Abstract
CD4+FoxP3+ regulatory T cells (Treg) play a critical role in maintaining self tolerance and inhibiting autoimmune disease. Despite being a major focus of modern immunological investigation, many aspects of Treg biology remain unknown. In a screen for novel candidate genes involved in human Treg function, we detected the expression of an autoimmune susceptibility gene, FcRL3, in Treg but not in conventional CD4+ T cells. FcRL3 is an orphan receptor of unknown function with structural homology to classical Fc receptors. Numerous genetic studies have demonstrated a link between a single nucleotide polymorphism in the FCRL3 promoter and both overexpression of FcRL3 and autoimmune diseases such as rheumatoid arthritis. Given the critical role of Treg in suppressing autoimmunity, we sought to ascertain how expression of FcRL3 relates to the phenotype, differentiation, and function of Treg. We show here that FcRL3 is expressed on a population of thymically derived Treg that exhibits a memory phenotype and high levels of programmed cell death-1 (PD-1). Purified FcRL3+ Treg are less responsive to antigenic stimulation in the presence of IL-2 than their FcRL3− counterparts, despite intact proximal and distal IL-2 signaling as determined by phosphorylation of Stat-5 and upregulation of Bcl2. In vitro suppression assays demonstrated that FcRL3+ Treg have reduced capacity to suppress the proliferation of effector T cells. These data suggest that FcRL3 expression is associated with Treg dysfunction that may, in turn, contribute to the loss of self tolerance and the development of autoimmunity.
Introduction
The survival of complex organisms is dependent upon the ability of an active immune system to recognize and fend off foreign invaders while simultaneously preventing an attack on self. Several mechanisms have evolved to accomplish this feat. On the one hand, tolerance to self can be enforced upon elimination of self-reactive T and B cells; on the other, peripheral mechanisms can actively curb the proliferation and/or function of self-reactive cells that escape deletion. In the latter instance, investigations from many independent laboratories using diverse models have firmly established the involvement of CD4+ regulatory T cells (Treg). Specialized in suppressing immune responses to self antigens, Treg play a crucial role in thwarting autoimmune disease (1,2).
The Treg compartment is comprised of two developmentally distinct populations. Natural Treg (nTreg) originate in the thymus and are specific for self antigens presented by thymic epithelial cells, whereas induced Treg (iTreg) are generated de novo in the periphery from conventional CD4+ T cells (Tconv) upon antigenic stimulation in the presence of TGF-β, and dampen immune responses to foreign antigens (2,3). Both of these Treg populations express FoxP3, a transcription factor critical for the homeostasis and suppressor functions of Treg (4), and without which dramatic autoimmune manifestations can arise (5–7).
Treg are characterized by a set of phenotypic and functional attributes that distinguish them from Tconv. In contrast to Tconv, for instance, Treg exhibit high levels of CD25 (the IL-2Rα chain) (8), low levels of CD127 (the IL-7Rα chain) (9,10), and constitutive expression of the B7 family co-stimulatory receptor, CTLA-4 (11). Treg characteristically do not proliferate when stimulated via the TCR, although IL-2 can overcome this anergy (12). The maintenance of self tolerance by Treg may involve any of several proposed immunosuppressive mechanisms, including: decreasing the co-stimulation or antigen presenting ability of antigen presenting cells (APC) (13,14), production of suppressive cytokines, IL-2 consumption, and the direct killing of target cells (2,15,16).
Even though Treg have been researched extensively for over a decade, many questions about their phenotype, differentiation, and function remain unanswered. Our laboratory has conducted a number of studies investigating the biology and clinical implications of Treg (9,17–20). During the course of this research, we discovered that Fc receptor-like 3 (FcRL3), the product of an autoimmune susceptibility gene, is expressed on Treg but not on conventional CD4+ T cells.
FcRL3 is part of a genetically-conserved gene family that encodes orphan cell surface receptors bearing high structural homology to classical Fc receptors, with multiple extracellular Ig domains and either ITAMs, ITIMs, or both in the intracellular domains. The natural ligands of the FcRL family members remain unknown but, given their signaling domains and expression on multiple immune cell types, these members likely modulate immune cell functions by affecting signaling pathways (21). FcRL3 is expressed predominantly in B lymphocytes in lymph nodes and germinal centers (22–24) and, although its function remains unknown, numerous genetic studies have revealed an association between a single nucleotide polymorphism (SNP) in FCRL3 and a variety of autoimmune diseases such as rheumatoid arthritis (RA) (22,25–31). This C>T polymorphism at position −169 in the promoter region of FCRL3 confers a higher affinity binding site for the NFκB transcription factor, resulting in increased promoter activity and up-regulation of FCRL3 mRNA transcription (22). This relationship implies that high expression of FcRL3 results in abnormal immune activation and loss of self tolerance.
Early studies documented that the C allele of this dimorphic SNP was significantly associated with RA in a Japanese population (22). Many subsequent studies followed, consistently finding a significant association between RA and the FCRL3 −169 C variant in Asian populations (25,26,30,31). In addition, associations have been reported between the FCRL3 gene and several other autoimmune diseases, e.g., systemic lupus erythematosus, Grave’s disease, and autoimmune thyroid disease (22,27,29,31). Although this association tends to be weaker in individuals of European descent, the −169 C allele was found to be significantly associated with autoimmune diseases within subgroups of patients stratified according to a functional NFkB1 polymorphism or to SNPs in other autoimmune susceptibility genes, e.g., PTPN22 or HLA-DRB1 (32–34).
The critical role of Treg in preventing autoimmunity and the genetic studies linking autoimmune diseases with the −169C/T FCRL3 polymorphism highlight the importance of investigating the biology of FcRL3+ Treg. Specifically, we sought to ascertain how the expression of FcRL3 on Treg relates to the phenotype, differentiation, and function of this important population of cells. We demonstrate that FcRL3+ Treg represent a subpopulation of thymically derived nTreg and exhibit an “exhausted” phenotype typically seen on chronically stimulated T cells. In accord with this phenotype, we provide evidence that, compared to their FcRL3− counterparts, purified FcRL3+ Treg are relatively non-responsive to antigenic stimulation in the presence of IL-2 in vitro and have a reduced capacity to suppress the proliferation of effector T cells.
Materials and Methods
Tissues and cell isolation
Adult peripheral blood samples were collected from healthy individuals, all of whom gave written informed consent using protocols approved by the UCSF Committee on Human Research. Adult blood buffy coats from healthy donors were obtained from Stanford Blood Center, Palo Alto, California. Peripheral blood mononuclear cells (PBMC) from blood or buffy coats were isolated by density centrifugation using Ficoll-HypaquePLUS (Amersham Biosciences). All phenotyping and functional analyses were performed on fresh samples. Lymphocytes were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS) (Gemini), 1% penicillin/streptomycin (Mediatech), and 2 mM L-glutamine (Mediatech) (hereafter referred to as R10 medium).
Human fetal tissues (mesentery, spleen, and thymus) from 18–23 gestational week specimens were obtained from San Francisco General Hospital, under the auspices of a protocol approved by the UCSF Committee on Human Research. Mesenteric lymph nodes (MLN) were dissected from the fetal mesentery. Single cell suspensions were prepared by passing physically disrupted tissue through a 40 μm cell strainer (BD Falcon). Thymic suspensions were washed in R10 medium and used directly. Splenic and MLN suspensions were first subjected to density centrifugation as above and washed in R10 medium before phenotypic analysis.
