Abstract
Coevolution between two antagonistic species follows the so-called ‘Red Queen dynamics’ when reciprocal selection results in an endless series of adaptation by one species and counteradaptation by the other. Red Queen dynamics are ‘genetically driven’ when selective sweeps involving new beneficial mutations result in perpetual oscillations of the coevolving traits on the slow evolutionary time scale. Mathematical models have shown that a prey and a predator can coevolve along a genetically driven Red Queen cycle. We found that embedding the prey–predator interaction into a three-species food chain that includes a coevolving superpredator often turns the genetically driven Red Queen cycle into chaos. A key condition is that the prey evolves fast enough. Red Queen chaos implies that the direction and strength of selection are intrinsically unpredictable beyond a short evolutionary time, with greatest evolutionary unpredictability in the superpredator. We hypothesize that genetically driven Red Queen chaos could explain why many natural populations are poised at the edge of ecological chaos. Over space, genetically driven chaos is expected to cause the evolutionary divergence of local populations, even under homogenizing environmental fluctuations, and thus to promote genetic diversity among ecological communities over long evolutionary time.
Keywords: adaptive dynamics, arms race, chaos, coevolution, genetic divergence, predator–prey
1. Introduction
Antagonistic coevolution describes the reciprocal evolutionary interactions between populations belonging to an ‘exploiter’ (such as a predator or a parasite) and a ‘victim’ (such as a prey or a host). It is a change in the genetic make-up of a population in response to a genetic change in the antagonistic population (Thompson 1994). Antagonistic interactions have the potential to drive coevolutionary dynamics of adaptive traits: an evolutionary advantage gained by one antagonist is often associated with a disadvantage for the other antagonist, and may therefore prompt a counteradaptation. This may drive stabilizing selection and evolutionary specialization with extreme refinement of the coevolving traits (convergence to an evolutionary equilibrium); or runaway selection and evolutionary escalation with the exaggeration of traits (with the possible extinction of some or all coevolving populations; Matsuda & Abrams 1994; Ferrière 2000); or fluctuating selection and the so-called ‘Red Queen dynamics' of perpetual reciprocal changes in the coevolving traits (convergence to a non-equilibrium evolutionary attractor; Van Valen 1973; Stenseth & Maynard Smith 1984; Vermeij 1994). It has been suggested that Red Queen dynamics underlie a large number of important biological processes, some of which are still poorly understood, such as genetic recombination and sexual reproduction (Hamilton et al. 1990), the extraordinary diversity of genes related to immune function, resistance and virulence (Salathe et al. 2008), and the spatial diversity and local adaptation of exploiter–victim systems (Gandon 2002).
An important dichotomy exists between two main types of Red Queen dynamics (Khibnik & Kondrashov 1997; Ebert 2008; Gaba & Ebert 2009): ecologically driven by negative frequency-dependent selection and genetically driven by beneficial mutations. This distinction is significant because the two types strongly differ in their mechanisms, their underlying genetic architecture, their ecological and evolutionary consequences, and the time scales on which they develop (Ebert 2008). With ecologically driven Red Queen dynamics, extant variants of the exploiter genotype that benefit the most from the numerically dominant victim genotypes are favoured, and, similarly, victim genotypes that best resist the numerically dominant exploiter genotypes are favoured. This pattern results in selection against common exploiter and victim genotypes in a time-lagged negative frequency-dependent fashion (ecological instability). A consequence of this form of fluctuating selection on extant genetic variation is that genetic polymorphism is maintained in the population for long periods (balanced selection) and that allele frequencies can oscillate considerably over time periods of a few generations.
In contrast, genetically driven Red Queen dynamics involve the repeated incidence, spread and fixation of new beneficial mutants in populations that stabilize at ecological equilibria. Mutants are driven to fixation by directional selection (selective sweeps). Thus, genetic polymorphism is transient only, and the evolutionary dynamics are slow—for two reasons. First, new mutations causing variation in the adaptive traits involved are rare events. Second, a new mutant starts with a very low frequency (1/N, where N is the number of wild-type alleles in the population); thus, empirically it can take hundreds of generations until the mutant becomes recognizable (e.g. 1%) at the population level (Elena et al. 1996). Therefore, genetically driven Red Queen dynamics develop on an evolutionary time scale that is several orders of magnitude slower than the time scale of ecological processes.
The slow time scale involved hampers the empirical investigation of genetically driven Red Queen dynamics, and mathematical models have been useful to seek conditions that could favour the Red Queen over specialization or escalation. So far, the majority of these models have focused on the two coevolving species and ignored the community context in which coevolution takes place. In this setting, genetically driven Red Queen dynamics develop as regular, predictable cycles in the adaptive trait space. However, pairs of coevolving species are inevitably embedded in community-level interactions of varying degrees of complexity. It is because most species interact with suites of other species that vary dynamically across geographical landscapes that coevolutionary processes can be important in shaping the structure and maintaining variability within specific pairwise interactions, such as predator–prey or host–parasite systems (Abrams 1991, 1996; Strauss et al. 2005; Thompson 2005; Thrall et al. 2007). For example, some trematode parasites have strong effects on the evolutionary dynamics of their snail hosts, but themselves are dependent upon waterflow for completion of their life cycle (Lively 1999). How the community context of coevolution affects the occurrence and manifestation of genetically driven Red Queen dynamics remains poorly known.
