Abstract
Socially acquired information improves the accuracy and efficiency of environmental assessments and can increase fitness. Public information may be especially useful during unpredictable food conditions, or for species that depend on resources made less predictable by human disturbance. However, the physiological mechanisms by which direct foraging assessments and public information are integrated to affect behaviour remain largely unknown. We tested for potential effects of public information on the behavioural and hormonal response to food reduction by manipulating the social environment of captive red crossbills (Loxia curvirostra). Red crossbills are irruptive migrants that are considered sensitive to changes in food availability and use public information in decision making. Here, we show that public information can attenuate or intensify the release of glucocorticoids (i.e. stress hormones) during food shortage in red crossbills. The observed modulation of corticosterone may therefore be a physiological mechanism linking public information, direct environmental assessments and behavioural change. This mechanism would not only allow for public information to affect individual behaviour, but might also facilitate group decision making by bringing group members into more similar physiological states. The results further suggest that stressors affecting entire populations may be magnified in individual physiology through social interactions.
Keywords: corticosterone, activity, red crossbill, food reduction, environmental predictability, migration
1. Introduction
Behaviour is a product of neurological processes that are sensitive to an array of environmental and physiological cues. Cue processing allows an animal to perform context-appropriate behaviours as behavioural needs change across the year (Wingfield 1983). For example, a seasonal change in photoperiod induces the growth of gonads and increases the production of circulating sex steroids in many animals. Sex steroids subsequently prime the neurological circuits that control secondary sexual behaviours and increase the probability that the animal will react to environmental stimuli with the appropriate reproductive behavioural response (Balthazart 1983). In the absence of hormonal change, the animal is less likely to successfully display relevant behaviours (Balthazart 1983). Thus, environmental cues that predict or describe conditions suitable for the expression of a behaviour can change the probability of behavioural expression through hormone effects on neurophysiology.
Glucocorticoids are a class of steroid hormones involved in energy balance, stress and behaviour. There is mounting evidence that glucocorticoids are involved in behavioural transitions that require increased activity and energetic expenditure such as migration, juvenile dispersal, facultative altitudinal movements, fledging and foraging or exploratory behaviour (Astheimer et al. 1992; Silverin 1997; Piersma et al. 2000; Breuner & Hahn 2003; Lohmus et al. 2003). These types of movements often occur in response to environmental changes that restrict food supply, such as storms (Astheimer et al. 1995; Pravosudov et al. 2001; Lynn et al. 2003; Wingfield 2003a,b). In captivity, birds given restricted or unpredictably scheduled food respond with increased corticosterone levels that correlate with increased activity (Elton 1942; Bock & Lepthien 1976; Pravosudov et al. 2001; Lynn et al. 2003; Newton 2006). Thus, changes in activity that are driven by energetic challenges appear likely to be mediated by corticosterone.
Extreme fluctuations in food abundance often lead to long-distance facultative migrations, or irruptions (Elton 1942; Newton 1972), which are common among highly mobile species of birds and mammals. Assessment of the local food supply contributes to the regulation of these migrations and is expected to be the most potent cue inducing irruptive migrations (Svardson 1957; Newton 1972). Inaccurate evaluations of local foraging conditions may result in suboptimal departure decisions, with possibly severe negative fitness consequences. Red crossbills (Loxia curvirostra) are irruptive, nomadic songbirds that specialize on the seeds of coniferous trees, a food supply that is distributed unpredictably in space and time (Bock & Lepthien 1976). North American and northern European red crossbills make annual, predictable nomadic migrations in search of developing cone crops but also make less predictable, facultative irruptive migrations to escape failed or poorly developed cone crops (Svardson 1957). It is thought that crossbills and other irruptive migrants respond directly to food supply to time-irruptive migrations. Poor food supply correlates well with observations of free-living irruptive species on the move (Bock & Lepthien 1976). However, research with captives suggests that red crossbills use public information to assess foraging conditions more accurately and efficiently than they could individually (Smith et al. 1999). An individual given a poor-quality food patch will depart the patch more quickly if other individuals in visual and acoustic proximity experience similarly poor foraging conditions. Public information, therefore, permits more efficient assessment of food availability (Smith et al. 1999).
