Abstract
In southwestern USA, the jimsonweed Datura wrightii and the nocturnal moth Manduca sexta form a pollinator–plant and herbivore–plant association. Because the floral scent is probably important in mediating this interaction, we investigated the floral volatiles that might attract M. sexta for feeding and oviposition. We found that flower volatiles increase oviposition and include small amounts of both enantiomers of linalool, a common component of the scent of hawkmoth-pollinated flowers. Because (+)-linalool is processed in a female-specific glomerulus in the primary olfactory centre of M. sexta, we hypothesized that the enantiomers of linalool differentially modulate feeding and oviposition. Using a synthetic mixture that mimics the D. wrightii floral scent, we found that the presence of linalool was not necessary to evoke feeding and that mixtures containing (+)- and/or (−)-linalool were equally effective in mediating this behaviour. By contrast, females oviposited more on plants emitting (+)-linalool (alone or in mixtures) over control plants, while plants emitting (−)-linalool (alone or in mixtures) were less preferred than control plants. Together with our previous investigations, these results show that linalool has differential effects in feeding and oviposition through two neural pathways: one that is sexually isomorphic and non-enantioselective, and another that is female-specific and enantioselective.
Keywords: olfaction, moth, oviposition, Manduca sexta
1. Introduction
Floral traits are remarkable examples of sensory stimuli that are important for mediating a variety of biological interactions, including interactions with pollinators (Stensmyr et al. 2002; Heiling et al. 2004; Raguso 2004). For instance, while providing a reproductive benefit for the plant, floral stimuli can be used by the pollinator for critical life-history functions such as oviposition (Holland & Fleming 1999; Pellmyr 2003).
In southwestern USA, the jimsonweed Datura wrightii (Solanaceae) and the nocturnal sphinx moth Manduca sexta (Sphingidae) form a pollinator–plant and herbivore–plant association (Alarcón et al. 2008; Bronstein et al. 2009). Their flowers, which bloom in a single night, are pollinated by adult moths (Alarcón et al. 2008; Riffell et al. 2008), and the plants serve as food resources for the moth larvae (Mechaber & Hildebrand 2000). Although this is not an obligatory association, as D. wrightii plants are highly self-compatible and set fruit in the absence of visitors or in greenhouse settings (Raguso et al. 2003; Bronstein et al. 2009), fruit set is increased in the presence of pollinators (Bronstein et al. 2009). Importantly, plants can tolerate high levels of defoliation and quickly regrow after herbivory (C. E. Reisenman 2009, personal observations). Moreover, upon herbivory D. wrightii plants reduce photosynthetic rates and redirect resources to storage in the roots (G. Barron-Gafford 2009, personal communication). Larvae of M. sexta develop well on D. wrightii and have physiological adaptations that allow them to feed on plants containing alkaloids that harm other insects (Glendinning 2002; Wink & Theile 2006).
While the simultaneous presence of attractive olfactory and visual stimuli is necessary to elicit nectar feeding in M. sexta (Raguso & Willis 2002, 2005), female moths rely primarily on olfactory cues to locate and identify host plants for oviposition (Sparks 1969, 1973; Ramaswamy 1988; Zhang et al. 1999). In the laboratory, females oviposit on non-flowering D. wrightii, indicating that vegetative volatiles mediate recognition and acceptance of host plants. It is possible, however, that in nature floral volatiles (FVs) play a role in mediating oviposition by aiding moths in long-distance olfaction-mediated orientation. This idea is supported by the fact that FVs are emitted at much higher rates than vegetative volatiles (Raguso et al. 2003) and therefore are likely to be detected at greater distances. In addition, females may mix nectar foraging and oviposition when visiting plants with feeding usually preceding oviposition (C. E. Reisenman 2009, personal observations). FVs might also play a role signalling plant quality to ovipositing females.
We thus conducted field, analytical–chemical and behavioural studies to test the components of the floral scent of D. wrightii that might mediate attraction for oviposition. We hypothesized that individual FVs, from among the more than 60 compounds found in the scent of D. wrightii flowers (Raguso et al. 2003), differentially modulate feeding and oviposition behaviours. We tested this idea focusing on linalool, a compound widespread in floral scents (Knudsen et al. 2006) and a common constituent of the floral scent of hawkmoth-pollinated flowers (Miyake et al. 1998; Raguso & Pichersky 1999). Although a minor component in the D. wrightii flower odour (Raguso et al. 2003), linalool is an important FV, mediating nectar foraging in M. sexta (Riffell et al. 2009a). In addition, projection neurons (PNs) associated with a female-specific glomerulus in the primary olfactory centres (the antennal lobes, ALs) of M. sexta, which may be involved in the sensory control of oviposition behaviour (Schneiderman et al. 1986), respond selectively to (+)-linalool (Reisenman et al. 2004; figure S1 in the electronic supplementary material). In contrast, linalool-responsive PNs associated with sexually isomorphic glomeruli (i.e. glomeruli probably involved in olfactory control of feeding behaviour) respond equally well to both enantiomers of linalool (Reisenman et al. 2004).
2. Material and methods
(a). Field experiments
In order to investigate whether flowers are attractive to M. sexta for oviposition in the field, a patch of D. wrightii was examined daily during the spring and summer of 2002 and 2004 in Tucson, Arizona, USA. Each morning, the number of flowers and M. sexta eggs oviposited on any part of the plant were recorded, and eggs and flowers were removed. To prevent leaf damage, the eggs were gently removed by rolling them between two fingers until they detached from the plant.