Seminal steps in the theoretical study of coevolutionary dynamics in the community context have been taken recently (Caldarelli et al. 1998; Loeuille et al. 2002; Gandon 2004; Nuismer & Doebeli 2004; Loeuille & Loreau 2005; Kisdi & Liu 2006; Bell 2007; Ferrière et al. 2007; Shoresh et al. 2008; Jones et al. 2009; Stegen et al. 2009), but models of genetically driven coevolutionary dynamics in which more than two species coevolve in a multi-dimensional trait space are still lacking. Here, we extend a simple two-species predator–prey coevolutionary system (Dieckmann et al. 1995; where genetically driven Red Queen cycles were first documented) to model coevolution in a three-dimensional trait space among three species forming a food chain. The function of each species in the food chain is determined by a continuous character subject to rare and small genetic mutations. One may expect that the addition of a coevolving species to a coevolving pair could stabilize the evolutionary process at an evolutionary equilibrium, thereby suppressing the Red Queen dynamics (Vermeij 1982; Futuyma 1983), or that the addition could destabilize the periodic evolutionary oscillation and drive the genetically driven Red Queen into chaos (Gavrilets 1997). Here we show that conditions leading to genetically driven periodic cycles in the two traits of coevolving predator and prey favour chaotic dynamics in the three coevolving traits of the three-species food chain.
2. Model construction
We focus on a single adaptive trait per species that characterizes the function of the species in the food chain. The trait determines competitive ability in the prey, and foraging success in the predator and superpredator. On the evolutionary time scale, de novo trait variation is caused by a rare genetic mutation. The current phenotypes determine the ecological equilibrium of the food chain, and hence the selective pressures acting on variants of the traits. Under the assumption that mutations have very small effects, the long-term coevolutionary process can be modelled as a trait substitution sequence in each species (Metz et al. 1992, 1996), the dynamics of which are captured by a set of three deterministic differential equations, one per trait (Dieckmann & Law 1996). When reduced to the classical two-trait, predator–prey coevolutionary model, the system is known to drive trait evolution towards a stable equilibrium or towards a Red Queen cycle (if not towards extinction; Dieckmann et al. 1995; Dercole et al. 2003, 2006).
As in Dieckmann et al. (1995), Lotka–Volterra equations are used to describe the ecological dynamics of the food chain:
| 2.1a |
| 2.1b |
| 2.1c |
where n1, n2 and n3 are prey, predator and superpredator densities, respectively; r and c are prey intrinsic per capita growth rate and sensitivity to intraspecific competition, respectively; and ai, ei and di are the attack rate, efficiency and intrinsic death rate in the predator (i = 2) and superpredator (i = 3), respectively. Each species is characterized by one genetic trait xi (i = 1–3). The genetic system is one-locus haploid; the genetic traits can influence the prey competition function c and the attack rates a2 and a3, and trait dependencies are modelled using the following functional forms:
| 2.2a |
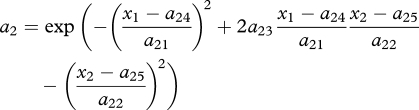 |
2.2b |
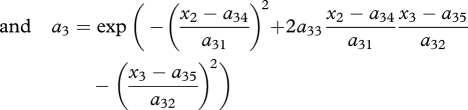 |
2.2c |
(with 0 < a23, a33 < 1 and c0, c2, a21, a22, a31, a32 all positive). Prey competition is minimum at x1 = c1, where prey are best adapted to their environment, while the attack rates a2 and a3 are bidimensional Gaussian functions with elliptic contour lines centred at (a24, a25) and (a34, a35), respectively, and controlled in amplitude and orientation by parameters a21–a23 and a31–a33, respectively. Differences (x1 − a24) and (x2 − a25) (respectively, (x2 − a34) and (x3 − a35)) measure the degree to which the predator (superpredator) ‘matches’ the prey (predator); that is, the attack rate is maximum when x1 = a24 and x2 = a25 (respectively, x2 = a34 and x3 = a35), while parameters a21–a23 (respectively, a31–a33) control the sensitivity of the attack rate to the mismatch.
When a mutation occurs in trait x1 and generates a new value 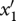 , the ecological system becomes
, the ecological system becomes
| 2.3a |
| 2.3b |
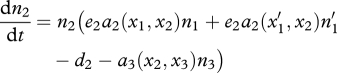 |
2.3c |
| 2.3d |
so that the fitness function of mutant 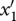 is given by
is given by
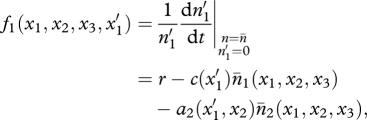 |
2.4 |
where n = (n1, n2, n3) and n̄ denotes the ecological equilibrium of model (2.1) at which the food chain stabilizes in the absence of mutants (§3).