Antibodies and flow cytometric phenotyping
The anti-FcRL3 antibody was kindly provided by Genentech (24). The antibody and a control irrelevant protein (human serum albumin, Sigma) were conjugated to biotin using the Fluoreporter mini-biotin protein labeling kit (Invitrogen), and were added to cell staining cocktails in conjunction with other surface markers. Secondary detection of FcRL3 or control was performed with a streptavidin-Qdot655 conjugate (Invitrogen). For phenotypic analysis and cell sorting by flow cytometry, the following Abs were used: anti-CD3 Al-700, anti-CD25 PE-Cy7, anti-CD25 APC-Cy7, anti-CCR7 PE-Cy7, anti-CD127 PE, anti-PD-1 APC, anti-CD28 APC, anti-CD31 FITC, anti-CD95/Fas APC, anti-CD62L FITC, anti-CD278 (ICOS) PE, anti-HLA-DR APC (all from BD Biosciences); anti-CD4 Texas Red, anti-CD8 PE-Cy5.5 (Caltag Laboratories); anti-CD4 Qdot605 (Invitrogen); and anti-CD45RA ECD and anti-CTLA-4 PE (Beckman Coulter). Dead cells were excluded from all flow cytometric analyses by cell surface staining with Aqua Live/Dead amine reactive dead cell stain reagent (Invitrogen).
For phenotyping, cells were incubated with the relevant antibodies diluted in PBS 2% FBS for 30 min on ice, followed by three washes with phosphate buffered saline (PBS) 2% FBS. Secondary staining for FcRL3 detection with streptavidin-Qdot655 was then performed, followed by an additional three washes in PBS 2% FBS. Cells were then fixed in 1% paraformaldehyde (Sigma) for flow cytometric analysis. Intracellular detection of FoxP3 was performed using anti-FoxP3 Pacific Blue (clone PCH101); the accompanying staining kit was provided by eBioscience and was used in accordance with the manufacturer’s protocol. The samples were acquired on an LSRII flow cytometer (BD Biosciences) and all data were analyzed using FlowJo software. Doublet discrimination based on forward scatter height versus area was performed to eliminate cellular aggregates.
RNA isolation and RT-PCR analysis
Total RNA was extracted using the RNeasy mini kit (Qiagen) and cDNA was prepared by reverse transcription using the Omniscript RT kit (Qiagen). Quantitative PCR was performed using FcRL3, FoxP3, or HPRT Taqman gene expression assays with Taqman Universal PCR Mix (Applied Biosystems), according to the manufacturer’s protocol. Amplification of cDNA was performed using the AB Step One Plus instrument (Applied Biosystems); the cycling conditions included a denaturation step for 10 min at 95°C, followed by 40 cycles of denaturation (95°C for 15 sec) and extension (60°C for 1 min).
SNP analysis
1 × 106 PBMC from 29 healthy adult donors were stored as dry pellets at −80°C until analysis. DNA was isolated from the cell pellets using the DNeasy Blood and Tissue kit (Qiagen), according to the manufacturer’s instructions. A PCR based 5′ nuclease genotyping assay was used to specifically discriminate between the −169C and −169T FcRL3 allele polymorphisms. This assay comprised unlabeled forward and reverse PCR primers (900 nM final concentration) and two allele-specific probes labeled with either VIC or FAM reporter dye (200 μM final concentration) (Applied Biosystems). Assay components were added to 20 ng DNA in a 20 μl reaction containing Taqman Universal PCR Mix. An AB Step One Plus instrument was used for amplification and detection, and AB system software was used for analysis.
FACS sorting
CD4+ T cells from buffy coats were pre-enriched by negative selection using the RosetteSep tetrameric complex system (StemCell Technologies) and density centrifugation. Following staining with antibodies for detection of T cell subpopulations, sorting was performed on a BD Aria flow cytometer (BD Biosciences). A lymphocyte gate was set on the basis of forward and side scatter, and both dead cells and doublets were excluded. Live CD3+CD4+CD8− T cells were gated and sorted based on expression of CD25, CD127, and FcRL3 to yield three populations: FcRL3+ Treg (CD25hiCD127loFcRL3+), FcRL3− Treg (CD25hiCD127loFcRL3−), and conventional CD4+ T cells (CD25loCD127hiFcRL3−).
In vitro induction of Treg
96-well round-bottomed culture wells were pre-coated with 0.68 μg/ml each of OKT3, SP34-2, and Hit3α clones of anti-CD3 antibodies (for a total of 2 μg/ml anti-CD3) in PBS for 5 hours at 37°C and then washed to remove excess antibody. Soluble anti-CD28 antibody (BD Biosciences) and recombinant human IL-2 (R&D) were added at a concentration of 1 μg/ml and 50 IU/ml, respectively. Sorted conventional CD4+ T cells were seeded at 100,000 cells/well in the presence of 0, 5, 12.5, or 50 ng/ml of recombinant human latent TGF-β (R&D). For inhibition of TGF-β signaling, the activin receptor–like kinase inhibitor SB-431542 (Sigma) was added at 1 μM.
Proliferation assays and Bcl2 detection
96-well round-bottomed culture wells were pre-coated with 0.68 μg/ml each of OKT3, SP34-2, and Hit3α clones of anti-CD3 antibodies (for a total of 2 μg/ml anti-CD3) in PBS for 5 hours at 37°C and then washed to remove excess antibody. Soluble anti-CD28 antibody (BD Biosciences) and recombinant human IL-2 (R&D) were added at a concentration of 1 μg/ml and 50 IU/ml, respectively. FACS-sorted T cell subpopulations were either stained immediately for Bcl2 as described below or seeded at 100,000 cells/well and incubated for 5 days. IL-2 was supplemented on day 3 at a concentration of 25 IU/ml. Following 5 days of culture, cells were recovered and stained with a live/dead marker, then fixed and permeabilized (Cytofix/Cytoperm, from BD Biosciences) prior to staining for Ki67 (BD Biosciences) or for Bcl2 (Dako), with an isotype control.
Stat-5 phosphorylation analysis
FACS-sorted T cell subpopulations were resuspended in 100 μl R10 medium and pre-warmed to 37°C prior to stimulation with either R10 medium alone or with 50 IU/ml of IL-2 for 30 min. Cells were immediately fixed and then permeabilized (Cytofix and PhosFlow Perm III). The phosphorylation status of Stat-5 was assessed using an anti-phospho-STAT-5 (Y694) antibody coupled to Pacific Blue, according to the manufacturer’s instructions. Control fluorescence was analyzed using a Pacific Blue-coupled control IgG antibody. All reagents were purchased from BD Biosciences.
Treg suppression assays
For stimulation, 96-well round-bottomed culture wells were pre-coated with 0.083 μg/ml each of OKT3 (eBioscience), SP34-2, and Hit3α (BD Biosciences) clones of anti-CD3 antibodies (for a total of 0.25 μg/ml anti-CD3) in PBS for 5 hours at 37°C and then washed to remove excess antibody. To obtain responder cells, PBMC were depleted of Treg using MACS anti-CD25 antibody-conjugated microbeads (Miltenyi Biotec) followed by magnetic column removal of the CD25-expressing cells, according to the manufacturer’s protocol. CD25-depleted PBMC were CFSE labeled by incubation in 1 μM CFSE (Invitrogen), diluted in PBS at a concentration of 10 × 106 cells/ml for 5 min at 37°C, washed three times with R10 medium, and then seeded in anti-CD3 coated wells at a density of 105 cells/well. Autologous FACS-sorted T cell subpopulations were added back at sorted cell: responder ratios varying from 1:5 (20,000 cells) to 1:25 (4,000 cells), volumes were normalized to 200 μl/well with R10 medium, and co-cultures were incubated for 5 days. After this time, cells were recovered, stained with a live/dead marker, and antibodies targeted to CD3, CD4, and CD8 for flow cytometric analysis. CFSE dilution (i.e., proliferation) of responder T cells was measured on live CD3+CD8+CD4− T cells. The frequency of CFSElo cells included all cells that had undergone at least one division.
Statistical analysis
Statistical analysis was performed in Prism software using either paired or unpaired Student’s t-test, as indicated in the text for relevant figures. For comparisons of FcRL3+ and FcRL3− Treg, statistically significant differences are indicated on the figures with asterisks. Results were considered significant at P < 0.05 (*), very significant at P < 0.01 (**), and extremely significant at P < 0.001 (***).