(b). Feeding experiments
Experiments were conducted to determine whether the enantiomers of linalool have differential effects in attracting female moths for feeding. No-choice feeding experiments were conducted in a wind tunnel (L × W × H = 4.0 × 1.5 × 1.5 m) with individual laboratory-reared, unmated female moths 3 days post-eclosion. Charcoal-filtered air (25 cm s−1) was forced into the upwind end of the wind tunnel and exhausted at the downwind end. FVs were diluted in odourless mineral oil (table 1). Twenty-five microlitres of FV solution or mineral oil alone was applied to an artificial flower made from odourless, white filter paper. An experimental (loaded with a stimulus solution) or control (loaded with mineral oil alone) artificial flower offering 1000 µl of 20 per cent sucrose solution was placed near the upwind end of the wind tunnel. Individual moths were placed in a screen cage on a release platform 0.7 m above the wind-tunnel floor near the downwind end and carefully released after 1 min. The behaviour of each moth was tracked by video recording. Each test finished after 5 min or when the moth extended its proboscis into the paper corolla to feed. In all experiments, moths and flowers were used only once, and each moth was tested with only one of the treatments. Treatments were conducted in a random order, alternating treatments every two tests; control tests were interspersed.
Table 1.
Liquid-phase constituent concentrations in mixtures. Mixture A (italic font) is as the five-component mixture except that it lacks linalool. Mixture B contained FVs different from those in the five-component mixture (terpenoids and aromatics), but was tested at an equal concentration (based on vapour pressure and verified by GC-FID). When linalool was not included in the mixtures, the other constituents were maintained at the same concentration. The purity of components (reported by the manufacturers or established by GC-FID) is indicated in the rightmost column.
| blend | odourant | concentration (μg μl−1) | purity |
|---|---|---|---|
| five-component D. wrightii mimic | |||
| (−/+)-linaloola | 0.036 | ≥99.0 (GC-FID) | |
| mixture A | benzaldehyde | 0.020 | ≥99.5 (Fluka) |
| benzyl alcohol | 4.890 | ≥99.8 (Sigma) | |
| nerol | 0.712 | ≥97.0 (Aldrich) | |
| geraniol | 28.976 | ≥99.0 (Fluka) | |
| mixture Bb | benzyl benzoate | 309.6 | ≥99.0 (Fluka) |
| methyl benzoate | 4.472 | ≥99.5 (Fluka) | |
| α-terpineol | 180 | ≥97.0 (Fluka) | |
| geranyl acetone | 34.4 | ≥98.0 (Fluka) | |
aThe same concentration of either (−) or (+) enantiomer was used in the odour mixture.
bThe (+) enantiomer of linalool was tested in this mixture (mixture B with (+)-linalool added) at the same concentration as in the five-component mixture.
(c). Oviposition experiments
Because we found that flowering plants attract moths for oviposition in field experiments (figure 1a), we tested which FVs might mediate this attraction in the laboratory, focusing on linalool. Dual-choice experiments were conducted in a screened flight cage (2 × 2 × 2 m). In each trial, two D. wrightii plants of the same cohort, and as similar as possible in terms of height, foliage and leaf size, were positioned 1.5 m apart. One plant presented a paper flower loaded with different blends of FVs (see below) and the other presented a paper flower loaded with mineral oil alone (figure S1c in the electronic supplementary material). Laboratory-reared female moths 1.5 days post-eclosion were mated and tested individually during the first 2–2.5 h of scotophase in the following night. Each moth was allowed to lay eggs during 10 min after taking flight, and the eggs oviposited in any part of each plant were carefully removed and counted. Moths that did not oviposit were discarded. Tests with fewer than five eggs, or cases in which eggs did not hatch, were discarded. The positions of the experimental and control plants were alternated between tests. Each plant was used only once (in a few cases twice) per night and reused only after at least one week.
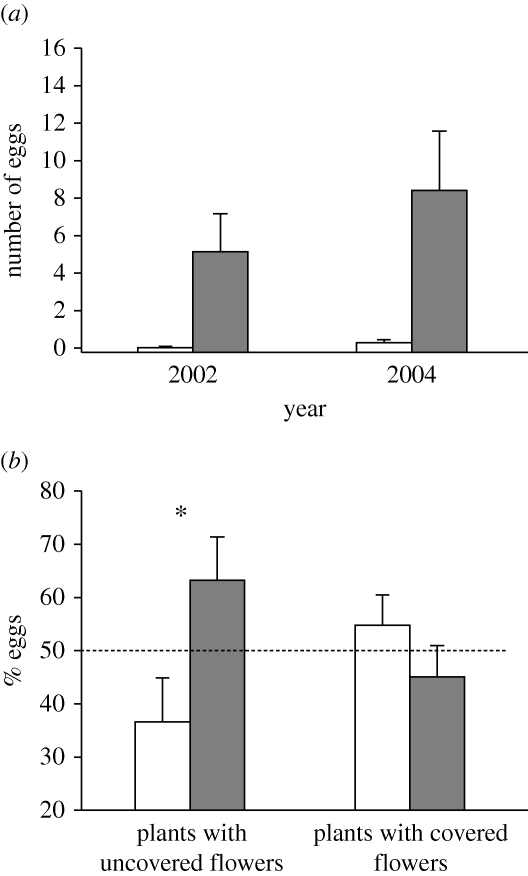
Figure 1.
The presence of flowers on D. wrightii plants attracts female M. sexta for oviposition. (a) A D. wrightii plant was examined daily, and the numbers of flowers and M. sexta eggs were recorded and removed. Shown are the number of eggs (mean ± s.e.) in days when the plant had flowers (n = 42 in 2002; n = 15 in 2004) and in days in which the plant did not have flowers (n = 99 in 2002; n = 56 in 2004). The number of flowers in flowering plants was, respectively, 4.16 ± 0.87 and 8.4 ± 3.17 (mean ± s.e.) in 2002 and 2004. White bars, plants without flowers; black bars, plants with flowers. (b) Individual laboratory-reared and mated female M. sexta moths were presented with a plant with a flower and a plant with an odourless paper flower, both uncovered (left, n = 12) or covered (right, n = 14) with transparent plastic bags. Data represent the percentage of eggs (mean ± s.e.) laid on each plant. The asterisk (*) indicates statistically significant differences (sign test, p < 0.05). White bars, plant with a paper flower; black bars, plant with a real flower.