Similar equations can be written when a mutation arises in the predator (trait x2) or superpredator (trait x3; see appendix S1 in the electronic supplementary material) and yields the fitness functions of mutants 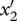 and
and 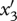 :
:
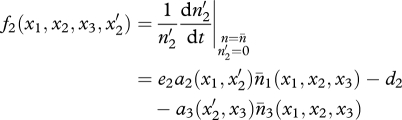 |
2.5 |
and
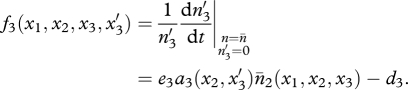 |
2.6 |
The long-term coevolution of traits x1, x2 and x3 on the evolutionary time scale obeys the so-called canonical equation of adaptive dynamics (Dieckmann & Law 1996); that is, the three-dimensional system of ODEs,
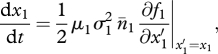 |
2.7a |
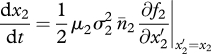 |
2.7b |
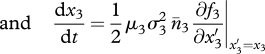 |
2.7c |
The right-hand sides are the product of mutation rates (μi, i = 1–3), mutational steps variances (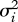 ), equilibrium densities (n̄i) and selection gradients (fitness derivatives). The latter explicit expressions are cumbersome and were always generated and handled by means of symbolic computation.
), equilibrium densities (n̄i) and selection gradients (fitness derivatives). The latter explicit expressions are cumbersome and were always generated and handled by means of symbolic computation.
3. Model analysis and results
The ecological model (2.1) has a unique non-trivial equilibrium,
| 3.1a |
| 3.1b |
| 3.1c |
which is positive if and only if n̄3 > 0. When positive, the equilibrium 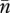 is globally stable (in the positive orthant). Thus, the ecological model (2.1) is only viable within the region of the trait space defined by the condition n̄3 > 0.
is globally stable (in the positive orthant). Thus, the ecological model (2.1) is only viable within the region of the trait space defined by the condition n̄3 > 0.
If the superpredator and the predator are able to simultaneously match the predator and the prey, respectively (i.e. a25 = a34), and if, at the same time, the prey is able to minimize its sensitivity to intraspecific competition (i.e. c1 = a24), then x̄1 = c1, x̄2 = a25, x̄3 = a35 is an equilibrium of the evolutionary model (2.7). Starting from these conditions, and fixing parameters at values corresponding to evolutionary cycles in the ditrophic model (Dieckmann et al. 1995), we performed the numerical continuation of the equilibrium 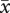 with respect to several parameters.
with respect to several parameters.
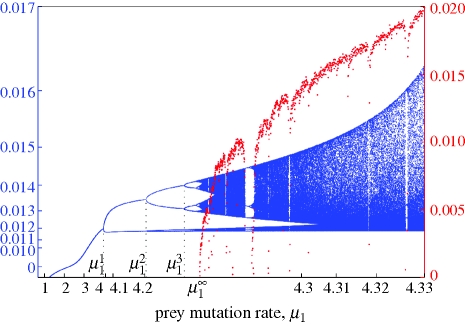
As expected, evolutionary stability was sensitive to the mutation rate μ1 of the prey. As μ1 increases, the evolutionary equilibrium loses stability through a supercritical Hopf bifurcation that yields a small-amplitude stable evolutionary cycle (see appendix S3 in the electronic supplementary material). Starting from the Hopf bifurcation, we numerically continued the cycle, while monitoring its stability through the computation of the associated Floquet multipliers (i.e. the three eigenvalues of the linearized Poincaré map associated with the cycle; one of them is structurally equal to 1, and therefore its estimated value is a measure of computation accuracy; the other two determine the stability of the cycle). Again, by increasing μ1, evolutionary stability was easily lost through a series of period-doubling bifurcations (a negative Floquet multiplier passing through −1; see appendix S3 in the electronic supplementary material). At each period-doubling bifurcation, the cycle becomes unstable, and a new stable cycle (which traces twice the bifurcating cycle) appears. Switching to the continuation of the new stable cycle allowed us to find the next period-doubling bifurcation. Because the sequence of bifurcation parameter values 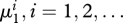 , accumulates geometrically at the frontier
, accumulates geometrically at the frontier 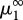 of the chaotic region of the Feigenbaum period-doubling cascade, only a limited number of bifurcations in the sequence could be detected (
of the chaotic region of the Feigenbaum period-doubling cascade, only a limited number of bifurcations in the sequence could be detected (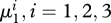 , are reported in figure 1). The robustness of the cascade has been checked through the continuation of the period-doubling bifurcations with respect to various pairs of parameters (details will be published elsewhere).
, are reported in figure 1). The robustness of the cascade has been checked through the continuation of the period-doubling bifurcations with respect to various pairs of parameters (details will be published elsewhere).