Results
FcRL3 is expressed on a subpopulation of Treg
An earlier microarray study in our laboratory comparing CD4+CD25hi Treg and CD25lo naïve CD4+ T cells revealed that the transcript for FcRL3 is ~100-fold more highly expressed in Treg than in naive CD4+ T cells (unpublished data). To confirm this observation, PCR quantification of transcripts from Treg and naïve CD4+ T cells was conducted for FcRL3 and for the Treg transcription factor, FoxP3 (Figure 1A). FcRL3 transcripts were amplified within the Treg fractions (R) but not from the naïve CD4+ fractions (N).
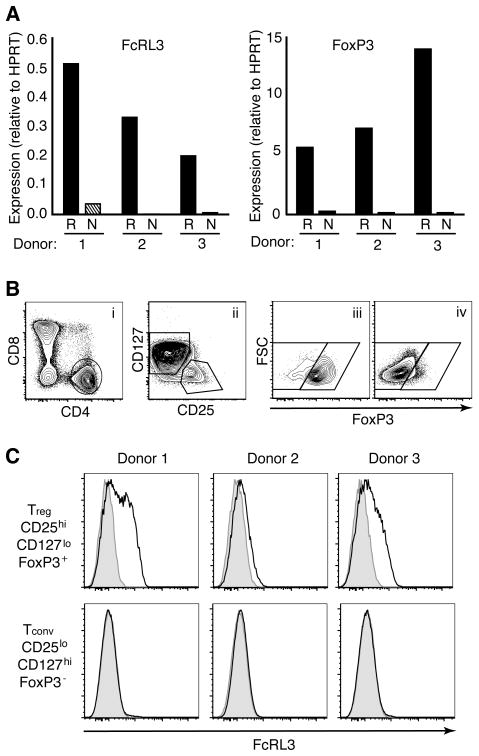
Figure 1. FcRL3 is expressed on a subpopulation of Treg.
A. CD4+CD25hi Treg (R) or CD4+CD25loCD45RA+ naive (N) T cells from three adult donors were FACS sorted and cDNA was analyzed for expression levels of FcRL3 and FoxP3 transcripts. Expression is shown relative to transcript levels of HPRT. B. Flow cytometry gating on live CD3+ adult T cells to determine FcRL3 expression on CD4+ Treg and conventional CD4+ T cells (Tconv). Treg were defined by the combination of CD4+CD8− (i), CD25hiCD127lo (ii) and FoxP3+ (iii) gates. Tconv were defined by the opposite gates, i.e., CD25loCD127hi in (ii) and FoxP3− in (iv). C. Expression levels of FcRL3 (black line) versus control staining (shaded histogram) on Treg or Tconv from three donors. The three donors in (A) do not correspond to the three donors in (C).
To determine the relationship between expression of FcRL3 mRNA and protein on Treg, surface expression levels of FcRL3 were detected by flow cytometry, using an anti-FcRL3 monoclonal antibody (24). Treg or Tconv were phenotyped by flow cytometry according to expression levels of FoxP3, CD25, and CD127 (Figure 1B). CD4+CD8− T cells were initially selected from the live CD3+ population (i). CD4+CD8− T cells were subsequently gated on either CD25hiCD127lo or CD25loCD127hi (ii), and then further segregated according to FoxP3 expression to document subpopulations that were either CD25hiCD127loFoxP3+ (Treg), (iii), or CD25loCD127hiFoxP3− (Tconv), (iv). Within the three donors analyzed, FcRL3 expression was detected on Treg but not on Tconv (Figure 1C). Expression levels on Treg were variable, ranging from approximately half of the Treg expressing FcRL3 in Donor 1 to barely detectable levels in Donor 2. On the highest expressing Donor 1, a separate ‘shoulder’ was evident, indicative of distinct FcRL3+ and FcRL3− populations. We conclude that the autoimmune susceptibility gene FcRL3 is expressed at the protein level on the surface of a subpopulation of CD4+ Treg.
Variability in Treg FcRL3 expression is associated with the −169C/T FCRL3 polymorphism
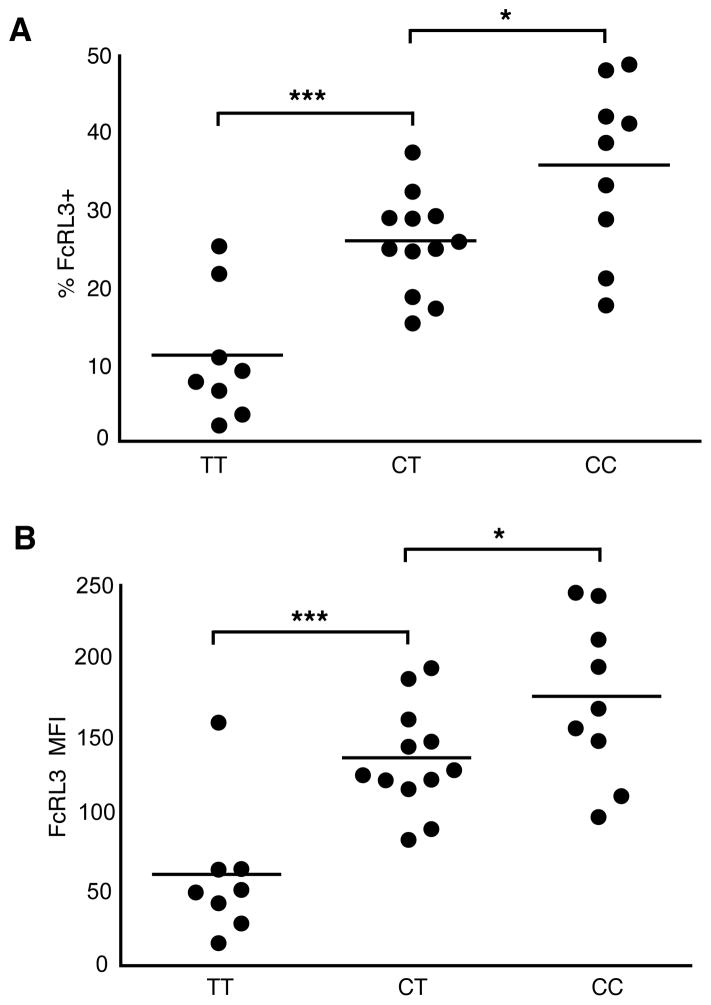
Given evidence that a C/T SNP in the promoter region of FCRL3 (position −169) is associated with expression levels of FcRL3 mRNA (with higher levels found with the C allele) (22), we postulated that this polymorphism was associated with the extreme variability in FcRL3 protein expression on Treg observed in Figure 1C. To test this hypothesis, blood samples were collected from 29 healthy adult donors and screened for FcRL3 expression levels by flow cytometry and for SNP −169C/T FCRL3 genotype by a PCR-based allelic discrimination assay. Regardless of whether the percentage of FcRL3+ Treg (Figure 2A) or the mean fluorescence intensity (MFI) of FcRL3 within Treg (Figure 2B) was analyzed, the data consistently showed significantly higher FcRL3 protein expression for each copy of the C allele present. Similar results have recently been reported in the case of FcRL3 expression on B cells in normal donors and SLE patients (35). Neither the variability in Treg FcRL3 expression, nor the −169C/T FCRL3 polymorphism were found to be significantly correlated with the expression of FoxP3 (Supplemental Figure 2).
Figure 2. Variability in Treg FcRL3 expression is associated with the −169C/T FCRL3 polymorphism.
FcRL3 expression was analyzed by flow cytometry as in Figure 1 in 29 healthy donors and the % FcRL3+ (A) in addition to the FcRL3 MFI (B) within Treg was determined. The FcRL3 MFI was calculated as [FcRL3 MFI – control stain MFI]. PBMC from the same donors were screened for the −169 C/T FCRL3 SNP genotype by a PCR allelic discrimination assay. Donors are grouped according to genotype and shown in the category scatter plots against the % FcRL3+ (A) or FcRL3 MFI (B) within Treg; horizontal bars show the mean of each group. Statistical significance was determined using unpaired Student’s t-test; * indicates that differences were significant (P < 0.05) and *** that differences were extremely significant (P < 0.001).
