Abstract
The influence of biodiversity on ecosystem functioning is a major concern of ecological research. However, the biodiversity–ecosystem functioning relationship has very often been studied independently from the mechanisms allowing coexistence. By considering the effects of dispersal and niche partitioning on diversity, the metacommunity perspective predicts a spatial scale-dependence of the shape of the relationship. Here, we present experimental evidence of such scale-dependent patterns. After approximately 500 generations of diversification in a spatially heterogeneous environment, we measured functional diversity (FD) and productivity at both local and regional scales in experimental source-sink metacommunities of the bacterium Pseudomonas fluorescens SBW25. At the regional scale, environmental heterogeneity yielded high levels of FD and we observed a positive correlation between diversity and productivity. At the local scale, intermediate dispersal increased local FD through a mass effect but there was no correlation between diversity and productivity. These experimental results underline the importance of considering the mechanisms maintaining biodiversity and the appropriate spatial scales in understanding its relationship with ecosystem functioning.
Keywords: biodiversity–ecosystem functioning relationship, spatial scale-dependence, source-sink metacommunity, mass effect, dispersal, productivity
1. Introduction
Under the current decline of biodiversity, understanding its influence on ecosystem properties such as productivity and stability has become a central issue in ecology (Loreau et al. 2001; Hooper et al. 2005). To this aim, ecologists have developed theoretical approaches and performed massive field and controlled laboratory experiments (Hooper et al. 2005; Balvanera et al. 2006). Such studies have revealed high variability in the shape of the biodiversity–ecosystem functioning (BEF) relationship. Some support a positive effect of biodiversity (i.e. positive BEF relationship), whereas others present evidence of negative and null patterns (Schwartz et al. 2000; Hooper et al. 2005; Srivastava & Vellend 2005; Jiang et al. 2008). Consequently, and despite the large body of work, controversy remains on the influence of biodiversity in ecosystem function (Srivastava & Vellend 2005; Smith 2007).
Previous studies have revealed how numerous factors such as the ecosystem type, the ecosystem function or type of diversity measured and the history of community assembly, may influence the shape of the BEF relationship, making it difficult to obtain a general pattern (Cardinale et al. 2000; Fukami & Morin 2003; Petchey et al. 2004; Van Ruijven & Berendse 2005; Balvanera et al. 2006; Smith 2007; Replansky & Bell 2009). Some studies have also pointed out a spatial scale-dependence in the shape of the BEF relationship (Bond & Chase 2002; Chase & Leibold 2002; Mouquet & Loreau 2003; Cardinale et al. 2004; Harrison et al. 2006), based on the multi-factorial nature of the mechanisms responsible for species coexistence (Mouquet et al. 2002; Cardinale et al. 2004). More specifically, the combined influence of local and regional mechanisms maintaining species diversity may alter the characteristics of communities and generate different BEF patterns at different spatial scales (Bond & Chase 2002).
Metacommunity ecology provides a general framework linking local and regional determinants of diversity maintenance and helps in understanding some aspects of the scale-dependence of the BEF relationship (Mouquet & Loreau 2003; Leibold et al. 2004). It is now well established that diversity results from the interaction between local and regional processes (Ricklefs 1987; Hubbell 2001; Leibold et al. 2004) and communities are represented as embedded within complex interactive networks connected by dispersal (Leibold et al. 2004). In a spatially heterogeneous environment, the source-sink perspective predicts that dispersal may increase local diversity through mass effects (Shmida & Wilson 1985; Loreau & Mouquet 1999). This is because sustained immigration from areas of high success (i.e. sources) may prevent the exclusion or extinction from unfavourable areas (i.e. sinks), allowing the establishment of species at sites where they cannot be self-maintaining without dispersal (Amarasekare & Nisbet 2001; Mouquet & Loreau 2002). This results in an increase in local diversity relative to what would be expected in a closed system. Too much dispersal, however, may be detrimental to diversity as the metacommunity becomes increasingly homogenized, reducing both local and regional diversity.
In a source-sink metacommunity, different mechanisms of diversity maintenance may be expressed at different scales, so it is likely that the shape of the BEF relationship will depend on the spatial scale considered (i.e. local or regional). At the regional scale, coexistence is based on niche differentiation, and thus it is likely that complementarity will lead to a positive BEF relationship (Venail et al. 2008). At the local scale, diversity strongly depends on immigration, there is no a priori reason for thinking that immigrants will increase functional complementarity, and a null or even a negative BEF relationship may arise when the proportion of the most productive species declines with increasing immigration (Loreau & Mouquet 1999; Mouquet & Loreau 2003). This does not mean that local positive BEF is impossible, indeed locally diverse resources can produce local positive diversity-functioning relationships (e.g. Gross & Cardinale 2007). But, we argue that if the only source of niche differentiation is regional (as in the source sink models), then the scale of the positive BEF should be regional. The scarce experiments conducted in source-sink systems have revealed a positive effect of diversity on productivity at both local (Matthiessen & Hillebrand 2006) and regional scales (Venail et al. 2008). We are unaware of any experimental study simultaneously addressing the BEF relationship at both local and regional scales.