Figure 1.
Period-doubling route towards genetically driven Red Queen chaos in a three-species food chain. Peak values of the superpredator trait, x3 (blue), in the corresponding evolutionary attractor and the largest Lyapunov exponent, L1 (red), as functions of the prey mutation rate, μ1. The value 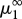 indicates the lower limit of the chaotic range. Parameter values: μ2 = 1, μ3 = 1,
indicates the lower limit of the chaotic range. Parameter values: μ2 = 1, μ3 = 1, 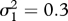 ,
, 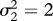 ,
, 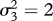 , r = 0.5, d2 = 0.05, d3 = 0.02, e2 = 0.14, e3 = 0.14, a21 = 0.22, a22 = 0.25, a23 = 0.6, a24 = 0, a25 = 0.04, a31 = 0.22, a32 = 0.25, a33 = 0.6, a34 = 0, a35 =− 0.04, c0 = 0.5, c1 = 0, c2 = 3.
, r = 0.5, d2 = 0.05, d3 = 0.02, e2 = 0.14, e3 = 0.14, a21 = 0.22, a22 = 0.25, a23 = 0.6, a24 = 0, a25 = 0.04, a31 = 0.22, a32 = 0.25, a33 = 0.6, a34 = 0, a35 =− 0.04, c0 = 0.5, c1 = 0, c2 = 3.
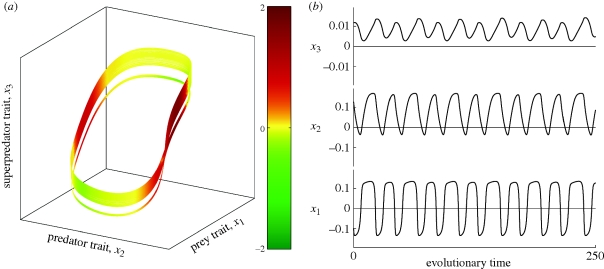
In order to estimate 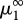 , we computed the full spectrum of the attractor's Lyapunov exponents L1 ≥ L2 ≥ L3 for finely incremented values of μ1 (step 10−5; see appendix S2 in the electronic supplementary material). L1 > 0 implies that μ1 is in the chaotic region, whereas L1 = 0 in periodic windows (figure 1); in the chaotic region, L2 is structurally equal to 0 (its estimated value measures computation accuracy), while L3 is negative. The attractor's fractal dimension then follows from the Kaplan–Yorke formula (figure 2). In this example, the dominant Lyapunov exponent equals +0.0081321 and the fractal dimension of the attractor is 2.0176 (the attractor lies roughly on a two-dimensional Möbius strip). Typically, the prey and predator characters oscillate with small irregular fluctuations in amplitude and frequency, while variation in the amplitude of the oscillations in the superpredator trait is more pronounced.
, we computed the full spectrum of the attractor's Lyapunov exponents L1 ≥ L2 ≥ L3 for finely incremented values of μ1 (step 10−5; see appendix S2 in the electronic supplementary material). L1 > 0 implies that μ1 is in the chaotic region, whereas L1 = 0 in periodic windows (figure 1); in the chaotic region, L2 is structurally equal to 0 (its estimated value measures computation accuracy), while L3 is negative. The attractor's fractal dimension then follows from the Kaplan–Yorke formula (figure 2). In this example, the dominant Lyapunov exponent equals +0.0081321 and the fractal dimension of the attractor is 2.0176 (the attractor lies roughly on a two-dimensional Möbius strip). Typically, the prey and predator characters oscillate with small irregular fluctuations in amplitude and frequency, while variation in the amplitude of the oscillations in the superpredator trait is more pronounced.
Figure 2.
Genetically driven chaotic Red Queen in a three-species food chain. (a) Evolutionary strange attractor. The estimated Lyapunov exponents are L1 = 8.1321 × 10−3, L2 =− 2.3923 × 10−6, L3 =− 4.6270 × 10−1, and the fractal dimension is 2 − L1/L3 = 2.0176 (Kaplan–Yorke formula). Colour codes the largest local Lyapunov exponent (see appendix S2 in the electronic supplementary material). (b) Chaotic time series of prey, predator and superpredator traits. Parameter values as in figure 1 and μ1 = 4.2667.
Our analysis shows that the genetically driven Red Queen turns chaotic under conditions similar to those leading to genetically driven Red Queen cycles, provided that the mutation time scale of the prey is short enough compared with the mutation time scales of the predator and the superpredator. That is (Dieckmann et al. 1995; Dercole et al. 2003), the predator efficiency should be great enough to drive the prey away from its genetic optimum; and there should be sufficient need for the predator to track the prey's character change. As the prey departs from its optimum, its population density drops, which causes a reversal of selection on the predator's trait, followed by a reversal of selection on the prey's character. If the prey evolves fast enough, it will not be ‘caught up’ by the predator and permanent trait oscillations will evolve; the system ends up in chaos because the predator is also engaged in a coevolutionary chase with the superpredator. Broad comparative analyses (e.g. Martin & Palumbi 1993) have established a strong relationship between nucleotide substitution rate and body size. For instance, rates of nuclear and mtDNA evolution are slow in whales, intermediate in primates and fast in rodents, and a similar effect of body size also exists in poikilothermic vertebrates. Thus, trophic chains with smaller prey (and hence faster mutagenesis) may be more prone to coevolutionary chaos.