FcRL3+ Treg are phenotypically distinct from FcRL3− Treg
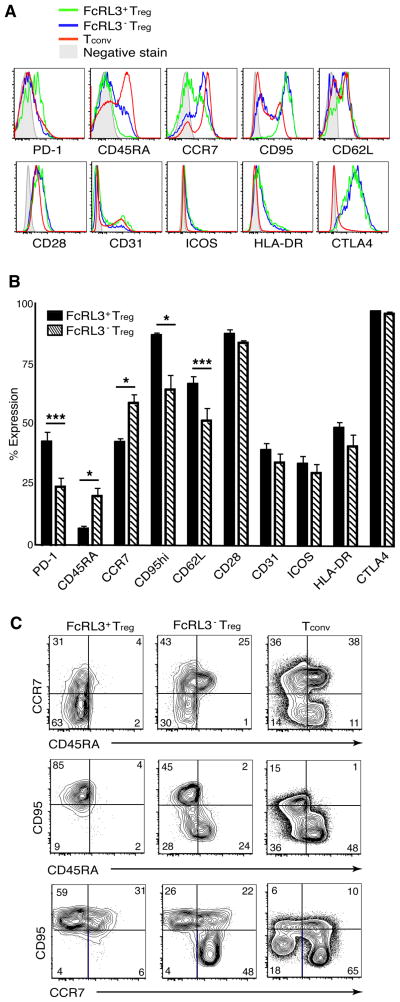
Several studies have suggested that the Treg population is heterogeneous and can be subdivided based on differential expression of markers such as CTLA-4, PD-1, CD45RA, CD62L, and CD278 (ICOS) (36–40). The expression of these and other markers was analyzed on three populations: FcRL3+ Treg, FcRL3− Treg, and Tconv (Figure 3A). PD-1, a marker of T cell exhaustion (40–42), was found to be expressed on nearly twice as many FcRL3+ Treg as FcRL3− Treg (mean 43% versus 24%, P < 0.001, n = 8). This correlated with FcRL3+ Treg exhibiting more of a CD45RA− memory phenotype: while the FcRL3− subpopulation was heterogeneous for CD45RA expression, significantly fewer CD45RA+ cells were detected within the FcRL3+ subpopulation (P < 0.05). FcRL3+ Treg also expressed low levels of CCR7 and were homogeneously high in expression of CD95/Fas whereas their FcRL3− counterparts generally exhibited higher levels of CCR7 and lower levels of CD95/Fas (P < 0.05). CD62L, which is expressed more highly on thymically derived nTreg (37), was expressed at higher levels on the FcRL3+ than on the FcRL3− subpopulation (P < 0.001). Finally, the two Treg subpopulations exhibited similar levels of CD28, CD31, ICOS, HLA-DR, and CTLA-4 (Figures 3A and B). The two parameter dot plots in Figure 3C showing co-expression of CCR7, CD45RA, and CD95/Fas within the Treg and Tconv subpopulations demonstrate that CD45RA+FcRL3− Treg express lower levels of CD95/Fas and higher levels of CCR7. We conclude that FcRL3+ and FcRL3− Treg exhibit distinct phenotypes: while FcRL3− Treg are more heterogeneous and comprise the majority of naïve Treg, the FcRL3+ Treg subpopulation is skewed more towards a memory phenotype expressing higher levels of PD-1.
Figure 3. FcRL3+ Treg are phenotypically distinct from FcRL3− Treg.
PBMC from healthy donors were phenotyped by flow cytometry to determine the expression levels of the indicated markers on FcRL3+ and FcRL3− Treg. A. Representative histograms from individual donors showing expression of the indicated markers on FcRL3+ Treg, FcRL3− Treg, or Tconv. Shaded grey histograms represent FMO control stains. B. Bar graph showing composite data of expression of the indicated markers on FcRL3+ (solid) or FcRL3− (striped) Treg. Data represent the mean +/− SEM from between 4–8 donors for each marker; statistically significant differences by paired Student’s t-test are marked with asterisks; * indicates that differences were significant (P < 0.05) and *** that differences were extremely significant (P < 0.001). C. Representative dot plots showing CD45RA versus CCR7 (top row), CD45RA versus CD95/Fas (middle row), and CCR7 versus CD95/Fas (bottom row) for FcRL3+ Treg, FcRL3− Treg, and Tconv.
FcRL3+ Treg are thymically derived and not inducible in vitro by TGF-β
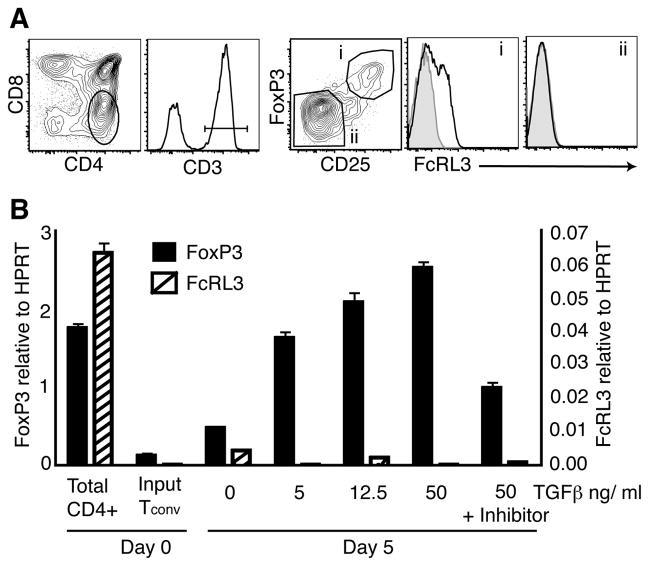
Treg can be generated either in the thymus or in peripheral lymphoid tissues (2). We evaluated fetal thymic Treg (CD25hiFoxP3+) and conventional CD4+CD8−CD3+CD25loFoxP3− single positive CD4+ (SP4) thymocytes for FcRL3 expression (Figure 4A), and found that the expression of FcRL3 was restricted to the Treg thymocyte population (i) with little or no expression on SP4 thymocytes (ii). To determine whether FcRL3+ Treg constitute a greater fraction of Treg in the thymus than in the periphery, paired samples of thymus and either mesenteric lymph node (MLN) or thymus and spleen were analyzed from the same fetal donor. Consistently, thymic Treg contained a higher fraction of FcRL3+ cells than Treg found in either the MLN (Supplemental Figure 3A) or the spleen (3B). Almost twice as many Treg expressed FcRL3 in the fetal thymus (32%) compared to Treg from peripheral lymphoid tissues (17%; MLN or spleen) in the six donors analyzed (P < 0.01).
Figure 4. FcRL3+ Treg are thymically derived and not inducible in vitro by TGF-β.
A. Flow cytometric analysis of a representative second trimester human fetal thymus showing FcRL3 expression on thymic Treg. Single positive CD4+ (SP4) cells were gated from total live thymocytes, subsequently selected for expression of CD3, and then Treg and conventional SP4 thymocytes were defined by FoxP3 and CD25 expression (gates i and ii, respectively). FcRL3 expression (black lines) and control stains (shaded histograms) are shown for these gates. B. Tconv were FACS sorted from total CD4+ T cells, and then cultured for 5 days with anti-CD3 and anti-CD28 antibodies (αCD3/CD28) and increasing concentrations of TGF-β. At the highest concentration of TGF-β, the TGF-β inhibitor SB-431542 was also added to the culture medium. At the end of the 5-day culture period, RT-PCR was performed to determine transcript levels of FoxP3 and FcRL3. Transcripts were normalized to HPRT and are expressed as the mean +/− SD of triplicate samples.