To explicitly test the hypothesis that the shape of the BEF relationship varies at different spatial scales, we experimentally measured functional diversity (FD) and productivity at both local and regional scales in a bacterial source-sink metacommunity. Bacteria are haploid and asexual organisms, and hence all the measured genetic diversity evolved during the experiment and each emerging genotype can be functionally considered as an ecological ‘species’. In a previous experiment (Venail et al. 2008), we allowed a single clone of the bacterium Pseudomonas fluorescens SBW25 to naturally diversify in a spatially heterogeneous environment under four different levels of dispersal. After approximately 500 generations of adaptive radiation, we observed a positive relationship between regional FD and productivity, with maximal values at intermediate levels of dispersal. In the present study, we measured the realized FD and productivity at the local (i.e. community) and regional (i.e. metacommunity) scales. At a local scale, our results support the action of a mass effect, resulting in a gradient of local diversity but with no consequences in terms of local productivity. On the contrary, the BEF relationship was positive at the regional scale as a result of regional niche differentiation. These results represent the first evidence of dispersal and spatial heterogeneity as main mechanisms explaining the predicted spatial scale-dependence in the diversity–productivity relationship.
2. Material and methods
(a). Selection experiment
A single clone of the bacterium P. fluorescens SBW25 was allowed to diversify for approximately 500 generations (40 days) over a highly heterogeneous environment (Biolog GN2 microplates) by controlling the amount of dispersal among communities (Venail et al. 2008). The Biolog system represents a highly structured environment composed of 95 wells (hereafter patches), each containing a different carbon substrate belonging to 11 different chemical families plus one blank control patch. Each patch also contains a dye (tetrazolium violet) that turns violet as the extant carbon substrate is metabolized. The strain we employed had previously evolved for approximately 900 generations in a complex environment of eight carbon sources (Barrett et al. 2005). Each of three replicates of this unique ‘ancestral’ strain was used to inoculate four independent Biolog microplates to be submitted to four different dispersal levels among the patches: 0%, 1%, 10% and 100% dispersal (i.e. 3 replicates × 4 dispersal treatments = 12 Biolog microplates). We transferred the bacteria to new Biolog GN2 microplates every 24 h to renew the available nutrients in each patch during a selection period of 40 days. This transfer technique (batch culture) enhances bacterial growth rates and allowed the control of dispersal between patches. After 24 h of incubation in the dark at 28°C, and prior to each transfer, we scored light absorbance at 590 nm for each patch using a FLUOstar Optima spectrophotometer (BMG). Absorbance is related to the metabolic capacities of bacteria and was used as a proxy of productivity. We allowed dispersal of bacteria between patches in each microplate such that 0%, 1%, 10% or 100% of the culture used to inoculate each patch of a new microplate came from a pool of immigrants derived from a mixture of all the 95 patches. At each transfer, each new recipient Biolog was inoculated with 1 µl of the content of a donor Biolog by using a pin replicator (Boekel 96 pin per well model no. 140500). After the 40 days of selection, we transferred 40 µl of the content of each patch of the Biolog microplates to new empty microplates and added 60 µl of 50 : 50 glycerol/M9 salts solution before storing them at −80°C.
(b). Phenotypic assays
A phenotypic assay consisted of measuring the performances of individual genotypes over the 95 carbon substrates of the Biolog system. We randomly sampled five genotypes from 10 evolved communities (i.e. patches containing a single carbon source) of the 95 available in the Biolog system after streaking out the content of the well in KB agar Petri dishes (120 communities in total: 10 wells × 4 dispersal levels × 3 replicates). For logistic reasons we were not able to include more communities in our analysis. We assayed a total of 600 evolved genotypes in eight blocks (i.e. days; 75 genotypes per day) using a randomized block design. Each genotype was first amplified in 1 ml of M9KB media for 24 h at 28°C under constant orbital shaking (200 r.p.m.) and then stored at −80°C in 30 per cent glycerol solution. For each genotype, 1 ml of culture was unfrozen, centrifuged (3 min at 8000 r.p.m) and then washed by eliminating the liquid media and adding 1 ml of M9 minimal salts. Of this solution, 125 µl was diluted into 25 ml of M9 minimal salts and starved for 2 h at 28°C before inoculation into the Biolog microplates (140 µl). After 24 h of incubation in the dark at 28°C in humid chambers, we scored absorbance at 590 nm for each genotype in each carbon source. As a control for any ‘block effect’, we also assayed five replicates of the ancestral clone each day (same protocol). Measured absorbances were corrected by the blank patch (with no carbon source, but with tetrazolium violet). Corrected absorbance on each patch measures genotypic catabolic performance.
