Abstract
Peripheral artery disease (PAD), which comprises atherosclerosis of the abdominal aorta, iliac, and lower-extremity arteries, is underdiagnosed, undertreated, and poorly understood by the medical community. Patients with PAD may experience a multitude of problems, such as claudication, ischemic rest pain, ischemic ulcerations, repeated hospitalizations, revascularizations, and limb loss. This may lead to a poor quality of life and a high rate of depression. From the standpoint of the limb, the prognosis of patients with PAD is favorable in that the claudication remains stable in 70% to 80% of patients over a 10-year period. However, the rate of myocardial infarction, stroke, and cardiovascular death in patients with both symptomatic and asymptomatic PAD is markedly increased. The ankle brachial index is an excellent screening test for the presence of PAD. Imaging studies (duplex ultrasonography, computed tomographic angiography, magnetic resonance angiography, catheter-based angiography) may provide additional anatomic information if revascularization is planned. The goals of therapy are to improve symptoms and thus quality of life and to decrease the cardiovascular event rate (myocardial infarction, stroke, cardiovascular death). The former is accomplished by establishing a supervised exercise program and administering cilostazol or performing a revascularization procedure if medical therapy is ineffective. A comprehensive program of cardiovascular risk modification (discontinuation of tobacco use and control of lipids, blood pressure, and diabetes) will help to prevent the latter.
ABI = ankle brachial index; ACE = angiotensin-converting enzyme; CAD = coronary artery disease; CI = confidence interval; CTA = computed tomographic angiography; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; MRA = magnetic resonance angiography; NHANES = National Health and Nutrition Examination Survey; PAD = peripheral artery disease
Peripheral artery disease (PAD) is underdiagnosed, undertreated, poorly understood, and much more common than previously thought.1,2 In the current article, the term peripheral artery disease will be used to denote vascular diseases caused by atherosclerosis of the abdominal aorta, iliac, and lower-extremity arteries leading to stenosis or occlusion.
In primary care practices across the United States, 29% of patients who are older than 70 years or who are older than 50 years with a history of smoking or diabetes have been reported to have PAD.1,3-5 Not only was the diagnosis of PAD frequently overlooked, but the cardiovascular risk factors were not treated as appropriately as in patients with CAD.
The diagnosis of PAD should not be overlooked for 2 important reasons. First, patients with PAD may experience many problems, such as claudication, ischemic rest pain, ischemic ulcerations, repeated hospitalizations, revascularizations, and limb loss.4 These lead to a poor quality of life and a high rate of depression.6,7 Even patients who have no leg symptoms have a poorer functional performance, poorer quality of life, smaller calf muscle area, and greater calf muscle fat than an age-matched group of patients without PAD.8 Second, patients with PAD have a greater likelihood of experiencing a myocardial infarction (MI), stroke, and cardiovascular death and have a higher rate of all-cause mortality compared with patients without PAD.9-11
EPIDEMIOLOGY
Approximately 12% of the adult population has PAD, and the prevalence is equal in men and women.12 A strong association exists between advancing age and the prevalence of PAD. Almost 20% of adults older than 70 years have PAD.13 In an elderly hypertensive population from the Systolic Hypertension in the Elderly Program, the prevalence of PAD was 38% in black men, 25% in white men, 41% in black women, and 23% in white women.14
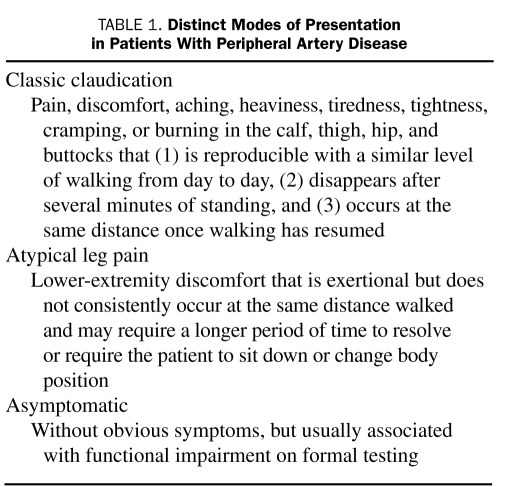
Claudication is the symptomatic expression of PAD; however, it occurs less frequently than has been reported previously. Patients may experience classic claudication, atypical leg pain, rest pain, ischemic ulcers, gangrene, or no symptoms at all (Table 1). In fact, asymptomatic disease may be present in up to 50% of patients with PAD.4 Of the 460 patients in the Walking and Leg Circulation Study, 19.8% had no exertional leg pain, 28.5% had atypical leg pain, 32.6% had classic intermittent claudication, and 19.1% had pain at rest.15 The Rotterdam Study identified a 19.1% prevalence of PAD in their cohort population; however, claudication was reported in only 6.3% in the PAD group.16 In the Edinburgh Artery Study, the prevalence of claudication among 1592 participants aged 55 to 74 years was 4.5%, whereas asymptomatic PAD occurred in 8.0% of enrollees.17
TABLE 1.
Distinct Modes of Presentation in Patients With Peripheral Artery Disease
RISK FACTORS
The most common risk factors associated with PAD are increasing age, diabetes, and smoking.18
Age
Persons aged 65 years or older in the Framingham Heart Study and persons aged 70 years or older in the National Health and Nutrition Examination Survey (NHANES) were at increased risk for the development of PAD.4 The prevalence was 4.3% in participants older than 40 years compared with 14.5% in those older than 70 years.19
Smoking
Smoking is the single most important modifiable risk factor for the development of PAD. It is unknown why the association between PAD and smoking is about twice as strong as that between PAD and coronary artery disease (CAD).20 Smokers have a risk of PAD that is 4 times that of nonsmokers and experience onset of symptoms almost a decade earlier. A dose-response relationship exists between pack-year history and PAD risk.20-22 Furthermore, smokers have poorer survival rates, a greater likelihood of progression to critical limb ischemia and amputation, and decreased artery bypass graft patency rates when compared with nonsmokers. Both former and current smokers are at increased risk of PAD. However, patients who are able to stop smoking are less likely to develop critical limb ischemia and have improved survival.23
Diabetes Mellitus
Diabetes increases the risk of developing symptomatic and asymptomatic PAD by 1.5- to 4-fold and leads to an increased risk of cardiovascular events and early mortality.24-26 In NHANES,22 26% of participants with PAD were identified as having diabetes, whereas in the Edinburgh Artery Study, the prevalence of PAD was greater in participants with diabetes or impaired glucose tolerance (20.6%) than in those with normal glucose tolerance (12.5%).27 Diabetes mellitus is a stronger risk factor for PAD in women than men, and the prevalence of PAD is higher in African American and Hispanic diabetic populations.26,28-30 Diabetes (and poor foot care) is the most common cause for amputation in the United States.26
Hyperlipidemia
In the Framingham Study, an elevated cholesterol level was associated with a 2-fold increased risk of claudication.28 In NHANES, more than 60% of patients with PAD had hypercholesterolemia, whereas in the PARTNERS (PAD Awareness, Risk, and Treatment: New Resources for Survival) program, the prevalence of hyperlipidemia in patients with known PAD was 77%.1,22 Hyperlipidemia increases the adjusted likelihood of developing PAD by 10% for every 10 mg/dL rise in total cholesterol (to convert to mmol/L, multiply by 0.0259).31 The 2001 National Cholesterol Education Program Adult Treatment Panel III considered PAD a CAD risk equivalent.32
Hypertension
Almost every study has shown a strong association between hypertension and PAD, and as many as 50% to 92% of patients with PAD have hypertension.33 The risk of developing claudication is increased 2.5- to 4-fold in both men and women with hypertension.28 In the Systolic Hypertension in the Elderly Program, 5.5% of the participants had an ankle brachial index (ABI) under 0.90.34 Cumulatively, these studies underscore the high prevalence of PAD in patients with hypertension.
Nontraditional Risk Factors
Other risk factors that are associated with an increased prevalence of PAD include race and ethnicity (African Americans and those of Hispanic origin are at higher risk), chronic kidney disease, the metabolic syndrome, and levels of C-reactive protein, β2-microglobulin, cystatin C, lipoprotein(a), and homocysteine.29,34-41 A full discussion of these nontraditional risk factors is beyond the scope of this review.