4. Discussion
Even though quantitative data on long-term predator–prey coevolutionary dynamics remain elusive (Barnosky 2001), the fossil record supports the view that predation is an important driver of evolutionary change (Kelley et al. 2003). Moreover, palaeontological and phylogenetic analyses gather increasing evidence for the role of three-level chain interactions in coevolution (Currie et al. 2003; Kelley et al. 2003). These empirical findings have been paralleled by extensions of coevolutionary theory beyond pairwise interactions (Abrams 1996; Caldarelli et al. 1998; Loeuille et al. 2002; Gandon 2004; Nuismer & Doebeli 2004; Loeuille & Loreau 2005; Kisdi & Liu 2006; Bell 2007; Ferrière et al. 2007; Shoresh et al. 2008; Jones et al. 2009; Stegen et al. 2009), but so far the complexity of evolutionary dynamics among more than two species coevolving in a multi-dimensional trait space has been little explored. As a step forward in that direction, we added a superpredator, as a third coevolving species, to coevolution between a prey and a predator.
Prey–predator–superpredator trophic chains have long attracted the attention of ecologists as they occur by diverse mechanisms, can cross ecosystem boundaries and have practical importance; for example, in the management of fisheries or biological control of crop pests (Cohen et al. 2009). Our model descends from the lineage of two-species models that addressed genetically driven predator–prey coevolution (Stenseth & Maynard Smith 1984; Rosenzweig et al. 1987; Rand & Wilson 1991; Marrow et al. 1992; Dieckmann et al. 1995; Doebeli 1997; Gavrilets 1997; Khibnik & Kondrashov 1997; Dercole et al. 2003, 2006) and specifically extends the analysis of Dieckmann et al. (1995), where stable cycles in adaptive dynamics were first documented.
We searched for strange attractors in the three-trait, three-species coevolutionary model by weaving intuition and theory. Theory was telling us that in third-order dynamical systems the most common route to chaos is the Feigenbaum period-doubling cascade (see appendix S3 in the electronic supplementary material), and we knew that evolutionary stability in predator–prey models was especially sensitive to the mutation rate of the prey (Dieckmann et al. 1995; Dercole et al. 2003). Thus, our analysis of the tritrophic evolutionary dynamics was organized by looking for parameters that caused evolutionary cycles in the lower ditrophic model (and such that increasing the prey mutation rate could trigger doubling of the cycle period) and then tracking the period-doubling cascade. The strategy was successful at detecting transitions towards evolutionary chaos in the three-species system.
Our analysis of three-species coevolution was intended as an extension of Dieckmann et al.'s (1995) two-species model. This is the technical motivation for our choice of the type I functional response to describe trophic interactions, hence the Lotka–Volterra structure of the ecological model. This has the important consequence of ensuring that the food chain always stabilizes at an equilibrium on the ecological time scale. Therefore, oscillations predicted by the evolutionary model could only be due to nonlinear interactions between selective pressures acting on genetic variation in the adaptive traits—not to trait variation induced by instabilities in the ecological dynamics (Abrams & Matsuda 1997a). More realistic food chain models with, for example, saturating (type II) functional responses or self-limitation at higher trophic levels can also stabilize at ecological equilibria, though ecological cycles and ecological chaos are also expected in viable regions of the trait space. This opens the possibility of Red Queen chaotic dynamics that would be ‘ecogenetically driven’ (sensu Khibnik & Kondrashov 1997; for the two-species case see Dercole et al. 2003; Dercole et al. 2006).
Another fundamental feature of the model is the definition of the adaptive traits. We keep the ‘matching model’ used in Dieckmann et al. (1995), which has long been popular in the theory of predator–prey coevolution (Cohen et al. 1993; Abrams 2000; Loeuille & Loreau 2005; Stegen et al. 2009). The matching model assumes that the traits of a species and its prey jointly determine the attack (and capture) rate on the former by the latter, and that the attack rate is maximized when the two traits match. Scaled body size is a commonly used surrogate measure for such traits (Williams & Martinez 2000). Defining the adaptive traits according to the matching model is known to promote genetically driven Red Queen cycles in the two-species predator–prey coevolutionary model (Marrow et al. 1992, 1996; Dieckmann et al. 1995; Abrams & Matsuda 1997b; Doebeli 1997; Gavrilets 1997), and thus provided us with the appropriate framework to answer our main question—how are two-species Red Queen cycles affected by the coevolution of a third species?
