Abstract
Understanding stopover decisions of long-distance migratory birds is crucial for conservation and management of these species along their migratory flyway. Recently, an increasing number of Barnacle geese breeding in the Russian Arctic have delayed their departure from their wintering site in the Netherlands by approximately one month and have reduced their staging duration at stopover sites in the Baltic accordingly. Consequently, this extended stay increases agricultural damage in the Netherlands. Using a dynamic state variable approach we explored three hypotheses about the underlying causes of these changes in migratory behavior, possibly related to changes in (i) onset of spring, (ii) potential intake rates and (iii) predation danger at wintering and stopover sites. Our simulations showed that the observed advance in onset of spring contradicts the observed delay of departure, whereas both increased predation danger and decreased intake rates in the Baltic can explain the delay. Decreased intake rates are expected as a result of increased competition for food in the growing Barnacle goose population. However, the effect of predation danger in the model was particularly strong, and we hypothesize that Barnacle geese avoid Baltic stopover sites as a response to the rapidly increasing number of avian predators in the area. Therefore, danger should be considered as an important factor influencing Barnacle goose migratory behavior, and receive more attention in empirical studies.
Introduction
In migratory species, flexibility allows dealing with a continuously changing environment. Illustratively, Sutherland [1] presented an overview of bird species that showed flexibility in their migratory behavior to changing environmental conditions. He described changes in the use of wintering, breeding and staging areas, occurring in a wide range of families. Recently, Jonzén et al. (2006) suggested a climate-driven evolutionary change in the timing of spring migration for a number of long-distance passerine migrants [2] but see [3]. Changes in migration can also be caused by factors other than climate. Gill et al. [4] for example, showed that an increasing population of Black-tailed godwits Limosa limosa islandica, wintering in the UK, established new wintering sites on less suitable sites than the original wintering sites. They suggested that the carrying capacity of the original sites was reached, forcing the Black-tailed godwits to winter elsewhere. Additionally, Klaassen et al. [5] adopted a dynamic state variable model and showed that Pink-footed geese Anser brachyrhynchus respond to scaring practices by farmers in Norway by changing their use of stopover sites. Alerstam & Lindström [6] discussed minimization of time, energy and predation during migration as the main drivers of evolution in migratory behavior. The aforementioned examples of migratory change might represent responses to changes in one or more of these factors. Identifying possible causes of these changes, is essential for understanding flexibility in migratory behavior.
Since the early 1990s, an increasing number of Barnacle geese Branta leucopsis breeding in the Russian Arctic have delayed their departure from their wintering site in the Netherlands by approximately one month. The geese reduced their staging duration in the next stopover area in the Baltic (traditionally used by the entire population) according to the delay from the Netherlands, such that some migrants virtually skip the Baltic stopover site altogether [7], [8]. Because of these changes, the question arose what has caused the delayed departure from the wintering site and decreased use of the Baltic stopover site. Compared to changes in (migration) phenology in other bird species [2], [9], [10], [11], the rate of change of approximately 3 days/year as observed in the Barnacle goose is unprecedently large. One important consequence of the delayed migration of Barnacle geese is an increased agricultural damage in the Netherlands of approximately €350,000 annually, and this figure is growing rapidly [12]. Successful management actions require the identification of factors and processes affecting departure and staging decisions. Therefore, we have formulated three possible explanations for the delay: Barnacle geese have delayed their departure as a consequence of changes in (i) onset of spring, (ii) potential food intake rates, and (iii) predation danger [13].
(i) Advanced onset of spring
Recently, several studies have found that migratory birds responded to climate-driven changes in plant phenology with advanced laying dates [14], advanced spring arrival dates [2], [10], [15] or increased rate of spring migration [9]. Climate change could result in higher spring temperatures in some regions, leading to earlier growth of the vegetation. Barnacle geese are thought to schedule their migration according to the “green wave” of fresh plant growth along the flyway [16]. However, this relationship might not be that straightforward, because geese may prioritize other factors, such as safety or food quality. Therefore, the potential effect of onset of spring is investigated in this study.
(ii) Decreased intake rate
The potential intake rate at a stopover site, i.e. the intake per day a goose can gain if foraging at maximum intensity, limits the rate at which geese can replenish their energy reserves [17]. Earlier studies have shown that decreased availability and reduced quality of food can make a stopover site less attractive [18]. Van der Graaf [19] reported lower intake rates in the Baltic as compared to the Netherlands. Moreover, as the total population of Barnacle goose passing through the Baltic has increased drastically over the past thirty years [20], the competition for food at the Baltic stopover site may also have intensified [21]. Additionally, desertion of farmland, and thus reduced facilitation by cattle grazing, in these regions may also have decreased intake rates [22]. For these reasons, decreased potential intake rates at the Baltic stopover site may cause Barnacle geese to reduce staging time or even completely skip this site. Then, the geese could fly directly to one of the next stopover sites in Russia; however, since food there becomes available only later in spring, they have to delay their departure from the Netherlands until spring starts in the arctic stopover sites in Russia.