(d). Olfactory stimuli
Different floral mixtures were used in both feeding and oviposition experiments to test the role of FVs in mediating these two behaviours. The enantiomeric composition of linalool in these mixtures was manipulated to test whether these compounds have differential effects in mediating feeding and oviposition. The identities and amounts of FVs used in these mixtures are listed in table 1. The five-component mixture (containing racemic linalool) has been shown to be an effective mimic of the D. wrightii floral scent in eliciting feeding behaviour in naive moths (Riffell et al. 2009a,b). This mixture thus served as the basis to determine the effect of the linalool enantiomers on oviposition. Mixture A was identical to the five-component mixture but without linalool (table 1). To further test the effectiveness of (+)-linalool as an attractant for oviposition, we prepared a synthetic mixture (mixture B, table 1) containing D. wrightii FVs that elicit responses in M. sexta olfactory receptor cells (Shields & Hildebrand 2000). The components of mixture B were pseudo-randomly selected from among those of the D. wrightii floral scent to be different from, but to represent the chemical classes of, the FVs in mixture A (i.e. terpenoid and aromatic components). They were present at levels corresponding to those of their counterparts in mixture A and not at their own concentrations in the actual floral scent.
(e). Collection and analysis of plant volatiles
Vegetative volatiles and FVs from D. wrightii were collected and analysed to determine which enantiomer(s) of linalool is (are) released by the plants. Flowers were sampled (16 flowers from 12 plants) from D. wrightii plants located in three sites in the Santa Rita Experimental Range, 45 km south of Tucson, Arizona, USA, at the base of the Santa Rita mountains (31°78′ N, 110°82′ W; 1320 m. a.s.l.; Riffell et al. 2008). In addition, eight flowers from eight greenhouse plants were sampled, yielding results consistent with those from field-collected flowers (data not shown). For collection of volatiles, living flowers in greenhouse and field populations of D. wrightii and single branches of potted intact greenhouse-grown D. wrightii plants were enclosed in transparent vinyl oven bags (Reynolds) cinched at 500 ml (Riffell et al. 2008). Portable diaphragm vacuum pumps (10D1125, Gast Manufacturing Inc., Benton Harbor, MI, USA) were used to pull headspace air through sorbent-cartridge traps at a flow rate of 250 ml air per minute. Collections began near sunset and continued overnight for up to 12 h. Traps were constructed by packing 100 mg of Super Q adsorbent (Alltech, Deerfield, IL, USA; mesh size 80–100) into borosilicate glass tubes (7 mm outer diameter) plugged with silanized glass wool. Trapped volatiles were eluted from cartridges using 400 µl of n-hexane and analysed using gas chromatography–time-of-flight mass spectrometry (GC-TOF-MS). Eluted volatiles were stored at −80°C until used. For analysis, 1 µl of the sample was subjected to GC-TOF-MS using a system comprising an HP 6890 (Agilent Technologies, Palo Alto, CA, USA) gas chromatograph and a TOF-MS (Waters Corporation, Milford, MA, USA). Two GC columns (J&W Scientific, Folsom, CA, USA) were used: DB1 (30 m, 0.25 mm, 0.25 µm) and Chiral SilB (30 m, 0.25 mm, 0.25 µm). Helium was used as a carrier gas at a constant flow rate of 1 ml m−1. For determination of volatile organic components using the DB1 column, the initial oven temperature was 50°C for 5 min followed by a heating rate of 6°C per min until 230°C was reached, and held isothermally for further 6 min. For the Chiral SilB column, peak identification was made using TOF-MS with 70 eV electron impact ionization. Chromatogram peaks were tentatively identified through use of the NIST mass spectral library (approx. 120 000 spectra) and verified by GC with authentic standards. Peak areas for each compound were integrated using Micromass MassLynx software (Waters Corporation) and are presented as percentage of total volatiles emitted. The peak area for each compound was quantified using either an internal standard (n-nonyl acetate) or through a five-point standard (0.1 ng to 1 mg) of the synthetic odourants, and expressed in units of nanograms per hour and as percentage of total emissions.
(f). Statistical analysis
Spearman rank order correlation tests (Zar 1999) were conducted to establish whether the number of eggs and flowers in field experiments were significantly correlated. Sign tests were conducted to establish whether females laid more eggs on one of the two plants. χ2-tests were used to compare the proportion of females that feed on flowers bearing different odour mixtures. A one-way ANOVA was conducted to compare the emission rates of linalool in flowers and in the synthetic blends. In all cases, statistical tests were considered significant if p < 0.05.
3. Results
(a). Datura wrightii flowers attract M. sexta for oviposition in field and laboratory experiments
While it is well known that D. wrightii plants attract M. sexta for oviposition (e.g. Mechaber et al. 2002), our results show that flowers also are important in mediating this attraction. In field experiments, we consistently found that the numbers of eggs oviposited on plants were significantly higher on nights when plants had flowers (figure 1a). The numbers of flowers and of M. sexta eggs were highly correlated (r = 0.55, n = 141 and r = 0.67, n = 71 for 2002 and 2004, respectively; in both years p < 0.0001). We therefore tested next whether FVs attract moths for oviposition (in this manuscript, the words ‘attractive’ and ‘repulsive/unattractive’ are used to refer to tests in which experimental plants receive, respectively, more or fewer eggs than control plants). Individual females were presented with a flowering D. wrightii plant (bearing one flower) and a plant with a scentless white-paper (artificial) flower (figure S2a and movie S1 in the electronic supplementary material), and allowed to lay eggs for 10 min. Females laid more eggs on the plant with the real flower (sign test, Z = 2.02, n = 12, p < 0.05; figure 1b). To test whether visual floral cues alone can attract moths for oviposition, real and paper flowers were covered with transparent plastic bags (figure S2b in the electronic supplementary material). Moths chose randomly between plants with real and paper flowers (sign test, Z = 0.27, n = 14, p > 0.5; figure 1b), supporting the hypothesis that floral scent attracts moths for oviposition.