Several well-studied antagonistic pairwise interactions seem to conform to the matching model. This includes parasitic cuckoos and their hosts, in which the probability that a parasitic egg be rejected depends on the similarity of host and parasite egg morphologies (Robert & Sorci 1999); crossbills and lodgepole pines, for which fitnesses are influenced by matching between bill size and cone structure (Benkman 1999); feather lice and dove hosts, in which louse fitness at least is influenced by matching size with host size (and host size correlates with parasite size across species; Clayton et al. 2003). Other equally well-studied systems, however, better fit an alternative model in which the strength of between-species interactions is a monotonic function of the difference between the predator and prey's traits. This is the case of parsnip web-worms and wild parsnips, in which feeding efficiency of defended plants increases with higher production of detoxifying enzymes (Berenbaum & Zangerl 1992). Likewise, the rate of successful attack in the Japanese-camellia–camellia-weevil system is a monotonic function of the difference between camelia fruit wall thickness and weevil mouthpart size (Toju & Sota 2006, 2009). The ‘difference model’ so defined also fits the trophic interaction between toxic newts as prey and potentially toxin-resistant garter snakes as predators (Brodie et al. 2002; Hanifin et al. 2008).
Nuismer et al.'s (2007) theoretical analysis of antagonistic coevolution under the difference model of attack rate shows that coevolutionary cycles are still possible with this model, provided that selection is strong enough and stabilizing selection acts on the traits. Thus, genetically driven coevolutionary cycles in pairwise antagonistic interactions appear to be at least possible under relatively broad conditions when the attack rate is described by the difference model. The question of whether coevolutionary cycles turn into chaos in the three-species food chain is open to investigation. Future models should also examine the coevolution of alternative or additional traits besides the attack rate. Dercole et al. (2003) and Kisdi & Liu (2006), for example, considered the evolution of handling time, a key factor of the functional response. As an extension of our model, it would be interesting to account for genetic variation in predator and superpredator handling times, track the evolution of the functional responses themselves as a byproduct and monitor the potential bifurcations experienced by the coevolutionary dynamics as a consequence.
The possibility that natural selection acting on extant genetic variation drives community dynamics into chaos has been known since early analyses of host–pathogen models (May & Anderson 1983), and is not unexpected given that competition between multiple species or genotypes can easily destabilize population dynamics (Hofbauer & Sigmund 1998; Turchin 2003). This type of chaotic evolutionary dynamics has been found in theoretical studies of genetic polymorphisms under frequency-dependent selection (e.g. May & Anderson 1983; Seger 1992; Ferrière & Fox 1995; Solé & Sardanyés 2007), strategy frequencies in evolutionary games (Nowak & Sigmund 2003) and rapid evolution of a continuous trait in interaction with population dynamics (Abrams & Matsuda 1997a). All these are instances of evolutionary chaos on the ecological time scale. The system considered here is different since the time scales of ecology and evolution are separated: the population dynamics of different alleles stabilize on a monomorphic state over a time scale that is fast compared with the slow evolutionary time scale over which the dynamics of the adaptive traits develop. Thus, our analysis uncovers the first example of genetically driven chaotic Red Queen.
The genetically driven chaotic Red Queen implies that nonlinear interactions of selective pressures can drive phenotypic changes that are unpredictable over the slow time scale of long-term evolution, even in a perfectly constant abiotic environment. (Note that with chaos in allele or strategy frequencies driven by negative frequency dependence, there is unpredictability in the dynamics of frequencies, but the identity of alleles or strategies never changes.) This has implications for our understanding of the role of ‘chance’ in evolution (Travisano et al. 1995; Beatty 2006). Chance manifests itself when the evolutionary trajectories of adaptive traits diverge between replicated populations that were initiated in similar phenotypic and genotypic states. Experimental tests on bacterial systems have provided some of the best evaluations of the role that chance may play in evolution. Although founded by the same clone, and evolving in identical conditions, replicate populations often diverge from one another in their relative growth rate, demographic traits, morphological features and performance in other environments (Elena & Lenski 2003 and references therein). The conventional explanation for evolutionary divergence ‘by chance’ involves genetic stochasticity (the randomness of mutation and drift owing to demographic stochasticity) and environmental stochasticity (random changes in environmental conditions; Lenormand et al. 2008). However, models of adaptive trait dynamics derived from individual-level ‘first principles’ have shown that the effect of genetic stochasticity is often ‘smoothed out’ in the long term, with traits converging towards the attractor of a deterministic dynamical system, provided that there is some minimal separation between the time scales of mutation and selection (Champagnat et al. 2006). The present study shows that even if the randomness of genetic stochasticity is smoothed out, uncertainty can arise from the selection component of the evolutionary process: adaptive trait trajectories converge towards a deterministic attractor, yet the chaotic nature of the attractor renders the trait dynamics unpredictable beyond a short evolutionary time horizon. Thus, the nonlinearity of the selection gradient offers an alternative to genetic or environmental stochasticity to explain the chance component of evolutionary trajectories in real populations.