To test the roles of food supply and public information in the migratory decision making of an irruptive nomad and to determine whether corticosterone is a possible mediator of this process, we restricted food for captive red crossbills, provided a neighbour from either the food-reduced group or the food ad libitum group as a social informant and measured the behavioural and physiological responses in a repeated-measures design. We expected food supply to have a strong effect on behaviour and physiology and public information to modify those responses to the food supply cue. We, therefore, made the following hierarchical predictions concerning changes in corticosterone and activity levels across sampling dates: (i) ad libitum individuals with unlimited access to food would show little or no change in patterns of corticosterone and behaviour, (ii) food-reduced individuals would show increases in both measures, and (iii) in both food-treatment groups, those housed next to food-reduced birds would show a larger change than those housed next to ad libitum birds. Thus, ΔA(a) < ΔA(f) ≪ ΔF(a) < ΔF(f); where A is the ad libitum individual and F is the food-reduced individual and lower case letters in parentheses represent the food supply of the social informant.
2. Material and methods
(a). Birds and food treatment
Twenty-eight adult red crossbills, L. curvirostra, were captured on the Olympic Peninsula in Washington State and transported to facilities at University of California-Davis six months prior to the experiment. The experiment was performed in January and February and birds were housed on naturally changing photoperiod. Performing the experiment in the winter allows for the study of response to a decline in food availability (as can happen as seeds disperse from cones beginning in late summer), but avoids possible confounding effects of the autumn molt on stress physiology (Romero 2002). Both sexes were used in the experiment and were balanced across treatment groups. All individuals were kept on an ad libitum diet of a pellet food and a daily allotment of two pine nuts per bird for one month prior to any manipulations. Data were collected from each individual in a repeated-measures design with pre-treatment (day 0), during-treatment (day 15) and post-treatment (day 24) sampling points. During the treatment period, experimental individuals were food-reduced to 75 per cent of their average daily intake of pellets and no pine nuts. After 78 h of food reduction, data were collected and all birds were returned to ad libitum pellets and the daily allotment of pine nuts.
(b). Social treatment
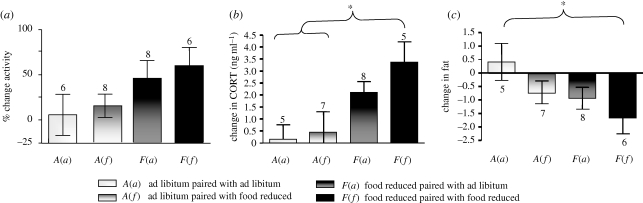
Individuals were housed on shelves in acoustic isolation chambers (IAC 250 ‘Mini’ Sound Shelters, 61 cm wide by 86 cm deep by 168 cm high inside dimensions; Industrial Acoustics Company, Bronx, New York, USA) next to another individual of the same sex that it could both see and hear. Four social and food-treatment groups were created by housing individuals in the following configurations: ad libitum with an ad libitum neighbour A(a), ad libitum with a food-reduced neighbour A(f), food-reduced with an ad libitum neighbour F(a) and food-reduced with a food-reduced neighbour F(f). Sample sizes between social treatments were unequal. We began the food supply acclimation period with 28 birds, six per pure social treatment group and eight per mixed social treatment group. However, two birds were lost during the treatment period sampling date (day 15) owing to arterial haemorrhage during bleeding (a problem that had not previously occurred in our experience bleeding red crossbills). Fat was not scored in the deceased individuals as changes in posture and loss of blood pressure may have influenced scoring methods. Activity data, however, were collected prior to the treatment period and are therefore included from these individuals (figure 2). One additional sample was lost during blood processing owing to a capillary tube seal failure. In the two cases where a bird was lost from the experiment, two unpaired birds remained. These unpaired individuals were re-paired together, but were not included in analyses of social treatment. Inclusion of these samples, however, did not change the statistical outcomes.
Figure 2.