(b). Analysis of plant volatiles
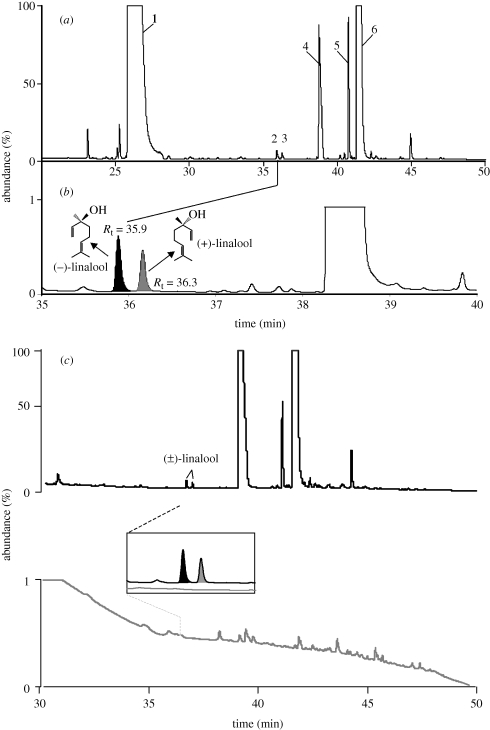
Because D. wrightii FVs attract moths for both feeding (Raguso & Willis 2002; Riffell et al. 2008, 2009a) and oviposition (figure 1b), we hypothesized that certain constituents of the floral scent—in particular linalool—differentially affect these two behaviours. As a first step in testing this possibility, we examined the enantiomeric composition of linalool emitted by D. wrightii flowers and foliage. We found that flowers produce small (less than 1 per cent of the total FV emissions) but consistent amounts of both enantiomers of linalool (figure 2a,b and table 2; figure S3 in the electronic supplementary material), whereas foliage of intact D. wrightii released no detectable linalool (figure 2c, bottom). These results indicate that linalool is a flower-specific scent component, at least during scotophase.
Figure 2.
Datura wrightii flowers, but not vegetation, produce small but significant amounts of both (+)- and (−)-linalool. Flower and vegetative volatiles were collected overnight and analysed via chiral gas chromatography and mass spectrometry. (a) Total ion chromatogram of the floral headspace. Peak labels are: (1) trans-β-ocimene, (2) (−)-linalool , (3) (+)-linalool, (4) benzyl alcohol, (5) nerol and (6) geraniol. (b) Close-up view of two minor peaks (Rt = 35.9 and 36.3 min) that correspond to the retention times of synthetic standards of (−)-linalool (black peak) and (+)-linalool (grey peak), respectively. (c) GC-MS chiral analysis of D. wrightii vegetative and floral headspace volatiles. Collection of vegetative and floral headspace volatiles was conducted simultaneously. Whereas the floral headspace (upper chromatogram) contains the both enantiomers of linalool, the vegetative scent (lower grey chromatogram) does not. Inset: close-up view of the diagnostic retention times of the linalool enantiomers and the lack of the peaks at similar retention times in the vegetative samples. Peak areas in grey denote the retention times corresponding to (+)-linalool; peak areas in black denote retention times corresponding to (−)-linalool. The flower emits a higher-intensity fragrance than the vegetation (emission rates of 206.1 and 16.3 ng h−1, respectively, for the two samples); note the different y-axis scales.
Table 2.
Quantification of linalool emission rates and enantiomeric composition in the D. wrightii flower and the synthetic mixture. Emission rates (ng h−1) of linalool in the synthetic mixtures were scaled to those of the D. wrightii flower (verified by GC-MS and GC-FID). n = 16 D. wrightii flowers, and 16 paper flowers loaded with the synthetic mixtures. Values in parentheses are s.e.m.
| odour source | emission rate (ng h−1) | percentage (%) |
|---|---|---|
| D. wrightii flower | 0.23 (0.04) | |
| (+)-linalool (%) | 44.22 (0.92) | |
| (−)-linalool (%) | 55.77 (0.92) | |
| five-component D. wrightii mimic | 0.26 (0.07)a | |
| (+)-linalool (%) | 45.25 (0.23) | |
| (−)-linalool (%) | 54.74 (0.23) |
aEmission rates were not significantly different (one-way ANOVA: F1,30 = 0.29, p = 0.59).
(c). Feeding and oviposition are differentially modulated by single floral components
Given that flowers produce both (+)-linalool and (−)-linalool, we tested the effects of these FVs on both the feeding and oviposition behaviours of females. We hypothesized that the enantiomeric composition of linalool does not differentially affect feeding, because many PNs probably involved in processing information about food-related volatile compounds do not discriminate between the enantiomers of linalool (Reisenman et al. 2004).