CLINICAL PRESENTATION
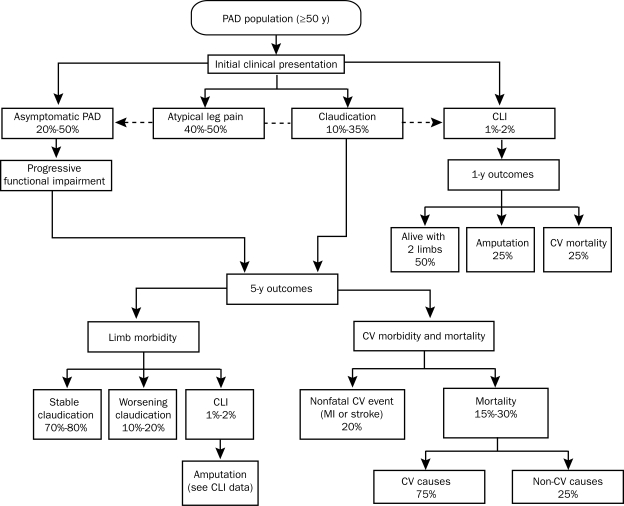
The clinical presentation, natural history, and outcomes in patients with PAD are summarized in Figure 1.4
FIGURE 1.
Natural history of peripheral artery disease (PAD). CLI = critical limb ischemia; CV = cardiovascular; MI = myocardial infarction.
From Circulation,4 with permission of the American Heart Association.
Symptoms
Peripheral artery disease has several distinct modes of presentation (Table 1). Because it is not uncommon for patients to deny that they have pain, it is helpful to reword the question to ask if they feel discomfort when walking. Patients with aortoiliac disease may experience exercise-induced hip, buttock, or thigh discomfort or simply a sense of power failure. If patients walk until the symptoms become so severe that they can no longer walk, they may not receive relief for 15 or 20 minutes (because of lactic acid accumulation in the muscles) and may need to sit down. The discomfort of claudication is usually experienced one level distal to the level of obstruction (ie, superficial femoral or popliteal obstruction causes calf claudication; aortoiliac disease causes thigh, hip, or buttock claudication).
From the standpoint of the limb, the prognosis of patients with PAD is favorable in that the claudication remains stable in 70% to 80% of patients over a 10-year period (Figure 1).4 In the remainder of patients, it may progress to disabling claudication, critical limb ischemia requiring revascularization, or (less commonly) amputation.4,42
The most common clinical manifestations of critical limb ischemia include pain at rest, ischemic ulcerations, and gangrene. Prognosis is particularly poor in patients in whom PAD progresses to critical limb ischemia, as demonstrated in Figure 1.4,42
Ischemic rest pain usually begins distally in the toes and foot, is worse with the leg elevated (eg, at night when the patient is in bed), and is relieved with dependency (hanging the leg over the side of the bed, standing or sitting in a chair). As the degree of ischemia worsens, patients may experience paresthesias, coldness of the extremity, muscular weakness, and stiffness of the foot and ankle joints.
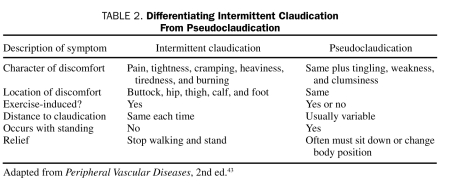
The most common conditions associated with symptoms that may be confused with claudication are spinal stenosis or lumbar radiculopathy. Furthermore, elderly patients may have both PAD from atherosclerosis and spinal stenosis (pseudoclaudication). It is only by a detailed history that one can distinguish which of these 2 common conditions is causing the symptoms in an individual patient (Table 2).43
TABLE 2.
Differentiating Intermittent Claudication From Pseudoclaudication
Physical Examination
Much information can be gained from a carefully performed cardiovascular physical examination. In patients with PAD, the blood pressure should be obtained from each arm because associated subclavian artery disease is frequently present in these patients. A blood pressure difference exceeding 20 mm Hg indicates innominate, subclavian, or axillary disease. In addition, one should listen for bruits over the carotid and subclavian arteries; if present, they should be described as systolic, diastolic, or both.44 Not only are bruits a clue to a potentially severe stenosis, but it has been shown in a recent meta-analysis involving 17,295 patients with 62,313 patient-years that the yearly MI rate and yearly cardiovascular death rate were 2 times greater in patients with than in those without carotid bruits.45 The abdominal aorta should be palpated in all patients; if enlarged, the patient should undergo abdominal ultrasonography. The femoral, popliteal, dorsalis pedis, and posterior tibial arteries should be palpated and described as normal [2+], diminished [1+], or absent [0].4 The presence of aneurysms in the femoral or popliteal artery should also be noted on the physical examination. The dorsalis pedis pulse may be absent in up to 12% of patients and thus is not considered an abnormal finding. However, it is never normal to have an absent posterior tibial pulse. Careful inspection of the feet should be undertaken to look for ulcerations, calluses, and tinea infection. Nail and foot care are important to help to prevent infection and amputation.
Physiology of Claudication
Claudication is a word derived from the Latin word claudicato, meaning to limp. The discomfort it causes results from reversible muscle ischemia. Blood flow is determined by the systemic blood pressure and the resistance to flow as represented by the formula (Flow = Pressure/Resistance). In healthy people, exercise causes vasodilatation, thereby decreasing peripheral vascular resistance and maintaining pressure distally. In patients with PAD, exercise causes increased demand for oxygen, yet only a fixed amount of blood can be delivered distally because of an obstruction to blood flow and vasodilatation that decreases outflow resistance. Thus, a fixed amount of blood is delivered to dilated capacitance vessels, causing a decrease in ankle pressure with exercise.46
Patients with PAD may experience not only hemodynamic abnormalities but also abnormalities of muscle structure and function. Muscle biopsy specimens from patients with PAD may show a decrease in the type II fast twitch fiber area. These findings have been associated with muscle weakness.47 Furthermore, patients with claudication may develop progressive denervation over time.48 These abnormalities have important clinical implications because patients with claudication have a slow walking speed, decreased step length and cadence, and impaired gait stability.46 Hiatt and Brass46 point out that reduced exercise capacity in patients with PAD cannot be explained by alterations in limb blood flow alone because of the presence of so many other abnormalities in muscle and nerve structure, function, and metabolism.
Differential Diagnosis of Claudication
A large number of conditions should be considered in patients who present with exercise-induced leg discomfort (Table 2). Several vascular conditions other than atherosclerotic PAD can cause claudication, including popliteal artery entrapment syndrome, cystic adventitial disease, fibromuscular dysplasia of the iliac or lower-extremity arteries, endofibrosis of the iliac artery associated with cycling, atheromatous embolization and vasculitis such as thromboangiitis obliterans (Buerger disease), Takayasu arteritis, or giant cell arteritis. Rarely, arthritis, myositis, and compartment syndrome may be mistaken for vascular claudication. Patients with iliac vein obstruction may develop venous claudication. Patients have described this as a burning pain when walking that feels like the leg is going to “burst.” The patient must sit or lie down to obtain relief.
Clinical Outcomes
The ABI is the ratio of the ankle systolic pressure to the arm systolic pressure; an ABI of less than 0.90 indicates that the patient has PAD. A low ABI has been shown to be an independent predictor of increased mortality.9,34,49-52 The 5-year mortality rate of patients with an ABI of less than 0.90 is approximately 25%.51 Patients with an ABI of less than 0.90 are twice as likely to have a history of MI, angina, and heart failure than patients with an ABI of 1.0 to 1.5.53,54 In a 10-year prospective study by Criqui et al,10 PAD patients with and without a history of cardiovascular disease had a significantly increased risk of dying of any cause or as a result of cardiovascular disease or CAD than age-matched controls.10 All-cause mortality was 3.1 times greater and cardiovascular disease mortality was 5.9 times greater in patients with than in those without PAD. The BARI (Bypass Angioplasty Revascularization Investigation) trial demonstrated that patients with multivessel CAD and PAD had a 4.9 times greater relative risk of death than those without PAD.55 In a pooled analysis of mortality in 8 large randomized trials involving 19,867 patients who underwent percutaneous coronary intervention, Saw et al56 demonstrated that the rates of death at 7 days, 30 days, 6 months, and 1 year and rates of MI were more than 2 times higher in patients with than in those without PAD.