(iii) Increased predation danger
Increased predation danger can reduce the attractiveness of a site because of its lethal and non-lethal effects [23], [24]. Although safety has long been acknowledged as potentially important for successful migration [6], it has received little attention so far and the few studies on the impact of predation danger on migration have not led to unambiguous conclusions [25], [26]. While a number of studies indeed demonstrated the effects of predators on body mass, stopover duration and site usage [27], [28], some of the results are difficult to interpret [29], and others even deny at least some of the suggested effects of predation danger [30].
In this study, we used a dynamic state variable model to analyze whether these three hypotheses can explain the observed changes in migratory behavior of Barnacle geese.
Methods
We used a dynamic state variable model to predict the migration strategy of the Barnacle goose that maximizes expected lifetime reproductive success under different environmental circumstances. This type of model is most suitable as it includes future goals (maximising long term reproductive success) when defining decisions that lead to achieving these goals [31], [32]. We used an already existing model (see for more details [5], [33], [34]) which we parameterized for the Barnacle goose. We shortly explain the model here to give insight in the logic of the used parameters and to facilitate understanding our predictions.
The dynamic state variable model
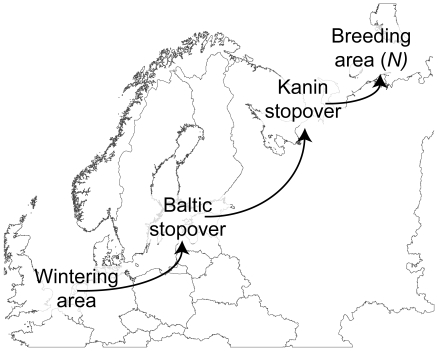
The state of the goose in the model was characterized by its energy stores x and its location i. At each time step of one day, t = 0,1…T, the state of body reserves was calculated, and according to state, location and time decisions for optimal migration was made. For computational reasons, x took only integer values between 0 and xmax = 100. One unit of x was equivalent to 232 kJ, representing 1% of the caloric value of the maximum body reserves (see table 1 for an overview of parameters). If the body reserves fell to zero, the goose died of starvation. We considered 4 different locations: a wintering site in the Netherlands, stopover sites in the Baltic sea region and at the Kanin peninsula in Russia, and a breeding site N at the Barents Sea coast in Russia [35] (figure 1). Breeding was only possible at the breeding site. At t = 0 (March 1) the goose started at the wintering site and simulations ended when it reached the breeding site or when t reached T, a predefined endpoint which was set to t = 121 (June 29), approximately 3 weeks after the optimal time window for breeding. The expected reproductive success of the goose, with body reserves x at time t at location i, was denoted by F(x,t,i).
Table 1. Parameterization of the model.
| Model parameters Barnacle geese | |||
| Parameter | unit | Reference | |
| Lean body mass | 1500 | g | Eichhorn 2008 |
| Maximum body mass | 2300 | g | Eichhorn 2008 |
| Potential mass reserves | 800 | g | |
| Energy density | 29 | kJ/g | Madsen and Klaassen, 2006 |
| Total energy reserves xmax | 23.2 | MJ | |
| Energy density per x | 232 | kJ | |
| Flight speed v | 18 | m/s | Green, 2001 |
| Average flight costs f | 6.23 | kJ/km | Butler et al., 2000; Nolet et al., 1992; Ward et al., 2002 |
| Daily energy expenditure e | 4.7 | kJ | Bruinzeel et al., 1997 |
| Model parameters of the staging areas of the Russian flyway | |||
| Wintering site The Netherlands | |||
| Distance to wintering site | 0 | km | |
| Maximum metabolizable energy intake g | 1397 | kJ/day | Eichhorn 2008 |
| Stop-over site Baltic | |||
| Distance to wintering site | 1270 | km | |
| Maximum metabolizable energy intake g | 1939 | kJ/day | Eichhorn 2008 |
| Peak date of food availability | May 14 | Van der Graaf et al., 2006 | |
| Stop-over site Kanin | |||
| Distance to wintering site | 2910 | km | |
| Maximum metabolizable energy intake g | 2296 | kJ/day | Eichhorn 2008 |
| Peak date of food availability | May 20 | ||
| Breeding site Kolokolkova Bay | Van der Jeugd et al., 2003 | ||
| Distance to wintering site | 3270 | km | |
| Time-window of arrival for optimal arrival K(t) | June 5–June 10 | Eichhorn et al., 2006 | |
Figure 1. Migration route of Russian Barnacle goose.
A schematic overview of the flyway of the Russian population of the Barnacle Goose. In spring (April–May), Barnacle geese depart from The Netherlands to stopover in the Baltic. After a stop of a few days to a few weeks they depart to pre-breeding areas in Northern Russia. The geese arrive at their arctic breeding grounds early June and start breeding immediately.
