar]
- 14.Nowrangi SK, Connolly HM, Freeman WK, Click RL. Impact of intraoperative transesophageal echocardiography among patients undergoing aortic valve replacement for aortic stenosis. J Am Soc Echocardiogr. 2001;14:863-866 [DOI] [PubMed] [Google Scholar]
- 15.Shapira Y, Vaturi M, Weisenberg DE, et al. Impact of intraoperative transesophageal echocardiography in patients undergoing valve replacement. Ann Thorac Surg. 2004;78(2):579-583 [DOI] [PubMed] [Google Scholar]
- 16.Sheikh KH, de Bruijn NP, Rankin JS, et al. The utility of transesophageal echocardiography and Doppler color flow imaging in patients undergoing cardiac valve surgery. J Am Coll Cardiol. 1990;15:363-372 [DOI] [PubMed] [Google Scholar]
- 17.Djaiani G, Ali M, Borger MA, et al. Epiaortic scanning modifies planned intraoperative surgical management but not cerebral embolic load during coronary artery bypass surgery. Anesth Analg. 2008;106:1611-1618 [DOI] [PubMed] [Google Scholar]
- 18.Gold JP, Torres KE, Maldarelli W, Zhuravlev I, Condit D, Wasnick J. Improving outcomes in coronary surgery: the impact of echo-directed aortic cannulation and perioperative hemodynamic management in 500 patients. Ann Thorac Surg. 2004;78:1579-1585 [DOI] [PubMed] [Google Scholar]
- 19.Murkin JM. Pathophysiological basis of CNS injury in cardiac surgical patients: detection and prevention. Perfusion 2006;21:203-208 [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger P, Shernan SK, Loffler M, et al. The influence of epiaortic ultrasonography on intraoperative surgical management in 6051 cardiac surgical patients. Ann Thorac Surg. 2008;85:548-553 [DOI] [PubMed] [Google Scholar]
- 21.Trehan N, Mishra M, Kasliwal RR, Mishra A. Reduced neurological injury during CABG in patients with mobile aortic atheromas: a five-year follow-up study. Ann Thorac Surg. 2000;70:1558-1564 [DOI] [PubMed] [Google Scholar]
- 22.Zingone B, Rauber E, Gatti G, et al. The impact of epiaortic ultrasonographic scanning on the risk of perioperative stroke. Eur J Cardiothorac Surg. 2006;29:720-728 [DOI] [PubMed] [Google Scholar]
- 23.Forrest AP, Lovelock ND, Hu JM, Fletcher SN. The impact of intraoperative transoesophageal echocardiography on an unselected cardiac surgical population: a review of 2343 cases. Anaesth Intensive Care 2002;30:734-741 [DOI] [PubMed] [Google Scholar]
- 24.Michel-Cherqui M, Ceddaha A, Liu N, et al. Assessment of systematic use of intraoperative transesophageal echocardiography during cardiac surgery in adults: a prospective study of 203 patients. J Cardiothorac Vasc Anesth. 2000;14:45-50 [DOI] [PubMed] [Google Scholar]
- 25.Minhaj M, Patel K, Muzic D, et al. The effect of routine intraoperative transesophageal echocardiography on surgical management. J Cardiothorac Vasc Anesth. 2007;21:800-804 [DOI] [PubMed] [Google Scholar]
- 26.Ommen SR, Park SH, Click RL, Freeman WK, Schaff HV, Tajik AJ. Impact of intraoperative transesophageal echocardiography in the surgical management of hypertrophic cardiomyopathy. Am J Cardiol. 2002;90:1022-1024 [DOI] [PubMed] [Google Scholar]
- 27.Qaddoura FE, Abel MD, Mecklenburg KL, et al. Role of intraoperative transesophageal echocardiography in patients having coronary artery bypass graft surgery. Ann Thorac Surg. 2004;78:1586-1590 [DOI] [PubMed] [Google Scholar]
- 28.Suvarna S, Smith A, Stygall J, et al. An intraoperative assessment of the ascending aorta: a comparison of digital palpation, transesophageal echocardiography, and epiaortic ultrasonography. J Cardiothorac Vasc Anesth. 2007;21:805-809 [DOI] [PubMed] [Google Scholar]
- 29.Aklog L, Filsoufi F, Flores KQ, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation 2001;104:I68-I75 [DOI] [PubMed] [Google Scholar]
- 30.Bach DS, Deeb GM, Bolling SF. Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol. 1995;76:508-512 [DOI] [PubMed] [Google Scholar]