DIAGNOSTIC EVALUATION
Exercise Treadmill Testing and ABI
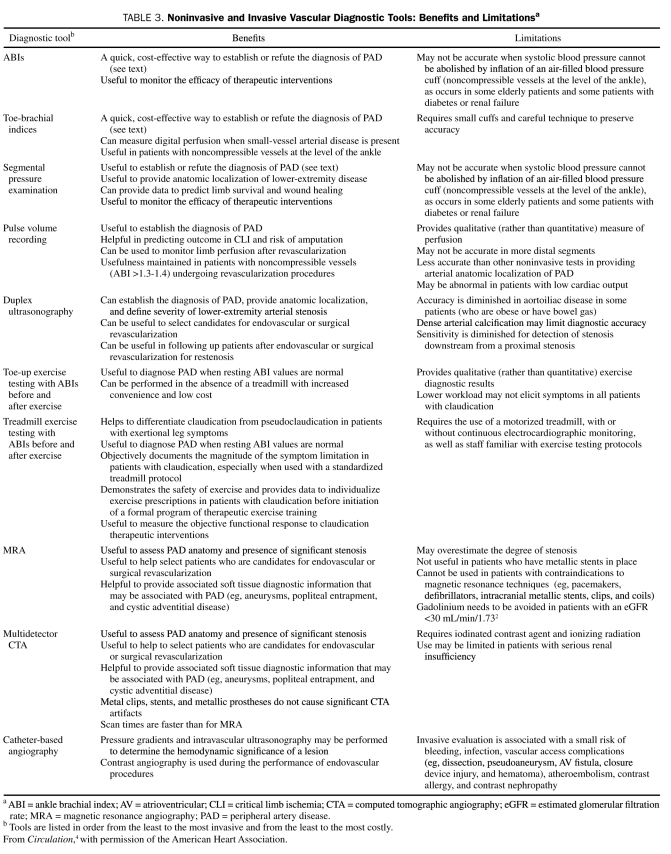
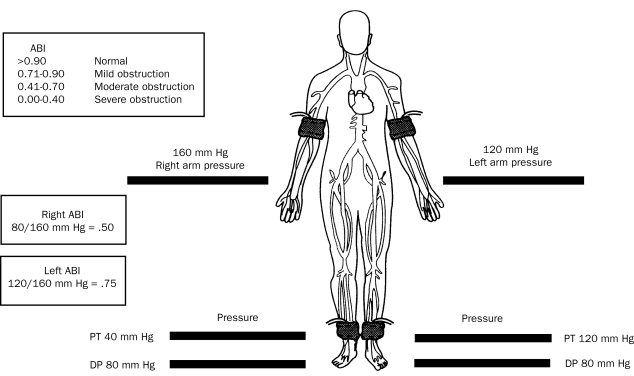
Of all of the noninvasive methods for the diagnosis of PAD (Table 3),4,57 the ABI, segmental blood pressure, and pulse volume waveform analysis are the only techniques that provide physiologic information about perfusion in the limb. Using a hand-held continuous wave Doppler ultrasound device, the higher systolic pressure measured from either the posterior tibial or dorsalis pedis (in each leg) is compared with the highest brachial pressure taken from either arm (Figure 2).4 A normal ABI is 0.90 to 1.40. A reduction in the ABI indicates reduced blood flow to the lower extremity.58,59 Measurement of the ABI does not define the level of obstructive disease, but it is accurate, simple to obtain, and correlates with the severity of the perfusion abnormality but not with the functional impairment that the patient may experience.
TABLE 3.
Noninvasive and Invasive Vascular Diagnostic Tools: Benefits and Limitationsa
FIGURE 2.
Calculation of the ankle brachial index (ABI). DP = dorsalis pedis; PT = posterior tibial artery.
Adapted from N Engl J Med,12 with permission. ©2001 Massachusetts Medical Society. All rights reserved.
The diagnostic value of the ABI is limited in disease states that lead to noncompressibility of blood vessels (eg, patients with diabetes or renal failure). In these circumstances, the increase in ABI (>1.40) may be an artifact. In the Strong Heart Study, an ABI of greater than 1.40 was associated with increased all-cause and cardiovascular mortality.9 In cases of noncompressibility at the ankle level, the toe brachial index (the ratio of the systolic pressure of the toe to that of the arm) may be used. Further details regarding segmental blood pressures, pulse volume recordings, and exercise ABIs are provided in Table 3.4
Duplex Ultrasonography
Duplex ultrasonography is a safe (no radiation or contrast agent) and cost-effective method of accurately determining the severity and location of stenosis and differentiating stenosis from occlusion. B-mode or gray-scale imaging displays a 2-dimensional image of the artery wall and lumen, permitting a rough evaluation of the lesion and atheroma characteristics. Color flow Doppler and pulsed wave Doppler allow an estimation of the stenosis severity on the basis of Doppler-derived velocity criteria.60 Duplex ultrasonography is an accurate method for determining the degree of stenosis or length of occlusion of the arteries supplying the lower extremity.61-63
Furthermore, duplex ultrasonography may be useful in the follow-up of patients who have undergone endovascular (percutaneous transluminal angioplasty/stent) or surgical revascularization. Some clinicians routinely place their patients into an ultrasound surveillance program after angioplasty or stent implantation, and most surgeons do so after lower-extremity bypass surgery. The goal of such a program is to identify a problem (and thus prevent occlusion) should it occur.64
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) of the aorta and peripheral vasculature can be performed rapidly with excellent image quality. Most vascular studies are performed with gadolinium-enhanced 3-dimensional MRA, which acquires angiographic-like images.65-68 The quality of MRA is so good that it (or computed tomographic angiography [CTA]) has virtually replaced diagnostic angiography in determining what type of intervention is feasible. The success of MRA in identifying small runoff vessels meets or exceeds that of traditional catheter-based angiography.69 With current technology, contrast-enhanced 3-dimensional MRA has a sensitivity of approximately 90% and a specificity of approximately 97% in the detection of hemodynamically significant stenoses in any of the lower-extremity arteries as compared with digital subtraction angiography.64
Computed Tomographic Angiography
Multidetector CTA provides high-resolution image quality quickly.70 Current multidetector-row scanners acquire up to 250 simultaneous interweaving helices. Computed tomographic angiography has several advantages over conventional angiography, including volumetric acquisition, which permits visualization of the anatomy from multiple angles and in multiple planes after a single acquisition; improved visualization of soft tissues and other adjacent anatomic structures; and less invasiveness and thus fewer complications.64,71,72 It also has several advantages over MRA, including higher spatial resolution, absence of flow-related phenomena that may distort MRA images, and the capacity to visualize calcification and metallic implants such as endovascular stents or stent grafts. The sensitivities and specificities are greater than 95% for identifying stenosis of greater than 50% and for correctly identifying occlusions.73
The main disadvantages of CTA compared with MRA are exposure to ionizing radiation and the need to use an iodinated contrast agent.
Digital Subtraction Angiography
Vascular imaging with ultrasonography, CTA, and MRA has replaced catheter-based techniques in the initial diagnostic evaluation of patients in most circumstances. Despite a paradigm shift away from catheter-based angiography as a purely diagnostic technique, its importance in intervention has increased dramatically.
The major advantage of digital subtraction angiography is the ability to selectively evaluate individual vessels, obtain physiologic information such as pressure gradients, and image the layers of the blood vessel wall with intravascular ultrasonography and as a platform for percutaneous intervention. Exposure to ionizing radiation, use of iodinated contrast agents, and risks related to vascular access and catheterization are limitations of this technique.
Table 34 summarizes the benefits, limitations, and differences of the various tests used to diagnose and follow up patients with PAD.