Terminal reward function
The terminal reward was defined as the reward at T, and served as a starting point for the backward iteration. Upon arrival at the breeding site N the expected reproductive success F(x,t,N) depended on the body stores at arrival as well as the timing of arrival [36]. Additionally, a component was added for expected future reproductive success BT because Barnacle geese are long-lived animals with many years of breeding attempts. Thus:
| (1) |
where K(t) was the function of the timing of arrival, K(x) the function of the body stores on arrival, and BT was set to 2, representing the expected future reproductive success given that an individual actually survived at any site until T. Both K(t) and K(x) result in 0 reward if an individual had not arrived at breeding site N at T. Subsequently, the effect of timing of arrival was incorporated by a step function, meaning that breeding was only possible if arriving at the breeding grounds within the set time-limits:
| (2) |
[20], [36]. The effect of body reserves on breeding success was described by a sigmoidal shape function based on data from the Pink-footed goose [36], indicating that the chance of successful breeding success increased if body stores upon arrival at the breeding site exceeded a certain threshold xc. We assumed a similar relationship for Barnacle geese. Thus:
| (3) |
where the shape parameter w was set to 0.028 and xc, the threshold for successful breeding, was set to 15080 kJ (xc = 65)
Backward iteration
At each time step a goose decided whether to stay at its present location and forage, or to depart to another location. When staying at location i, the potential intake rate (defined as metabolizable energy intake according to [37]) of the goose was site- and time-dependent and had predefined stochasticity [g(i,t), kJ day−1]. However, the actual intake rate depended on the foraging intensity u, ranging from 0 (no foraging) to 1 (continuous foraging). The actual intake rate minus the energy expenditure e [kJ day−1] resulted in the energy available for the storage of reserves. However, foraging with a particular intensity and storing reserves had a cost in terms of predation risk, defined by β(x,u):
| (4) |
where a, the mass-dependent escape performance exponent, was set to 2 and the site-specific constant attack rate [33] mβ(i) is set to 10−8. The parameter mβ(i) is the predation danger according to the definition by [13]. Thus, the goose foraged with the intensity that maximized its expected reproductive success F:
| (5) |
Alternatively, when departing to another site j, the goose chose the site j that maximized F:
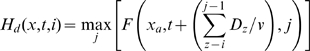 |
(6) |
This choice depended on the distance between the sites [Dz (km)], the speed of flight [v (km day−1)], and the reserves upon arrival (xa) at site j. The latter was defined by
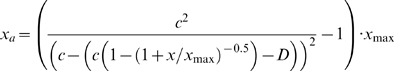 |
(7) |
where D was the distance covered. The constant c in this equation was defined by
| (8) |
where xf was the level of body reserves available for flight, which equaled xmax for Barnacle goose, and Dmax was the maximum flight distance defined by
| (9) |
where f was the average flight cost [kJ km−1] [38], [39], [40]. To find the fitness-maximizing decision, we calculated the fitness consequences of the behavioral alternatives, i.e., to forage or depart, for all combinations of state, location and time and chose the one with the highest fitness. The thus obtained optimal decision matrix showed the best decision for each time step and for all possible levels of body reserves and sites, namely:
| (10) |
Forward simulation
Based on the decision matrix, optimal migration was simulated for each goose. The simulations started at t = 0, each goose started with a random amount of body reserves between 4640 kJ≤x≤11600 kJ, and ended when the bird reached the breeding site, died, or passed the time limit T at any other site. In the simulations, we assumed geese had full knowledge of the environment, i.e. the geese experienced the same conditions in the forward simulation for which the optimal decisions were calculated in the backward calculation. The actual experienced potential intake rate g(i,t) for each individual was drawn from a distribution with a predefined stochasticity.
Scenarios
We analyzed the three different hypotheses by step-wisely changing the relevant model-parameters, i.e., onset of spring, intake rates and predation danger. For all scenarios, both backward iteration and forward simulations were run. First, we changed onset of spring in the Baltic staging site from 24 April to 3 June in steps of 5 days. Onset of spring was defined as the point in time when food availability g(i,t) first reached its highest value. Second, we changed food availability in the wintering and Baltic stop-over site from 1392 kJ d−1 to 2784 kJ d−1 in steps of 232 kJ d−1, and in all possible combinations for both sites.
Third, we increased predation danger (mβ(i)) in the Baltic site from 10−10 to 10−6 with 16 logarithmically equal steps (10−10, 10−9.75, 10−9.5, …, 10−6.5, 10−6.25, 10−6). We choose this range of values based on the value of 10−8 used by Klaassen et al. [5] and the value of 2·10−6 used by Weber et al. [33].
