Abstract
The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for regulation of nociceptive transmission by both local segmental and supraspinal mechanisms. Descending control of spinal nociception originates from many brain regions and plays a critical role in determining the experience of both acute and chronic pain. The earlier concept of descending control as an “analgesia system” is now being replaced with a more nuanced model in which pain input is prioritized relative to other competing behavioral needs and homeostatic demands.
Descending control arises from a number of supraspinal sites, including the midline periaqueductral gray-rostral ventromedial medulla (PAG-RVM) system, and the more lateral and caudal dorsal reticular nucleus (DRt) and ventrolateral medulla (VLM). Inhibitory control from the PAG-RVM system preferentially suppresses nociceptive inputs mediated by C-fibers, preserving sensory-discriminative information conveyed by more rapidly conducting A-fibers. Analysis of the circuitry within the RVM reveals that the neural basis for bidirectional control from the midline system is two populations of neurons, ON-cells and OFF-cells, that are differentially recruited by higher structures important in fear, illness and psychological stress to enhance or inhibit pain. Dynamic shifts in the balance between pain inhibiting and facilitating outflows from the brainstem play a role in setting the gain of nociceptive processing as dictated by behavioral priorities, but are also likely to contribute to pathological pain states.
Keywords: pain modulation, pronociception, antinociception, brainstem, periaqueductal gray, raphe, caudal ventrolateral medulla
1. Introduction
The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for the regulation of nociceptive transmission by both local segmental and supraspinal mechanisms. Supraspinal (or descending) control of spinal nociception originates from many brain regions and plays a critical role in determining the experience of both acute and chronic pain. Initial reports in the 1970’s and 1980’s were of inhibitory influences from sites in the midbrain periaqueductal grey (PAG) and from the midline nucleus raphe magnus and adjacent reticular regions in the pons and medulla, the rostral ventromedial medulla (RVM, see Fields et al.24 and Heinricher and Ingram49 for recent reviews). For many decades attention focused on these areas as sources of descending inhibitory control, with a role in endogenous analgesia (antinociception) in states of extreme stress10, 119 or in creating contrast in sensory signals that sharpened the signalling of pain by ascending pathways.68
It is now evident that descending control can be facilitatory as well as inhibitory. Indeed, facilitatory and inhibitory influences on spinal events are often reported to emanate from a single brain region (e.g., Zhuo and Gebhart134). Some descending influences are tonically active, but the balance between inhibition and facilitation is dynamic, and can be altered in different behavioral, emotional and pathological states. As already noted, it has long been recognized that intense stress and fear are associated with hypoalgesia (a decreased responsiveness to noxious stimuli) that reflects a shift towards descending inhibition. By contrast, inflammation and nerve injury, sickness, and chronic opioid administration are associated with hyperalgesia (an increased responsiveness to noxious stimuli) that in part reflects a shift towards descending facilitation. Of clinical importance, there is much evidence to suggest that descending facilitation of spinal nociception is a major contributor to central sensitisation and the development of secondary hyperalgesia, indicating that the balance shifts in favor of facilitation in the transition from acute to chronic pain.
Descending control arises from a number of supraspinal sites, but the best studied is the PAG-RVM system mentioned above (Fig. 1). The PAG is heavily interconnected with the hypothalamus and limbic forebrain structures including the amygdala, and also receives direct spinomesencephalic input. The PAG projects to the RVM, which in turn sends its output to dorsal horn laminae important in nociceptive function. This system has a pivotal role in organising strategies for coping with intrinsic and extrinsic stressors, and is also recognized as the central site of action of analgesic agents including opioids, cyclooxygenase inhibitors, and cannabinoids.52, 70, 128 Understanding the PAG-RVM system is thus of considerable importance from both a behavioral and therapeutic point of view. Spinal mechanisms that mediate descending control from the PAG are discussed in Section 2, and intrinsic organization of the RVM and recruitment of PAG-RVM system are considered in Section 3. Additional sources of descending modulation include pontine noradrenergic cell groups95 and two areas of the caudal medulla discussed in Section 4, the dorsal reticular nucleus (DRt) and ventrolateral medulla (VLM).118
Fig. 1. Schematic illustrates main topics of this review.
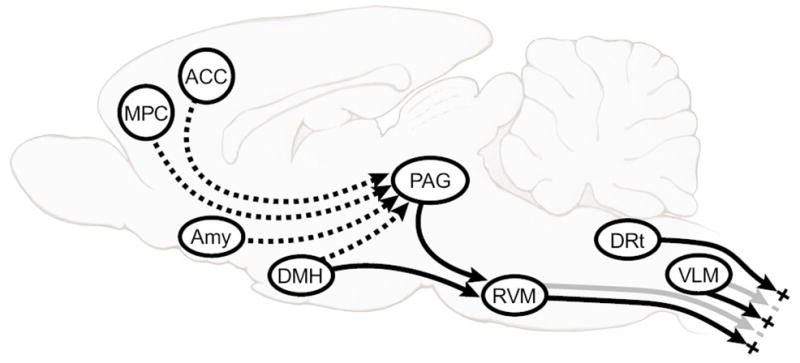
midline PAG-RVM system, which exerts bidirectional control over dorsal horn nociceptive processing, and the DRt and VLM in the caudal medulla. DRt is thought to be facilitating, and VLM primarily inhibitory, although it may, like the RVM, have both an inhibitory and facilitatory influence. The PAG especially, but also the RVM, DRt and VLM (not shown) receive important direct and indirect inputs from limbic forebrain areas including anterior cingulate cortex (ACC), amygdala (AMY), dorsomedial nucleus of the hypothalamus (DMH), and medial prefrontal cortex (MPC).
2. Descending control from the PAG distinguishes between the spinal processing of different sensory qualities, including different components of the pain signal
In the 40 years since Reynolds first described the phenomenon of stimulation-produced analgesia,107 the therapeutic potential of descending control has fuelled intense investigation of how descending systems interface with nociceptive circuitry of the dorsal horn. There is nevertheless much conflicting information, and many unknowns: to what extent and under what conditions are descending controls mediated by presynaptic versus postsynaptic mechanisms; what neurotransmitters/neuromodulators prevail under different conditions and what are the interactions between them; and finally, do descending controls discriminate between different sensory qualities including different components of the pain signal and, if so, is this control dynamically regulated? Issues relating to the last question are the subject of this part of the review, which will consider descending control by the PAG of spinal processing of noxious versus non-noxious inputs, and of different components of the pain signal.
Initial reports of behavioral analgesia following stimulation in the PAG concluded that the effects of central stimulation were highly selective for behaviors evoked by noxious stimuli, and that animals continued to respond to non-noxious, tactile, stimuli and other non-aversive cues.78 This finding was at odds with early electrophysiological studies in which activation of the PAG was often found to produce a non-selective inhibition of both non-nociceptive and nociceptive responses of dorsal horn neurons.9, 18, 33, 59 It is likely that non-selective effects of electrical stimulation reflected activation of fibers of passage and/or antidromic activation of spinal neurons that project to the PAG. This is because activation of neuronal cell bodies in other studies revealed that PAG control of dorsal horn responses is highly selective for noxious inputs: comparison of electrical and chemical stimulation at the same sites in the PAG revealed non-selective and selective effects, respectively.124 From a behavioral perspective it was concluded that selective descending control might operate as part of an integrated response to stressful or threatening stimuli. Selective suppression of nociception would allow an organism to respond in an appropriate manner to a life-threatening situation without the distraction or counterproductive motor responses that might be evoked by noxious input. The likelihood of survival would be further heightened as responses to potentially important non-noxious cues would be left intact.