TREATMENT
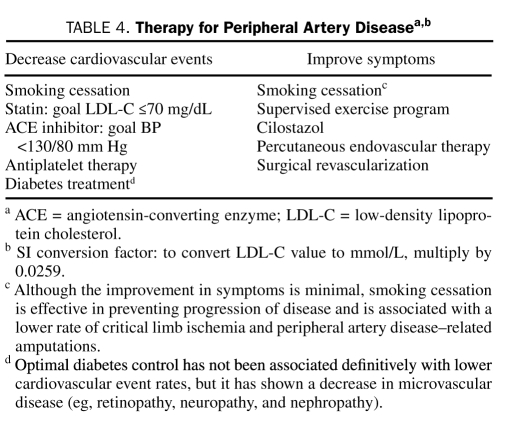
The 2 primary treatment goals in patients with PAD are to decrease cardiovascular morbidity and mortality and to improve limb-related symptoms (ie, claudication) and quality of life (Table 4).
TABLE 4.
Therapy for Peripheral Artery Diseasea,b
Lowering Cardiovascular Morbidity and Mortality
Aggressively managing risk factors such as tobacco use, high lipid levels, and hypertension is an essential component in lowering cardiovascular risk.
Smoking Cessation. It has been clearly shown that patients who successfully quit smoking have decreased rates of PAD progression, critical limb ischemia, amputation, MI, and stroke, as well as increased long-term survival.23 Although the details of an effective smoking cessation program are beyond the scope of this article, it is important to convey to the patient that discontinuation of smoking is extremely important to overall well-being, preservation of the limb, and survival.74,75 Because discontinuation of smoking or use of tobacco in any form is so important, it is the first item to be discussed with the patient during each office visit. In a nonjudgmental way, the clinician should convey to the patient how important discontinuing tobacco use is for cardiovascular health in general and for PAD in particular.
Lipid-Lowering Therapy. According to the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), PAD is a CAD risk equivalent, and thus the goal low-density lipoprotein cholesterol (LDL-C) level is less than 100 mg/dL (to convert to mmol/L, multiply by 0.0259).32 Although many large-scale prospective clinical trials on the efficacy of LDL-C reduction in patients with CAD and stroke have been conducted, no prospective randomized trials have been conducted in patients with PAD.76-78 Furthermore, intensive cholesterol lowering in patients with LDL-C levels at a baseline of less than 130 mg/dL (median value, 108 mg/dL) and increased C-reactive protein levels of greater than 2.0 mg/L (median value, 4.2 mg/L) (to convert to nmol/L, multiply by 9.524) significantly reduced the incidence of MI, stroke, revascularization, hospitalization for unstable angina, or death from cardiovascular causes in patients without clinical evidence of cardiovascular disease (hazard ratio, 0.56; P<.001).79
In the Heart Protection Study, which randomized 20,536 high-risk participants to 40 mg/d of simvastatin or placebo, a 24% relative risk reduction was observed in first-time cardiovascular events in patients who received simvastatin.76 The subgroup of patients with PAD had similar cardiovascular benefits regardless of history of MI or CAD. Even the subgroup population who had LDL-C levels less than 100 mg/dL at baseline benefited from statin therapy.76
Independent of cholesterol-lowering effects, statin use improved walking distance and speed in patients with PAD80; indeed, patients with PAD who take statins have been shown to have less annual decline in lower-extremity performance than those who do not.81 Several studies have evaluated the role of statins on claudication symptoms and walking duration and have shown that these agents may have a modest effect at best.82,83
The current recommendations advocate a goal LDL-C level of less than 100 mg/dL for patients with PAD; for very high-risk patients, the goal is an LDL-C level of less than 70 mg/dL.4 Because all patients with PAD are at very high risk, lowering the LDL-C level to less than 70 mg/dL in all patients with PAD is reasonable.
Hypertension Management. Antihypertensive therapy should be administered to hypertensive patients with PAD to achieve a goal of less than 140/90 mm Hg for nondiabetic patients or of less than 130/80 mm Hg for patients with diabetes or chronic renal disease to reduce the risk of MI, stroke, congestive heart failure, and cardiovascular death.4
Although angiotensin-converting enzyme (ACE) inhibitors are considered the initial drug class of choice by some investigators, it is probably more important to treat to achieve goal blood pressure levels than to insist on a specific antihypertensive agent.33,84 With that caveat, and unless there are reasons to prefer another blood pressure–lowering agent, ACE inhibitors are an attractive first-line agent. They have favorable effects on the cardiovascular system well beyond their blood pressure–lowering capabilities.85,86 In the HOPE (Heart Outcomes Prevention Evaluation) trial, patients with known vascular disease or diabetes and 1 other cardiovascular risk factor were randomized to ramipril or placebo. Patients treated with ramipril experienced a 22% reduction in the primary composite end point of MI, stroke, or cardiovascular death despite little blood pressure lowering.87 Similar cardiovascular event reductions were observed with perindopril (20% relative risk reduction) in 12,218 patients with stable CAD, 883 of whom had PAD.88
Although there continues to be the opinion that β-blockers worsen claudication symptoms in patients with PAD, a meta-analysis of 11 randomized controlled trials by Radack and Deck89 clearly showed that β-blockers do not worsen claudication in patients with PAD and may be used if clearly indicated.33
The role of diabetes management in patients with PAD is discussed in detail elsewhere.26
Antithrombotic Therapy. Aspirin. Antiplatelet agents such as aspirin are indicated for secondary prevention in high-risk cardiovascular patients. Although the benefits of aspirin in patients with CAD and carotid artery disease have been demonstrated by large-scale clinical trials,90,91 several recent studies have questioned the efficacy of aspirin in patients with PAD.92,93 Yet, the American College of Cardiology/American Heart Association Guidelines for the Management of Patients With Peripheral Arterial Disease (class I, level of evidence A) and the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) (grade A [symptomatic patients] and C [asymptomatic patients without CAD or carotid artery disease]) support aspirin use in patients with PAD.4,94
The Antithrombotic Trialists' Collaboration analyzed 287 randomized trials including more than 135,000 patients and reported that the odds of a vascular event (vascular death, nonfatal MI, or nonfatal stroke) were reduced by 22% in high-risk patients receiving antiplatelet therapy.90 In the 9214 patients with PAD, antiplatelet medications reduced serious vascular events by 23%. A similar reduction was seen in patients with intermittent claudication and in patients undergoing peripheral bypass graft procedures or angioplasty.90
In a recent meta-analysis by Berger et al92 evaluating 18 trials and 5269 participants, cardiovascular events were experienced by 251 (8.9%) of 2823 patients taking aspirin (alone or with dipyridamole) and by 269 (11.0%) of 2446 participants in the control group (pooled relative risk, 0.88; 95% confidence interval [CI], 0.76-1.04). It should be noted that the study was designed to detect a difference of 25% and was not powered to detect a smaller difference. Although not statistically significant, the point estimate (a relative risk reduction of 12.0%) showed a favorable trend.
Moreover, it must be acknowledged that aspirin therapy was associated with a reduction in the secondary outcome of nonfatal stroke (52 [1.8%] of 2823 vs 76 [3.1%] of 2446; relative risk, 0.66; 95% CI, 0.47-0.94; P=.02). This meta-analysis has a number of limitations, the most important of which is that the study that contributed the largest number of patients to the meta-analysis (24%) used an ABI of 0.91 to 0.99 to denote PAD, a range much higher than used in any other clinical trial.95
The AAA (Aspirin for Asymptomatic Atherosclerosis) trial screened 28,980 people; of these, 3350 had an ABI of less than 0.95 and were eligible for entry into the trial.93 Participants were randomly assigned to receive 100 mg/d of aspirin or placebo and were followed up for a mean of 8.2 years. The primary end point was the composite of an initial fatal or nonfatal coronary event, stroke, revascularization, angina, claudication, transient ischemic attack, and all-cause mortality. No difference was noted in the event rate between the group receiving aspirin and the group receiving placebo. The aspirin group had more adverse events compared with the placebo group (hemorrhage, 2.0% vs 1.2%; gastrointestinal ulcer, 0.8% vs 0.5%; hazard ratio, 1.71; 95% CI, 0.99-2.97). However, this study has several important methodological problems, the most important of which is that 40% of the patients were nonadherent and did not take the aspirin as prescribed for the duration of the trial. Therefore, on the basis of class I, level A evidence, aspirin is still recommended as an antiplatelet agent for patients with PAD.4,18
Thienopyridines. Thienopyridine medications, such as ticlopidine and clopidogrel, inhibit the activation of platelets by adenosine diphosphate. Clopidogrel has been used as an alternative medication to aspirin in patients with PAD.90,96,97
The efficacy of clopidogrel has been directly compared with that of aspirin in the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial.98 Of the 19,185 high-risk cardiovascular patients (recent MI, recent ischemic stroke, PAD) recruited for the study, 6452 had PAD. The patients were randomized to either clopidogrel (75 mg/d) or aspirin (325 mg/d). After 3 years, an 8.7% relative risk reduction in MI, stroke, or cardiovascular death was observed in the group assigned to clopidogrel (P=.043). The PAD subgroup had the greatest benefit in favor of clopidogrel, with a 23.8% relative risk reduction over aspirin (95% CI, 8.9-36.2; P=.003).98
Although the combination of aspirin and clopidogrel was effective in decreasing cardiovascular events in patients with unstable angina,99 the combination of clopidogrel and aspirin vs aspirin alone in a high-risk group of patients including those with PAD (CHARISMA [Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance] trial) demonstrated no benefit of combination therapy.100 The combination of clopidogrel and aspirin is commonly used in patients undergoing infrainguinal angioplasty and stenting; however, no clear evidence exists to support such a practice.