(i). Feeding experiments
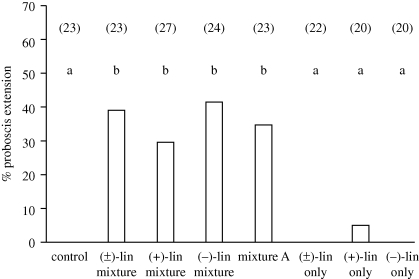
We studied the odour-modulated upwind flight of female moths in a wind tunnel (movie S2 in the electronic supplementary material). We used a synthetic mixture of five FVs (hereafter referred to as five-component mixture; table 1) that is as effective as the natural floral scent in mediating nectar feeding by male M. sexta (Riffell et al. 2009a,b). In each test, a paper flower offering sucrose solution was impregnated with the solvent alone (control) or one of the synthetic scents. Individual unmated females were tested with the following scents (table 1): (i) five-component mixture including both enantiomers of linalool, as in the real flower; (ii) five-component mixture including (+)-linalool; (iii) five-component mixture including (−)-linalool; (iv) four-component mixture (the five-component mixture without linalool; mixture A, table 1); (v) (±)-linalool only; (vi) (+)-linalool only; and (vii) (−)-linalool only. In each case, a majority of moths (n = 151/159; 94.5%) flew upwind, but when they came into contact with the paper flower, their feeding behaviour differed in response to different scents. Of females presented with one of the five-component mixtures (containing one or both enantiomers of linalool) or mixture A, 30 to 40 per cent exhibited feeding behaviour that was different from that evoked by control flowers (figure 3; 2 × 2 χ2-tests, p < 0.05). By contrast, (+)-linalool, (−)-linalool or the mixture of enantiomers presented alone elicited feeding responses in 5 (1/20), 0 (0/20) and 0 per cent (0/22) of the moths (figure 3), respectively, which was comparable to results with the control group (0/20 moths). These results indicate that floral mixtures containing either or both enantiomers of linalool were equally effective in mediating odour-mediated feeding, although the presence of linalool was not critical for this behaviour.
Figure 3.
The chirality of linalool in the five-component mixture had no effect on the feeding behaviour of M. sexta females. In no-choice experiments, individual virgin females were exposed to a paper flower that offered sucrose solution and was impregnated with one of the following: solvent only (control); the five-component mixture with (±)-linalool; the five-component mixture with (+)-linalool; the five-component mixture with (−)-linalool; mixture A; (±)-linalool only; (+)-linalool only; or (−)-linalool only. Data represent the proportion of females within each experimental group that extended their proboscis and contacted the paper corolla. Numbers above bars indicate the number of females tested in each group; different letters indicate statistically significant differences (χ2 2 × 2 tests, p < 0.05).
(ii). Oviposition experiments
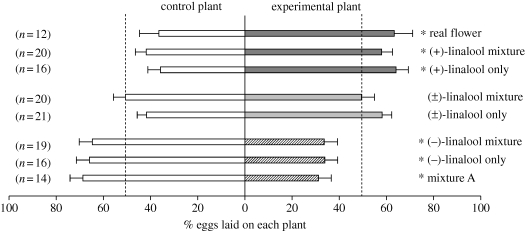
We next tested whether the enantiomers of linalool have differential effects on mediating oviposition, because female-specific PNs respond selectively to (+)-linalool (Reisenman et al. 2004; figure S1 in the electronic supplementary material). Individual mated females were presented with a plant bearing a paper flower impregnated with a scent (as in the feeding experiments) and a plant with a control paper flower (figure S2c in the electronic supplementary material). We found that plants with a paper flower with the mixture containing (+)-linalool received more eggs than control plants (sign test, Z = 2.29, n = 20, p < 0.05; figure 4), while those with the mixture containing (−)-linalool received fewer eggs than control plants (Z = 2.25, n = 16, p < 0.05; figure 4). Similar results were obtained when females were allowed to oviposit overnight (figure S4 in the electronic supplementary material). The attractive effect of (+)-linalool is further demonstrated by the finding that plants bearing a paper flower with a mixture from which this compound was omitted (i.e. use of mixture A; table 1) received significantly fewer eggs than control plants (Z = 2.40, n = 14, p < 0.05; figure 4). Moreover, plants with paper flowers with (+)-linalool alone received significantly more eggs than control plants (Z = 2.99, n = 19, p < 0.005; figure 4). In contrast, plants with paper flowers emitting (−)-linalool alone (linalool enantiomers were tested at the concentration emitted from a living flower; table 2) received fewer eggs than control plants (Z = 3.09, n = 16, p < 0.005; figure 4). When we tested females with the five-component mixture containing both linalool enantiomers or simply with a mixture of linalool enantiomers at the natural ratio, moths oviposited on experimental and control plants randomly (Z = 0.67, n = 20, p > 0.5 and Z = 1.27, n = 21, p > 0.1). Thus, the presence of (+)-linalool could partially reverse the adverse effect of (−)-linalool on oviposition.
Figure 4.
The chirality of linalool in the five-component mixture had opposite effects on the oviposition behaviour of M. sexta. Individual mated females were presented with a control plant (plant with a paper flower impregnated with solvent only) and an experimental plant (plant with a paper flower impregnated with an FV solution) and allowed to lay eggs during 10 min. Results from the experiment with real flowers (figure 1c) are shown for comparison (top). Data represent the percentage of eggs (mean ± s.e.) laid on each plant; numbers in parentheses indicate the number of insects tested in each group. Asterisks indicate statistically significant differences between the control and the experimental plants (sign tests, p < 0.05). The dotted lines indicate the percentage of eggs expected on each plant if oviposition site selection were random.
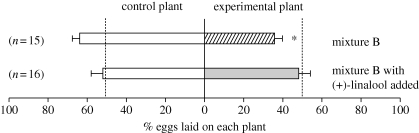
To further test the effectiveness of (+)-linalool as an attractant for oviposition, we used a mixture that lacks linalool (mixture B; table 1) and comprised four D. wrightii FVs different from those in mixture A but at the same total concentration. As with mixture A, which also lacked linalool, moths avoided plants with paper flowers bearing mixture B (sign test, Z = 2.06, n = 15, p < 0.05; figure 5). When (+)-linalool was added to mixture B, however, moths chose experimental and control plants equally (Z = −0.25, n = 16, p > 0.8; figure 5). This experiment confirmed that addition of (+)-linalool to an unattractive floral scent stimulates oviposition.
Figure 5.