Effects of public information on changes in behaviour and physiology during the food-treatment period. (a) Activity. (b) Baseline corticosterone and (c) fat deposition were only significantly altered by food reduction if the public informant was similarly food-reduced (F(f) group). Social pairing with a well-fed control individual attenuated the response to food reduction (F(a) group). Bars represent group averages with s.e.m. Asterisks denote p < 0.05 by Tukey comparison of means test.
(c). Activity data
Activity data were collected using Hi-8 Sony camcorders. Activity levels were later scored on video by dividing the cage into quadrants and recording the number of times the bird entered a new quadrant during a 90 min filming session. Final activity levels reflect an average across 3 filming days for each of the three sampling periods. Time of filming for each individual was held constant across all sampling periods. Activity was scored blind with respect to subject identification and sampling period. Intra-observer error was tested by re-scoring 10 randomly chosen videos and was less than 2 per cent.
(d). Body condition and blood samples
Body mass was measured to the nearest 0.1 g using a Pesola spring scale and furcular and abdominal fat was ranked on a scale of 0 (no fat) to 5 (bulging; after Helms & Drury 1960). Blood samples were collected by puncture of the alar vein with a 26 gauge needle and 80–100 μl was collected into heparinized microhaematocrit capillary tubes. Plasma corticosterone concentrations rise significantly in small songbirds within 3 min of capture (Wingfield et al. 1982). We therefore collected baseline blood samples within 3 min of opening the door of the isolation chamber and took samples from both shelf neighbours at the same time. Time of day of sampling was standardized for each individual across the pre-, during- and post-treatment bleeds, and all individuals were sampled prior to 12.00. Samples were stored on ice until centrifugation, at which point plasma was separated from cellular fraction. Plasma samples were then kept at −20°C until the assays were performed.
(e). Corticosterone assay
Plasma corticosterone concentrations were determined using Enzyme Immunoassay kits (Cat. no. 901-097) purchased from Assay Designs. Plasma dilution and steroid displacement buffer (SDB) concentrations were optimized for red crossbills at 1 : 40 dilution and 1 per cent (per raw plasma volume) SDB. Samples were completely randomized and run in duplicate in a single assay using 11 plates, each with a separate standard curve and hormone standard. Intra-assay coefficient of variation was calculated at 5.2 per cent and inter-plate coefficient of variation was calculated at 4.5 per cent. Sensitivity was determined separately for each of the 11 plates by taking 2 s.d. away from the mean of blank wells. Using data directly from the curves to calculate sensitivity is a more conservative and plate-specific method than that provided by the kit manufacturer. Values that were below the limit of detection for each plate were assigned the minimum sensitivity of that plate. Average sensitivity was 2.25 pg per well (0.9 ng ml−1) and actual sensitivities were 0.60, 0.60, 0.60, 0.62, 0.62, 0.80, 0.80, 0.96, 1.20 and 2.50 ng ml−1. Exclusion of samples from the least sensitive plate (2.50 ng ml−1) does not change the results of the experiment and are therefore included.
(f). Statistical analyses
Treatment effects on physiology and behaviour were first determined in a general linear model. Effects of food treatment on CORT, fat and activity were then analysed using a repeated-measures ANOVA followed by Tukey–Kramer HSD comparisons of means. Owing to lower sample sizes, social treatment effects were analysed using the non-parametric Jonkheere test for ordered alternatives. We selected the Jonkheere method because it tests the null hypothesis of equality among treatments against the alternative in which order is specified and can therefore test our predicted hierarchy. However, because the Jonkheere test is prone to Type 1 error and because the data are normally distributed, we also performed an ANOVA and post hoc Tukey–Kramer comparison of means.