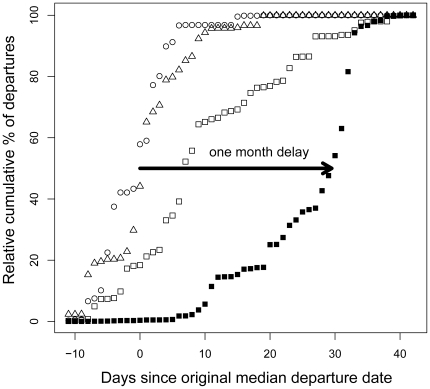
We compared the model predictions of the three scenarios with passage data from the Ottenby bird observatory (56°11′45″N, 16°23′56″E) from 1970 until 2004 (adapted from [41], see figure 2). Ottenby is situated on a main migratory corridor for Barnacle geese traveling from the Netherlands to Baltic stopover sites [42]. Because the total population of Barnacle geese also greatly increased during that period, we used the relative cumulative percentage of passed dates. The most plausible predictions were those that showed a delay in departure equivalent to the observed delay of one month. All results were analyzed with R.2.8.1 [43].
Figure 2. Observed delay in onset of spring migration.
The departure dates from the wintering grounds in the Netherlands, shown as the relative cumulative percentage of departure as a function of days since the median departure date in the 1970's. Data points represent per day the mean relative cumulative passage count at Ottenby bird observatory over a certain period (circles: 1970–1979, triangles: 1980–1989, open squares: 1990–1999, solid squares: 2000–2004). The median departure date in the 1970's was April 12.
Results
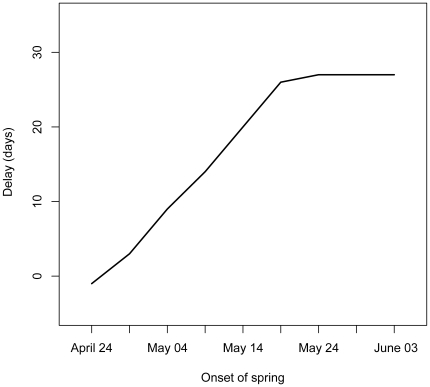
Advancing the onset of spring in the Baltic by a given unit of time led to an equally advanced departure date from the wintering site for most of the range tested in our simulations (figure 3). Additionally, the simulations showed that the geese always depart from the Dutch wintering site just before the onset of spring in the Baltic.
Figure 3. Predicted delay in onset of spring scenario.
The delay in departure (in days since April 12, which was the median departure date in the 1970's) from the wintering site in the Netherlands as a function of onset of spring. In the model, the geese responded to a change in the peak date of intake rate such that they advance departures with an earlier spring and vice versa, they would depart later from the wintering site if spring in the Baltic would be delayed.
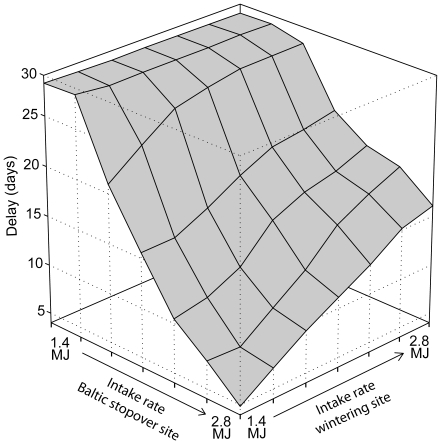
Decreasing intake rates in the Baltic stopover sites by 1392kJ/day led to a delay in departure date from the wintering site of 29 days (mid April–mid May) (figure 4). If, alternatively, the intake rates in the wintering site increased, the geese delayed their departure date by only 16 days (figure 4).
Figure 4. Predicted delay in intake rate scenario.
The predicted delay in departure date (in days since April 12, which was the median departure date in the 1970's) from the wintering site in the Netherlands to a changed intake rate, ranging from 1.4 MJ to 2.8 MJ, at the wintering site and the Baltic stopover site.
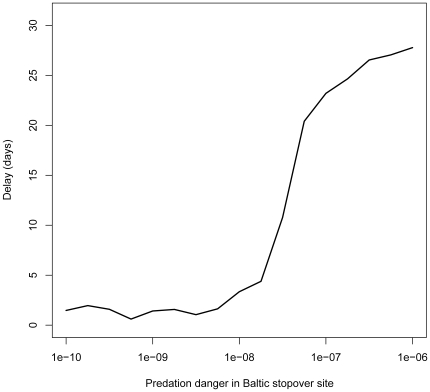
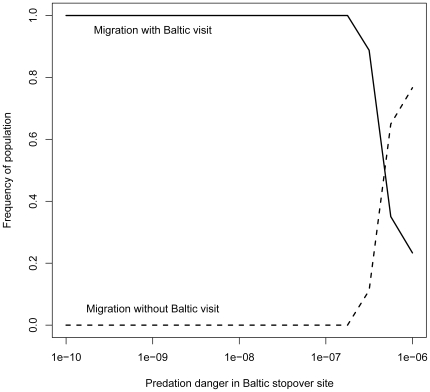
Increasing predation danger in the Baltic above the predation danger of the other sites led to a rapid delay of 28 days (mid April–mid May) in departure date from the wintering site (figure 5). When predation danger was further increased, a growing proportion of geese stopped using the Baltic stopover site (figure 6). However, a small proportion geese still visited the Baltic, and stayed for a few days only. They had low energy reserves, and apparently, could not skip this site as they were in dire need of replenishing their body stores.