Newer Antiplatelet Agents. Several new agents have either been recently approved (prasugrel, a thienopyridine)97,101 or are undergoing clinical investigation (SCH 530348, a thrombin receptor antagonist).102,103 Their usefulness as antiplatelet agents in treating patients with PAD remains to be determined.
Warfarin. In the WAVE (Warfarin Antiplatelet Vascular Evaluation) trial, 2161 patients with PAD were randomly assigned to combination therapy with an antiplatelet agent and warfarin (goal international normalized ratio, 2-3) or an antiplatelet agent alone.104 The combination therapy was no more effective than antiplatelet therapy alone and was associated with an increase in life-threatening bleeding (4.0% with combination vs 1.2% with antiplatelet therapy alone [relative risk, 3.41; P<.001]).
Medical Treatment of Claudication
An approach to the treatment of patients with claudication is shown in Table 5. Unfortunately, few randomized trials have been conducted to help guide therapy. Because the results of iliac stenting are good and the restenosis rate is low, stenting may be offered as first-line therapy in patients with iliac disease–related claudication that interferes with lifestyle (Table 5).105,106 The CLEVER (Claudication: Exercise Vs. Endoluminal Revascularization) study, which was funded by the Heart, Lung, and Blood Institute of the National Institutes of Health, is a prospective, multicenter, randomized, controlled clinical trial evaluating the relative efficacy, safety, and health economic impact of 3 treatment strategies for people with aortoiliac disease and claudication. The treatment arms are: optimal medical care (claudication pharmacotherapy)2; optimal medical care and supervised exercise3; and optimal medical care and stent.107-109 It is hoped that the CLEVER study will definitively establish the most appropriate and effective therapy for patients with aortoiliac disease.
TABLE 5.
Approach to the Management of Claudicationa,b
Exercise Therapy. Several randomized prospective trials have demonstrated that supervised exercise is an effective method of treating patients with claudication.110-113 The magnitude of effect from a supervised exercise program exceeds that achieved with any of the pharmacologic agents available.
A meta-analysis of 21 studies by Gardner and Poehlman,110 which included both nonrandomized and randomized trials, showed that pain-free walking time improved by an average of 180% and maximal walking time by 120% in patients with claudication who underwent exercise training. Furthermore, a meta-analysis from the Cochrane Collaboration that included only randomized, controlled trials showed that exercise improved maximal walking ability by an average of 150% (range, 74%-230%).114
The PAD guidelines state that a program of supervised exercise training is recommended as an initial treatment modality for patients with claudication (class I, level of evidence A) and that supervised exercise training should be performed for a minimum of 30 to 45 minutes, in sessions performed at least 3 times per week for a minimum of 12 weeks (class I, level of evidence A).4
Although exercise has many positive effects, the exact mechanism by which exercise therapy improves walking distance is unknown.112 No convincing evidence supports the often stated claim that exercise promotes the growth of collateral vessels. Several comprehensive sources discuss the potential mechanisms of improvement.46,112 Furthermore, McDermott et al115 have shown that patients who walk more (3 times weekly) experience a slower rate of functional decline within the next year.
An exercise program has several important limitations.115 First, patients must be motivated, a difficult task because they experience discomfort every time they walk. Second, the best results occur when patients go to a center for supervised exercise, as with cardiac rehabilitation; however, lack of reimbursement for supervised training prevents its widespread use. Finally, patients who are told to “go home and walk” do not achieve the same improvement as patients in a supervised program.116
Pharmacologic Treatments. Two drugs have been approved by the Food and Drug Administration for the treatment of intermittent claudication: pentoxifylline and cilostazol. No randomized trial has compared the combination of exercise therapy with pharmacotherapy vs either one alone.117 However, our approach is to use exercise and cilostazol at the outset for patients with infrainguinal disease and claudication (Table 5).
Pentoxifylline. Pentoxifylline is a methylxanthine derivative with hemorheological properties. It is thought to act by improving red blood cell and leukocyte flexibility, inhibiting neutrophil adhesion and activation, decreasing fibrinogen concentrations, and reducing blood viscosity.118-120 However, a recent study failed to support this hypothesis in blood samples taken from patients with moderate to severe claudication.121
The beneficial response to pentoxifylline is small in most patients, and the overall data are insufficient to support its widespread use in patients with claudication.12 Pentoxifylline should be reserved for patients who cannot take cilostazol, have not responded adequately to an exercise program, and/or are not candidates for revascularization procedures or clinical trials.117,122-125
Cilostazol. The mechanism by which cilostazol, a phosphodiesterase type 3 inhibitor, improves claudication is unknown, but the medication has the following properties: antiplatelet activity, vasodilatory properties, and in vitro inhibition of vascular smooth muscle cells. It may also cause an increase in high-density lipoprotein cholesterol levels and a decrease in triglyceride levels.126
Because cilostazol is a phosphodiesterase inhibitor similar to milrinone, it is contraindicated (black box warning) in patients with a history of congestive heart failure or in patients with an ejection fraction of less than 40%.4 Long-term use of oral milrinone in cardiomyopathic patients was associated with increased mortality.127 Cilostazol was administered at a dose of 100 mg twice daily. Total patient-years of exposure during treatment were 1046 for cilostazol and 1090 for placebo. During treatment, 18 deaths occurred among those taking cilostazol vs 19 deaths among those receiving placebo, for a hazard ratio of 0.99 (95% CI, 0.52-1.88). Cardiovascular deaths during treatment occurred in 14 patients who were taking cilostazol and 14 who were receiving placebo. Little difference was noted in the incidence of serious bleeding events in the 2 groups (affecting 18 patients taking cilostazol and 22 taking placebo). The rates of bleeding events were similar in patients who used aspirin, aspirin plus clopidogrel, or anticoagulants at any time during the course of the study.
In a meta-analysis of 8 randomized, double-blinded, placebo-controlled trials, cilostazol increased maximal and pain-free walking distances by 50% and 67%, respectively.126 Cilostazol was superior to placebo in most studies performed to date. Dawson et al128 compared the efficacy and safety of cilostazol (100 mg twice daily) to pentoxifylline (400 mg 3 times daily) in patients with intermittent claudication. After 24 weeks, cilostazol significantly increased walking distance compared with pentoxifylline and placebo.128 It should be noted that walking distance progressively increased during the 24 weeks of the study. Therefore, patients should be given an adequate trial of at least 4 months before a decision is made about whether the medication is working.