Peripheral FoxP3+ Treg can be induced by TCR activation in the presence of TGF-β (43). To assess whether FcRL3+ Treg can be induced from Tconv in this manner, sorted CD25loCD127hiCD4+ T cells were stimulated for 5 days with anti-CD3 and anti-CD28 antibodies (αCD3/CD28), IL-2, and increasing concentrations of TGF-β. The resulting cell cultures were then screened for FcRL3 and FoxP3 transcripts by RT-PCR. TGF-β treatment was clearly able to induce transcription of FoxP3 but not FcRL3 (Figure 4B). In aggregate, these data indicate that FcRL3+ Treg are thymically derived and that FoxP3 expression per se is not sufficient to induce FcRL3 expression.
FcRL3+ Treg exhibit defective proliferation in response to αCD3/CD28 activation in the presence of IL-2
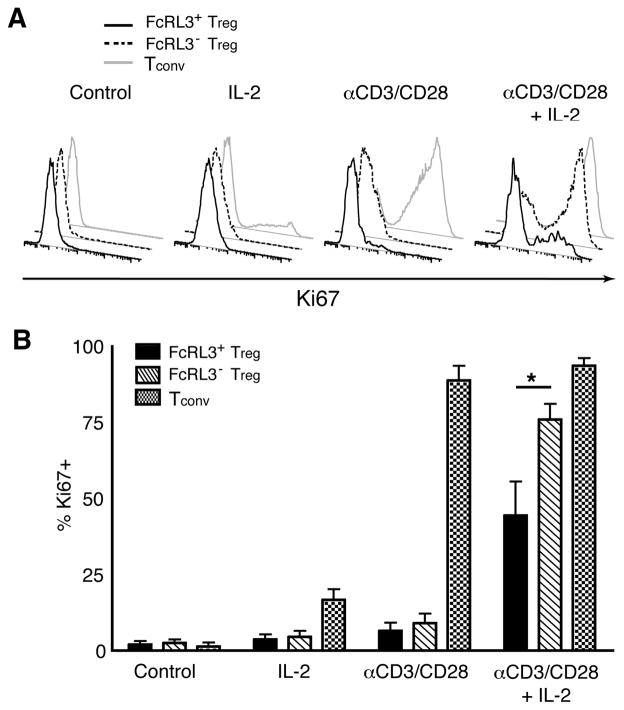
Numerous lines of evidence indicate that PD-1 expression on T cells is associated with limited proliferative capacity (40–42,44,45). Since the FcRL3+ Treg population expressed significantly higher levels of PD-1 compared to the population of FcRL3− Treg (Figures 3A and 3B), we postulated that FcRL3+ Treg would demonstrate defective proliferation compared to their FcRL3− counterparts when provided TCR stimulation in the presence of IL-2 (12). FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were cultured for 5 days with medium alone, IL-2, αCD3/CD28, or a combination of αCD3/CD28 and IL-2. The purity of sorted populations prior to activation is represented in Supplemental Figure 1. As shown in the scatter plots of Supplemental Figure 4, no blast formation was observed in any subpopulation after culture in medium alone or in the presence of IL-2, while αCD3/CD28 activation resulted in blast formation in Tconv but not in either Treg subpopulation. The addition of IL-2 to αCD3/CD28 stimulation (far right column) induced blast formation in Tconv and FcRL3− Treg but to a lesser degree in FcRL3+ Treg, which exhibited a reduced cell size compared to FcRL3− Treg as seen by the forward scatter (FSC) parameter (mean FSC 62700 versus 67000, respectively, n = 4).
To more accurately detect the induction of cell cycling, cells were stained for the proliferation antigen, Ki67 (Figure 5A). No Ki67+ cells were detected in any subpopulation after culture in medium alone and only a small proportion (22%) of Tconv were Ki67+ after IL-2 stimulation. αCD3/CD28 activation resulted in Ki67 expression in nearly all of Tconv (83%), whereas less than 15% of the Treg populations were Ki67+ under this condition. The addition of IL-2 to αCD3/CD28 activation was sufficient to induce Ki67 in the majority of FcRL3− Treg (64%) whereas only half this number stained positive for the proliferation antigen in the FcRL3+ subpopulation (32%). The composite data from four different donors shown in Figure 5B indicates that the hypoproliferation of FcRL3+ Treg versus FcRL3− Treg in response to αCD3/CD28 + IL-2 is significant (P < 0.05), confirming the hypothesis that FcRL3+ Treg are relatively defective in their proliferative responses.
Figure 5. FcRL3+ Treg exhibit defective proliferation in response to αCD3/CD28 activation in the presence of IL-2.
FcRL3+ Treg, FcRL3− Treg, and Tconv were FACS sorted from adult PBMC and cultured in the presence of R10 medium alone, IL-2, αCD3/CD28 antibodies, or a combination of αCD3/CD28 antibodies and IL-2. After 5 days, cells were fixed, permeabilized, and stained for the intracellular proliferation antigen, Ki67. A. Histogram overlays of Ki67 expression from one representative donor. B. Composite data from 3 separate experiments comprising 4 different donors showing the mean +/− SEM of % Ki67+ cells; statistical significance comparing FcRL3+ and FcRL3− Treg was determined using paired Student’s t-test; * indicates that differences were significant (P < 0.05).
FcRL3+ and FcRL3− Treg demonstrate intact proximal and distal signaling responses to IL-2
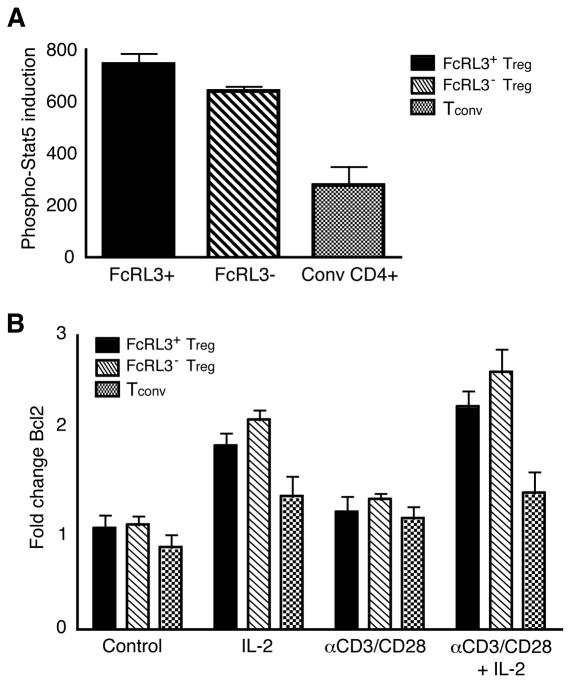
To ascertain whether FcRL3+ Treg are refractory to a stimulus from IL-2, both proximal and distal readouts of IL-2 signaling were monitored. The transcription factor, Stat-5, is rapidly phosphorylated following IL-2 binding to the IL-2 receptor, resulting in Stat-5 dimerization and nuclear entry to facilitate transcription of IL-2 response genes, including Bcl2 (46). FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were stimulated with either IL-2 or medium alone for 30 minutes and stained intracellularly using an antibody specific to a phosphorylated epitope at the tyrosine 694 residue of Stat-5 (P-Stat-5). Figure 6A shows composite data of P-Stat-5 induction by IL-2 from three individual donors for each T cell subpopulation. As expected, IL-2 induced higher levels of P-Stat-5 in both Treg subpopulations compared to Tconv (47). FcRL3+ and FcRL3− Treg, demonstrated similar levels of Stat-5 phosphorylation in response to IL-2 (P > 0.05).
Figure 6. FcRL3+ and FcRL3− Treg demonstrate intact proximal and distal signaling responses to IL-2.
A. FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were incubated with either medium alone or IL-2 for 30 minutes, fixed, and then stained for Stat-5 using an antibody specific for the phosphorylated Y694 Stat-5 epitope. The specific induction of phospho-Stat-5 is shown (MFI after IL-2 stimulation minus MFI after R-10 medium alone); data represent mean +/− SEM of 3 separate experiments comprising 3 different donors. B. FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were cultured in the presence of medium alone, IL-2, αCD3/CD28 antibodies, or a combination of αCD3/CD28 antibodies and IL-2. After 5 days, cells were fixed, permeabilized, and stained for Bcl2. The fold change in Bcl2 expression from day 0 to analysis at day 5 is shown; data represent mean +/− SEM of 3 separate experiments comprising 4 different donors.