The realization that descending control from the midline PAG-RVM system is specific for noxious relative to non-noxious input raises the question of whether selectivity in descending control extends further, to different aspects of the noxious signal. Information about actual or potential tissue damage in the periphery is conveyed to the spinal dorsal horn in A- and C-fiber nociceptors. These two classes of nociceptor have different electrophysiological properties, various chemical phenotypes (see Lawson67 for review), signal different qualities of acute pain109, 121 and have distinct roles in the development and maintenance of chronic pain.28, 75, 94 Given the importance of descending control in defining the pain experience, together with the different roles of A- and C-fiber nociceptors in acute and chronic pain, it is important to determine how information flow in pathways activated by these distinct afferents is modulated from supraspinal sites.
The question of descending control of A- versus C-nociceptor-evoked responses in the spinal dorsal horn has been the subject of a number of studies (for example, Jurna58). However, most of these studies have employed electrical stimulation to activate afferents, which could confound interpretation of the data. Electrical stimulation of peripheral nerves evokes un-physiological, synchronous inputs to the spinal cord, which may be resistant to modulation. It also simultaneously activates afferents innervating excitatory and inhibitory receptive fields of spinal neurons. One approach to overcoming these limitations is to establish the profile of A- and C-fiber input to an individual neuron using electrical stimulation, which enables assumptions about the fiber types mediating the naturally evoked responses of that cell. Thus, dorsal horn neurons can be classified as “C-positive” (C+ve, those showing a response at C-fiber latency, in addition to A-fiber responses) or “C-negative” (C-ve, those in which there is no evidence of C-fiber-evoked activity) on the basis of their responses to percutaneous electrical stimulation. If it is then assumed that pinch-evoked responses of C+ve neurons are mediated, at least in part, by C-fibers and that those of C-ve cells are mediated by A-fiber nociceptors alone, it is possible to gain insights into any selectivity in descending control of dorsal horn activity evoked by A- or C-fiber nociceptors. This approach revealed that pinch-evoked responses of C+ve cells are generally depressed, whilst those of C-ve cells show a net facilitation following activation of the PAG,125 indicating that descending control from the PAG distinguishes between neurons with and without C-fiber inputs. Although post-synaptic excitation of dorsal horn neurons from medullary pain control centers has been reported,72, 131 there is reason to think that the facilitatory effects on C-ve neurons reflect removal of segmental inhibition normally exerted by the C+ve neurons that were suppressed by the PAG activation.125
A limitation of the approach described above is that it relies on the assumption that pinch-evoked responses of C+ve are mediated by C- and/or A-fiber nociceptors, and C-ve neurons solely by A-fibers. It would be desirable to identify a more direct approach in which A- or C-fiber nociceptors were activated differentially using natural stimulation of the cutaneous receptive field. One way to do this is to use different rates of skin heating to preferentially activate A- or C-heat nociceptors, as first described by Yeomans and colleagues.129, 130 This technique has been further refined and, in a number of experimental paradigms, has been shown reliably to activate these distinct groups of nociceptors.70, 81 Fast rates of heating (7.5 ± 1 °C·s−1) are used to preferentially activate A-fiber (myelinated, capsaicin-insensitive) heat nociceptors, whereas slow rates of heating (2.5 ± 1 °C·s−1) activate C-fiber (unmyelinated, capsaicin-sensitive) heat nociceptors.
Taking this approach, Lumb and colleagues examined whether withdrawal reflexes evoked by fast and slow rates of skin heating were differentially modulated by PAG activation. C-fiber mediated withdrawals were found to be inhibited, and A-fiber evoked reflexes unaffected.74, 83, 111 Similar differential effects on A- versus C-fiber evoked spinal reflexes were also described following inhibition of cyclo-oxygenase (COX) in the PAG.70 Suppression of C-fiber mediated reflexes was consistent with the inhibition of C+ve dorsal horn neurons. However, the lack of effect on A-nociceptor-evoked withdrawal reflexes was unexpecteded, given the strong facilitation of C-ve neurons described above. The explanation may be that reflexes evoked by A-heat nociceptors are presumably mediated by both C+ve and C-ve neurons. Since the former would have been inhibited and the latter facilitated by PAG stimulation, the net effect on the withdrawal reflex would be null.
To show that the differential PAG modulation of reflexes evoked by A- versus C-fibers reflected an action at the dorsal horn, Lumb and colleagues next performed parallel experiments recording activity of deep dorsal horn C+ve neurons evoked by fast and slow rates of heating. Interestingly, although the thresholds for activation of the neurons by A- and C-heat nociceptors were raised to a similar extent by PAG stimulation, coding of suprathreshold stimuli was differentially affected. Stimulus-response functions of C+ve neurons to fast and slow rates of skin heating are remarkably similar to those of peripheral A- and C-heat nociceptors respectively82, 130 in that fast rates of skin heating are encoded faithfully well into the tissue-damaging range whereas slow rates of skin heating are only poorly encoded. PAG activation causes a rightward shift in the stimulus-response relationship for A-evoked activity, but the linear relationship between skin temperature and firing rate is maintained. Such a relationship between stimulus temperature and cell activity as existed with the slow ramp was disrupted by PAG activation. Differential regulation of A- versus C-fiber inputs is further supported by the observation that the degree to which the heat-evoked activity of a given C+ve dorsal horn neuron is depressed by PAG activation is correlated with the strength of the C-fiber input to that neuron. Neurons with robust C-fiber inputs (“strong” C+ves) are more strongly inhibited by PAG stimulation than neurons that are less responsive to C-fiber stimulation (“weak” C+ves).69 It remains to be determined whether A-heat nociceptor-evoked responses of C-ve neurons are facilitated by descending control from the PAG.125
2.1. Mechanisms of differential descending control of C- versus A-fiber-evoked spinal nociception
The following model (Fig. 2) builds on what is known of C- and A-nociceptive input to the dorsal horn in order to explain how the magnitude of descending control of deep dorsal horn neurons could be directly proportional to the degree of their C-fiber input.
Fig. 2. A simplified model to explain how descending control from the PAG, which targets different populations of superficial dorsal horn neurons, could produce an inhibition of deep dorsal horn neurons that is proportional to their C-fiber input, but a facilitation of other neurons with weak or no C-fiber input.
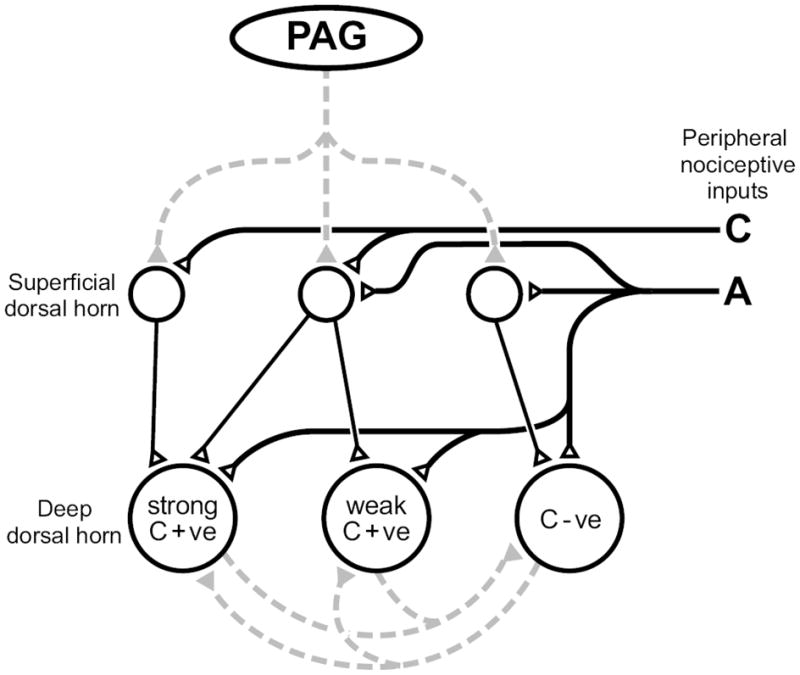
Solid lines represent direct (monosynaptic) connections, dotted lines represent indirect (polysynaptic) connections between neurons; open triangles/black lines are excitatory synapses and gray lines/filled triangles are inhibitory connections. The PAG inhibits superficial dorsal horn neurons that relay information carried by C-fibers to the deep dorsal horn. The net inhibitory or facilitatory effect of PAG stimulation is also a function of reciprocal inhibition between C+ve and C-ve neurons at the segmental level. C+ve: C-fiber input positive, C-ve: C-fiber input negative.