3. Results
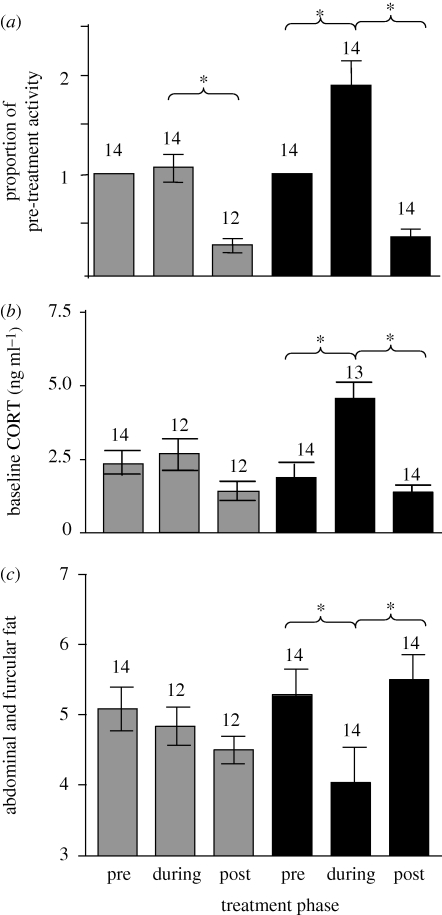
Food reduction caused increased activity levels and baseline levels of circulating corticosterone, and decreased fat stores (figure 1; repeated-measures ANOVA: activity (F2,21 = 2.19, p < 0.05), corticosterone (F2,21 = 5.51, p < 0.05), fat (F2,21 = 7.73, p < 0.005)). In food-reduced birds, corticosterone and activity levels increased significantly during food reduction (Tukey–Kramer HSD, p < 0.05) and returned to pre-treatment levels following return to ad libitum food. Fat deposition decreased significantly during food reduction and returned to pre-treatment levels following return to ad libitum food (Tukey–Kramer HSD, p < 0.05). Ad libitum birds showed no significant changes in fat deposition, activity levels or corticosterone during the treatment phase, but did significantly decrease activity levels in the post-treatment phase of the experiment (Tukey–Kramer HSD, p < 0.05).
Figure 1.
Behavioural and physiological adjustments in captive red crossbills before, during and after food reduction. Food reduction increased activity levels (a) and baseline levels of circulating corticosterone (b), and decreased fat stores (c). During the post-treatment period, all birds were returned to ad libitum food availability, and baseline corticosterone and fat deposition returned to pre-treatment levels in response. Activity was reduced in both treatment groups during the post-treatment sampling period, potentially reflecting effects of public information and the costs of a repeated-measures blood sampling protocol. Bars represent group averages with s.e.m. Asterisks denote significant differences by a Tukey comparison of means test. Grey bars, ad libitum; black bars, food-reduced.
Social treatment significantly predicted baseline total CORT (F3,23 = 5.2, r2 = 0.44, p = 0.008) and fat (F3,23 = 3.15, r2 = 0.32, p = 0.04) in a general linear model. Corticosterone and fat deposition changed in the predicted direction during food and social treatment: A(a) < A(f) ≪ F(a) < F(f) (figure 2; corticosterone: Jonkheere test for ordered alternatives J* = 3.0, p < 0.001, ANOVA F3,23 = 5.21, p = .008; fat: Jonckheere test for ordered alternatives J*=1.65, p < 0.05, ANOVA F3,23 = 3.15, p < 0.05). Activity levels matched the predicted relationships but not significantly so (ANOVA F3,23 = 2.02, r2 = 0.23, p = 0.14). Baseline corticosterone and fat deposition of food-reduced birds were only significantly different from ad libitum birds during food treatment if paired with a food-reduced social informant (figure 2; Tukey–Kramer HSD, p < 0.05).
4. Discussion
In agreement with our predictions, and consistent with the hypothesis that food-supply cues induce irruptive migratory behaviour, we found that captive red crossbills limited to 75 per cent of their ad libitum daily intake of pellet food, and denied access to favoured food items significantly increased their activity levels (figure 1a). Baseline plasma levels of corticosterone significantly increased in food-restricted birds (figure 1b) and fat deposits decreased (figure 1c), suggesting that the food restriction resulted in negative energy balance and increased fat mobilization. This suggests that there are relationships between declining body condition, increasing corticosterone, and behavioural response, and supports the hypothesis that corticosterone is involved in the behavioural response to reduced food supply (Astheimer et al. 1992; Breuner & Hahn 2003). However, exogenous corticosterone manipulations are necessary to determine whether corticosterone is playing a central mechanistic role in irruptive behaviour.