To assay distal IL-2 signaling responses, FACS-sorted T cell subpopulations were stimulated under the same conditions as in Figure 5 and then stained for Bcl2. Figure 6B shows composite data from four different donors of the fold change in Bcl2 levels from day 0 to day 5. In response to IL-2, Bcl2 was upregulated on both FcRL3+ Treg and FcRL3− Treg (P < 0.01). Both Treg subpopulations also showed significant upregulation of Bcl2 in response to the combination of αCD3/CD28 + IL-2 compared to αCD3/CD28 alone (P < 0.01 for FcRL3+ Treg and P < 0.05 for FcRL3− Treg). There was, however, no significant difference in Bcl2 upregulation between FcRL3+ and FcRL3− Treg (P > 0.05). These data demonstrate that FcRL3+ Treg have intact proximal and distal IL-2 signaling responses that are comparable in magnitude to FcRL3− Treg.
FcRL3+ Treg are dysfunctional in their ability to suppress Teff proliferation
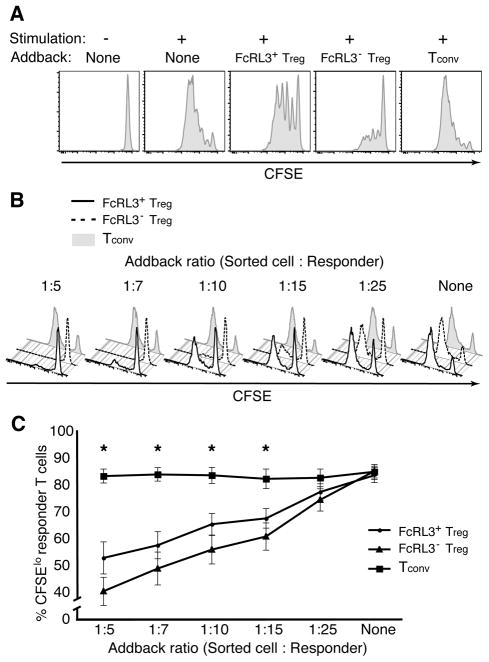
Given that FcRL3+ Treg exhibit an exhausted phenotype with high levels of PD-1 (Figure 3) and defective proliferative responses (Figure 5) compared to FcRL3− Treg, we postulated that they would be dysfunctional in their regulatory capacity, i.e., the ability to suppress the proliferation of Teff. To test this hypothesis, FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were added to CFSE-labeled autologous responders in a co-culture proliferation assay. The addition of FcRL3+ Treg was found to suppress the proliferation of CD8+ Teff to a far lesser extent than the addition of FcRL3− Treg (Figure 7A). As expected, the addback of Tconv had no effect.
Figure 7. FcRL3+ Treg are dysfunctional in their ability to suppress Teff cell proliferation.
FACS-sorted FcRL3+ Treg, FcRL3− Treg, and Tconv were added back to CFSE-labeled responder T cells (CD25-depleted PBMC). Co-cultures were stimulated with αCD3 antibodies for 5 days, stained with antibodies directed against CD3, CD4, and CD8, and then analyzed by flow cytometry for CFSE dilution of CD8+ T cells. A. CFSE dilution of Teff after five days co-culture with the indicated addback populations. Unstimulated responder T cells are shown as a control in the left histogram. B. CFSE dilution of responder T cells after five days co-culture with the indicated addback populations at varying ratios of sorted cells to responders. C. Composite data showing the frequency of divided (CFSElo) responder cells at each addback ratio from 7 separate experiments comprising 14 different donors. Data represent mean +/− SEM of the 14 individuals for each ratio; statistically significant differences (P < 0.05) between FcRL3+ Treg and FcRL3− Treg by paired Student’s t-test are marked with an asterisk (*).
For some donors, the addback ratio used in Figure 7A (1:5) resulted in complete inhibition of Teff proliferation by both Treg populations (e.g., see left panel of Figure 7B). To better observe differences in the suppressive function of FcRL3+ and FcRL3− Treg subpopulations, the same suppression assay was performed with cells from 14 individual donors, using different ratios of sorted addback to responder cells ranging from 1:5 to 1:25 (Figures 7B and C). The CFSE profiles of one representative donor (Figure 7B) show that, as the proportion of Treg in the co-culture diminishes, the proliferation of the responders increases. Notably, for ratios at which differences in the suppressive capacity of FcRL3+ and FcRL3− Treg can clearly be observed (i.e., at 1:10 and 1:15 addback ratios for this donor), the FcRL3+ subpopulation was less able to inhibit responder T cell proliferation.
Composite data from all 14 donors are shown in Figure 7C, demonstrating that FcRL3+ Treg are less capable of suppressing proliferation at every addback ratio from 1:5 to 1:15. Accordingly, FcRL3+ Treg appear to be dysfunctional, exhibiting a reduced capacity to suppress responder T cell proliferation compared to their FcRL3− counterparts.
Discussion
We report here that FcRL3 is expressed on a subpopulation of human CD4+CD25hiFoxP3+ Tregs, with inter-individual variation in expression that correlates with a SNP at promoter position 169. FcRL3+ Treg appear to emanate from the thymus and express a unique phenotype characteristic of “exhausted” memory T cells. Although they can signal across the IL-2 receptor, FcRL3+ Treg demonstrate a proliferative defect and are also less capable of suppressing Teff proliferation in vitro relative to their FcRL3− Treg counterparts. Given the many reports linking expression of FcRL3 with various autoimmune disorders, we speculate that such disorders may be due in part to FcRL3-mediated inhibition of Treg function.
Dysfunctional suppressive ability of FcRL3+ Treg
These data are consistent with those reported by Nagata et al (48), especially with respect to the proliferative defect of FcRL3+ Treg in response to antigenic stimulation in the presence of IL-2. We show here, however, that FcRL3+ and FcRL3− Treg exhibit differences in phenotype and function. Most importantly, and in contrast to the data from Nagata et al, our study demonstrates that FcRL3+ Treg are dysfunctional and do not inhibit the proliferation of responder T cells to the same extent as do FcRL3− Treg. It is possible that this difference in results is reflective of the heterogeneity of FcRL3 expression on Treg and the fact that we screened cells from a large number of donors (14 as compared to three in Nagata et al). However, it is also important to consider that different Treg suppression assays were used in our experiments and that methodologic distinctions may have also played a role.
The studies conducted by Nagata et al used bead-coupled anti-CD3 and anti-CD28 antibodies to stimulate highly purified CD4+CD25loCD127hi Teff in the complete absence of APC. In our experiments, a more physiologic suppression assay closer to mimicking in vivo cellular interactions was employed. Specifically, CD25-depleted autologous PBMC were utilized as responders with anti-CD3 antibodies supplying the exogenous stimulation. The use of CD25-depleted PBMC provides a more physiologically representative mixture of cells, including APC, that can directly or indirectly influence or mediate Treg function (2,13–16). Indeed, it is becoming increasingly recognized within the Treg community that different in vitro suppression assays may measure different aspects of Treg cell-suppressor function (15). It is also noteworthy that we analyzed suppression exclusively on CD8+ Teff, whereas Nagata et al measured suppression on CD4+ Teff. Assessing suppression on CD4+ Teff can be problematic as, after 4–5 days proliferation, the diluted CFSE signal on CD4+ Teff can begin to overlap with the negative CFSE signal of unlabeled CD4+ Treg addback cells. Contamination of the CD4+ Teff CFSElo/− cells with CD4+ Treg CFSE− cells and skewing of the data may therefore occur. We analyzed exclusively CD8+ Teff to avoid this potential problem, such that CFSE would only be measured on pure effector cells.