Because C-fibers terminate primarily in the superficial dorsal horn, the C-fiber evoked responses of neurons in the deep dorsal horn must be received on superficially directed dendrites or relayed via superficial interneurons.89 A-fiber nociceptors also terminate heavily in the superficial dorsal horn, although some also provide direct input to the deep dorsal horn. Individual neurons in the superficial dorsal horn may be dominated by C-fiber inputs, A-nociceptive inputs, or by a mix of A- and C-fiber inputs.1, 17 All deep dorsal horn neurons receive indirect and/or direct inputs from A-nociceptors. Like the C-fiber primary afferents, descending modulatory pathways terminate heavily, although not exclusively, within the superficial dorsal horn.6, 7, 27, 108 Hence, although the activity of deep dorsal horn cells may be influenced directly by descending pathways, much of the descending influence is likely to be secondary to modulation in the superficial dorsal horn.
The model developed by Lumb and coworkers focuses on the interneurons as both the source of C-fiber input to the deep dorsal horn and the target for descending control from the PAG. It postulates that “strong” C+ve neurons in the deep dorsal horn receive C-fiber input relayed by numerous superficial C-receptive neurons, whilst “weak” C+ve neurons are targeted by relatively few C-receptive interneurons, and that C-ve neurons in the deep dorsal horn receive no projections from superficial C-receptive interneurons. In addition, evidence has been provided for reciprocal segmental inhibition of deep dorsal horn neurons, whereby activity in C+ve neurons inhibits activity in C-ve neurons and vice versa.125 Descending inhibition of C+ve neurons will thus disinhibit weak C+ve and C-ve neurons. Given that descending control spares ongoing and non-noxious-evoked activity of class 2 deep dorsal horn neurons,125 the inhibition of noxious-evoked activity is unlikely to result entirely from postsynaptic inhibition in the deep dorsal horn. However unlike in the deep dorsal horn, inhibition exerted in the superficial dorsal horn from the PAG is not selective for C-fiber input,63 although it is likely to be at least in part post-synaptic (see review in Fields, et al24).
Taken together, these lines of evidence suggest that the differential effect of activating descending inhibition from the PAG on C-evoked activity in the deep dorsal horn is mediated by a direct post-synaptic action in the superficial dorsal horn. The strength of inhibitory control of the C-evoked activity in a given deep neuron reflects the degree to which that neuron receives C-fiber input relayed through the superficial layers. Because the strong C+ve deep dorsal horn neurons receive multiple inputs from the superficial dorsal horn, descending control produces a large net inhibition of nociceptive activity (both A- and C-fiber-evoked). By the same argument, activity of weak C+ve neurons, which receive a small input from the superficial dorsal horn, is only modestly depressed by PAG stimulation. Strong inhibitory influences on C+ve neurons will lift weak C+ve cells and C-ve cells from segmental inhibition, resulting in a net facilitatory effect. Importantly, direct A-nociceptor inputs to the deep dorsal horn that are not subject to descending control may act to further protect A-nociceptor-evoked responses. Together these mechanisms could account for the range in descending modulation of deep dorsal horn neurons, from strong inhibition to significant facilitation.
2.2. Functional significance of differential descending control of spinal nociception mediated by C-fibers and A-fibers
Our recent data have demonstrated clear differences in the descending control of C-versus A-fiber mediated spinal nociception from the PAG. C-fiber evoked activity is powerfully suppressed, whereas A-fiber nociception is unaffected or even enhanced. Such differential control is of considerable behavioral significance given the central role of the PAG in coordinating survival strategies. Potentially distracting input arising from C-fiber activation would be suppressed, preserving rapidly conducted sensory-discriminative information carried by A-fiber nociceptors.
These findings may also be relevant to development and maintenance of chronic pain states, since C-fiber inputs play a significant role in the sensitization of dorsal horn neurons.28, 75 Descending inhibitory control of those inputs may in many cases limit development of a central sensitized state, and failure of this inhibition may permit recruitment of descending facilitation (see sections 3 and 4 below).
3. Organization and recruitment of pain modulating circuitry of the RVM
The PAG does not project directly to the spinal cord. Instead, its principle descending projection is to the RVM, which can be considered the output of the midline pain-modulation system. The RVM is defined functionally, as the midline pontomedullary area in which electrical stimulation or opioid microinjection produces behavioral antinociception. It includes the nucleus raphe magnus and adjacent reticular formation, and projects diffusely to dorsal horn laminae important in nociceptive processing, including superficial layers and deep dorsal horn.22
3.1. ON-cells and OFF-cells as the neural basis for bidirectional control from the RVM
The ability of the PAG-RVM system to suppress nociception is well documented,24 but as noted above (Section 1), descending control can be facilitatory as well as inhibitory. Functionally opposing facilitation and inhibition may in some instances arise from distinct brain regions, as occurs in the caudal medulla (Section 4, below). However, in the case of the RVM, facilitatory and inhibitory influences have been found to overlap. Non-selective stimulation or inactivation of RVM neurons can suppress or enhance nociception, depending on the functional context. Electrical stimulation can produce facilitation or inhibition with different thresholds, or over the course of a developing inflammatory response.106, 132–134 Focal application of opioids in the RVM evokes analgesia, whereas the neuropeptide cholecystokinin produces behavioral hyperalgesia.43, 47, 64 Whether neurotensin microinjection in the RVM produces analgesia or hyperalgesia varies with dose, and presumably, the receptor type activated.11, 92, 112, 122 The effects of RVM inactivation are similarly complex. Lesion or general inactivation of RVM neurons may produce modest hyperalgesia or have no effect under basal conditions, but raise the nociceptive threshold in acute and chronic hyperalgesic states.37, 60, 102
It is difficult to understand how the RVM could produce both analgesia and hyperalgesia when approaching the problem at the level of the region as a whole, using c-fos expression, bulk labelling, or non-specific pharmacological manipulations. However, the increasing appreciation of the RVM as mediating bidirectional control of nociception has been paralleled by a growing understanding of the functional physiology of RVM neurons. In 1983, Fields and colleagues described RVM neurons that exhibited abrupt state changes associated with nocifensive withdrawal and named these “ON-cells” and “OFF-cells”. ON-cells entered a period of activity, and OFF-cells a period of silence.20 (The remaining neurons were classified by exclusion, and referred to as NEUTRAL-cells.) The validity of this categorization for classifying RVM neurons has been repeatedly confirmed by the distinct pharmacological profiles exhibited by the different cell classes.34, 38, 47, 84, 85, 92, 110 At least some cells of each class project to the spinal cord, and specifically to the dorsal horn.23, 123 Within 15 years of describing the ON/OFF/NEUTRAL cell classes, it became evident that it is the OFF-cells that function as the antinociceptive output from the RVM (see Heinricher & Ingram49 for comprehensive review).