Figure 5. Predicted delay in danger scenario.
The delay in departure (in days since April 12, which was the median departure date in the 1970's) from the wintering site in the Netherlands as a function of predation danger at the Baltic stopover site. Above a predation danger of 3·10−8, the geese adjusted their migration by abruptly delaying their departure date from the wintering site by up to 28 days.
Figure 6. Predicted use of Baltic stopover site in danger scenario.
The predicted response to increased predation danger, described as the proportion of the geese that make use of the Baltic as a stopover site. With low predation danger all geese are predicted to use the Baltic stopover site (solid line), i.e. no skipping of the Baltic (broken line). However, with increasing predation danger the majority (+/−75%) of the geese skip the site while some geese with (very) low body reserves continue to use the Baltic stopover site for a few days to build up extra reserves.
Discussion
Our simulations showed that the delayed departure of Barnacle geese from their wintering grounds by up to one month can be explained by either decreased potential intake rates or increased predation danger in the Baltic stopover site. In contrast, an advanced onset of spring fails to explain such a delay. The predicted response to an advanced spring growth is opposite to a delayed departure actually observed in the field. According to our simulations, an advancement of spring of 8 days (as predicted by [19] based on growing degree days) should advance departure by 8 days too. Interestingly, also the Barnacle geese breeding on Spitsbergen have not advanced their departure from Scottish wintering grounds despite an advanced onset of spring at their Norwegian stopover site, in contrast to Pink-footed geese, which largely share the same flyway and have advanced their spring migration [44]. Tombre et al. [44] suggest that Barnacle geese breeding at Spitsbergen cannot predict spring in Norway from their wintering site in the United Kingdom because of the large overseas crossing. The Russian breeding Barnacle geese, however, do not have such a large overseas crossing, and prioritize other factors than responding to advanced onset of spring in the Baltic. Thus, although the timing of high quality food during migration is important for Barnacle geese [16], this result suggests that Barnacle geese may prioritize other factors above the onset of plant growth in spring, and that the observed delay in migration cannot be caused by climatic changes. Theory also predicts that birds should not advance their timing of migration as much as spring advances, because the timing of migration has not only evolved to match the peak of food availability but also in response to many other factors, such as competition for territories and predation risk [45].
Our assumptions on decreased potential intake rates are supported by empirical studies [16], [41]. Both studies suggested a recent decrease in intake rates in a Baltic stopover site. Additionally, Barnacle geese have been observed to colonize new staging sites at several locations in the Baltic. Populations staging at traditional sites remained approximately constant [46], indicating that the traditional sites reached capacity, especially because the total population of geese increased much more than the population staging in the Baltic [7]. Besides, the ongoing urbanization in the Baltic region has led to a general decline in agricultural practice, e.g., cattle farming. Consequently, intake rates may also have decreased as facilitation by large grazers decreased. Altogether, decreased intake rates can be a plausible explanation for the observed delay.
In addition to the importance of food en route, our simulations showed a particularly strong effect of predation danger on the departure date from the wintering site. When predation danger in the Baltic was only slightly higher compared to the other sites, the geese immediately started delaying departure from the wintering site, reducing staging time at the dangerous site and ultimately, skipping the site with higher predation risk. This is in line with theoretical predictions that a migratory bird should minimize the time spent in a dangerous area [47] and that the loss of future reproductive success by predation is traded off against the benefit of increasing reserves by foraging [48]. Predators can have a strong influence on migratory strategies, e.g. by causing migrants to avoid the predator abundance peak [28]. If the whole Baltic area has become more dangerous due to the recovery of predator populations, we expect the geese to minimize the time spent in that area. The strong increase in predator numbers such as White-tailed eagles in the Baltic; a fourfold increase in Estonia (from 40 to 150–170 [49]), Latvia and Finland and expansion into Gotland, Sweden [50], indicates that the Baltic has indeed become a more dangerous place for Barnacle geese compared to the Netherlands. For example, on the island of Saaremaa (2,672km2), Estonia, which is a major stopover site in the Baltic, there are 28 known White-tailed eagle territories (pers. comm. V. Völke). Contrastingly, there is currently only a single breeding pair in the Netherlands (41,528km2). For this breeding pair it has been confirmed that it preys on Greylag geese Anser anser [51].
Additionally, predation danger caused birds to not take full advantage of available resources, as they take the danger into account in their decision of where to forage [52]. These non-lethal effects of predation can potentially be larger than the lethal effects [24]. Hence, increased predation danger can reinforce the already existing effects of decreased intake rates. The influence of density-dependent effects on this trade-off are not immediately clear. Potentially, danger can cause many geese to shift to safer areas, thereby decreasing the competition for food in the dangerous areas. However, it is known that Barnacle geese facilitate each other while grazing [53]. Consequently, a dangerous and less grazed area does not necessarily lead to better feeding conditions. Our model did not take these density-dependent effects into account.