(c). Statistical analysis
Before any analysis on the effect of dispersal on diversity, we tested for an eventual ‘block effect’ in absorbance measurements made on different days by comparing the eight sessions of the five replicated assays of the ancestral strain.
In order to quantify the FD within each evolved community, we partitioned the total phenotypic variance into genotype, environment and genotype-by-environment interaction components (Bell 1990). This partitioning technique requires a complete genotype by environment matrix (i.e. common garden experiment). The interaction component (G × E) indicates the occurrence of genotypes adapted to different environments (i.e. niche diversification). The interaction was further decomposed into inconsistency and responsiveness (Bell 1990; Barrett et al. 2005; Venail et al. 2008). Inconsistency (I) indicates the lack of correlation among genotypes over environments:
with G being the number of genotypes tested, σEi and σEj the standard deviations of environmental responses for each genotype and ρEiEj the environmental correlation among each pair of genotypes tested. The lack of correlation implies that reaction norms are negatively correlated, revealing niche complementarity among genotypes. We used the inconsistency proportion of total phenotypic variance in performances over the 95 carbon substrates of the Biolog system as a measure of FD. We estimated local FD by calculating inconsistency using the corrected absorbance of the five randomly selected genotypes from each community. We measured regional FD by including in the calculation of inconsistency the 50 genotypes belonging to the same metacommunity (10 communities × 5 genotypes).
We discriminated among ‘source’ and ‘sink’ genotypes by using their corrected absorbance in the patch from which they were sampled. Positive-corrected absorbances represent genotypes able to grow in the patch from which they were isolated and were considered as ‘sources’. On the contrary, a negative-corrected absorbance means the genotype cannot exploit the patch from which they were sampled. As a consequence, ‘source’ and ‘sink’ genotypes are defined only with respect to the assayed environmental conditions. We calculated the proportion of source and sink genotypes for each community.
As a measure of local productivity, we employed the mean absorbance over the three last transfers from our previous dataset (Venail et al. 2008). When calculating regional productivity we averaged local productivity over the 54 ‘informative’ carbon substrates after 41 were excluded, because repeated measurements of the ancestor were statistically unreliable (coefficient of variation >1; Cooper & Lenski 2000). Performing the same analysis using the 95 substrates did not change the results.
We used an analysis of covariance (ANCOVA) to test for dispersal (covariate) and community (random) effects on local FD. We used a one-way analysis of variance to test for an effect of dispersal on regional FD and also to compare the proportion of sink genotypes (after angular transformation) among dispersal treatments. We fitted quadratic models to the dispersal–diversity relationships, a linear model for the effect of dispersal on the proportion of sink genotypes, and linear and log-linear models to the FD–productivity relationship. We used r and jmp5 software for statistical analyses.
3. Results
(a). No block effect on assays
We did not observe any ‘block effect’ on the replicated absorbance measurements of the ancestral strain (F7,39 = 0.7, p = 0.67), allowing all the assayed genotypes (n = 600) to be pooled for a global analysis of the effect of dispersal on diversity.
(b). Regional diversity peaks at intermediate dispersal rates
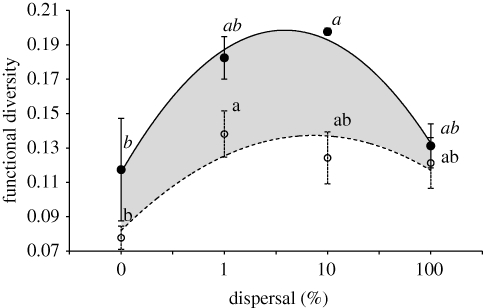
Dispersal significantly affected the amount of regional FD after approximately 500 generations of diversification in our source–sink metacommunities (r2 = 0.65, F3,8 = 4.987, p = 0.0308). Regional FD was maximal for intermediate levels of dispersal (figure 1) as revealed by quadratic regression (F2,9 = 8.15, p = 0.0096), quadratic parameter (t = −3.96, p = 0.0033) and multiple comparison tests (figure 1). These results confirm a previous study (Venail et al. 2008), where we employed a different sampling technique of genotypes for regional FD calculation. In that study, we measured regional diversity after sampling 16 genotypes from a mixture of the content of the entire metacommunity (95 different evolved communities). The estimation of local diversity was not possible with this sampling technique as the origin of each genotype was unknown.
Figure 1.
Effect of dispersal rate on regional and local FD after approximately 500 generations of diversification. Both local diversity (empty circles) and regional diversity (filled circles) peaked for intermediate dispersal levels (local quadratic regressions: F2,37 = 4.8479, p = 0.0135, quadratic parameter: t = −2.40, p = 0.0213 and F2,9 = 8.15, p = 0.0096, see also table 1; regional quadratic parameter: t = −3.96, p = 0.0033). For regional FD, each point represents the averaged diversity and standard error bars over three independent replicates, each containing 50 genotypes. For local FD, each point represents the averaged diversity and standard error bars over the 10 assayed communities, each containing five genotypes. Lines represent quadratic model fits. Any two data points that do not share the same letter have significantly different FD (p < 0.05), as determined by a Tukey test. We tested for differences between local (regular characters) and regional FD (italic characters).