Addition of (+)-linalool to an unattractive floral mixture increases oviposition. Individual females were presented with a control plant and an experimental plant with a paper flower impregnated with mixture B or mixture B with (+)-linalool added (table 1). Data represent the percentage of eggs (mean ± s.e.) laid on each plant; numbers in parentheses indicate the number of insects tested in each group. Asterisks indicate statistically significant differences (sign tests, p < 0.05). The dotted lines indicate the percentage of eggs expected on each plant if oviposition site selection were random.
Comparison of experimental series showed that plants with real flowers, with the five-component mixture containing (+)-linalool or with (+)-linalool alone, were equally attractive. In contrast, plants with either of the two mixtures lacking linalool, with the five-component mixture containing (−)-linalool or with (−)-linalool alone were similarly avoided (figure S5a in the electronic supplementary material). In all experimental series, females laid similar average numbers of eggs (figure S5b in the electronic supplementary material), indicating that in all cases females actively oviposited on plants.
4. Discussion
Taking advantage of the plant–pollinator and plant–herbivore association between D. wrightii and M. sexta, we investigated the effects of FVs on feeding and oviposition behaviours. We tested the hypothesis that certain FVs have distinct effects on moth behaviour. In field and laboratory experiments, we found that the presence of flowers on D. wrightii plants attracts moths for oviposition (figure 1) and that the floral-specific volatiles (+)- and (−)-linalool have different effects on feeding and oviposition behaviours (figures 3–5). Plants bearing (+)- or (−)-linalool were, respectively, attractive and repellent to moths for oviposition, while the presence of either or both enantiomers of linalool in mixtures had a neutral effect on nectar feeding.
Our results suggest that (+)-linalool might be an oviposition attractant. For the silkworm Bombyx mori, it has been reported that a highly female-biased olfactory receptor responds to (±)-linalool (Anderson et al. 2009). Linalool is a common component of the scent of moth-pollinated flowers (Miyake et al. 1998; Raguso & Pichersky 1999), and (+)-linalool is the major floral component in the moth-pollinated Clarkia breweri (Raguso & Pichersky 1999). While foliage volatiles from host plants are sufficient to elicit oviposition in laboratory experiments (Mechaber et al. 2002), it is possible that in nature floral (+)-linalool acts in concert with foliage volatiles to attract M. sexta from a distance and/or at close range to elicit oviposition behaviour. If so, then the effects of (+)-linalool parallel the reported scale-dependent effects of CO2 on visits of hungry M. sexta moths to D. wrightii flowers (Goyret et al. 2008). Because it is produced only by flowers, (+)-linalool might indicate plant quality to the moths.
Although D. wrightii flowers emit both enantiomers of linalool (figure 2) and are attractive for oviposition (figure 1), we found that moths avoided ovipositing on plants emitting (−)-linalool (figure 4). It is possible that in our experiments (−)-linalool interferes with the attractiveness of foliage volatiles from host plants (Chapman et al. 1981; Thiéry & Visser 1986) or that (−)-linalool is repellent (Dethier et al. 1960; Hori 1998). Plants defend themselves against insect herbivory by producing volatiles (including linalool; see below) that provide host-location cues for insects that are natural enemies of the herbivores (De Moraes et al. 1998; Baldwin & Preston 1999; Paré & Tumlinson 1999; Schnee et al. 2006). Indeed, feeding by M. sexta larvae on tobacco plants induces systemic production of enantiomerically uncharacterized linalool (De Moraes et al. 2001; Kessler & Baldwin 2001) and decreases oviposition by the closely related moth Manduca quinquemaculata (Kessler & Baldwin 2001). For tomato, a favourite host plant of M. sexta, a monoterpene synthase that produces only (−)-linalool was reported to be induced in response to herbivory (van Schie et al. 2007). Feeding of Nalepella sp. mites on spruce induces release of (−)-linalool (Kännaste et al. 2008). This suggests that (−)-linalool might be a general insect oviposition deterrent produced by larva-damaged foliage, although whether larva-damaged D. wrightii plants produce (−)-linalool remains to be investigated. Larvae of M. sexta grow to be so large that a single larva can completely defoliate its host by the end of its last instar (McFadden 1968). For example, an individual M. sexta larva can process approximately 1700 cm2 of leaves (Heinrich 1971; Casey 1976), which is greater than the size of many D. wrightii plants. Thus, an ovipositing female should avoid plants where potential competitors of her offspring are already present, and we propose that (−)-linalool produced by larva-damaged plants might signal larval presence.
We found that plants with real flowers or paper flowers with the floral mixture containing (+)-linalool were preferred over control plants, while plants with the mixture containing both enantiomers of linalool were as preferred as control plants (figure 4). These results indicate that the reduced five-component floral mixture containing (−)-linalool (which is a negative stimulus) is not as effective as the complete floral odour. Thus, it is likely that the presence of other FVs in combination with both enantiomers of linalool is necessary to mediate oviposition attraction.
Given the opposite behavioural effects and likely functions of (+)- and (−)-linalool, one might ask why D. wrightii flowers produce both enantiomers of linalool. Linalool is produced from geranyl diphosphate, the universal precursor of monoterpenes, in a reaction catalysed by monoterpene synthases that produce either (+)-linalool (Raguso & Pichersky 1999) or (−)-linalool (van Schie et al. 2007). One possibility is that D. wrightii flowers have co-opted the enzymatic pathway producing (−)-linalool to reduce oviposition by moths, a scenario that would not preclude flowers from attracting moths for nectar feeding, and hence pollination. Another possibility is that the relative concentrations of (+)- and (−)-linalool change throughout the season. It has been shown that the rearing environment can have an important effect on floral scent composition, including monoterpenes (Majetic et al. 2009).