In conclusion, predation danger, in addition to food availability, can be a key factor in explaining the observed changes in migratory behavior of Barnacle geese. This study only approached the problem from a theoretical point of view, but identified critical factors to be studied empirically in the field. These new insights also suggest that challenging geese with natural predators in the Netherlands, e.g. by creating suitable nesting places for White-tailed Eagles, may improve management of the agricultural conflict. Future empirical research needs to test our predictions by measuring the direct and indirect effects of predator activities on goose behavior. Although this study focused on the case of the Barnacle goose, its conclusions are not limited to goose migration. It is often assumed that timing of migration is synchronized with the phenology of resources [11], resulting in potential mismatches and associated population declines as a result of climate change [54]. These two studies state respectively that looking at predation in addition to resources as explanatory factor is very difficult or do not even mention predation at all as potential explanatory factor. We want to emphasize that in addition to currently well studied factors such as food availability and climatic change, predation danger should be considered in the suite of potential explanatory variables for changes in the migratory behavior of birds.
Acknowledgments
We are grateful to Ron Ydenberg, Herbert Prins, Sean Rands, Zoltan Barta and an anonymous reviewer for comments on predation danger and for improving earlier versions of the manuscript. We thank Tom van der Have and Jan Gert Vink from the Dutch Faunafonds for information on agricultural damage. This is publication 4784 of the Netherlands Institute of Ecology (NIOO-KNAW).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Rudy Jonker was funded by the Dutch Faunafonds and the Koninklijke Nederlandse Jagersvereniging. Götz Eichhorn was supported by ESF travel grants and scholarships from the ‘Marianne und Dr. Fritz Walter-Fischer Stiftung’, Germany, and the ‘Ubbo Emmius Programme’ of Groningen University. Silke Bauer received support from The Netherlands Organisation for Scientific Research, Earth & Life Sciences (NWO-ALW) project grant 816.01.007. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sutherland WJ. Evidence for flexibility and constraint in migration systems. Journal of Avian Biology. 1998;29:441–446. [Google Scholar]
- 2.Jonzén N, Linden A, Ergon T, Knudsen E, Vik JO, et al. Rapid advance of spring arrival dates in long-distance migratory birds. Science. 2006;312:1959–1961. doi: 10.1126/science.1126119. [DOI] [PubMed] [Google Scholar]
- 3.Both C. Comment on “Rapid advance of spring arrival dates in long-distance migratory birds”. Science. 2007;315 doi: 10.1126/science.1136920. [DOI] [PubMed] [Google Scholar]
- 4.Gill JA, Norris K, Potts PM, Gunnarsson TG, Atkinson PW, et al. The buffer effect and large-scale population regulation in migratory birds. Nature. 2001;412:436–438. doi: 10.1038/35086568. [DOI] [PubMed] [Google Scholar]
- 5.Klaassen M, Bauer S, Madsen J, Ingunn T. Modelling behavioural and fitness consequences of disturbance for geese along their spring flyway. Journal Of Applied Ecology. 2006;43:92–100. [Google Scholar]
- 6.Alerstam T, Lindström A. Optimal bird migration: the relative importance of time, energy and safety. In: Gwinner E, editor. Bird migration, physiology and eco-physiology. Berlin: Springer-Verlag; 1990. pp. 331–350. [Google Scholar]
- 7.Eichhorn G, Drent RH, Stahl J, Leito A, Alerstam T. Skipping the Baltic: the emergence of a dichotomy of alternative spring migration strategies in Russian barnacle geese. Journal of Animal Ecology. 2009;78:63–72. doi: 10.1111/j.1365-2656.2008.01485.x. [DOI] [PubMed] [Google Scholar]
- 8.Eichhorn G, Afanasyev V, Drent RJ, van der Jeugd HP. Spring stopover routines in Russian Barnacle geese Branta leucopsis tracked by resightings and geolocation. Ardea. 2006;94 [Google Scholar]
- 9.Marra PP, Francis CM, Mulvihill RS, Moore FR. The influence of climate on the timing and rate of spring bird migration. Oecologia. 2005;142:307–315. doi: 10.1007/s00442-004-1725-x. [DOI] [PubMed] [Google Scholar]
- 10.Stervander M, Lindström Á, Jonzén N, Andersson A. Timing of spring migration in birds: long-term trends, North Atlantic Oscillation and the significance of different migration routes. Journal of Avian Biology. 2005;36:210–221. [Google Scholar]
- 11.Visser ME, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proceedings of the Royal Society B-Biological Sciences. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faunafonds. Dordrecht: Agricultural damage by Barnacle geese in the months April and May. 2009. (in Dutch: Faunaschade door brandganzen in de maanden april en mei.)