Further examples of genetically driven chaotic Red Queen dynamics are likely to be discovered in models of long-term evolution in which the adaptive process operates in a three- (or more) dimensional trait space—even if all traits (e.g. behavioural or life-history traits) pertain to the same single species. Genetically driven chaos might also arise in two-trait adaptive dynamics models, or even in one-trait systems showing ecological multi-stability (Dercole et al. 2002), that are subject to externally driven periodic fluctuations in mutation or selection. Besides its conceptual value, the genetically driven chaotic Red Queen suggests three new hypotheses (discussed below) about coevolutionary dynamics. Each hypothesis opens an avenue for future theoretical work.
(a). The intrinsic unpredictability of coevolutionary dynamics is widespread
In view of the general theory of dynamical systems, the existence of chaotic evolutionary attractors over some parameter region can affect the coevolutionary dynamics broadly outside that region. Even when the coevolutionary attractor of the food chain is an equilibrium or a cycle, the ‘shadow’ of evolutionary chaos will be seen in the form of long erratic transients (Hastings 2004). Genetic noise—owing, for example, to random drift or stochastic gene flow—or stochastic environmental fluctuations on the slow evolutionary time scale may actually maintain these transients for arbitrarily long evolutionary times. Such ‘noise-induced chaos’ illustrates the general fact that small amounts of exogenous noise can have disproportionate qualitative impacts on the long-term dynamics of a nonlinear system in which chaotic structures exist for some parameter values (Rand & Wilson 1991; Lai et al. 2003; Ellner & Turchin 2005).
(b). Coevolution can drive population dynamics to the edge of chaos
Looking at evolution on a slow time scale, in contrast with, or even completely separated from, the fast time scale of ecology, does not mean that the coevolutionary process has no effect on the ecological state of the system. In fact, the genetically driven chaotic Red Queen implies that the population size of each species also fluctuates chaotically, but these fluctuations develop on the slow, evolutionary time scale, because at each point in evolutionary time, the food chain model analysed here is at ecological equilibrium. In other food chain models, ecological cycles and chaos occur readily (Hastings & Powell 1991; Gross et al. 2005). In the light of this and other studies (Khibnik & Kondrashov 1997; Dercole et al. 2006), the trait domain corresponding to ecological chaos may contain part or all of the coevolutionary attractor (ecogenetically driven Red Queen). A sharp change in the selective regime at the boundary between chaotic and non-chaotic ecological dynamics is expected in general (Ferrière & Gatto 1995; Dercole et al. 2006), and may poise the food chain near that boundary for long evolutionary times, in a process called ‘evolutionary sliding’ (Dercole et al. 2006). This would provide an evolutionary explanation for the standing puzzle that the abundance of many natural populations seemingly fluctuates ‘at the edge of chaos’ (Ellner & Turchin 1995; Turchin 2003).
(c). The chaotic Red Queen promotes genetic divergence in metacommunities
There is considerable interest in better understanding how coevolutionary processes work in geographically structured habitats (Thompson 2005). The arising of genetically driven chaos has direct implications for the origin and maintenance of genetic diversity in spatially extended communities. Let us consider the metaphor of a fragmented landscape in which all patches are identical and isolated. Genetically driven chaotic Red Queen dynamics imply that each local trophic chain evolves along the same strange attractor, but small ancestral differences in the genetic make-up of local communities will result in permanent genetic differences between patches. The magnitude of these differences will vary over time and sometimes be as large as the coevolutionary attractor. In contrast, small ancestral differences remain small in the case of periodic Red Queen dynamics (and the same would be true if the Red Queen were ecologically driven). In other words, local genetically driven coevolutionary chaos promotes spatial genetic divergence, even in the absence of environmental differences between patches. Red Queen dynamics in general can explain phenotypic mismatches between coevolving species even in the absence of spatial structure, gene flow or genetic drift (Berenbaum et al. 1986; Hanifin et al. 2008); the chaotic Red Queen, in particular, predicts the persistence of different degrees of mismatches between local communities, even if environmental conditions are spatially uniform.
Furthermore, general results on the synchronization of dynamical systems subject to common fluctuating exogenous forces warn that the genetic divergence between local populations can be lost in the presence of long-term environmental fluctuations (this is known in ecology as the Moran effect; see Royama 1992 for a review). However, recent results (Colombo et al. 2008) show in great generality that this is possible only if environmental fluctuations are large and tuned specifically to the endogenous dynamics of the system. Genetically driven coevolutionary chaos could therefore play an important role in promoting genetic diversity in ecological communities threatened by environmental homogenization (Olden et al. 2004). We conclude that genetically driven Red Queen chaos might explain genetic differentiation of local communities without invoking local adaptation to different habitat conditions or to multiple steady states of local populations in the metacommunity. This points to the possibility that, in sexual species, the genetic divergence of local populations induced by complex adaptive dynamics might favour the evolution of reproductive isolation and hence parapatric speciation—even across relatively uniform habitats, as in marine species (Palumbi 1994). Extension of speciation models along ecological gradients (Doebeli & Dieckmann 2003) will help examine this hypothesis further.