We previously found that the presence of a monoterpene with an alcohol functional group is necessary to mediate feeding in male M. sexta, with linalool being highly effective (Riffell et al. 2009b). Although we have not explicitly tested whether this is also the case in females, we found that addition of one or both enantiomers of linalool to floral mixtures that contain other monoterpenoids with an alcohol functional group does not differentially affect feeding. In contrast, we found that oviposition responses are enantioselective, with (+)- and (−)-linalool, respectively, causing attraction and repellence. These findings correlate well with our previous investigations showing that most sexually isomorphic linalool-responsive AL PNs are non-enantioselective, while certain female-specific AL PNs are highly selectively responsive to (+)-linalool (Reisenman et al. 2004). It is possible that the antenna of female M. sexta has different populations of olfactory receptor cells specifically tuned to (+)-linalool and (−)-linalool that project to distinct glomeruli involved in controlling oviposition behaviour, although this remains to be investigated. These results suggest that processing of sensory information about linalool via two distinct olfactory pathways leads to behavioural effects that are context-specific, such that (+)-linalool is attractive for oviposition and (−)-linalool is unattractive or repellent for oviposition but not for feeding.
Acknowledgements
This work was supported by NSF grant IOS 0822709 to C.E.R. and J.A.R. and NIH grant R01-DC-02751 to J.G.H. The authors thank A. Beyerlein, T. A. Christensen, A. Dacks, J. P. Martin, D. Papaj and M. W. Nachman for critically reading the manuscript; A. Zeeger for maintaining the plants; A. Pesque and K. Duffy for help with some of the behavioural experiments; and S. Mackzum and M. Marez for rearing M. sexta. We also thank two anonymous reviewers for their very valuable comments and suggestions.
References
- Alarcón R., Davidowitz G., Bronstein J.2008Nectar usage in a southern Arizona hawkmoth community. Ecol. Entomol. 33, 503–509 (doi:10.1111/j.1365-2311.2008.00996.x) [Google Scholar]
- Anderson A. R., Wanner K. W., Trowell S. C., Warr C. G., Jaquin-Joly E., Zagatti P., Robertson H., Newcomb R. D.2009Molecular basis of female-specific odorant responses in Bombyx mori. Insect Biochem. Mol. Biol. 39, 189–197 (doi:10.1016/j.ibmb.2008.11.002) [DOI] [PubMed] [Google Scholar]
- Baldwin I. T., Preston C. A.1999The eco-physiological complexity of plant responses to insect herbivores. Planta 208, 137–145 (doi:10.1007/s004250050543) [Google Scholar]
- Bronstein J. L., Huxman T., Horvath B., Farabee M., Davidowitz G.2009Reproductive biology of Datura wrightii: the benefits of a herbivorous pollinator. Ann. Bot. 103, 1435–1443 (doi:10.1093/aob/mcp053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey T. M.1976Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae). Ecology 57, 485–497 (doi:10.2307/1936433) [Google Scholar]
- Chapman R. F., Bernays E. A., Simpson S. J.1981Attraction and repulsion of the aphid, Cavariella aegopodii, by plant odors. J. Chem. Ecol. 7, 881–888 (doi:10.1007/BF00992385) [DOI] [PubMed] [Google Scholar]
- De Moraes C. M., Lewis W. J., Paré P. W., Alborn H. T., Tumlinson J.1998Hervibore-infested plants selectively attract parasitoids. Nature 393, 570–573 (doi:10.1038/31219) [Google Scholar]
- De Moraes C. M., Mescher M. C., Tumlinson J. H.2001Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580 (doi:10.1038/35069058) [DOI] [PubMed] [Google Scholar]
- Dethier V. G., Browne B., Smith C. N.1960The designation of chemicals in terms of the responses they elicit from insects. J. Ecol. Entomol. 53, 134–136 [Google Scholar]
- Glendinning J. I.2002How do herbivorous insects cope with noxious secondary plant compounds in their diet? Entomol. Exp. Appl. 104, 15–25 (doi:10.1023/A:1021271717409) [Google Scholar]
- Goyret J., Markwell P. M., Raguso R. A.2008Context- and scale-dependent effects of floral CO2 on nectar foraging by Manduca sexta. Proc. Natl Acad. Sci. USA 105, 4565–4570 (doi:10.1073/pnas.0708629105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiling A. M., Cheng K., Herberstein M. E.2004Exploitation of floral signals by crab spiders (Thomisus spectabilis, Thomisidae). Behav. Ecol. 15, 321–326 (doi:10.1093/beheco/arh012) [Google Scholar]
- Heinrich B.1971The effect of leaf geometry on the feeding behavior of the caterpillar of Manduca sexta (Sphingidae). Anim. Behav. 19, 119–124 (doi:10.1016/S0003-3472(71)80145-8) [Google Scholar]
- Holland J. N., Fleming T. H.1999Mutualistic interactions between Upiga virescens (Pyralidae), a pollinating seed-consumer, and Lophocereus schottii (Cactaceae). Ecology 80, 2074–2084 [Google Scholar]
- Hori M.1998Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 24, 1425–1432 (doi:10.1023/A:1020947414051) [Google Scholar]
- Kännaste A., Vongvanich N., Borg-Karlson A.-K.2008Infestation by a Nalepella species induces emissions of α- and β-farnesenes, (−)-linalool and aromatic compounds in Norway spruce clones of different susceptibility to the large pine weevil. Arthropod Plant Interact. 2, 31–41 (doi:10.1007/s11829-008-9029-4) [Google Scholar]
- Kessler A., Baldwin I. T.2001Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 (doi:10.1126/science.291.5511.2141) [DOI] [PubMed] [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J., Stål B.2006Diversity and distribution of floral scent. Bot. Rev. 72, 1–120 (doi:10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2) [Google Scholar]