- 13.Lank DB, Ydenberg RC. Death and danger at migratory stopovers: problems with “predation risk”. Journal of Avian Biology. 2003;34:225–228. [Google Scholar]
- 14.Crick HQP, Dudley C, Glue DE, Thomson DL. UK birds are laying eggs earlier. Nature. 1997;388:526–526. [Google Scholar]
- 15.Gordo O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Research. 2007;35:37–58. [Google Scholar]
- 16.Van der Graaf AJ, Stahl J, Klimkowska A, Bakker JP, Drent RH. Surfing on a green wave - how plant growth drives spring migration in the Barnacle Goose. Ardea. 2006;94:567–577. [Google Scholar]
- 17.Beekman JH, Nolet BA, Klaassen M. Skipping swans: Fuelling rates and wind conditions determine differential use of migratory stopover sites of Bewick's Swans Cygnus bewickii. Ardea. 2002;90:437–460. [Google Scholar]
- 18.Van der Graaf AJ, Stahl J, Veen GF, Havinga RM, Drent RH. Patch choice of avian herbivores along a migration trajectory - From Temperate to Arctic. Basic and Applied Ecology. 2007;8:354–363. [Google Scholar]
- 19.Van der Graaf AJ. Geese on a green wave: Flexible migrants in a changing world. [Phd Thesis] Groningen: Rijksuniversiteit Groningen; 2006. 224 [Google Scholar]
- 20.Van der Jeugd HP, Eichhorn G, Litvin KE, Stahl J, Larsson K, et al. Keeping up with early springs: Rapid range expansion in an avian herbivore incurs a mismatch between reproductive timing and food supply. Global Change Biology. 2009;15:1057–1071. [Google Scholar]
- 21.Forslund P, Larsson K. Breeding Range Expansion Of The Barnacle Goose Branta leucopsis In The Baltic Area. Ardea. 1991;79:342–346. [Google Scholar]
- 22.Prins HHT, Gordon IJ. Introduction: Grazers and Browsers in a Changing World. In: Gordon IJ, Prins HHT, editors. THe Ecology of Browsing and Grazing. Berlin Heidelberg: Springer; 2008. pp. 5–9. [Google Scholar]
- 23.Ydenberg RC, Dill LM. The Economics of Fleeing from Predators. Advances in the Study of Behavior. 1986;16:229–249. [Google Scholar]
- 24.Cresswell W. Non-lethal effects of predation in birds. Ibis. 2008;150:3–17. [Google Scholar]
- 25.Alerstam T, Hedenström A, Ákesson S. Long-distance migration: evolution and determinants. Oikos. 2003;103:247–260. [Google Scholar]
- 26.Ydenberg RC, Butler RW, Lank DB. Effects of predator landscapes on the evolutionary ecology of routing, timing and molt by long-distance migrants. Journal of Avian Biology. 2007;38:523–529. [Google Scholar]
- 27.Pomeroy AC. Tradeoffs between food abundance and predation danger in spatial usage of a stopover site by western sandpipers, Calidris mauri. Oikos. 2006;112:629–637. [Google Scholar]
- 28.Lank DB, Butler RW, Ireland J, Ydenberg RC. Effects of predation danger on migration strategies of sandpipers. Oikos. 2003;103:303–319. [Google Scholar]
- 29.Fransson T, Weber TP. Migratory fuelling in blackcaps (Sylvia atricapilla) under perceived risk of predation. Behavioral Ecology and Sociobiology. 1997;41:75–80. [Google Scholar]
- 30.Dierschke V. Predation hazard during migratory stopover: are light or heavy birds under risk? Journal of Avian Biology. 2003;34:24–29. [Google Scholar]
- 31.Clark CW, Mangel M. Dynamic state variable models in Ecology; Methods and applications. In: May RM, Pagel MD, editors. Oxford, New York: Oxford University Press; 2000. 288 [Google Scholar]
- 32.Houston AI, McNamara JM. Models of Adaptive Behaviour - An approach based on state. Cambridge: Cambridge University Press; 1999. 378 [Google Scholar]
- 33.Weber TP, Ens BJ, Houston AI. Optimal avian migration: A dynamic model of fuel stores and site use. Evolutionary Ecology. 1998;12:377–401. [Google Scholar]
- 34.Bauer S, Dinther MV, Høgda K-A, Klaassen M, Madsen J. The consequences of climate-driven stop-over sites changes on migration schedules and fitness of Arctic geese. Journal of Animal Ecology. 2008;77:654–660. doi: 10.1111/j.1365-2656.2008.01381.x. [DOI] [PubMed] [Google Scholar]
- 35.Van der Jeugd HP, Gurtovaya E, Eichhorn G, Litvin KY, Mineev OY, et al. Breeding barnacle geese in Kolokolkova Bay, Russia: number of breeding pairs, reproductive success and morphology. Polar Biology. 2003;26:700–706. [Google Scholar]