(c). Local diversity peaks at intermediate dispersal rates
Both dispersal and community had an effect on local FD (table 1). Local FD peaked at intermediate dispersal rates (figure 1) as revealed by ANCOVA (table 1). At 100 per cent dispersal, local and regional FD converged (figure 1; t-test: t = −0.590, p = 0.5876).
Table 1.
ANCOVA testing for effects of dispersal and community on local functional diversity. This table shows the results of an ANCOVA on the amount of local FD treating community as a random effect and dispersal as a covariate. m.s. = mean square. Bold p-values represent significant effects on diversity (p < 0.05).
| effect | m.s. | d.f. | F | p-value |
|---|---|---|---|---|
| dispersal | 0.0204 | 1 | 8.5146 | 0.0044 |
| community | 0.0080 | 9 | 3.3413 | 0.0014 |
| dispersal × community | 0.0055 | 9 | 2.3015 | 0.0224 |
| dispersal2 | 0.0301 | 1 | 12.579 | 0.0006 |
| dispersal2 × community | 0.0021 | 9 | 0.8941 | 0.5339 |
| residual | 0.0024 | 90 |
(d). Dispersal limits local adaptation
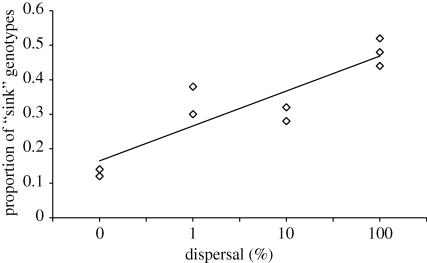
The proportion of ‘sink’ genotypes linearly increased with dispersal (figure 2; r2 = 0.76, F1,10 = 32.47, p = 0.0002) starting at 0.127 (±0.0067) for 0% dispersal and increasing up to 0.48 (±0.023) for 100 per cent dispersal; thus, dispersal prevented local adaptation in our experimental system.
Figure 2.
Effect of dispersal rate on mean proportion of sink genotypes (as defined in §2) after approximately 500 generations of evolution in highly heterogeneous environments. The mean proportion of sink genotypes linearly increased with dispersal (r2 = 0.76, F1,10 = 32.47, p = 0.0002). Plotted points show the mean (±s.e.m; n = 3) proportion of sink genotypes in each community across dispersal levels.
(e). The BEF relationship is scale-dependent
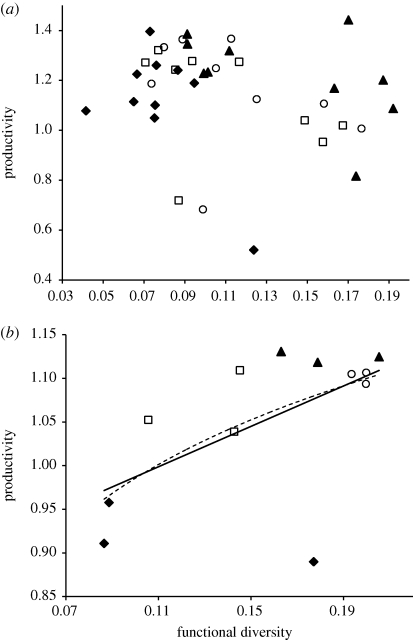
The observed gradient of diversity created by dispersal had different effects on productivity depending on the spatial scale considered (figure 3). At the local scale, FD had no effect on productivity (figure 3a; r2 = 0.0078, F1,38 = 0.2983, p = 0.5881), whereas at the regional scale, FD positively affected productivity (figure 3b; linear fit: r2 = 0.3369, F1,10 = 5.08, p = 0.0479; log-linear fit: r2 = 0.3628, F1,10 = 5.6947, p = 0.0381).
Figure 3.
FD and productivity relationship at (a) local and (b) regional scales. Local FD and local productivity are not related (r2 = 0.0078, F1,38 = 0.2983, p = 0.5881). Regional FD and productivity are positively related (linear fit: r2 = 0.3369, F1,10 = 5.08, p = 0.0479; log-linear fit: r2 = 0.3628, F1,10 = 5.6947, p = 0.0381). At the local scale, each point represents the mean community values over three replicates. At the regional scale, points represent average metacommunity values over three replicates. Filled diamonds, filled triangles, empty circles and empty squares represent 0%, 1%, 10% and 100% dispersal, respectively. The continuous line represents a linear fit and the dashed line represents log-linear fit.