- 31.Chaliki HP, Click RL, Abel MD. Comparison of intraoperative transesophageal echocardiographic examinations with the operative findings: prospective review of 1918 cases. J Am Soc Echocardiogr. 1999;12:237-240 [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry FA, Upadya SP, Singh VP, et al. Identifying patients with degenerative mitral regurgitation for mitral valve repair and replacement: a transesophageal echocardiographic study. J Am Soc Echocardiogr. 2004;17:988-994 [DOI] [PubMed] [Google Scholar]
- 33.Fanshawe M, Ellis C, Habib S, Konstadt SN, Reich DL. A retrospective analysis of the costs and benefits related to alterations in cardiac surgery from routine intraoperative transesophageal echocardiography. Anesth Analg. 2002;95:824-827 [DOI] [PubMed] [Google Scholar]
- 34.Freeman WK, Schaff HV, Khandheria BK, et al. Intraoperative evaluation of mitral valve regurgitation and repair by transesophageal echocardiography: incidence and significance of systolic anterior motion. J Am Coll Cardiol. 1992;20:599-609 [DOI] [PubMed] [Google Scholar]
- 35.Gisbert A, Souliere V, Denault AY, et al. Dynamic quantitative echocardiographic evaluation of mitral regurgitation in the operating department. J Am Soc Echocardiogr. 2006;19:140-146 [DOI] [PubMed] [Google Scholar]
- 36.Grewal KS, Malkowski MJ, Piracha AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000;85:199-203 [DOI] [PubMed] [Google Scholar]
- 37.Ionescu AA, West RR, Proudman C, Butchart EG, Fraser AG. Prospective study of routine perioperative transesophageal echocardiography for elective valve replacement: clinical impact and cost-saving implications. J Am Soc Echocardiogr. 2001;14:659-667 [DOI] [PubMed] [Google Scholar]
- 38.Klein AL, Obarski TP, Stewart WJ, et al. Transesophageal Doppler echocardiography of pulmonary venous flow: a new marker of mitral regurgitation severity. J Am Coll Cardiol. 1991;18:518-526 [DOI] [PubMed] [Google Scholar]
- 39.Monin JL, Dehant P, Roiron C, et al. Functional assessment of mitral regurgitation by transthoracic echocardiography using standardized imaging planes: diagnostic accuracy and outcome implications. J Am Coll Cardiol. 2005;46:302-309 [DOI] [PubMed] [Google Scholar]
- 40.Morehead AJ, Firstenberg MS, Shiota T, et al. Intraoperative echocardiographic detection of regurgitant jets after valve replacement. Ann Thorac Surg. 2000;69:135-139 [DOI] [PubMed] [Google Scholar]
- 41.Neuman YM, Brasch AV, Kobal S, et al. Comparison of transthoracic and intraoperative transesophageal color flow Doppler assessment of mitral and aortic regurgitation. Cardiology 2003;99:145-152 [DOI] [PubMed] [Google Scholar]
- 42.Saiki Y, Kasegawa H, Kawase M, Osada H, Ootaki E. Intraoperative TEE during mitral valve repair: does it predict early and late postoperative mitral valve dysfunction? Ann Thorac Surg. 1998;66:1277-1281 [DOI] [PubMed] [Google Scholar]
- 43.Willett DL, Hall SA, Jessen ME, Wait MA, Grayburn PA. Assessment of aortic regurgitation by transesophageal color Doppler imaging of the vena contracta: validation against an intraoperative aortic flow probe. J Am Coll Cardiol. 2001;37:1450-1455 [DOI] [PubMed] [Google Scholar]
- 44.Fix J, Isada L, Cosgrove D, et al. Do patients with less than ‘echo-perfect’ results from mitral valve repair by intraoperative echocardiography have a different outcome? Circulation 1993;88:II39-II48 [PubMed] [Google Scholar]
- 45.O'Rourke DJ, Palac RT, Malenka DJ, Marrin CA, Arbuckle BE, Plehn JF. Outcome of mild periprosthetic regurgitation detected by intraoperative transesophageal echocardiography. J Am Coll Cardiol. 2001;38:163-166 [DOI] [PubMed] [Google Scholar]
- 46.Schroder JN, Williams ML, Hata JA, et al. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation 2005;112:I293-I298 [DOI] [PubMed] [Google Scholar]
- 47.Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998;116:734-743 [DOI] [PubMed] [Google Scholar]
- 48.Applebaum RM, Cutler WM, Bhardwaj N, et al. Utility of transesophageal echocardiography during port-access minimally invasive cardiac surgery. Am J Cardiol. 1998;82:183-188 [DOI] [PubMed] [Google Scholar]