Public information modified the physiological response to food treatment as predicted (figure 2). All food-reduced birds increased corticosterone and activity levels in response to food reduction, however, corticosterone levels in food-reduced birds differed significantly from ad libitum groups only in the food-reduced: food-reduced group (figure 2b). This was also true for fat deposition (figure 2c). Food-reduced birds housed next to food-reduced birds lost more fat on average, despite having similar food restrictions as those housed next to ad libitum individuals. Activity levels in the four treatment groups showed similar trends that followed our hierarchical predictions (figure 2a) and may account for these differences in fat use. These data suggest that the public information gained from a neighbour can either attenuate or intensify the hormonal response to food stress, thereby bringing neighbours into more similar physiological and behavioural states.
The observed adjustments in corticosterone release may represent the translation of public information into a physiological signal that can subsequently change the probability of behaviour. An individual that responds to poorly foraging flock members by elevating corticosterone would be hormonally primed to respond more quickly to declining food resources. Alternatively, an individual that slows corticosterone release in response to the cues of successfully foraging flock members would be less likely to escalate its behavioural response to the level of an irruption if it experiences a bout of poor foraging. Essentially then, group members influence each others' physiologies through public information, in ways that either confirm or refute individual foraging assessments. Such a system can theoretically increase the accuracy and efficiency of detecting genuine food crises and responding appropriately.
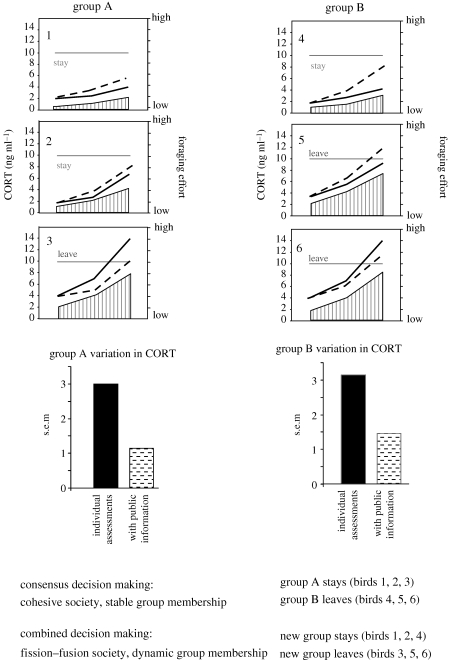
The implications of this effect on group membership and dynamics are dependent on decision-making strategies (figure 3). If public information informs decisions but individuals make autonomous decisions (i.e. combined decisions) (Conradt & Roper 2005), then the result is a fission–fusion society in which group membership changes depending on the final decision of each individual. Alternatively, in a consensus decision-making society, hormonal response to public information may be a mechanism that enhances group cohesiveness and promotes group stability. The public information effect would also serve to decrease the variability in voting opinion (figure 3). This may increase individual fitness as democratic group decisions are thought to produce less extreme decisions and generally result in lower costs of trial and error (Conradt & Roper 2003).
Figure 3.
Public information modifies individual corticosterone response to foraging success and alters group composition dependent on decision making strategies. As foraging effort theoretically changes across time (vertical stripes), so do corticosterone (CORT) concentrations for six individuals evaluating foraging conditions individually (thick black lines) and with public information (dashed lines). CORT concentrations above theoretical threshold (thin solid line) result in a vote to leave. Variation in circulating corticosterone in groups A and B is decreased when foraging assessments include public information. Public information decreases the hormonal variation in each group, promoting group cohesiveness and decision making in consensus decision making societies. In a consensus society group membership is stable, and decisions to migrate are made as cohesive, democratic units. Thus, in group A the majority vote is stay whereas in group B the majority vote is leave. Group composition is stable in this case and groups maintain a diversity of stress responsiveness within them. In a combined society group membership is dynamic. Decisions to migrate are made individually but are influenced by public information. Thus, individuals 3, 5 and 6 form a new leave group and individuals 1, 2 and 4 form a new stay group. In these societies, similar physiologies or foraging aptitudes will tend to group together as environmental conditions change.