Determining a role for the ON-cells proved more challenging. ON-cells were at first relegated to a role as inhibitory interneurons mediating the reflex-related pause in firing that characterizes OFF-cells. In the absence of functional evidence for descending facilitation from the RVM, the suggestion that these neurons could have a permissive or possibly facilitatory influence received little attention.22 However, it subsequently became clear that ON-cells are not inhibitory interneurons in the RVM.16, 41 Moreover, growing evidence pointed to a pain facilitatory role for the RVM under a variety of conditions in which behavioral hyperalgesia was correlated with increased activity of ON-cells and suppression of OFF-cell firing.4, 8, 21, 35, 80, 87, 88, 93 However, reports that noxious-evoked activation of apparent ON-cells is correlated with analgesia rather than hyperalgesia,2, 71, 120 and claims that ON- and OFF-cells had no role in pain modulation,77 underscored the limitations of correlative methods for defining the function of these neurons.
A much stronger approach is to determine the net behavioral effect (facilitatory or inhibitory) of targeted activation or inactivation of each population. To achieve this, a combined single-cell recording/microinjection approach in which neuronal activity within the RVM and nocifensor reflex threshold are recorded before, during and after focal application of a drug within the RVM was developed.40 When appropriate pharmacological tools are available, this approach permits differential manipulation of the activity of the different cell classes, and allows the determination of the net behavioral effect of altering the firing of each class in the intact animal. Using this approach, it was possible to show that ON-cells are the facilitating output from the RVM.47, 49, 61, 92, 127
3.2. NEUTRAL-cells, serotonin and descending control
Whether NEUTRAL-cells have any role in modulation of pain has been an important unresolved question ever since the term was first applied to all cells that were neither ON-cells nor OFF-cells. NEUTRAL-cells do not respond during nocifensor withdrawals or during acute inflammation.61, 127 NEUTRAL-cell firing is also unchanged following focal microinjection of μ-opioids, cannabinoids, α2-agonists, and the neuropeptides cholecystokinin and neurotensin, all at doses that have unambiguous effects on activity of ON-cells and/or OFF-cells as well as on behavioral threshold.44, 47, 85, 92 The failure of NEUTRAL-cells to respond in a way that can be linked to nociceptive behavior provides no obvious hypothesis as to how these neurons might contribute to descending control, and their distinct pharmacology further corroborates their segregation into a class distinct from ON- and OFF-cells. However, one possibility is that NEUTRAL-cells are recruited to become ON- or OFF-cells during development of chronic pain states.86 Although apparently inconsistent with the distinct pharmacology of NEUTRAL-cells, this latter idea may be related to the wide variation in excitability of ON-and OFF-cells under basal conditions.35 In addition, at least some NEUTRAL-cells, but apparently no ON-cells or OFF-cells are serotonergic.29, 30, 103, 126 Given the widely accepted importance of serotonin in nociceptive modulation,114 the role(s) of NEUTRAL-cells in pain modulation remains an open question of significant interest.
3.3. Recruitment of RVM ON-cells and OFF-cells in positive feedback loops
ON- and OFF-cells appear to exert a “mass-action” regulation of dorsal horn function, and nociceptive threshold varies with the balance between the two populations. Cells within each class fire in phase, with the two classes out of phase. In lightly anesthetized animals, the two populations alternate spontaneously, with active periods in each class lasting seconds to many minutes. Nocifensive reflexes such as the tail flick or paw withdrawal to noxious heat are also marked by a shift in the balance between the populations such that ON-cells more or less synchronously enter an active phase, whereas OFF-cells show the opposite response, and become silent.5, 35 Nociceptive threshold is lowest when the ON-cell population is active and OFF-cells are silent.35, 36
The equilibrium between the ON- and OFF-cell populations under basal conditions likely reflects a role for the RVM in mediating subtle shifts in the priority of nociceptive responding relative to other behavioral tasks. Thus, for example, it has long been recognized that the pain threshold is elevated when hungry animals are given access to food.14, 15 Moreover, there appears to be an equilibrium between responding to noxious inputs and the need to maintain energy balance. Feeding is suppressed in favor of pain behaviors during the first phase of the formalin response, generally thought to represent a relatively intense sensation. By contrast, pain behaviors are reduced in favor of feeding during the second, less intense, phase of the formalin response.66 There is anecdotal evidence that this is mediated by OFF-cells in the RVM.25 Similar elevations in nociceptive threshold are observed during micturition, and this presumably allows the bladder to be emptied without disturbance by reflexive movements evoked by noxious stimulation.3 Analgesic drugs such as opioids and the novel agent improgan take advantage of this system, and produce their effects by causing OFF-cells to become continuously active,39, 42, 44, 45, 90 an action that may be pharmacological rather than physiological. Conversely, an encounter with a noxious stimulus increases nociceptive vigilance, at least temporarily, and activation of ON-cells and inhibition of OFF-cells triggered by a discrete noxious experience lowers the behavioral threshold for subsequent noxious inputs.105 Reflex-related ON-cell activity apparently plays a role in the magnitude or intensity of the behavioral response to noxious input.57
A shift in the balance ON- and OFF-cell populations such that ON-cells predominate for extended periods likely underlies the pro-nociceptive influence that this region develops during chronic inflammatory and nerve injury states.46, 102 ON-cells enter periods of prolonged activation during acute inflammation,61 and the increase in c-fos expression in the RVM following acute inflammation of the ankle joint100 likely reflects activation of these pro-nociceptive neurons. Interestingly, the pattern is quite different in chronic arthritis (kaolin and carrageenan in the knee joint) or following nerve injury (spinal nerve ligation, spared nerve injury). With chronic arthritis, both ON- and OFF-cell classes display a modest increase in spontaneous activity, and there is no change in the threshold for withdrawal to noxious heat.97 The lack of behavioral change may reflect co-activation of the pro-nociceptive and anti-nociceptive outflow from the RVM. The finding that chronic ankle joint inflammation (Complete Freund’s adjuvant) increased c-fos expression in the RVM with no change at the level of the dorsal horn100 could be considered consistent with the increase in ongoing activity of both ON- and OFF-cell classes reported by Pinto-Ribeiro and colleagues.97 Interestingly, responses of both ON- and OFF-cells to noxious pinch are reported to be reduced during chronic inflammation,97 although pinch-evoked c-fos expression in RVM neurons is enhanced, and associated with decreased expression at the level of the dorsal horn.100 Further work will clearly be required to link findings obtained using the c-fos method with functionally distinct cell populations in the RVM.
In neuropathic models, mechanical allodynia and thermal hyperalgesia are maintained by the RVM.102 Surprisingly, the ongoing activity of ON- and OFF-cells does not predict behavioral hypersensitivity in this situation. However, both ON- and OFF-cells developed novel responses to innocuous mechanical stimuli, and enhanced responses to noxious heat and mechanical stimulation of the nerve-injured limb.13, 32 This suggests that there are important differences in dynamic reorganization of the RVM in inflammation as compared to nerve injury, or that compensatory processes attempt to realign this system as a chronic pain state progresses.