- 49.Bach DS. Subvalvular left ventricular outflow obstruction for patients undergoing aortic valve replacement for aortic stenosis: echocardiographic recognition and identification of patients at risk. J Am Soc Echocardiogr. 2005;18:1155-1162 [DOI] [PubMed] [Google Scholar]
- 50.Lee KS, Stewart WJ, Lever HM, Underwood PL, Cosgrove DM. Mechanism of outflow tract obstruction causing failed mitral valve repair: anterior displacement of leaflet coaptation. Circulation 1993;88:II24-II29 [PubMed] [Google Scholar]
- 51.Mascagni R, Al Attar N, Lamarra M, et al. Edge-to-edge technique to treat post-mitral valve repair systolic anterior motion and left ventricular outflow tract obstruction. Ann Thorac Surg. 2005;79:471-473 [DOI] [PubMed] [Google Scholar]
- 52.Moisa RB, Zeldis SM, Alper SA, Scott WC. Aortic regurgitation in coronary artery bypass grafting: implications for cardioplegia administration. Ann Thorac Surg. 1995;60:665-668 [DOI] [PubMed] [Google Scholar]
- 53.Oh CC, Click RL, Orszulak TA, Sinak LJ, Oh JK. Role of intraoperative transesophageal echocardiography in determining aortic annulus diameter in homograft insertion. J Am Soc Echocardiogr. 1998;11:638-642 [DOI] [PubMed] [Google Scholar]
- 54.Weisenberg D, Sahar Y, Sahar G, et al. Atherosclerosis of the aorta is common in patients with severe aortic stenosis: an intraoperative transesophageal echocardiographic study. J Thorac Cardiovasc Surg. 2005;130:29-32 [DOI] [PubMed] [Google Scholar]
- 55.Kallmeyer IJ, Collard CD, Fox JA, Body SC, Shernan SK. The safety of intraoperative transesophageal echocardiography: a case series of 7200 cardiac surgical patients. Anesth Analg. 2001;92:1126-1130 [DOI] [PubMed] [Google Scholar]
- 56.Lennon MJ, Gibbs NM, Weightman WM, Leber J, Ee HC, Yusoff IF. Transesophageal echocardiography-related gastrointestinal complications in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2005;19:141-145 [DOI] [PubMed] [Google Scholar]
- 57.Ducharme A, Courval JF, Dore A, Leclerc Y, Tardif JC. Severe aortic regurgitation immediately after mitral valve annuloplasty. Ann Thorac Surg. 1999;67:1487-1489 [DOI] [PubMed] [Google Scholar]
- 58.Kumano H, Suehiro S, Shibata T, Hattori K, Kinoshita H. Stuck valve leaflet detected by intraoperative transesophageal echocardiography. Ann Thorac Surg. 1999;67:1484-1485 [DOI] [PubMed] [Google Scholar]
- 59.Masiello P, Mastrogiovanni G, Leone R, et al. One leaflet immobilization after mitral valve replacement with a bileaflet prosthesis. J Heart Valve Dis. 1996;5:114-116 [PubMed] [Google Scholar]
- 60.Tavilla G, Pacini D. Damage to the circumflex coronary artery during mitral valve repair with sliding leaflet technique. Ann Thorac Surg. 1998;66:2091-2093 [DOI] [PubMed] [Google Scholar]
- 61.Couture P, Denault AY, McKenty S, et al. Impact of routine use of intraoperative transesophageal echocardiography during cardiac surgery. Can J Anaesth. 2000;47:20-26 [DOI] [PubMed] [Google Scholar]
- 62.Bernard Y, Meneveau N, Vuillemenot A, et al. Planimetry of aortic valve area using multiplane transoesophageal echocardiography is not a reliable method for assessing severity of aortic stenosis. Heart 1997;78:68-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen GI, Duffy CI, Klein AL, Miller DP, Cosgrove DM, Stewart WJ. Color Doppler and two-dimensional echocardiographic determination of the mechanism of aortic regurgitation with surgical correlation. J Am Soc Echocardiogr. 1996;9:508-515 [DOI] [PubMed] [Google Scholar]
- 64.Enriquez-Sarano M, Freeman WK, Tribouilloy CM, et al. Functional anatomy of mitral regurgitation: accuracy and outcome implications of transesophageal echocardiography. J Am Coll Cardiol. 1999;34:1129-1136 [DOI] [PubMed] [Google Scholar]
- 65.Heinle SK, Hall SA, Brickner ME, Willett DL, Grayburn PA. Comparison of vena contracta width by multiplane transesophageal echocardiography with quantitative Doppler assessment of mitral regurgitation. Am J Cardiol. 1998;81:175-179 [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann R, Flachskampf FA, Hanrath P. Planimetry of orifice area in aortic stenosis using multiplane transesophageal echocardiography. J Am Coll Cardiol. 1993;22:529-534 [DOI] [PubMed] [Google Scholar]