The most common adverse effects with cilostazol are headache, palpitations, and diarrhea. The CASTLE study129 was a randomized, double-blinded, placebo-controlled safety study of cilostazol. A total of 717 patients received cilostazol, and 718 received placebo. This study demonstrated no safety signal for cilostazol on all-cause mortality or cardiovascular mortality. No increased bleeding was observed in those randomized to cilostazol. However, adherence to cilostazol therapy was poor. More than 60% of participants discontinued cilostazol by 36 months of treatment.130
The optimal dose of cilostazol is 100 mg twice daily; it should be given on an empty stomach (a half hour before or 2 hours after breakfast and dinner). Because of the inhibitory effects of cilostazol on metabolism, the dose should be halved in patients who are taking medications (eg, erythromycin, diltiazem, and omeprazole) that inhibit the cytochrome P450 isoenzymes CYP3A4 and CYP2C19.131
Other Agents. A whole host of therapies have been used in the treatment of claudication. Naftidrofuryl, a 5-hydroxytryptamine serotonin receptor inhibitor, has been available in Europe for a number of years and has shown some efficacy in improving claudication symptoms.132,133 This benefit has not been confirmed by other reports using a 5-hydroxytryptamine antagonist.134 Numerous therapies have been tested and found to be ineffective, including propionyl-L-carnitine, gingko biloba extract, L-arginine, oral vasodilators, prostaglandins, avasimibe, and chelation therapy. A number of trials have used gene or cell-based therapy to treat patients with claudication, and their findings have been nicely summarized by Sneider et al.135
Revascularization
The 3 clear indications for revascularization in patients with PAD are ischemic rest pain, ischemic ulcers or gangrene, and claudication that interferes with the patient's lifestyle. Although the specific methods of endovascular (angioplasty, stent, atherectomy) or surgical therapy are beyond the scope of this article, certain principles should be adhered to when caring for patients with claudication.105,106,136 These are summarized in Table 6.
TABLE 6.
Guiding Principles for Revascularization in Patients With PAD
CONCLUSION
Patients with PAD may experience claudication or critical limb ischemia or may have no symptoms at all. Both symptomatic and asymptomatic patients with PAD have a markedly increased rate of MI, stroke, and cardiovascular events. The 2 major strategies for treatment are: (1) to improve symptoms and quality of life with medical therapy alone (exercise, cilostazol) or percutaneous or surgical revascularization and (2) to prevent cardiovascular events with a comprehensive program that includes smoking cessation, an exercise program, control of blood pressure, achievement of goal LDL-C, antiplatelet therapy, and control of diabetes.
Supplementary Material
On completion of this article, you should be able to (1) identify the signs and symptoms of peripheral artery disease (PAD) and distinguish them for other diseases that can mimic PAD; (2) diagnose PAD using the history, findings on physical examination, and ankle brachial index; and (3) formulate an integrated treatment program to improve the symptoms and quality of life and decrease the high cardiovascular event rate.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286(11):1317-1324 [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Kerwin DR, Liu K, et al. Prevalence and significance of unrecognized lower extremity peripheral arterial disease in general medicine practice*. J Gen Intern Med. 2001;16(6):384-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott MM, Mehta S, Ahn H, Greenland P. Atherosclerotic risk factors are less intensively treated in patients with peripheral arterial disease than in patients with coronary artery disease. J Gen Intern Med. 1997;12(4):209-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463-e654 [DOI] [PubMed] [Google Scholar]
- 5.McDermott MM, Hahn EA, Greenland P, et al. Atherosclerotic risk factor reduction in peripheral arterial disease: results of a national physician survey. J Gen Intern Med. 2002;17(12):895-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott MM, Greenland P, Guralnik JM, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18(6):461-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13(1):15-24 [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Guralnik JM, Ferrucci L, et al. Asymptomatic peripheral arterial disease is associated with more adverse lower extremity characteristics than intermittent claudication. Circulation 2008;117(19):2484-2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004;109(6):733-739 [DOI] [PubMed] [Google Scholar]
- 10.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381-386 [DOI] [PubMed] [Google Scholar]
- 11.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300(2):197-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344(21):1608-1621 [DOI] [PubMed] [Google Scholar]
- 13.Regensteiner JG, Hiatt WR. Current medical therapies for patients with peripheral arterial disease: a critical review. Am J Med. 2002;112(1):49-57 [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Sutton-Tyrrell K, Kuller LH. Lower-extremity arterial disease in older hypertensive adults. Arterioscler Thromb. 1993;13(4):555-562 [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med. 2002;136(12):873-883 [DOI] [PubMed] [Google Scholar]
- 16.Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18(2):185-192 [DOI] [PubMed] [Google Scholar]
- 17.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20(2):384-392 [DOI] [PubMed] [Google Scholar]
- 18.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASC II Working Group Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5-S67 [DOI] [PubMed] [Google Scholar]
- 19.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71(3):510-515 [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Shurtleff D. The Framingham Study: cigarettes and the development of intermittent claudication. Geriatrics 1973;28(2):61-68 [PubMed] [Google Scholar]
- 21.Powell JT, Edwards RJ, Worrell PC, Franks PJ, Greenhalgh RM, Poulter NR. Risk factors associated with the development of peripheral arterial disease in smokers: a case-control study. Atherosclerosis 1997;129(1):41-48 [DOI] [PubMed] [Google Scholar]
- 22.Selvin E, Hirsch AT. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999-2004. Atherosclerosis 2008;201(2):425-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication: effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221(3):253-260 [PubMed] [Google Scholar]
- 24.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421-431 [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162(1):33-41 [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care 2003;26(12):3333-3341 [DOI] [PubMed] [Google Scholar]
- 27.MacGregor AS, Price JF, Hau CM, Lee AJ, Carson MN, Fowkes FG. Role of systolic blood pressure and plasma triglycerides in diabetic peripheral arterial disease: The Edinburgh Artery Study. Diabetes Care 1999;22(3):453-458 [DOI] [PubMed] [Google Scholar]
- 28.Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33(1):13-18 [DOI] [PubMed] [Google Scholar]
- 29.Smith SC, Jr, Milani RV, Arnett DK, et al. Atherosclerotic vascular disease conference: writing group II: risk factors. Circulation 2004;109(21):2613-2616 [DOI] [PubMed] [Google Scholar]
- 30.Wattanakit K, Folsom AR, Selvin E, et al. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2005;180(2):389-397 [DOI] [PubMed] [Google Scholar]
- 31.Hiatt WR, Hoag S, Hamman RF, San Luis Valley Diabetes Study Effect of diagnostic criteria on the prevalence of peripheral arterial disease. Circulation 1995;91(5):1472-1479 [DOI] [PubMed] [Google Scholar]
- 32.Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285(19):2486-2497 [DOI] [PubMed] [Google Scholar]
- 33.Olin JW. Hypertension and peripheral arterial disease. Vasc Med. 2005;10(3):241-246 [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Tyrrell KS, Kuller LH. Mortality over four years in SHEP participants with a low ankle-arm index. J Am Geriatr Soc. 1997;45(12):1472-1478 [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA 2001;285(19):2481-2485 [DOI] [PubMed] [Google Scholar]