4. Discussion
We initiated our experiment with a single clone of P. fluorescens SBW25, allowing emergent communities to naturally assemble by only controlling the amount of dispersal between them, so all the observed diversity exclusively emerged by mutation during the experiment. Migration has contrasting effects on local specialization and diversity. On the one hand, the increase in mutation supply rate provided by immigration increases the rate of adaptation to poor environments, resulting in an increase in productivity in patches that support poor growth of the ancestral clone. At higher dispersal, however, the massive arrival of maladapted mutants was detrimental for local adaptation (i.e. migration load), and the composition of the entire metacommunity becomes homogenized, resulting in a decrease in diversity and productivity (Venail et al. 2008).
(a). Evidence for source-sink dynamics
The consequences of dispersal on species coexistence have previously been tested (Cadotte 2006a). However, the predicted positive effect of dispersal on local diversity has only been well documented under homogeneous environmental conditions (Kneitel & Miller 2003; Cadotte 2006b; Matthiessen & Hillebrand 2006). Empirical evidence of true source-sink dynamics (i.e. a combination of mass effects and regional species sorting) is still scarce (Forbes & Chase 2002; Cottenie et al. 2003). Here, we present clear evidence of source-sink dynamics in experimental metacommunities based on combined actions of regional environmental heterogeneity, species sorting and dispersal as mechanisms for species coexistence.
Local diversity was shown to be highly correlated with regional diversity, exhibiting the same hump-shaped pattern as a function of dispersal. Given that local communities experienced single homogeneous environments (i.e. only one carbon source per patch), no local niche partitioning was expected. It is therefore likely that the hump-shaped pattern observed at this scale was because of immigration from the regional pool. This is confirmed by an increase in the proportion of ‘sink’ genotypes with dispersal, showing that continuous dispersal from ‘sources’ maintained genotypes not adapted to local conditions. At higher dispersal, local and regional diversity converged, confirming the homogenization pattern predicted from source-sink theory (Mouquet & Loreau 2003).
Our results differ from purely ecological models of source-sink metacommunities that do not include speciation dynamics (Mouquet & Loreau 2002, 2003), which predict maximal regional diversity in closed communities at equilibrium. In these models, higher regional diversity is based on higher differentiation among communities (i.e. beta diversity). Our experiment addressed the emergence of diversity by starting with a single clone and by measuring diversity after approximately 500 generations of evolution. It is probable that our communities were not at evolutionary equilibrium (i.e. complete regional niche differentiation). Differentiation among closed communities is slower when compared with open ones, and thus we suggest that the hump-shaped dispersal–regional diversity pattern observed may be evolutionarily transient. Despite this transient pattern, we found evidence for ‘ecological’ source-sink dynamics. This illustrates that source-sink dynamics can also result in more complex patterns for the relationship between local (i.e. alpha) and regional (i.e. gamma) diversity than expected from equilibrium ecological models.
Predictions of the relationship between alpha, beta and gamma diversity exist for alternative metacommunity models such as the neutral model (Economo & Keitt 2008). The mechanisms generating the patterns are, however, fundamentally different. In neutral metacommunities, the differentiation between communities observed without dispersal is a consequence of the action of both local and regional stochastic effects (i.e. drift). As dispersal increases, the communities converge because gamma diversity decreases and alpha diversity increases because of mass effect (Economo & Keitt 2008). Our experimental results, while sharing some of the tendencies found for neutral metacommunities, differ from this perspective because we found clear evidence of local specialization and niche differentiation at the regional scale (Venail et al. 2008), as well as the presence of non-adapted genotypes increased with dispersal. In the future, it would be interesting to compare our results with experiments performed with purely neutral metacommunities based on the same experimental design but with a homogeneous environment at the regional scale (e.g. a single carbon source in every patch).
(b). Scaling up the BEF relationship
We addressed the BEF relationship at both regional and local scales. If the mechanisms determining species assemblages vary with spatial scale, it is likely that different BEF relationships will be obtained for each scale considered (Bond & Chase 2002; Mouquet & Loreau 2003; Cardinale et al. 2004; Gross & Cardinale 2007). Our results strongly support this prediction. In our experimental metacommunities, local coexistence is based on mass effects, while regional coexistence is based on niche differentiation (i.e. specialization on different resources). As expected, we found a positive BEF relationship only at the regional scale where niche differentiation occurs. Under the complementarity hypothesis (Tilman et al. 1997; Loreau 1998), multiple resources are required for a positive biodiversity–productivity relationship; and the more potential niches available, the more the positive effects of diversity on productivity should be expressed (Cardinale et al. 2000; Wacker et al. 2008; Weis et al. 2008; Replansky & Bell 2009). In our experiment, it is at the regional scale that most diversity has evolved and that niches have differentiated. Thus, even if by increasing diversity, dispersal shifted the effects of regional habitat heterogeneity to the local scale, this did not result in a positive relationship between diversity and productivity.