3.4 Top-down recruitment of the RVM in illness and stress
Changes in the dynamics of the RVM during inflammation or following nerve injury doubtless represent important components of central positive feedback loops engaged by noxious input. However, the PAG particularly, but also the RVM, receive substantial afferent input from higher centers, particularly the hypothalamus, amygdala and prefrontal cortex (Fig. 1). The amygdala is known to be a critical relay to the PAG-RVM system in the analgesic states associated with intense fear,50, 51 and opioid action in the basolateral nucleus of the amygdala recruits OFF-cells in the RVM.79 Higher centers may also be important in hyperalgesia, as well as analgesia. For example, stimulation in the ventrolateral orbital cortex activates RVM ON-cells, and hyperalgesia produced by stimulation in the anterior cingulate requires the RVM.12, 54 Prostaglandin E2 (PGE2) in the medial preoptic area similarly activates ON-cells and produces hyperalgesia.48 This is of interest because the medial preoptic area is a primary site at which PGE2 acts to organize autonomic, neuroendocrine and behavioral elements of the sickness response.19, 55, 62 Activation of the dorsomedial nucleus of the hypothalamus, a region implicated in autonomic aspects of the response to psychological stress, also evokes behavioral hyperalgesia mediated by ON-cells.76 Importantly, associated fever and tachycardia are not mediated by ON-cells, arguing against the proposal that autonomic and pain-modulating function are conflated in the RVM,77 and instead pointing to segregation of these functions of the RVM at the level of individual neurons.
Evidence for top-down control of the RVM provides a possible neural substrate for the influence of cognitive and emotional factors on pain. Just as suppression of pain could be advantageous in highly stressful or dangerous situations where other behaviors must pre-empt pain responses and recuperative behaviors in order to ensure survival, facilitation of pain could promote recuperative behaviors during illness, and enhance vigilance in situations where threat is possible, but not imminent.
4. Descending control from the caudal medulla
In addition to the PAG-RVM system, two areas of the caudal medulla, the dorsal reticular nucleus (DRt) and caudal lateral ventrolateral medulla (VLM), have also been implicated in descending control of dorsal horn nociceptive processing. The DRt is reciprocally connected with dorsal horn laminae important in nociception, and experimental stimulation of the DRt facilitates behavioral measures of nociception, implicating this region in a positive feedback loop that is closely tied to processing of nociceptive information.73 The VLM also participates in a closed loop with dorsal horn nociceptive processing laminae.117 Based on the importance of spinobulbospinal loops in central sensitization,115 the reciprocal connections of the DRt and VLM with the spinal cord provide an important anatomical background for the participation of those areas in central sensitization during chronic pain. Additionally, the VLM shows close parallels with the more rostral areas, namely the RVM and the A5 noradrenergic cell group, the latter being relevant for pain modulation.116 Stimulation in the VLM potently inhibits behavioral and dorsal horn nociceptive responses, whilst lesions result in apparent disinhibition. This suggests that the VLM exerts a tonic inhibitory control of dorsal horn nociception.26, 31, 56 Nevertheless, the VLM may, like the RVM, exert a facilitatory influence, as neurons with features of ON and OFF cells have been identified in this region.96
Studies using c-fos detection as a functional anatomical method point to dynamic changes in both DRt and VLM in acute and chronic inflammatory models. Acute inflammation induced by intra-articular injection of a solution of PGE2 and bradykinin induces a strong neuronal activation both at the VLM and the spinal cord. This suggests that at the initial phases of inflammation, descending inhibition from the VLM fails to inhibit the strong nociceptive transmission arising from the spinal cord (Pinto et al., 2007). With chronic inflammation (Complete Freund’s adjuvant in the knee joint), innocuous stimulation of the affected paw gives rise to an inverse correlation between c-fos expression in the VLM and dorsal horn, suggesting that descending inhibition is sufficient to suppress spinal activation. However, when intense pinch is applied to the same limb, strong c-fos expression is seen at both levels, implying either that the c-fos expression represents activated pain-facilitating neurons or, if expression is in pain-inhibiting neurons, that descending inhibition is insufficient to suppress activation of the dorsal horn neurons.98, 100
The imbalance between inhibition and facilitation during chronic pain is likely to be due to complex changes at the pain control centers. At the RVM, the activity of ON- and OFF-cells is affected during the course of chronic pain installation.61 Recent studies suggest that in other areas besides the RVM, the neuronal activity is strongly affected, as recently shown by increased activity of ON-cells and OFF-cells at the VLM of monoarthritic rats.97 At the DRt, neurochemical changes have been described during chronic inflammatory pain. The pronociceptive effects of the nucleus are maintained during chronic pain,113 which is probably associated with a decreased inhibitory tone. The expression of μ-opioid receptors (MOR) and δ-opioid receptors (DOR) is decreased at the DRt during chronic pain without changes in local levels of opioids.91, 99 This is likely to directly affect descending modulation from the nucleus, since spinally-projecting DRt neurons express MOR receptors101 and the instillation of the MOR agonist DAMGO ([D-Ala2, N-MePhe4Gly-ol5]-enkephalin) at the DRt has opposite effects in non-inflamed and monoarthritic rats.99 The changes described at the DRt are opposite from those described in other pain control centers, namely at the RVM, where the local efficacy of opioids increases without changes in opioid receptor expression.53 This shows that different components of the supraspinal pain-control system are differentially affected by the installation of chronic pain. Strategies for manipulation of the supraspinal pain control system should take into account the regional changes induced by chronic pain and could be based on vector delivery of the suitable transgenes using gene therapy approaches.118
5. Concluding remarks
Our understanding of pain mechanisms and pain control has in large part focused on the properties of primary afferent and dorsal horn nociceptive neurons and ascending pathways. The role of supraspinal processing has undergone a recent renaissance with the advent of functional imaging techniques, which have pointed to an interacting cortical “matrix”, rather than a “pain center” as underlying the pain experience. Nevertheless, a complete understanding of the neural basis of pain requires recognition that the brain is not a passive receiver of a “pain message”. Rather, there is an active regulation of sensory transmission at the level of the dorsal horn by means of descending projections from the brainstem. Although brain control of sensory input is by no means unique to pain, it seems to have a particularly important role in this system. The need to regulate nociceptive input likely reflects the imperative of responding to stimuli that harm or threaten to harm the body. Depending on the behavioral context, signals related to noxious or potentially noxious input could receive enhanced attention or be subordinated to other bodily needs of higher priority.10, 65, 104 Understanding how the descending control systems interface with dorsal horn nociceptive processes, how they are recruited to effect changes in the priority of pain relative to other behaviors, and how the dynamics of these systems are altered and contribute to pathological pain states, are important questions that are only now starting to be fully addressed.
Acknowledgments
BML was supported by the BBSRC and The Wellcome Trust. JLL was a BBSRC Case Student. MMH was supported by grants from NINDS (NS052364) and NIDA (DA05608). IT was supported by a grant from FCT (PTDC/SAU-OSM/64643/2006). We thank Andy Rekito for providing illustrations.