It is unknown what specific cues constitute the public information used by red crossbills. Visual presence of food can lower corticosterone levels in food-reduced birds despite a lack of actual food intake (Harvey et al. 1983). In this study, food in the adjacent cage was visually obscured by opaque covered food cups, although olfactory cues remain a possibility. However, the use of inadvertent social information, or cues provided by an individual actively engaged in a relevant behaviour, is a form of public information that is common among animals (Danchin et al. 2004). Inadvertent public information is reliable because visual, aural or olfactory cues are produced unintentionally through performance of the behaviour and thus are not prone to cheating. Acquiring inadvertent public information (particularly if the cues are visual) may be incompatible with active foraging, thus creating a trade-off between gaining public information and gaining personal information (Giraldeau et al. 2002). Crossbills forage in dense evergreen foliage on food items that require substantial handling effort, and may be especially disadvantaged by this trade-off. It is probable that at least some public information is gathered using inadvertent social information; however, information may also be produced intentionally by group members. For example, captive migratory silvereyes (Zosterops lateralis) can induce migratory behaviours in non-migrant birds, apparently through stimulation by night-time vocalizations (Chan 1994). Observations in captive and free-living environments suggest that crossbills experiencing poor foraging conditions vocalize with greater intensity (Smith et al. 1999). Production of this signal may function as a recruitment tool for group members to move to an alternate patch. We did not measure calling rates during this study, but direct communication may have been involved as well as public information (e.g. observations of increased activity and foraging success). It would be interesting to determine whether pairs that used a greater degree of vocal communication interactions converged more in physiology and activity than those doing less of this, but we cannot evaluate this possibility with the available data.
This study suggests that public information modifies the endocrine response to food limitation, thereby altering behaviour and possibly facilitating group decision making by reducing inter-individual variation. As this is a novel finding, these results should be considered incentive to rigorously test the relationship with experimental manipulations of corticosterone and to determine its generality, especially in species that deal with different degrees of unpredictability in resources. The ability to respond to public information by modifying a physiological response to food shortage may be a specific adaptation found in nomadic or irruptive species. These species deal with a high level of spatial and temporal unpredictability in resources and may therefore rely particularly heavily on public information. However, the effects of public information on movement behaviour are widespread in animals and our discovery that public information can modify the stress response is a plausible physiological link between public information and behaviour. This mechanism suggests that population-wide stressors may have cumulative effects on individual stress physiology, and as human-induced environmental change disrupts historical patterns of food availability and increases unpredictability, it may have particularly important implications for conservation biology.
Acknowledgements
Animals' care was in accordance with institutional guidelines at the University of California-Davis under approved animal care protocol no. 12095.
We would like to thank Hugh Dingle, James Millam, Barbara Clucas, Heather Watts and two anonymous reviewers for valuable content and editing suggestions. Kendra Sewall, Rodd Kelsey and Taylor Chapple provided invaluable assistance during the experiment. Funding was partially provided by National Science Foundation grant to T.P.H. and American Ornithologists Union and Society of Integrative and Comparative Biology research grants to J.M.C.