It is interesting to speculate why FcRL3+ Treg have diminished suppressor activity. Treg-mediated suppression may involve a number of mechanisms of action attributed to Treg cells. These can be broadly divided into those that target T cells (e.g., suppressor cytokines, IL-2 consumption, and cytolysis) and those that primarily target APC (e.g., decreased co-stimulation or decreased antigen presentation). APC may also modulate the activity of Treg directly or indirectly (15,16). Given the discrepancies between our data and those of Nagata et al, it would be logical to speculate that the presence of APC in our suppression assay likely allowed the unmasking of differences in function between FcRL3+ and FcRL3− Treg. Interestingly, we find that the FcRL3+ Treg population expresses higher levels of PD-1. PD-1 acts as an inhibitory molecule on Tconv and Treg after interacting with its ligands, PDL1 and PDL2, expressed primarily on activated dendritic cells and macrophages, cell types that are present in our suppressor assay (44). PD-1 is upregulated on exhausted T cells and plays a causative role in limiting proliferation and other functions such as cytokine secretion and cytolytic activity upon ligation by PDL1/2 (40–42,44,45).
Other mechanisms may be at work in preventing FcRL3+ Treg from suppressing Teff expansion. FcRL3+ Treg also exhibit higher levels of the apoptosis-inducing TNF-R family member, CD95/Fas, than FcRL3− Treg, suggestive of an increased predisposition to cell death. This is supported by a recent article demonstrating that high expression of PD-1 is associated with increased spontaneous and CD95/Fas induced apoptosis in T cells (49). A reduced number of active suppressor cells due to increased apoptosis could also account for the dysfunction observed in FcRL3+ Treg. It would be interesting to determine whether siRNA knockdown of FcRL3+ Treg modulates the suppressive function of these cells, as it is presently unknown whether this functional defect is caused by, or merely associated with, FcRL3 expression.
Purified FcRL3+ Treg exhibit defective proliferation
While FcRL3+ Treg demonstrate reduced suppressive ability in the presence of activated PBMC, purified FcRL3+ Treg exhibit defective proliferation in response to αCD3/CD28 activation in the presence of IL-2 without the presence of any other cell types ((48) and Figure 5). Although Nagata et al attributed the reduced expansion of FcRL3+ Treg to IL-2 non-responsiveness, we ascertained that these cells exhibit intact proximal and distal signaling responses to IL-2 (as determined by phosphorylation of Stat-5 and upregulation of Bcl2, respectively). Therefore, it is more likely that FcRL3+ Treg have a defect in their ability to respond to αCD3/CD28 activation. Consistent with this hypothesis, PD-1hi T cells are known to have reduced proliferative capacity and to possess shorter telomeres than PD-1lo T cells (50,51). Moreover, our analysis of six donors showed a trend for FcRL3+ Treg to express lower levels of cyclin B2 transcripts than FcRL3− Treg (data not shown). Future experiments to determine whether blocking antibodies to PD-1 restore the proliferative defect in FcRL3+ Treg may establish whether this molecule is itself a causative factor in FcRL3+ Treg defective proliferation.
Thymic development of FcRL3+ Treg
Our analysis of fetal tissues indicates that FcRL3 is expressed by a subpopulation of CD4+FoxP3+CD25hi SP4 thymocytes, but not by FoxP3+ Treg induced from conventional T cells by TCR stimulation and TGF-β. These observations suggest that FcRL3 may only be present on thymically-derived nTreg and not on peripherally induced iTreg. Despite their detection in the thymus, the FcRL3+ Treg subpopulation exhibits a memory phenotype with reduced CD45RA and CCR7 expression, suggestive of more rapid memory conversion upon migration to the periphery than FcRL3− nTreg. The switch from naïve to memory phenotype in T cells is associated with proliferation and activation triggered by recognition of cognate antigen, suggesting that FcRL3+ Treg may recognize self-antigen with greater specificity or affinity than their FcRL3− counterparts. As has been observed in the case of PD-1 upregulation in the context of antigenic stimulation (40–42), chronic stimulation of FcRL3+ Treg by autoantigens could result in the exhausted phenotype and the responses observed in this study.
Perspectives: FcRL3 as a contrasuppressor?
Given the association between various autoimmune diseases and the −169 C/T SNP that leads to overexpression of FcRL3, any discussion of FcRL3 expression on Treg must also consider the mechanisms by which this protein may have a causal impact on aberrant immune activation. In vitro signaling assays have demonstrated that immunotyrosine inhibitory motifs (ITIMs) in the cytosolic domain of FcRL3 can be phosphorylated and bind to SHP family phosphatases, proteins known to function as negative regulators in TCR signaling (52). In addition, FcRL5, a closely related FcRL family member, was shown to inhibit B cell activation via SHP-1 tyrosine phosphatase recruitment (53). Another FcRL member, FcRL4, acts as a negative regulator of BCR signaling (54). FcRL4 expression is associated with exhaustion in dysfunctional memory B cells and poor proliferation in response to B cell stimuli, consistent with high-level expression of multiple inhibitory receptors (55). We observe striking similarities in the phenotype and function of the FcRL3+ Treg.
Given these findings, we hypothesize that FcRL3 may negatively regulate TCR signaling in Treg cells. A recent publication supports this hypothesis with the demonstration that co-ligation of the FcRL3 intracellular domain in a B cell line inhibited tyrosine phosphorylation and calcium mobilization mediated by BCR signaling (56). It is, however, difficult to reconcile high expression of a potential inhibitory molecule like FcRL3 on B cells with predisposition to autoimmunity, particularly as it is low expression of inhibitory molecules such as FcγRIIB on B cells that increases the risk for loss of self-tolerance (57,58). A role for FcRL3 as an inhibitory co-receptor is more readily explained in the context of its link with autoimmune manifestations if it acts as a negative regulator of otherwise immunosuppressive T cell functions. Treg can have both beneficial and deleterious effects. Although Treg prevent autoimmune responses and excessive inflammation, they can also suppress useful immunity and thus, in turn, require regulation and brakes to restrict their effects. As Walter B. Cannon, the father of the concept of homeostasis, declared in 1932: “When a factor is known which can shift a homeostatic state in one direction, it is reasonable to look for a factor or factors having an opposing effect” (59). As early as 1981, Gershon adopted this concept in the arena of regulatory T cells, postulating that “contrasuppressor” T cells may control the activity of “suppressor” T cells (60). Additional precedents exist for negative regulators of Treg, such as PD-1 and TLR8-mediated inhibition of Treg function (40,61). We accordingly postulate that FcRL3 represents a novel mechanism of “contrasuppression” which, under conditions of high expression induced by the −169 C/T SNP, contributes to greater inhibition of Treg function, higher levels of immune activation, and the loss of tolerance to self.
In sum, the results of this study demonstrate that Treg expressing the FcRL3 autoimmune susceptibility gene exhibit an exhausted memory phenotype and aberrant suppressive function. Future elucidation of FcRL3+ Treg differentiation, signaling, and function will likely expand upon our understanding of immune tolerance and homeostasis, and may create opportunities for the development of new therapeutic interventions in disease settings.
Supplementary Material
Acknowledgments
Support for this work was provided by grants from the National Institutes of Health, including R37 AI40312 and DPI OD00329 to J.M.M., the UCSF-GIVI Center for AIDS Research (P30 MH59037), and the UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131). Support was also provided by the Harvey V. Berneking Living Trust. L.S. is supported by the Human Frontier Science Program (HFSP). U.B. is a UCSF Rheumatology Fellow and is funded by a NIAMS Academic Rheumatology Training Grant and the Rosalind Russell Arthritis Center. J.M.M. is a recipient of the NIH Director’s Pioneer Award Program, part of the NIH Roadmap for Medical Research, through grant DPI OD00329.