Abbreviations
- COX
cyclo-oxygenase
- C+ve
C-fiber input positive
- C-ve
C-fiber input negative
- DOR
δ opioid receptor
- DRt
dorsal reticular nucleus of the caudal medulla
- MOR
μ opioid receptor
- PAG
periaqueductal gray
- PGE2
prostaglandin E2
- RVM
rostral ventromedial medulla
- VLM
ventrolateral quadrant of the caudal medulla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Andrew D, Craig AD. Responses of spinothalamic lamina I neurons to maintained noxious mechanical stimulation in the cat. J Neurophysiol. 2002;87:1889–1901. doi: 10.1152/jn.00577.2001. [DOI] [PubMed] [Google Scholar]
- 2.Azami J, Green DL, Roberts MH, Monhemius R. The behavioural importance of dynamically activated descending inhibition from the nucleus reticularis gigantocellularis pars alpha. Pain. 2001;92:53–62. doi: 10.1016/s0304-3959(00)00471-1. [DOI] [PubMed] [Google Scholar]
- 3.Baez MA, Brink TS, Mason P. Roles for pain modulatory cells during micturition and continence. J Neurosci. 2005;25:384–94. doi: 10.1523/JNEUROSCI.3536-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–10. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- 5.Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–25. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- 6.Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978;178:209–24. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Ralston DD, Ralston HJ., 3rd Bulbospinal projections in the primate: a light and electron microscopic study of a pain modulating system. J Comp Neurol. 1986;250:311–23. doi: 10.1002/cne.902500305. [DOI] [PubMed] [Google Scholar]
- 8.Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 9.Bennett GJ, Mayer DJ. Inhibition of spinal cord interneurons by narcotic microinjection and focal electrical stimulation in the periaqueductal central gray matter. Brain Res. 1979;172:243–57. doi: 10.1016/0006-8993(79)90536-5. [DOI] [PubMed] [Google Scholar]
- 10.Bolles RC, Fanselow MS. A perceptual-defensive-recuperative model of fear and pain. Behav Brain Sci. 1980;3:291–301. [Google Scholar]
- 11.Buhler AV, Choi J, Proudfit HK, Gebhart GF. Neurotensin activation of the NTR1 on spinally-projecting serotonergic neurons in the rostral ventromedial medulla is antinociceptive. Pain. 2005;114:285–94. doi: 10.1016/j.pain.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Calejesan AA, Kim SJ, Zhuo M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain. 2000;4:83–96. doi: 10.1053/eujp.1999.0158. [DOI] [PubMed] [Google Scholar]
- 13.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–31. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey KL, Morrow TJ. Supraspinal nocifensive responses of cats: spinal cord pathways, monoamines, and modulation. J Comp Neurol. 1988;270:591–605. doi: 10.1002/cne.902700412. [DOI] [PubMed] [Google Scholar]
- 15.Casey KL, Morrow TJ. Effect of medial bulboreticular and raphe nuclear lesions on the excitation and modulation of supraspinal nocifensive behaviors in the cat. Brain Res. 1989;501:150–61. doi: 10.1016/0006-8993(89)91036-6. [DOI] [PubMed] [Google Scholar]
- 16.Cleary DR, Neubert MJ, Heinricher MM. Are opioid-sensitive neurons in the rostral ventromedial medulla inhibitory interneurons? Neuroscience. 2008;151:564–71. doi: 10.1016/j.neuroscience.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig AD, Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. J Neurophysiol. 2002;87:1902–1914. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- 18.Duggan AW, Morton CR. Periaqueductal grey stimulation: an association between selective inhibition of dorsal horn neurones and changes in peripheral circulation. Pain. 1983;15:237–48. doi: 10.1016/0304-3959(83)90059-3. [DOI] [PubMed] [Google Scholar]
- 19.Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–70. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- 20.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- 22.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans of the R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 23.Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–59. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 24.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5. Elsevier; London: 2006. pp. 125–142. [Google Scholar]
- 25.Foo H, Mason P. Sensory suppression during feeding. Proc Natl Acad Sci USA. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foong FW, Duggan AW. Brain-stem areas tonically inhibiting dorsal horn neurones: studies with microinjection of the GABA analogue piperidine-4-sulphonic acid. Pain. 1986;27:361–71. doi: 10.1016/0304-3959(86)90160-0. [DOI] [PubMed] [Google Scholar]
- 27.Fritschy JM, Lyons WE, Mullen CA, Kosofsky BE, Molliver ME, Grzanna R. Distribution of locus coeruleus axons in the rat spinal cord: a combined anterograde transport and immunohistochemical study. Brain Res. 1987;437:176–80. doi: 10.1016/0006-8993(87)91541-1. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–9. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 29.Gao K, Mason P. Serotonergic raphe magnus cells that respond to noxious tail heat are not ON or OFF cells. J Neurophysiol. 2000;84:1719–25. doi: 10.1152/jn.2000.84.4.1719. [DOI] [PubMed] [Google Scholar]
- 30.Gao K, Mason P. Physiological and anatomic evidence for functional subclasses of serotonergic raphe magnus cells. J Comp Neurol. 2001;439:426–39. doi: 10.1002/cne.1360. [DOI] [PubMed] [Google Scholar]
- 31.Gebhart GF, Ossipov MH. Characterization of inhibition of the spinal nociceptive tail-flick reflex in the rat from the medullary lateral reticular nucleus. J Neurosci. 1986;6:701–13. doi: 10.1523/JNEUROSCI.06-03-00701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves L, Almeida A, Pertovaara A. Pronociceptive changes in response properties of rostroventromedial medullary neurons in a rat model of peripheral neuropathy. Eur J Neurosci. 2007;26:2188–2195. doi: 10.1111/j.1460-9568.2007.05832.x. [DOI] [PubMed] [Google Scholar]
- 33.Gray BG, Dostrovsky JO. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:932–47. doi: 10.1152/jn.1983.49.4.932. [DOI] [PubMed] [Google Scholar]
- 34.Heinricher MM, Haws CM, Fields HL. Opposing actions of norepinephrine and clonidine on single pain-modulating neurons in rostral ventromedial medulla. In: Dubner R, Gebhart GF, Bond MR, editors. Pain Res Clin Management. Vol. 3. Elsevier; Amsterdam: 1988. pp. 590–594. [Google Scholar]
- 35.Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–39. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 36.Heinricher MM, Haws CM, Fields HL. Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res. 1991;8:215–25. doi: 10.3109/08990229109144745. [DOI] [PubMed] [Google Scholar]
- 37.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–113. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- 38.Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–43. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- 39.Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 40.Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–46. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 41.Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: Implications for circuitry. Pain. 1998;75:247–255. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 42.Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain. 1999;81:57–65. doi: 10.1016/s0304-3959(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 43.Heinricher MM, Morgan MM. Supraspinal mechanisms of opioid analgesia. In: Stein C, editor. Opioids and Pain Control. Cambridge University Press; Cambridge: 1999. pp. 46–69. [Google Scholar]
- 44.Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol. 2001;85:280–286. doi: 10.1152/jn.2001.85.1.280. [DOI] [PubMed] [Google Scholar]
- 45.Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-D-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain. 2001;92:129–138. doi: 10.1016/s0304-3959(00)00480-2. [DOI] [PubMed] [Google Scholar]
- 46.Heinricher MM, Pertovaara A, Ossipov MH. Descending modulation after injury. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Progress in Pain Research and Management. Vol. 24. IASP Press; Seattle: 2003. pp. 251–260. [Google Scholar]