References
- Astheimer L. B., Buttemer W. A., Wingfield J. C.1992Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Orn. Scand. 23, 355–365 (doi:10.2307/3676661) [Google Scholar]
- Astheimer L. B., Buttemer W. A., Wingfield J. C.1995Seasonal and acute changes in adrenocortical responsiveness in an Arctic-breeding bird. Horm. Behav. 29, 442–457 (doi:10.1006/hbeh.1995.1276) [DOI] [PubMed] [Google Scholar]
- Balthazart J.1983Hormonal correlates of behavior. In Avian biology VII (eds Farner D. S., King J. R., Parkes K. C.). New York, NY: Academic Press [Google Scholar]
- Bock C. E., Lepthien L. W.1976Synchronous eruptions of boreal seed-eating birds. Am. Nat. 110, 559–571 (doi:10.1086/283091) [Google Scholar]
- Breuner C. W., Hahn T. P.2003Integrating stress physiology, environmental change, and behavior in free-living sparrows. Horm. Behav. 43, 115–123 (doi:10.1016/S0018-506X(02)00020-X) [DOI] [PubMed] [Google Scholar]
- Chan K.1994Nocturnal activity of caged resident and migrant silvereyes (Zosteropidae: Aves). Ethology 96, 313–321 [Google Scholar]
- Conradt L., Roper T. J.2003Group decision-making in animals. Nature 421, 155–158 (doi:10.1038/nature01294) [DOI] [PubMed] [Google Scholar]
- Conradt L., Roper T. J.2005Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456 (doi:10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- Danchin E., Giraldeau L.-A., Valone T. J., Wagner R. H.2004Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- Elton C. S.1942Voles, mice and lemmings: problems in population dynamics Oxford, UK: Oxford University Press [Google Scholar]
- Giraldeau L.-A., Valone T. J., Templeton J. J.2002Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S., Klandorf H., Pinchasov Y.1983Visual and metabolic stimuli cause adrenocortical suppression in fasted chickens during refeeding. Neuroendocrinology 37, 59–63 (doi:10.1159/000123516) [DOI] [PubMed] [Google Scholar]
- Helms C. W., Drury W. H. J.1960Winter migratory weight and fat field studies on some North American buntings. Bird Banding 31, 1–40 [Google Scholar]
- Lohmus M., Sandberg R., Holberton R. L., Moore F. R.2003Corticosterone levels in relation to migratory readiness in red-eyed vireos (Vireo olivaceus). Behav. Ecol. Sociobiol. 54, 233–239 (doi:10.1007/s00265-003-0618-z) [Google Scholar]
- Lynn S. E., Breuner C. W., Wingfield J. C.2003Short-term fasting affects locomotor activity, corticosterone, and corticosterone binding globulin in a migratory songbird. Horm. Behav. 43, 150–157 (doi:10.1016/S0018-506X(02)00023-5) [DOI] [PubMed] [Google Scholar]
- Newton I.1972Finches London, UK: Williams Collins Sons & Co Ltd [Google Scholar]
- Newton I.2006Advances in the study of irruptive migration. Ardea 94, 433–460 [Google Scholar]
- Piersma T., Reneerkens J., Ramenofsky M.2000Baseline corticosterone peaks in shorebirds with maximal energy stores for migration: a general preparatory mechanism for rapid behavioral and metabolic transitions? Gen. Comp. Endocrinol. 120, 118–126 (doi:10.1006/gcen.2000.7543) [DOI] [PubMed] [Google Scholar]
- Pravosudov V. V., Kitaysky A. S., Wingfield J. C., Clayton N. S.2001Long-term unpredictable foraging conditions and physiological stress response in mountain chickadees (Poecile gambeli). Gen. Comp. Endocrinol. 123, 324–331 (doi:10.1006/gcen.2001.7684) [DOI] [PubMed] [Google Scholar]
- Romero L. M.2002Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- Silverin B.1997The stress response and autumn dispersal behaviour in willow tits. Anim. Behav. 53, 451–459 (doi:10.1006/anbe.1996.0295) [Google Scholar]
- Smith J. W., Benkman C. W., Coffey K.1999The use and misuse of public information by foraging red crossbills. Behav. Ecol. 10, 54–62 (doi:10.1093/beheco/10.1.54) [Google Scholar]
- Svardson G.1957The ‘invasion’ type of bird migration. Br. Birds 50, 314–343 [Google Scholar]
- Wingfield J. C.1983Environmental and endocrine control of reproduction: an ecological approach. In Avian endocrinology: environmental and ecological aspects (eds Mikami S. I., Ishii S., Wada M.), pp. 265–288 Berlin, Germany: Springer-Verlag [Google Scholar]
- Wingfield J. C.2003aAvian migration: regulation of facultative-type movements. In Avian migration (eds Berthold P., Gwinner E., Sonnenshein E.), pp. 113–125 Berlin, Germany:Springer-Verlag [Google Scholar]
- Wingfield J. C.2003bControl of behavioural strategies for capricious environments. Anim. Behav. 66, 807–815 (doi:10.1006/anbe.2003.2298) [Google Scholar]
- Wingfield J. C., Smith J. P., Farner D. S.1982Endocrine responses of white-crowned sparrows to environmental stress. Condor 84, 399–409 [Google Scholar]