- Majetic C. J., Raguso R. A., Ashman T. L.2009Sources of floral scent variation. Can environment define floral scent phenotype? Plant Signal. Behav. 4, 129–131 (doi:10.4161/psb.4.2.7628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden M. W.1968Observations on feeding and movement of tobacco hornworm larvae. J. Econ. Entomol. 61, 352–356 [Google Scholar]
- Mechaber W., Hildebrand J. G.2000Novel, non-solanaceous host-plant record for Manduca sexta (Lepidoptera: Sphingidae) in the southwestern United States. Ann. Entomol. Soc. Am. 93, 447–451 (doi:10.1603/0013-8746(2000)093[0447:NNSHRF]2.0.CO;2) [Google Scholar]
- Mechaber W., Capaldo C. T., Hildebrand J. G.2002Behavioral responses of adult female tobacco hornworms, Manduca sexta, to hostplant volatiles change with age and mating status. J. Insect Sci. 2, 5 See http://www.insectscience.org/2.5/ [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Yamaoka R., Yahara T.1998Floral scents of hawkmoth-pollinated flowers in Japan. J. Plant Res. 111, 199–205 (doi:10.1007/BF02512170) [Google Scholar]
- Paré P. W., Tumlinson J. H.1999Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–331 (doi:10.1104/pp.121.2.325) [PMC free article] [PubMed] [Google Scholar]
- Pellmyr O.2003Yuccas, yucca moths, and coevolution: a review. Ann. Mo. Bot. Gard. 90, 35–55 (doi:10.2307/3298524) [Google Scholar]
- Raguso R. A.2004Flowers as sensory billboards: progress towards an integrated understanding of floral advertisement. Curr. Opin. Plant Biol. 7, 434–440 (doi:10.1016/j.pbi.2004.05.010) [DOI] [PubMed] [Google Scholar]
- Raguso R. A., Pichersky E.1999A day in the life of a linalool molecule: chemical communication in a plant–pollinator system. Part 1: linalool biosynthesis in flowering plants. Plant Spec. Biol. 14, 95–120 (doi:10.1046/j.1442-1984.1999.00014.x) [Google Scholar]
- Raguso R. A., Willis M. A.2002Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Anim. Behav. 64, 685–695 (doi:10.1006/anbe.2002.4010) [Google Scholar]
- Raguso R. A., Willis M. A.2005Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Anim. Behav. 69, 407–418 (doi:10.1016/j.anbehav.2004.04.015) [Google Scholar]
- Raguso R. A., Henzel C., Buchmann S. L., Nabhan G. P.2003Trumpet flowers of the Sonoran desert: floral biology of Peniocereus cacti and sacred Datura. Int. J. Plant Sci. 164, 877–892 (doi:10.1086/378539) [Google Scholar]
- Ramaswamy S. B.1988Host finding by moths: sensory modalities and behavior. J. Insect Phys. 34, 235–249 (doi:10.1016/0022-1910(88)90054-6) [Google Scholar]
- Reisenman C. E., Christensen T. A., Francke W., Hildebrand J. G.2004Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J. Neurosci. 24, 2602–2611 (doi:10.1523/JNEUROSCI.5192-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell J. A., Alarcón L., Abrell J. L., Bronstein J., Davidowitz G., Hildebrand J. G.2008Behavioral consequences of innate preferences and olfactory learning in hawkmoth–flower interactions. Proc. Natl Acad. Sci. USA 105, 3404–3409 (doi:10.1073/pnas.0709811105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell J. A., Lei H., Christensen T. A., Hildebrand J. G.2009aCharacterization and coding of behaviorally significant odor mixtures. Curr. Biol. 19, 335–340 (doi:10.1016/j.cub.2009.01.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffell J. A., Lei H., Hildebrand J. G.2009bNeural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc. Natl Acad. Sci. USA 106, 19 219–19 226 (doi:10.1073/pnas.0910592106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C., Kollner T. G., Held M., Turlings T. C. J., Gershenzon J., Degenhardt J.2006The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl Acad. Sci. USA 103, 1129–1134 (doi:10.1073/pnas.0508027103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman A. M., Hildebrand J. G., Brennan M. M., Tumlinson J. H.1986Trans-sexually grafted antennae alter pheromone-directed behaviour in a moth. Nature 323, 801–803 (doi:10.1038/323801a0) [DOI] [PubMed] [Google Scholar]
- Shields V., Hildebrand J. G.2000Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. A 186, 1135–1151 (doi:10.1007/s003590000165) [DOI] [PubMed] [Google Scholar]
- Sparks M. R.1969A surrogate leaf for oviposition by the tobacco hornworm. J. Econ. Entomol. 63, 537–540 [Google Scholar]
- Sparks M. R.1973Physical and chemical stimuli affecting oviposition preference of Manduca sexta (Lepidoptera: Sphingidae). Ann. Entomol. Soc. Am. 66, 571–573 [Google Scholar]
- Stensmyr M., Giordano E., Balloi A., Angioy A.-M., Hansson B. S.2003Novel natural ligands for Drosophila olfactory receptor neurons. J. Exp. Biol. 206, 715–724 (doi:10.1242/jeb.00143) [DOI] [PubMed] [Google Scholar]
- Thiéry D., Visser J. H.1986Masking of host plant odour in the olfactory orientation of the Colorado potato beetle. Entomol. Exp. Appl. 41, 165–172 (doi:10.1007/BF00195573) [Google Scholar]
- van Schie C. C., Haring M. A., Schuurink R. C.2007Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 64, 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M., Theile V.2006Alkaloid tolerance in Manduca sexta and phylogenetically related sphingids (Lepidoptera: Sphingidae). Chemoecology 12, 29–46 (doi:10.1007/s00049-002-8324-2) [Google Scholar]
- Zar J. H.1999Biostatistical analysis, 4th edn.Upper Saddle River, NJ: Prentice-Hall Inc [Google Scholar]
- Zhang A. J., Linn C., Wright S., Propoky R., Reissig W., Roelofs W.1999Identification of a new blend of apple volatiles attractive to apple maggot, Rhagoletis pomonella. J. Chem. Ecol. 25, 1221–1232 (doi:10.1023/A:1020910305873) [Google Scholar]