- 47.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–9. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 48.Heinricher MM, Neubert MJ, Martenson ME, Gonçalves L. Prostaglandin E2 in the medial preoptic area produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Neuroscience. 2004;128:389–398. doi: 10.1016/j.neuroscience.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 49.Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, A Comprehensive Reference vol 5 Pain. Academic Press; San Diego: 2008. pp. 593–626. [Google Scholar]
- 50.Helmstetter FJ. Contribution of the amygdala to learning and performance of conditional fear. Physiol Behav. 1992;51:1271–6. doi: 10.1016/0031-9384(92)90320-2. [DOI] [PubMed] [Google Scholar]
- 51.Helmstetter FJ, Tershner SA. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. J Neurosci. 1994;14:7099–108. doi: 10.1523/JNEUROSCI.14-11-07099.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 53.Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal μ opioid receptor agonists after inflammatory injury. J Neurosci. 2001;21:2536–45. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutchison WD, Harfa L, Dostrovsky JO. Ventrolateral orbital cortex and periaqueductal gray stimulation-induced effects on on- and off-cells in the rostral ventromedial medulla in the rat. Neuroscience. 1996;70:391–407. doi: 10.1016/0306-4522(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 55.Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- 56.Janss AJ, Gebhart GF. Brainstem and spinal pathways mediating descending inhibition from the medullary lateral reticular nucleus in the rat. Brain Res. 1988;440:109–22. doi: 10.1016/0006-8993(88)91163-8. [DOI] [PubMed] [Google Scholar]
- 57.Jinks SL, Carstens EE, Antognini JF. Glutamate receptor blockade in the rostral ventromedial medulla reduces the force of multisegmental motor responses to supramaximal noxious stimuli. Neurosci Lett. 2007;426:175–180. doi: 10.1016/j.neulet.2007.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jurna I. Effect of stimulation in the periaqueductal grey matter on activity in ascending axons of the rat spinal cord: selective inhibition of activity evoked by afferent Aδ and C fibre stimulation and failure of naloxone to reduce inhibition. Brain Res. 1980;196:33–42. doi: 10.1016/0006-8993(80)90714-3. [DOI] [PubMed] [Google Scholar]
- 59.Kajander KC, Ebner TJ, Bloedel JR. Effects of periaqueductal gray and raphe magnus stimulation on the responses of spinocervical and other ascending projection neurons to non-noxious inputs. Brain Res. 1984;291:29–37. doi: 10.1016/0006-8993(84)90647-4. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan H, Fields HL. Hyperalgesia during acute opioid abstinence: evidence for a nociceptive facilitating function of the rostral ventromedial medulla. J Neurosci. 1991;11:1433–9. doi: 10.1523/JNEUROSCI.11-05-01433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- 62.Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koutsikou S, Parry DM, MacMillan FM, Lumb BM. Laminar organization of spinal dorsal horn neurones activated by C- vs. A-heat nociceptors and their descending control from the periaqueductal grey in the rat. Eur J Neurosci. 2007;26:943–952. doi: 10.1111/j.1460-9568.2007.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- 65.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102:12950–5. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaGraize SC, Borzan J, Rinker MM, Kopp JL, Fuchs PN. Behavioral evidence for competing motivational drives of nociception and hunger. Neurosci Lett. 2004;372:30–4. doi: 10.1016/j.neulet.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Aδ- or A-α/β fibres. Exp Physiol. 2002;87:239–44. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 68.Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Rev. 2002;40:29–44. doi: 10.1016/s0165-0173(02)00186-8. [DOI] [PubMed] [Google Scholar]
- 69.Leith J, Martindale J, LFD, Lumb B. Descending control produced by cyclooxygenase-1 inhibition in the periaqueductal grey targets dorsal horn neurones with strong C-fibre inputs. Proc Physiol Soc. 2008;11:PC110. [Google Scholar]
- 70.Leith JL, Wilson AW, Donaldson LF, Lumb BM. Cyclooxygenase-1-derived prostaglandins in the periaqueductal gray differentially control C- versus A-fiber-evoked spinal nociception. J Neurosci. 2007;27:11296–305. doi: 10.1523/JNEUROSCI.2586-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li HS, Monhemius R, Simpson BA, Roberts MH. Supraspinal inhibition of nociceptive dorsal horn neurones in the anaesthetized rat: tonic or dynamic? J. Physiol. 1998;506:459–69. doi: 10.1111/j.1469-7793.1998.459bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Light AR, Casale EJ, Menetrey DM. The effects of focal stimulation in nucleus raphe magnus and periaqueductal gray on intracellularly recorded neurons in spinal laminae I and II. J Neurophysiol. 1986;56:555–71. doi: 10.1152/jn.1986.56.3.555. [DOI] [PubMed] [Google Scholar]
- 73.Lima D, Almeida A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Prog Neurobiol. 2002;66:81–108. doi: 10.1016/s0301-0082(01)00025-9. [DOI] [PubMed] [Google Scholar]
- 74.Lu Y, Sweitzer SM, Laurito CE, Yeomans DC. Differential opioid inhibition of C- and Aδ-fiber mediated thermonociception after stimulation of the nucleus raphe magnus. Anesth Analg. 2004;98:414–419. doi: 10.1213/01.ANE.0000094334.12027.06. [DOI] [PubMed] [Google Scholar]
- 75.Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–64. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- 76.Martenson ME, Heinricher MM. Stress-induced hyperalgesia: recruitment of the rostral ventromedial medulla by stimulation of the dorsomedial hypothalamus. Neuroscience Meeting Planner; San Diego, CA. 2007. Society for Neuroscience, Program No. 723.11, Online. [Google Scholar]
- 77.Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- 78.Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–4. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- 79.McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 80.McGaraughty S, Chu KL, Bitner RS, Martino B, Kouhen RE, Han P, Nikkel AL, Burgard EC, Faltynek CR, Jarvis MF. Capsaicin infused into the PAG affects rat tail flick responses to noxious heat and alters neuronal firing in the RVM. J Neurophysiol. 2003;90:2702–2710. doi: 10.1152/jn.00433.2003. [DOI] [PubMed] [Google Scholar]
- 81.McMullan S, Simpson DAA, Lumb BM. A reliable method for the preferential activation of C- or A-fibre heat nociceptors. J Neurosci Meth. 2004;138:133–139. doi: 10.1016/j.jneumeth.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 82.McMullan S, Lumb BM. Spinal dorsal horn neuronal responses to myelinated versus unmyelinated heat nociceptors and their modulation by activation of the periaqueductal grey in the rat. J Physiol. 2006;576:547–56. doi: 10.1113/jphysiol.2006.117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McMullan S, Lumb BM. Midbrain control of spinal nociception discriminates between responses evoked by myelinated and unmyelinated heat nociceptors in the rat. Pain. 2006;124:59. doi: 10.1016/j.pain.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–3. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- 85.Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–93. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–60. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 87.Morgan MM, Fields HL. Pronounced changes in the activity of nociceptive modulatory neurons in the rostral ventromedial medulla in response to prolonged thermal noxious stimuli. J Neurophysiol. 1994;72:1161–70. doi: 10.1152/jn.1994.72.3.1161. [DOI] [PubMed] [Google Scholar]
- 88.Morgan MM, Heinricher MM, Fields HL. Inhibition and facilitation of different nocifensor reflexes by spatially remote noxious stimuli. J Neurophysiol. 1994;72:1152–60. doi: 10.1152/jn.1994.72.3.1152. [DOI] [PubMed] [Google Scholar]
- 89.Morris R, Cheunsuang O, Stewart A, Maxwell D. Spinal dorsal horn neurone targets for nociceptive primary afferents: do single neurone morphological characteristics suggest how nociceptive information is processed at the spinal level. Brain Res Rev. 2004;46:173–90. doi: 10.1016/j.brainresrev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res. 2004;1021:248–55. doi: 10.1016/j.brainres.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 91.Neto F, Carvalhosa A, Ferreira-Gomes J, Reguenga C, Castro-Lopes J. Delta-opioid receptor mRNA expression is changed in the thalamus of monoarthritic rats. J Chem Neuroanat. doi: 10.1016/j.jchemneu.2008.05.004. in press. [DOI] [PubMed] [Google Scholar]