The source-sink metacommunity model developed by Mouquet & Loreau (2003) predicts a positive BEF relationship at the regional scale, while a more complex pattern may emerge at the local scale with both positive and negative relationships. In their model, maximal local productivity is expected at equilibrium for closed communities when species optimally fit environmental conditions. The arrival of poor competitors through the mass effect hinders local productivity resulting in a negative BEF relationship. Our results confirm a previous experiment (Mouquet et al. 2004) showing that even if the mass effect can be strong enough to increase local species diversity, it will not necessarily result in the expected decrease of local productivity.
Other scale-dependent relationships have been proposed between species richness and productivity (Loreau et al. 2001; Chase & Leibold 2002; Schmid 2002; Chalcraft et al. 2004). For instance, it has been hypothesized that by considering feedbacks between productivity (e.g. fertility) and biodiversity, some counterintuitive scaling relationships might emerge (Waide et al. 1999; Chase & Leibold 2002; Gross & Cardinale 2007). At the local scale, diversity ought to be maximal for intermediate productivity, while diversity and productivity are positively linked at larger scales (Dodson et al. 2000; Chase & Leibold 2002; Chase & Ryberg 2004). Even if these empirical results consider another definition of productivity (i.e. closer to fertility), they also suggest that the BEF relationship cannot be understood without considering the appropriate scale for the mechanism controlling biodiversity and productivity. In the future, it would be interesting to investigate how all these different scale-dependent relationships interact to shape the realized BEF relationship observed in the field.
The experiment presented in this paper is obviously an oversimplification of real environments. Controlled experimentation was central however in disentangling the combined actions of regional species sorting and mass effect in shaping the BEF relationship. Moreover, we have considered only one aspect of the BEF relationship (i.e. productivity–diversity), while many other ecosystem functions could be associated with species diversity (Hooper et al. 2005). For example, the ‘insurance hypothesis’ proposes that species which are functionally redundant for an ecosystem process might show some kind of complementarity in temporally fluctuating environments, increasing long-term community productivity and stability (Yachi & Loreau 1999). It is thus likely that the local diversity maintained in our experiment through the mass effect could buffer local communities from environmental fluctuations. This could lead to a positive relationship between local diversity and mean temporal productivity, such as that suggested in the spatial and temporal insurance hypothesis (Loreau et al. 2003).
Two decades of intense work have revealed that there is no single pattern linking biodiversity to ecosystem functioning. Our study supports the spatial scale-dependence of the BEF relationship in source-sink metacommunities by manipulating the mechanisms of species coexistence and allowing communities to assemble through natural ecological and evolutionary processes. Spatial heterogeneity generated regional diversification, while dispersal produced a hump-shaped pattern of FD at both local and regional scales. We found that the relationship between biodiversity and productivity was null at the local scale and positive at the regional scale, where complementarity among species occurred. More complex experiments combining other factors controlling species diversity over short and large scales are now needed. For instance, understanding diversity organization also requires encompassing different temporal scales (Johnson & Stinchcombe 2007; Fussmann et al. 2007; Urban et al. 2008). Our experiment suggests a transient evolutionary state for the relationship between regional and local species richness. We expect interesting results to emerge from experiments combining longer ecological and evolutionary time scales.
Acknowledgements
We thank D. Mouillot for helpful discussion. P. A. Venail and N. Mouquet are supported by the ‘Programme National EC2CO’. N. Mouquet is supported by the ‘Centre National de la Recherche Scientitique’ and a grant from the ‘Agence Nationale pour la Recherche’ (ANR-09-JCJC-0110-01).
References
- Amarasekare P., Nisbet R. M.2001Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am. Nat. 158, 572–584 (doi:10.1086/323586) [DOI] [PubMed] [Google Scholar]
- Balvanera P., Pfisterer A. B., Buchmann N., He J. S., Nakashizuka T., Raffaelli D., Schmid B.2006Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156 (doi:10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- Barrett R. D. H., MacLean R. C., Bell G.2005Experimental evolution of Pseudomonas fluorescens in simple and complex environments. Am. Nat. 166, 470–480 (doi:10.1086/444440) [DOI] [PubMed] [Google Scholar]