We are grateful to Dr. Andy Polson and Genentech for kindly providing the anti-FcRL3 antibody, without which this study would not have been possible. Helpful assistance in FACS sorting was provided by Bill Hyun and Terence Ho of the DEM flow core. We also wish to thank members of the McCune lab for valuable comments and suggestions throughout the course of this work and for critical review of the manuscript.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 7.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 9.Hartigan-O’Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 14.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 15.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, Baskin CR, Palermo R, Pandrea I, Miller CJ, Katze MG, McCune JM. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartigan-O’Connor DJ, Abel K, McCune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J Exp Med. 2007;204:2679–2692. doi: 10.1084/jem.20071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–5748. doi: 10.4049/jimmunol.176.10.5741. [DOI] [PubMed] [Google Scholar]
- 20.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis RS. Fc receptor-like molecules. Annu Rev Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 22.Kochi Y, Yamada R, Suzuki A, Harley JB, Shirasawa S, Sawada T, Bae SC, Tokuhiro S, Chang X, Sekine A, Takahashi A, Tsunoda T, Ohnishi Y, Kaufman KM, Kang CP, Kang C, Otsubo S, Yumura W, Mimori A, Koike T, Nakamura Y, Sasazuki T, Yamamoto K. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat Genet. 2005;37:478–485. doi: 10.1038/ng1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller I, Hatzivassiliou G, Cattoretti G, Mendelsohn C, Dalla-Favera R. IRTAs: a new family of immunoglobulinlike receptors differentially expressed in B cells. Blood. 2002;99:2662–2669. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- 24.Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, Yen L, Tan C, Hongo JA, Koeppen H, Ebens A. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 25.Ikari K, Momohara S, Nakamura T, Hara M, Yamanaka H, Tomatsu T, Kamatani N. Supportive evidence for a genetic association of the FCRL3 promoter polymorphism with rheumatoid arthritis. Ann Rheum Dis. 2006;65:671–673. doi: 10.1136/ard.2005.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YH, Woo JH, Choi SJ, Ji JD, Song GG. Fc receptor-like 3 −169 C/T polymorphism and RA susceptibility: a meta-analysis. Rheumatol Int. 2009 doi: 10.1007/s00296-009-1082-5. [DOI] [PubMed] [Google Scholar]
- 27.Mao C, Pan H, Chen Q, Wang X, Ye D, Qiu L. Association between Fc receptor-like 3 C169T polymorphism and risk of systemic lupus erythematosus: a meta-analysis. Mol Biol Rep. 2009 doi: 10.1007/s11033-009-9591-6. [DOI] [PubMed] [Google Scholar]
- 28.Thabet MM, Wesoly J, Slagboom PE, Toes RE, Huizinga TW. FCRL3 promoter 169 CC homozygosity is associated with susceptibility to rheumatoid arthritis in Dutch Caucasians. Ann Rheum Dis. 2007;66:803–806. doi: 10.1136/ard.2006.064949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium (TASC); Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Ferreira T, Pereira-Gale J, Hallgrimsdo’ttir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M Biologics in RA Genetics and Genomics Study Syndicate (BRAGGS) Steering Committee; Breast Cancer Susceptibility Collaboration (UK) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begovich AB, Chang M, Schrodi SJ. Meta-analysis evidence of a differential risk of the FCRL3 −169T-->C polymorphism in white and East Asian rheumatoid arthritis patients. Arthritis Rheum. 2007;56:3168–3171. doi: 10.1002/art.22857. [DOI] [PubMed] [Google Scholar]
- 31.Chistiakov DA, Chistiakov AP. Is FCRL3 a new general autoimmunity gene? Hum Immunol. 2007;68:375–383. doi: 10.1016/j.humimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Martínez A, Sánchez E, Valdivia A, Orozco G, López-Nevot MA, Pascual-Salcedo D, Balsa A, Fernández-Gutiérrez B, de la Concha EG, García-Sánchez A, Koeleman BP, Urcelay E, Martín J. Epistatic interaction between FCRL3 and NFkappaB1 genes in Spanish patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1188–1191. doi: 10.1136/ard.2005.048454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez A, Núñez C, Martín MC, Mendoza JL, Taxonera C, Díaz-Rubio M, de la Concha EG, Urcelay E. Epistatic interaction between FCRL3 and MHC in Spanish patients with IBD. Tissue Antigens. 2007;69:313–317. doi: 10.1111/j.1399-0039.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 34.Newman WG, Zhang Q, Liu X, Walker E, Ternan H, Owen J, Johnson B, Greer W, Mosher DP, Maksymowych WP, Bykerk VP, Keystone EC, Amos CI, Siminovitch KA. Rheumatoid arthritis association with the FCRL3 −169C polymorphism is restricted to PTPN22 1858T-homozygous individuals in a Canadian population. Arthritis Rheum. 2006;54:3820–3827. doi: 10.1002/art.22270. [DOI] [PubMed] [Google Scholar]
- 35.Gibson AW, Li FJ, Wu J, Edberg JC, Su K, Cafardi J, Wiener H, Tiwari H, Kimberly RP, Davis RS. The FCRL3 −169CT promoter single-nucleotide polymorphism, which is associated with systemic lupus erythematosus in a Japanese population, predicts expression of receptor protein on CD19+B cells. Arthritis Rheum. 2009;60:3510–3512. doi: 10.1002/art.24915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–39. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radziewicz H, Dunham RM, Grakoui A. PD-1 tempers Tregs in chronic HCV infection. J Clin Invest. 2009;119:450–453. doi: 10.1172/JCI38661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 42.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 45.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 46.Lord JD, McIntosh BC, Greenberg PD, Nelson BH. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 47.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagata S, Ise T, Pastan I. Fc receptor-like 3 protein expressed on IL-2 nonresponsive subset of human regulatory T cells. J Immunol. 2009;182:7518–7526. doi: 10.4049/jimmunol.0802230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrovas C, Chaon B, Ambrozak DR, Price DA, Melenhorst JJ, Hill BJ, Geldmacher C, Casazza JP, Chattopadhyay PK, Roederer M, Douek DC, Mueller YM, Jacobson JM, Kulkarni V, Felber BK, Pavlakis GN, Katsikis PD, Koup RA. Differential association of programmed death-1 and CD57 with ex vivo survival of CD8+ T cells in HIV infection. J Immunol. 2009;183:1120–1132. doi: 10.4049/jimmunol.0900182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hokey DA, Johnson FB, Smith J, Weber JL, Yan J, Hirao L, Boyer JD, Lewis MG, Makedonas G, Betts MR, Weiner DB. Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol. 2008;38:1435–1445. doi: 10.1002/eji.200737857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichterfeld M, Mou D, Cung TD, Williams KL, Waring MT, Huang J, Pereyra F, Trocha A, Freeman GJ, Rosenberg ES, Walker BD, Yu XG. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008;112:3679–3687. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu MJ, Zhao R, Cao H, Zhao ZJ. SPAP2, an Ig family receptor containing both ITIMs and ITAMs. Biochem Biophys Res Commun. 2002;293:1037–1046. doi: 10.1016/S0006-291X(02)00332-7. [DOI] [PubMed] [Google Scholar]
- 53.Haga CL, Ehrhardt GR, Boohaker RJ, Davis RS, Cooper MD. Fc receptor-like 5 inhibits B cell activation via SHP-1 tyrosine phosphatase recruitment. Proc Natl Acad Sci U S A. 2007;104:9770–9775. doi: 10.1073/pnas.0703354104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehrhardt GR, Davis RS, Hsu JT, Leu CM, Ehrhardt A, Cooper MD. The inhibitory potential of Fc receptor homolog 4 on memory B cells. Proc Natl Acad Sci U S A. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kochi Y, Myouzen K, Yamada R, Suzuki A, Kurosaki T, Nakamura Y, Yamamoto K. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J Immunol. 2009;183:5502–5510. doi: 10.4049/jimmunol.0901982. [DOI] [PubMed] [Google Scholar]
- 57.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 58.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannon . The Wisdom of the Body. w.w. Norton; New York: 1932. [Google Scholar]
- 60.Gershon RK, Eardley DD, Durum S, Green DR, Shen FW, Yamauchi K, Cantor H, Murphy DB. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981;153:1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–1384. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.