- 36.Prop J, Black JM, Shimmings P. Travel schedules to the high arctic: barnacle geese trade-off the timing of migration with accumulation of fat deposits. Oikos. 2003;103:403–414. [Google Scholar]
- 37.Bruinzeel LW, Eerden MRV, Drent RH, Vulink JT. Scaling metabolisable energy intake and daily energy expenditure in relation to the size of herbivorous waterfowl: limits set by available foraging time and digestive performance. In: Eerden MRV, editor. Patchwork. Lelystad: Rijkswaterstaat Directie IJsselmeergebied; 1997. pp. 111–132. [Google Scholar]
- 38.Ward S, Bishop CM, Woakes AJ, Butler PJ. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). Journal of Experimental Biology. 2002;205:3347–3356. doi: 10.1242/jeb.205.21.3347. [DOI] [PubMed] [Google Scholar]
- 39.Nolet BA, Butler PJ, Masman D, Woakes AJ. Estimation of Daily Energy-Expenditure from Heart-Rate and Doubly Labeled Water in Exercising Geese. Physiological Zoology. 1992;65:1188–1216. [Google Scholar]
- 40.Butler PJ, Woakes AJ, Bevan RM, Stephenson R. Heart rate and rate of oxygen consumption during flight of the barnacle goose, Branta leucopsis. Comparative Biochemistry and Physiology a-Molecular and Integrative Physiology. 2000;126:379–385. doi: 10.1016/s1095-6433(00)00221-x. [DOI] [PubMed] [Google Scholar]
- 41.Eichhorn G. Travels in a Changing World: Flexibility and Constraints in Migration and Breeding of the Barnacle Goose [Ph.D. Thesis] Groningen: University of Groningen; 2008. [Google Scholar]
- 42.Ganter B, Larsson K, Syroechkovsky EV, Litvin KE, Leito A, et al. Barnacle Goose Branta Leucopsis: Russia/Baltic. In: Madsen J, Cracknell G, Fox T, editors. Goose population of the Western Palaearctic: A review of status and distribution. Rönde, Denmark: National Environmental Research Institute; 1999. pp. 270–283. [Google Scholar]
- 43.R Development Core Team. R: A language and environment for statistical computing. 2009. Vienna, Austria R Foundation for Statistical Computing http://www.R-project.org.
- 44.Tombre IM, Høgda KA, Madsen J, Griffin LR, Kuijken E, et al. The onset of spring and timing of migration in two arctic nesting goose populations: The pink-footed goose Anser bachyrhynchus and the barnacle goose Branta leucopsis. Journal of Avian Biology. 2008;39:691–703. [Google Scholar]
- 45.Jonzén N, Hedenström A, Lundberg P. Climate change and the optimal arrival of migratory birds. Proceedings of the Royal Society B-Biological Sciences. 2007;274:269–274. doi: 10.1098/rspb.2006.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leito A. The Barnacle Goose in Estonia. Estonia Maritima. 1996;1:1–103. [Google Scholar]
- 47.Houston AI. Models of optimal avian migration: state, time and predation. Journal of Avian Biology. 1998;29:395–404. [Google Scholar]
- 48.McNamara JM, Houston AI. The effect of a change in foraging options on intake rate and predation rate. American Naturalist. 1994;144:978–1000. [Google Scholar]
- 49.Hermann C, Krone O, Stjernberg T, Helander B. 2009. Population Development of Baltic Bird Species: White-tailed Sea Eagle (Haliaeetus albicilla) HELCOM Indicator Fact Sheets Online http://www.helcom.fi/environment2/ifs/ifs2009/en_GB/White-tailedSeaEagle/Accessed 2010 March 26.
- 50.Helander B, Marquiss M Bowerman We. SEA EAGLE. Proceedings from an international conference at Björkö, Sweden, 13–17 September 2000; 2003; 45 Tryckeri, AB. Stockholm: Swedish Society for Nature Conservation/SNF & Åtta; 2000. [Google Scholar]
- 51.Roder F, Bijlsma R, Klomp J. Second breeding case of White-tailed Eagle Haliaeetus albicilla in The Netherlands (In Dutch with English abstract: Tweede broedgeval van de Zeearend Haliaeetus albicilla in Nederland). De Takkeling. 2008;16:100–123. [Google Scholar]
- 52.Pomeroy AC, Butler RW, Ydenberg RC. Experimental evidence that migrants adjust usage at a stopover site to trade off food and danger. Behavioral Ecology. 2006;17:1041–1045. [Google Scholar]
- 53.Ydenberg RC, Prins HHT. Spring Grazing and the Manipulation of Food Quality by Barnacle Geese. Journal of Applied Ecology. 1981;18:443–453. [Google Scholar]
- 54.Jones T, Cresswell W. The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology. 2010;79:98–108. doi: 10.1111/j.1365-2656.2009.01610.x. [DOI] [PubMed] [Google Scholar]