- Bell G.1990The ecology and genetics of fitness in Chlamydomonas. 1. Genotype-by-environment interaction among pure strains. Proc. R. Soc. B 240, 295–321 (doi:10.1098/rspb.1990.0039) [Google Scholar]
- Bond E. M., Chase J. M.2002Biodiversity and ecosystem functioning at local and regional spatial scales. Ecol. Lett. 5, 467–470 (doi:10.1046/j.1461-0248.2002.00350.x) [Google Scholar]
- Cadotte M. W.2006aDispersal and species diversity: a meta-analysis. Am. Nat. 167, 913–924 (doi:10.1086/504850) [DOI] [PubMed] [Google Scholar]
- Cadotte M. W.2006bMetacommunity influences on community richness at multiple spatial scales: a microcosm experiment. Ecology 87, 1008–1016 (doi:10.1890/0012-9658(2006)87[1008:MIOCRA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Cardinale B. J., Ives A. R., Inchausti P.2004Effects of species diversity on the primary productivity of ecosystems: extending our spatial and temporal scales of inference. Oikos 104, 437–450 (doi:10.1111/j.0030-1299.2004.13254.x) [Google Scholar]
- Cardinale B. J., Nelson K., Palmer M. A.2000Linking species diversity to the functioning of ecosystems: on the importance of environmental context. Oikos 91, 175–183 (doi:10.1034/j.1600-0706.2000.910117.x) [Google Scholar]
- Chalcraft D. R., Williams J. W., Smith M. D., Willig M. R.2004Scale dependence in the species-richness–productivity relationship: the role of species turnover. Ecology 85, 2701–2708 (doi:10.1890/03-0561) [Google Scholar]
- Chase J. M., Leibold M. A.2002Spatial scale dictates the productivity–biodiversity relationship. Nature 416, 427–430 (doi:10.1038/416427a) [DOI] [PubMed] [Google Scholar]
- Chase J. M., Ryberg W. A.2004Connectivity, scale-dependence, and the productivity–diversity relationship. Ecol. Lett. 7, 676–683 (doi:10.1111/j.1461-0248.2004.00622.x) [Google Scholar]
- Cooper V. S., Lenski R. E.2000The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407, 736–739 (doi:10.1038/35037572) [DOI] [PubMed] [Google Scholar]
- Cottenie K., Michels E., Nuytten N., De Meester L.2003Zooplankton metacommunity structure: regional vs. local processes in highly interconnected ponds. Ecology 84, 991–1000 (doi:10.1890/0012-9658(2003)084[0991:ZMSRVL]2.0.CO;2) [Google Scholar]
- Dodson S. I., Arnott S. E., Cottingham K. L.2000The relationship in lake communities between primary productivity and species richness. Ecology 81, 2662–2679 (doi:10.1890/0012-9658(2000)081[2662:TRILCB]2.0.CO;2) [Google Scholar]
- Economo E. P., Keitt T. H.2008Species diversity in neutral metacommunities: a network approach. Ecol. Lett. 11, 52–62 [DOI] [PubMed] [Google Scholar]
- Forbes A. E., Chase J. N.2002The role of habitat connectivity and landscape geometry in experimental zooplankton metacommunities. Oikos 96, 433–440 (doi:10.1034/j.1600-0706.2002.960305.x) [Google Scholar]
- Fukami T., Morin P. J.2003Productivity–biodiversity relationships depend on the history of community assembly. Nature 424, 423–426 (doi:10.1038/nature01785) [DOI] [PubMed] [Google Scholar]
- Fussmann G. F., Loreau M., Abrams P. A.2007Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477 (doi:10.1111/j.1365-2435.2007.01275.x) [Google Scholar]
- Gross K., Cardinale B. J.2007Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am. Nat. 170, 207–220 (doi:10.1086/518950) [DOI] [PubMed] [Google Scholar]
- Harrison S., Safford H. D., Grace J. B., Viers J. H., Davies K. F.2006Regional and local species richness in an insular environment: serpentine plants in California. Ecol. Monogr. 76, 41–56 (doi:10.1890/05-0910) [Google Scholar]
- Hooper D. U., et al. 2005Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (doi:10.1890/04-0922) [Google Scholar]
- Hubbell S. P.2001The unified neutral theory of biodiversity and biogeography Princeton, NJ: Princeton University Press [Google Scholar]
- Jiang L., Pu Z., Nemergut D. R.2008On the importance of the negative selection effect for the relationship between biodiversity and ecosystem functioning. Oikos 117, 488–493 (doi:10.1111/j.0030-1299.2008.16401.x) [Google Scholar]
- Johnson M. T. J., Stinchcombe J. R.2007An emerging synthesis between community ecology and evolutionary biology. Trends Ecol. Evol. 22, 250–257 (doi:10.1016/j.tree.2007.01.014) [DOI] [PubMed] [Google Scholar]
- Kneitel J. M., Miller T. E.2003Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. Am. Nat. 162, 165–171 (doi:10.1086/376585) [DOI] [PubMed] [Google Scholar]
- Leibold M. A., et al. 2004The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613 (doi:10.1111/j.1461-0248.2004.00608.x) [Google Scholar]