- 36.Bartholomew JR, Olin JW. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleve Clin J Med. 2006;73(suppl 4):S8-S14 [DOI] [PubMed] [Google Scholar]
- 37.Fung ET, Wilson AM, Zhang F, et al. A biomarker panel for peripheral arterial disease. Vasc Med. 2008;13(3):217-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson AM, Kimura E, Harada RK, et al. ß2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation 2007;116(12):1396-1403 [DOI] [PubMed] [Google Scholar]
- 39.McDermott MM, Green D, Greenland P, et al. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92(2):194-199 [DOI] [PubMed] [Google Scholar]
- 40.McDermott MM, Greenland P, Green D, et al. D-dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation 2003;107(25):3191-3198 [DOI] [PubMed] [Google Scholar]
- 41.Albert MA, Ridker PM. The role of C-reactive protein in cardiovascular disease risk. Curr Cardiol Rep. 1999;1(2):99-104 [DOI] [PubMed] [Google Scholar]
- 42.Dormandy JA, Rutherford RB, TASC Working Group. TransAtlantic Inter-Society Concensus (TASC) Management of peripheral arterial disease (PAD). J Vasc Surg. 2000;31(1, pt 2):S1-S296 [PubMed] [Google Scholar]
- 43.Krajewski LP, Olin JW. Atherosclerosis of the aorta and lower-extremity arteries. In: Young JR, Olin JW, Bartholomew JR, eds. Peripheral Vascular Diseases 2nd ed.Philadelphia, PA: CV Mosby; 1996:208-233 [Google Scholar]
- 44.Olin JW. Evaluation of the peripheral circulation. In: Izzo JL, Jr, Sica DA, Black HR, eds. Hypertension Primer Philadelphia, PA: Lippincott Williams &Wilkins; 2008:374-378 [Google Scholar]
- 45.Pickett CA, Jackson JL, Hemann BA, Atwood JE. Carotid bruits as a prognostic indicator of cardiovascular death and myocardial infarction: a meta-analysis. Lancet 2008;371(9624):1587-1594 [DOI] [PubMed] [Google Scholar]
- 46.Hiatt WR, Brass EP. Pathophysiology of intermittent claudication. In: Creager MA, Dzau VJ, Loscalzo J, eds. Vascular Medicine, A Companion to Braunwald's Heart Disease Philadelphia, PA: Saunders, Elsevier; 2006:239-247 [Google Scholar]
- 47.Regensteiner JG, Wolfel EE, Brass EP, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 1993;87(2):413-421 [DOI] [PubMed] [Google Scholar]
- 48.England JD, Ferguson MA, Hiatt WR, Regensteiner JG. Progression of neuropathy in peripheral arterial disease. Muscle Nerve. 1995;18(4):380-387 [DOI] [PubMed] [Google Scholar]
- 49.Vogt MT, Cauley JA, Newman AB, Kuller LH, Hulley SB. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA 1993;270(4):465-469 [PubMed] [Google Scholar]
- 50.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991;87(2-3):119-128 [DOI] [PubMed] [Google Scholar]
- 51.Newman AB, Shemanski L, Manolio TA, et al. Cardiovascular Health Study Group Ankle-arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19(3):538-545 [DOI] [PubMed] [Google Scholar]
- 52.Criqui MH, Coughlin SS, Fronek A. Noninvasively diagnosed peripheral arterial disease as a predictor of mortality: results from a prospective study. Circulation 1985;72(4):768-773 [DOI] [PubMed] [Google Scholar]
- 53.Newman AB, Siscovick DS, Manolio TA, et al. Cardiovascular Heart Study (CHS) Collaborative Research Group Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation 1993;88(3):837-845 [DOI] [PubMed] [Google Scholar]
- 54.Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 1997;131(1):115-125 [DOI] [PubMed] [Google Scholar]
- 55.Burek KA, Sutton-Tyrrell K, Brooks MM, et al. Prognostic importance of lower extremity arterial disease in patients undergoing coronary revascularization in the Bypass Angioplasty Revascularization Investigation (BARI). J Am Coll Cardiol. 1999;34(3):716-721 [DOI] [PubMed] [Google Scholar]
- 56.Saw J, Bhatt DL, Moliterno DJ, et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006;48(8):1567-1572 [DOI] [PubMed] [Google Scholar]
- 57.Olin JW. Claudication. In: Garcia MJ, ed. Noninvasive Cardiovascular Imaging: A Multimodality Approach Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010:252-268 [Google Scholar]
- 58.Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation 1968;37(4):624-637 [DOI] [PubMed] [Google Scholar]
- 59.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA 1969;207(10):1869-1874 [PubMed] [Google Scholar]
- 60.Jager KA, Ricketts HJ, Strandness DE., Jr Duplex scanning for the evaluation of lower limb arterial disease. In: Bernstein EF, ed. Noninvasive Diagnostic Techniques in Vascular Disease St Louis, MO: CV Mosby; 1985:619-631 [Google Scholar]
- 61.Kohler TR, Nance DR, Cramer MM, Vandenburghe N, Strandness DE., Jr Duplex scanning for diagnosis of aortoiliac and femoropopliteal disease: a prospective study. Circulation 1987;76(5):1074-1080 [DOI] [PubMed] [Google Scholar]
- 62.Moneta GL, Yeager RA, Antonovic R, et al. Accuracy of lower extremity arterial duplex mapping. J Vasc Surg. 1992;15(2):275-283 [PubMed] [Google Scholar]
- 63.Whelan JF, Barry MH, Moir JD. Color flow Doppler ultrasonography: comparison with peripheral arteriography for the investigation of peripheral vascular disease. J Clin Ultrasound 1992;20(6):369-374 [DOI] [PubMed] [Google Scholar]
- 64.Olin JW, Kaufman JA, Bluemke DA, et al. Atherosclerotic Vascular Disease Conference. American Heart Association, Imaging, Writing Group IV. Circulation 2004;109(21):2626-2633 [DOI] [PubMed] [Google Scholar]
- 65.Prince MR, Meaney JF. Expanding role of MR angiography in clinical practice. Eur Radiol. 2006;16(suppl 2):B3-B8 [DOI] [PubMed] [Google Scholar]
- 66.Ersoy H, Zhang H, Prince MR. Peripheral MR angiography. J Cardiovasc Magn Reson 2006;8(3):517-528 [DOI] [PubMed] [Google Scholar]
- 67.Prince MR. Peripheral vascular MR angiography: the time has come. Radiology 1998;206(3):592-593 [DOI] [PubMed] [Google Scholar]
- 68.Prince MR, Narasimham DL, Stanley JC, et al. Breath-hold gadolinium-enhanced MR angiography of the abdominal aorta and its major branches. Radiology 1995;197(3):785-792 [DOI] [PubMed] [Google Scholar]
- 69.Grist TM. MRA of the abdominal aorta and lower extremities. J Magn Reson Imaging 2000;11(1):32-43 [DOI] [PubMed] [Google Scholar]
- 70.Fleischmann D, Hallett RL, Rubin GD. CT angiography of peripheral arterial disease. J Vasc Interv Radiol. 2006;17(1):3-26 [DOI] [PubMed] [Google Scholar]
- 71.Rubin GD, Shiau MC, Leung AN, Kee ST, Logan LJ, Sofilos MC. Aorta and iliac arteries: single versus multiple detector-row helical CT angiography. Radiology 2000;215(3):670-676 [DOI] [PubMed] [Google Scholar]
- 72.Rubin GD, Schmidt AJ, Logan LJ, Sofilos MC. Multi-detector row CT angiography of lower extremity arterial inflow and runoff: initial experience. Radiology 2001;221(1):146-158 [DOI] [PubMed] [Google Scholar]
- 73.Sun Z. Diagnostic accuracy of multislice CT angiography in peripheral arterial disease. J Vasc Interv Radiol. 2006;17(12):1915-1921 [DOI] [PubMed] [Google Scholar]
- 74.Hobbs SD, Wilmink AB, Adam DJ, Bradbury AW. Assessment of smoking status in patients with peripheral arterial disease. J Vasc Surg. 2005;41(3):451-456 [DOI] [PubMed] [Google Scholar]
- 75.Hobbs SD, Bradbury AW. Smoking cessation strategies in patients with peripheral arterial disease: an evidence-based approach. Eur J Vasc Endovasc Surg. 2003;26(4):341-347 [DOI] [PubMed] [Google Scholar]
- 76.Heart Protection Study Collaborative Group MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 77.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559 [DOI] [PubMed] [Google Scholar]
- 78.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344(8934):1383-1389 [PubMed] [Google Scholar]
- 79.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207 [DOI] [PubMed] [Google Scholar]
- 80.McDermott MM, Guralnik JM, Greenland P, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation 2003;107(5):757-761 [DOI] [PubMed] [Google Scholar]
- 81.Giri J, McDermott MM, Greenland P, et al. Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol. 2006;47(5):998-1004 [DOI] [PubMed] [Google Scholar]
- 82.Mohler ER, III, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003;108(12):1481-1486 [DOI] [PubMed] [Google Scholar]
- 83.Mondillo S, Ballo P, Barbati R, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114(5):359-364 [DOI] [PubMed] [Google Scholar]
- 84.Clement DL, De Buyzere ML, Duprez DA. Hypertension in peripheral arterial disease. Curr Pharm Des. 2004;10(29):3615-3620 [DOI] [PubMed] [Google Scholar]