- 92.Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 93.Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron. 2000;26:515–522. doi: 10.1016/s0896-6273(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 94.Pertovaara A. A neuronal correlate of secondary hyperalgesia in the rat spinal dorsal horn is submodality selective and facilitated by supraspinal influence. Exp Neurol. 1998;149:193–202. doi: 10.1006/exnr.1997.6688. [DOI] [PubMed] [Google Scholar]
- 95.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Pinto-Ribeiro F, Ansah O, Almeida A, Pertovaara A. Descending pain modulatory influence from the hypothalamic paraventricular nucleus through the caudal ventrolateral medulla in arthritic and control rats. FENS Abstr. 2006;3:A004.13. [Google Scholar]
- 97.Pinto-Ribeiro F, Ansah OB, Almeida A, Pertovaara A. Influence of arthritis on descending modulation of nociception from the paraventricular nucleus of the hypothalamus. Brain Res. 2008;1197:63–75. doi: 10.1016/j.brainres.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 98.Pinto M, Lima D, Tavares I. Correlation of noxious evoked c-fos expression in areas of the somatosensory system during chronic pain: involvement of spino-medullary and intra-medullary connections. Neurosci Lett. 2006;409:100–5. doi: 10.1016/j.neulet.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 99.Pinto M, Tschudy F, MS, Wilson SP, Lima D, Tavares I. Opioid-mediated control of pain modulation from the medullary dorsal reticularnucleus: a gene therapy and pharmacological study in the monoarthritic rat. Soc. Neuroscience Meeting Planner; Atlanta, GA. 2006. p. 249.21. Online. [Google Scholar]
- 100.Pinto M, Lima D, Tavares I. Neuronal activation at the spinal cord and medullary pain control centers after joint stimulation: a c-fos study in acute and chronic articular inflammation. Neuroscience. 2007;147:1076–89. doi: 10.1016/j.neuroscience.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 101.Pinto M, Sousa M, Lima D, Tavares I. Expression of μ-opioid, GABAB and NK1 receptors in spinally-projecting neurons of the caudal medulla oblongata: implications for descending modulation of nociceptive transmission. J Comp Neurol. doi: 10.1002/cne.21793. in press. [DOI] [PubMed] [Google Scholar]
- 102.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–25. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 103.Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–65. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quevedo AS, Coghill RC. Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci. 2007;27:11635–40. doi: 10.1523/JNEUROSCI.3356-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramirez F, Vanegas H. Tooth pulp stimulation advances both medullary off-cell pause and tail flick. Neurosci Lett. 1989;100:153–6. doi: 10.1016/0304-3940(89)90676-9. [DOI] [PubMed] [Google Scholar]
- 106.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 107.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;154:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 108.Ruda MA, Allen B, Gobel S. Ultrastructural analysis of medial brain stem afferents to the superficial dorsal horn. Brain Res. 1981;205:175–80. doi: 10.1016/0006-8993(81)90729-0. [DOI] [PubMed] [Google Scholar]
- 109.Schady WJ, Torebjork HE, Ochoa JL. Peripheral projections of nerve fibres in the human median nerve. Brain Res. 1983;277:249–61. doi: 10.1016/0006-8993(83)90932-0. [DOI] [PubMed] [Google Scholar]
- 110.Selden NR, Carlson JD, Cetas J, Close LN, Heinricher MM. Purinergic actions on neurons that modulate nociception in the rostral ventromedial medulla. Neuroscience. 2007;146:1808–16. doi: 10.1016/j.neuroscience.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 111.Simpson DA, Headley PM, Lumb BM. Selective inhibition from the anterior hypothalamus of C- versus A-fibre mediated spinal nociception. Pain. 2008;136:305–12. doi: 10.1016/j.pain.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 112.Smith DJ, Hawranko AA, Monroe PJ, Gully D, Urban MO, Craig CR, Smith JP, Smith DL. Dose-dependent pain-facilitatory and -inhibitory actions of neurotensin are revealed by SR 48692, a nonpeptide neurotensin antagonist: influence on the antinociceptive effect of morphine. J Pharmacol Exp Ther. 1997;282:899–908. [PubMed] [Google Scholar]
- 113.Sotgiu ML, Valente M, Storchi R, Caramenti G, Mario Biella GE. Contribution by DRt descending facilitatory pathways to maintenance of spinal neuron sensitization in rats. Brain Res. 2008;1188:69–75. doi: 10.1016/j.brainres.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 114.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 115.Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14:175–81. doi: 10.1159/000087656. [DOI] [PubMed] [Google Scholar]
- 116.Tavares I, Lima D, Coimbra A. The pontine A5 noradrenergic cells which project to the spinal cord dorsal horn are reciprocally connected with the caudal ventrolateral medulla in the rat. Eur J Neurosci. 1997;9:2452–61. doi: 10.1111/j.1460-9568.1997.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 117.Tavares I, Lima D. The caudal ventrolateral medulla as an important inhibitory modulator of pain transmission in the spinal cord. J Pain. 2002;3:337–46. doi: 10.1054/jpai.2002.127775. [DOI] [PubMed] [Google Scholar]
- 118.Tavares I, Lima D. From neuroanatomy to gene therapy: searching for new ways to manipulate the supraspinal endogenous pain modulatory system. J Anat. 2007;211:261–8. doi: 10.1111/j.1469-7580.2007.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Terman GW, Shavit Y, Lewis JW, Cannon JT, Liebeskind JC. Intrinsic mechanisms of pain inhibition: activation by stress. Science. 1984;226:1270–7. doi: 10.1126/science.6505691. [DOI] [PubMed] [Google Scholar]
- 120.Thurston CL, Randich A. Responses of on and off cells in the rostral ventral medulla to stimulation of vagal afferents and changes in mean arterial blood pressure in intact and cardiopulmonary deafferented rats. Pain. 1995;62:19–38. doi: 10.1016/0304-3959(94)00223-2. [DOI] [PubMed] [Google Scholar]
- 121.Torebjork HE, Ochoa JL. New method to identify nociceptor units innervating glabrous skin of the human hand. Exp Brain Res. 1990;81:509–14. doi: 10.1007/BF02423499. [DOI] [PubMed] [Google Scholar]
- 122.Urban MO, Smith DJ. Localization of the antinociceptive and antianalgesic effects of neurotensin within the rostral ventromedial medulla. Neurosci Lett. 1994;174:21–5. doi: 10.1016/0304-3940(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 123.Vanegas H, Barbaro NM, Fields HL. Tail-flick related activity in medullospinal neurons. Brain Res. 1984;321:135–41. doi: 10.1016/0006-8993(84)90689-9. [DOI] [PubMed] [Google Scholar]
- 124.Waters AJ, Lumb BM. Inhibitory effects evoked from both the lateral and ventrolateral periaqueductal grey are selective for the nociceptive responses of rat dorsal horn neurones. Brain Res. 1997;752:239–49. doi: 10.1016/s0006-8993(96)01462-x. [DOI] [PubMed] [Google Scholar]
- 125.Waters AJ, Lumb BM. Descending control of spinal nociception from the periaqueductal grey distinguishes between neurons with and without C-fibre inputs. Pain. 2008;134:32–40. doi: 10.1016/j.pain.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 126.Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- 127.Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–62. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yaksh TL, Yeung JC, Rudy TA. Systematic examination in the rat of brain sites sensitive to the direct application of morphine: observation of differential effects within the periaqueductal gray. Brain Res. 1976;114:83–103. doi: 10.1016/0006-8993(76)91009-x. [DOI] [PubMed] [Google Scholar]
- 129.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 130.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 131.Yezierski RP. Effects of midbrain and medullary stimulation on spinomesencephalic tract cells in the cat. J Neurophysiol. 1990;63:240–55. doi: 10.1152/jn.1990.63.2.240. [DOI] [PubMed] [Google Scholar]
- 132.Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Pain. 1990;42:337–50. doi: 10.1016/0304-3959(90)91147-B. [DOI] [PubMed] [Google Scholar]
- 133.Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol. 1992;67:1599–614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 134.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–58. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]