- Loreau M.1998Biodiversity and ecosystem functioning: a mechanistic model. Proc. Natl Acad. Sci. USA 95, 5632–5636 (doi:10.1073/pnas.95.10.5632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M., Mouquet N.1999Immigration and the maintenance of local species diversity. Am. Nat. 154, 427–440 (doi:10.1086/303252) [DOI] [PubMed] [Google Scholar]
- Loreau M., et al. 2001Ecology–biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808 (doi:10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- Loreau M., Mouquet N., Gonzalez A.2003Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl Acad. Sci. USA 100, 12 765–12 770 (doi:10.1073/pnas.2235465100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthiessen B., Hillebrand H.2006Dispersal frequency affects local biomass production by controlling local diversity. Ecol. Lett. 9, 652–662 (doi:10.1111/j.1461-0248.2006.00916.x) [DOI] [PubMed] [Google Scholar]
- Mouquet N., Loreau M.2002Coexistence in metacommunities: the regional similarity hypothesis. Am. Nat. 159, 420–426 (doi:10.1086/338996) [DOI] [PubMed] [Google Scholar]
- Mouquet N., Loreau M.2003Community patterns in source-sink metacommunities. Am. Nat. 162, 544–557 (doi:10.1086/378857) [DOI] [PubMed] [Google Scholar]
- Mouquet N., Moore J. L., Loreau M.2002Plant species richness and community productivity: why the mechanism that promotes coexistence matters. Ecol. Lett. 5, 56–65 (doi:10.1046/j.1461-0248.2002.00281.x) [Google Scholar]
- Mouquet N., Leadley P., Meriguet J., Loreau M.2004Immigration and local competition in herbaceous plant communities: a three-year seed-sowing experiment. Oikos 104, 77–90 (doi:10.1111/j.0030-1299.2004.12644.x) [Google Scholar]
- Petchey O. L., Downing A. L., Mittelbach G. G., Persson L., Steiner C. F., Warren P. H., Woodward G.2004Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 104, 467–478 (doi:10.1111/j.0030-1299.2004.13257.x) [Google Scholar]
- Replansky T., Bell G.2009The relationship between environmental complexity, species diversity and productivity in a natural reconstructed yeast community. Oikos 118, 233–239 (doi:10.1111/j.1600-0706.2008.16948.x) [Google Scholar]
- Ricklefs R. E.1987Community diversity—relative roles of local and regional processes. Science 235, 167–171 (doi:10.1126/science.235.4785.167) [DOI] [PubMed] [Google Scholar]
- Schmid B.2002The species richness–productivity controversy. Trends Ecol. Evol. 17, 113–114 (doi:10.1016/S0169-5347(01)02422-3) [Google Scholar]
- Schwartz M. W., Brigham C. A., Hoeksema J. D., Lyons K. G., Mills M. H., van Mantgem P. J.2000Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 122, 297–305 (doi:10.1007/s004420050035) [DOI] [PubMed] [Google Scholar]
- Shmida A., Wilson M. V.1985Biological determinants of species-diversity. J. Biog. 12, 1–20 (doi:10.2307/2845026) [Google Scholar]
- Smith V. H.2007Microbial diversity–productivity relationships in aquatic ecosystems. Fems Micro. Ecol. 62, 181–186 (doi:10.1111/j.1574-6941.2007.00381.x) [DOI] [PubMed] [Google Scholar]
- Srivastava D. S., Vellend M.2005Biodiversity–ecosystem function research: is it relevant to conservation? Ann. Rev. Ecol. Evol. Syst. 36, 267–294 (doi:10.1146/annurev.ecolsys.36.102003.152636) [Google Scholar]
- Tilman D., Lehman C. L., Thomson K. T.1997Plant diversity and ecosystem productivity: theoretical considerations. Proc. Natl Acad. Sci. USA 94, 1857–1861 (doi:10.1073/pnas.94.5.1857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban M. C., et al. 2008The evolutionary ecology of metacommunities. Trends Ecol. Evol. 23, 311–317 (doi:10.1016/j.tree.2008.02.007) [DOI] [PubMed] [Google Scholar]
- Van Ruijven J., Berendse F.2005Diversity–productivity relationships: initial effects, long-term patterns, and underlying mechanisms. Proc. Natl Acad. Sci. USA 102, 695–700 (doi:10.1073/pnas.0407524102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venail P. A., MacLean R. C., Bouvier T., Brockhurst M. A., Hochberg M. E., Mouquet N.2008Diversity and productivity peak at intermediate dispersal rate in evolving metacommunities. Nature 452, 210–257 (doi:10.1038/nature06554) [DOI] [PubMed] [Google Scholar]
- Waide R. B., Willig M. R., Steiner C. F., Mittelbach G., Gough L., Dodson S. I., Juday G. P., Parmenter R.1999The relationship between productivity and species richness. Ann. Rev. Ecol. Evol. Syst. 30, 257–300 (doi:10.1146/annurev.ecolsys.30.1.257) [Google Scholar]
- Wacker L., Baudois O., Eichenberger-Glinz S., Schmid B.2008Environmental heterogeneity increases complementarity in experimental grassland communities. B. Appl. Ecol. 9, 467–474 (doi:10.1016/j.baae.2007.08.003) [Google Scholar]
- Weis J. J., Madrigal D. S., Cardinale B. J.2008Effects of algal diversity on the production of biomass in homogeneous and heterogeneous nutrient environments: a microcosm experiment. PLOS ONE 3, e2825 (doi:10.1371/journal.pone.0002825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachi S., Loreau M.1999Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 (doi:10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]