- 85.Lonn E, Yusuf S, Dzavik V, et al. Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001;103(7):919-925 [DOI] [PubMed] [Google Scholar]
- 86.Stumpe KO. Antihypertensive therapy: new strategies beyond blood pressure control. J Cardiovasc Pharmacol. 1992;20(suppl 6):S1-S4 [PubMed] [Google Scholar]
- 87.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145-153 [DOI] [PubMed] [Google Scholar]
- 88.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362(9386):782-788 [DOI] [PubMed] [Google Scholar]
- 89.Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease: a meta-analysis of randomized controlled trials. Arch Intern Med. 1991;151(9):1769-1776 [PubMed] [Google Scholar]
- 90.Antithrombotic Trialist Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324(7329):71-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(6)(suppl):815S-843S [DOI] [PubMed] [Google Scholar]
- 92.Berger JS, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA 2009;301(18):1909-1919 [DOI] [PubMed] [Google Scholar]
- 93.Fowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA 2010;303(9):841-848 [DOI] [PubMed] [Google Scholar]
- 94.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(suppl 1):S1-S75 [DOI] [PubMed] [Google Scholar]
- 95.Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiatt WR, Krantz MJ. Masterclass series in peripheral arterial disease: antiplatelet therapy for peripheral arterial disease and claudication. Vasc Med. 2006;11(1):55-60 [DOI] [PubMed] [Google Scholar]
- 97.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015 [DOI] [PubMed] [Google Scholar]
- 98.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348(9038):1329-1339 [DOI] [PubMed] [Google Scholar]
- 99.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494-502 [DOI] [PubMed] [Google Scholar]
- 100.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706-1717 [DOI] [PubMed] [Google Scholar]
- 101.Bhatt DL. Prasugrel in clinical practice. N Engl J Med. 2009;361(10):940-942 [DOI] [PubMed] [Google Scholar]
- 102.Becker RC, Moliterno DJ, Jennings LK, et al. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet 2009;373(9667):919-928 [DOI] [PubMed] [Google Scholar]
- 103.Morrow DA, Scirica BM, Fox KA, et al. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2 degrees P)-TIMI 50 trial. Am Heart J. 2009;158(3):335-341 [DOI] [PubMed] [Google Scholar]
- 104.Anand S, Yusuf S, Xie C, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357(3):217-227 [DOI] [PubMed] [Google Scholar]
- 105.White CJ, Gray WA. Endovascular therapies for peripheral arterial disease: an evidence-based review. Circulation 2007;116(19):2203-2215 [DOI] [PubMed] [Google Scholar]
- 106.Gray BH, Conte MS, Dake MD, et al. Atherosclerotic peripheral vascular disease symposium II: lower-extremity revascularization: state of the art. Circulation 2008;118(25):2864-2872 [DOI] [PubMed] [Google Scholar]
- 107.Murphy TP, Hirsch AT, Cutlip DE, et al. Claudication: exercise vs endoluminal revascularization (CLEVER) study update. J Vasc Surg. 2009;50(4):942-945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bronas UG, Hirsch AT, Murphy T, et al. Design of the multicenter standardized supervised exercise training intervention for the claudication: exercise vs endoluminal revascularization (CLEVER) study. Vasc Med. 2009;14(4):313-321 [DOI] [PubMed] [Google Scholar]
- 109.Murphy TP, Hirsch AT, Ricotta JJ, et al. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: rationale and methods. J Vasc Surg. 2008;47(6):1356-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain: a meta-analysis. JAMA 1995;274(12):975-980 [PubMed] [Google Scholar]
- 111.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23(1):104-115 [DOI] [PubMed] [Google Scholar]
- 112.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med. 2002;347(24):1941-1951 [DOI] [PubMed] [Google Scholar]
- 113.Regensteiner JG, Gardner A, Hiatt WR. Exercise testing and exercise rehabilitation for patients with peripheral arterial disease: status in 1997. Vasc Med. 1997;2(2):147-155 [DOI] [PubMed] [Google Scholar]
- 114.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;(2):CD000990 [DOI] [PubMed] [Google Scholar]
- 115.McDermott MM, Liu K, Ferrucci L, et al. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med. 2006;144(1):10-20 [DOI] [PubMed] [Google Scholar]
- 116.Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2006;(2):CD005263 [DOI] [PubMed] [Google Scholar]
- 117.Girolami B, Bernardi E, Prins MH, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Intern Med. 1999;159(4):337-345 [DOI] [PubMed] [Google Scholar]
- 118.Strano A, Davi G, Avellone G, Novo S, Pinto A. Double-blind, cross-over study of the clinical efficacy and the hemorheological effects of pentoxifylline in patients with occlusive arterial disease of the lower limbs. Angiology 1984;35(7):459-466 [DOI] [PubMed] [Google Scholar]
- 119.Rao KM, Simel DL, Cohen HJ, Crawford J, Currie MS. Effects of pentoxifylline administration on blood viscosity and leukocyte cytoskeletal function in patients with intermittent claudication. J Lab Clin Med. 1990;115(6):738-744 [PubMed] [Google Scholar]
- 120.Franzini E, Sellak H, Babin-Chevaye C, Hakim J, Pasquier C. Effects of pentoxifylline on the adherence of polymorphonuclear neutrophils to oxidant-stimulated human endothelial cells: involvement of cyclic AMP. J Cardiovasc Pharmacol. 1995;25(suppl 2):S92-S95 [DOI] [PubMed] [Google Scholar]
- 121.Dawson DL, Zheng Q, Worthy SA, Charles B, Bradley DV., Jr Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication. Angiology 2002;53(5):509-520 [DOI] [PubMed] [Google Scholar]
- 122.Hood SC, Moher D, Barber GG. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized controlled trials. CMAJ 1996;155(8):1053-1059 [PMC free article] [PubMed] [Google Scholar]
- 123.Porter JM, Cutler BS, Lee BY, et al. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J. 1982;104(1):66-72 [DOI] [PubMed] [Google Scholar]
- 124.Gallus AS, Gleadow F, Dupont P, et al. Intermittent claudication: a double-blind crossover trial of pentoxifylline. Aust N Z J Med. 1985;15(4):402-409 [DOI] [PubMed] [Google Scholar]
- 125.De Sanctis MT, Cesarone MR, Belcaro G, et al. Treatment of intermittent claudication with pentoxifylline: a 12-month, randomized trial–walking distance and microcirculation. Angiology 2002;53(suppl 1):S7-S12 [PubMed] [Google Scholar]
- 126.Thompson PD, Zimet R, Forbes WP, Zhang P. Meta-analysis of results from eight randomized, placebo-controlled trials on the effect of cilostazol on patients with intermittent claudication. Am J Cardiol. 2002;90(12):1314-1319 [DOI] [PubMed] [Google Scholar]
- 127.Packer M, Carver JR, Rodeheffer RJ, et al. PROMISE Study Research Group Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325(21):1468-1475 [DOI] [PubMed] [Google Scholar]
- 128.Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109(7):523-530 [DOI] [PubMed] [Google Scholar]
- 129.Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-Term Effects). J Vasc Surg. 2008;47(2):330-336 [DOI] [PubMed] [Google Scholar]
- 130.Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51(13):1292-1298 [DOI] [PubMed] [Google Scholar]
- 131.Dobesh PP, Stacy ZA, Persson EL. Pharmacologic therapy for intermittent claudication. Pharmacotherapy 2009;29(5):526-553 [DOI] [PubMed] [Google Scholar]
- 132.Lehert P, Comte S, Gamand S, Brown TM. Naftidrofuryl in intermittent claudication: a retrospective analysis. J Cardiovasc Pharmacol. 1994;23(suppl 3):S48-S52 [PubMed] [Google Scholar]
- 133.Lehert P, Riphagen FE, Gamand S. The effect of naftidrofuryl on intermittent claudication: a meta-analysis. J Cardiovasc Pharmacol. 1990;16(suppl 3):S81-S86 [PubMed] [Google Scholar]
- 134.Hiatt WR, Hirsch AT, Cooke JP, Olin JW, Brater DC, Creager MA. Randomized trial of AT-1015 for treatment of intermittent claudication: a novel 5-hydroxytryptamine antagonist with no evidence of efficacy. Vasc Med. 2004;February;9(1):18-25 [DOI] [PubMed] [Google Scholar]
- 135.Sneider EB, Nowicki PT, Messina LM. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem. 2009;108(4):753-761 [DOI] [PubMed] [Google Scholar]
- 136.White C. Clinical practice: intermittent claudication. N Engl J Med. 2007;356(12):1241-1250 [DOI] [PubMed] [Google Scholar]
- 137.Kalish J, Hamdan A. Management of diabetic foot problems. J Vasc Surg. 2010;51(2):476-486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.