- 67.Konstadt SN, Louie EK, Shore-Lesserson L, Black S, Scanlon P. The effects of loading changes on intraoperative Doppler assessment of mitral regurgitation. J Cardiothorac Vasc Anesth. 1994;8:19-23 [DOI] [PubMed] [Google Scholar]
- 68.Maslow AD, Regan MM, Haering JM, Johnson RG, Levine RA. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol. 1999;34:2096-2104 [DOI] [PubMed] [Google Scholar]
- 69.Meloni L, Aru GM, Abbruzzese PA, Cardu G, Martelli V, Cherchi A. Localization of mitral periprosthetic leaks by transesophageal echocardiography. Am J Cardiol. 1992;69:276-279 [DOI] [PubMed] [Google Scholar]
- 70.Sato Y, Kawazoe K, Kamata J, et al. Clinical usefulness of the effective regurgitant orifice area determined by transesophageal echocardiography in patients with eccentric aortic regurgitation. J Heart Valve Dis. 1997;6:580-586 [PubMed] [Google Scholar]
- 71.Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127-132 [DOI] [PubMed] [Google Scholar]
- 72.Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation 2005;111:975-979 [DOI] [PubMed] [Google Scholar]
- 73.Daniel WG, Erbel R, Kasper W, et al. Safety of transesophageal echocardiography: a multicenter survey of 10,419 examinations. Circulation 1991;83:817-821 [DOI] [PubMed] [Google Scholar]
- 74.Mohty-Echahidi D, Malouf JF, Girard SE, et al. Impact of prosthesis-patient mismatch on long-term survival in patients with small St Jude Medical mechanical prostheses in the aortic position. Circulation 2006;113:420-426 [DOI] [PubMed] [Google Scholar]
- 75.Goland S, Trento A, Czer LS, et al. Thoracic aortic arteriosclerosis in patients with degenerative aortic stenosis with and without coexisting coronary artery disease. Ann Thorac Surg. 2008;85:113-119 [DOI] [PubMed] [Google Scholar]
- 76.Cohn LH, Kowalker W, Bhatia S, et al. Comparative morbidity of mitral valve repair versus replacement for mitral regurgitation with and without coronary artery disease, 1988: updated in 1995. Ann Thorac Surg. 1995;60:1452-1453 [DOI] [PubMed] [Google Scholar]
- 77.Dagum P, Green GR, Glasson JR, et al. Potential mechanism of left ventricular outflow tract obstruction after mitral ring annuloplasty. J Thorac Cardiovasc Surg. 1999;117:472-480 [DOI] [PubMed] [Google Scholar]
- 78.Schunemann HJ, Oxman AD, Brozek J, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med. 2008;13:162-163 [DOI] [PubMed] [Google Scholar]
- 79.Grewal J, Mankad S, Freeman WK, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr. 2009;22:34-41 [DOI] [PubMed] [Google Scholar]
- 80.Kronzon I, Sugeng L, Perk G, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J Am Coll Cardiol. 2009;53:1543-1547 [DOI] [PubMed] [Google Scholar]
- 81.Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106-1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Gara PT, Messe SR, Tuzcu EM, Catha G, Ring JC. Percutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials: a science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2009;53(21):2014-2018 [DOI] [PubMed] [Google Scholar]
- 83.Krasuski RA, Hart SA, Allen D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009;302:290-297 [DOI] [PubMed] [Google Scholar]
- 84.Cahalan MK, Abel M, Goldman M, et al. American Society of Echocardiography and Society of Cardiovascular Anesthesiologists task force guidelines for training in perioperative echocardiography. Anesth Analg. 2002;94:1384-1388 [DOI] [PubMed] [Google Scholar]
- 85.Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777-802 [DOI] [PubMed] [Google Scholar]
- 86.Sukernik MR, Bennett-Guerrero E. The incidental finding of a patent foramen ovale during cardiac surgery: should it always be repaired? a core review. Anesth Analg. 2007;105:602-610 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.