5. Concluding Remarks
Here, we have extended Dieckmann et al.'s (1995) model of predator–prey genetically driven coevolution by adding a coevolving superpredator to the system. When Red Queen periodic cycles develop in the two-species model, the adaptive dynamics of the three coevolving species are often chaotic. A general condition for this to happen is that the evolutionary rate of the prey be large enough. The greatest irregularity is then predicted in the dynamics of the superpredator trait. Because the ecological model of the food chain is always at equilibrium throughout the trait space, instability in the ecological dynamics plays no role in generating this chaotic Red Queen, which is thus entirely driven by nonlinear interactions between the selective pressures acting on rare genetic variation of the traits.
The specificities of the model and the new hypotheses arising from the results call for continued theoretical investigation of chaotic dynamics in genetically driven coevolutionary processes. This theoretical endeavour should be paralleled by an empirical effort focusing on the patterns of temporal unpredictability and spatial heterogeneity of antagonistic coevolution and the consequences for population dynamics, genetic differentiation in metacommunities and macroevolutionary processes, including speciation.
A key difference between coevolutionary cycles and coevolutionary chaos lies in the expectation that geographically distinct communities subject to homogenizing factors of their environment (e.g. large-scale climatic fluctuations) should exhibit similar degrees of phenotype mismatching when coevolving cyclically, and persistently dissimilar degrees of mismatching when coevolving chaotically. Spatially heterogeneous mismatches have been documented recently in the camellia–weevil (Toju 2009) and newt–garter snake (Hanifin et al. 2008) systems. In the light of our results, the fine-scale divergence of coevolution in the former may not require geographical variation of environmental factors (Toju 2009). Molecular data supporting the role of beneficial mutations, rather than standing genetic variation, as fuelling coevolution between newts and their snake predators (Feldman et al. 2009) offer promising evidence for the relevance of genetically driven Red Queen models to deepen our understanding of geographical patterns of coevolution in nature.
Besides trophic interactions, the Red Queen is expected to reign in many exploiter–victim systems (Lythgoe & Read 1998). Biomedical science has already revealed the potential ubiquity of the Red Queen in parasitic and pathogenic interactions (Moya et al. 2004). Experimental coevolution in host–pathogen systems is being used successfully to evidence the patterns and dissect the processes of ecologically driven Red Queen dynamics in laboratory systems (Koskella & Lively 2009) and in nature (Decaestecker et al. 2007). On the evolutionary time scale, antagonistic coevolutionary dynamics fuelled by de novo genetic variation have been studied experimentally using bacterial systems (Lenski & Levin 1985; Bohannan & Lenski 2000; Buckling & Rainey 2002; Gallet et al. 2009). The time-shift experimental design (Gaba & Ebert 2009) implemented to study ecologically driven Red Queen dynamics could be applied to measure how predictable genetically driven coevolutionary trajectories are under different experimental treatments, and thus to search for the essential property of chaotic dynamics—exponentially declining predictability of trajectories. Combining experiments with sufficiently detailed mathematical models of the study systems will be instrumental to identify relevant experimental treatments, to design data collection and analysis and to interpret the results (Decaestecker et al. 2007). If it were supported by such experiments on microbial systems, the genetically driven chaotic Red Queen might contribute to our understanding of the rapid and indeterminate evolution of viral pathogens (Kirkwood & Bangham 1994; Moya et al. 2004), and perhaps influence the study and control of emergent pathogens on large temporal and spatial scales.
Ultimately, the important question raised by the genetically driven chaotic Red Queen is unlikely to be whether or not long-term evolution in any specific ecological system is chaotic—a question that makes sense only in the realm of mathematical models. Population ecologists have long gone beyond that question—chaos versus non-chaos—to draw stunning insights from the nonlinear dynamics theory into how environmental forces and internal dynamics shape species abundance and distribution in nature (Allen et al. 1993; Ellner & Turchin 1995; Dixon et al. 1999; Turchin 2003). The same move could take place in evolutionary biology, as genetically driven Red Queen chaos challenges our ability to measure, compare and interpret coevolutionary patterns and processes in the real world.
Acknowledgments
We thank M. E. Evans, M. Herron, S. Levin, M. Loreau, J. Masel, J. Stegen, P. Turchin and M. Worobey for comments and discussions. Reviews by Associate Editor T. Day and two anonymous referees greatly helped improve an earlier version of the manuscript. This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca and the International Institute for Applied Systems Analysis (to F.D. and S.R.). R.F. acknowledges funding from the Institut Universitaire de France, the French Ministry of Research (ANR grant Stochastic Models in Evolution) and the National Science Foundation (Frontiers in Integrative Biological Research programme, grant EF-0623632).
References
- Abrams P. A.1991The effects of interacting species on predator–prey coevolution. Theor. Popul. Biol. 39, 241–262 (doi:10.1016/0040-5809(91)90022-8) [Google Scholar]
- Abrams P. A.1996Evolution and the consequences of species introduction and deletions. Ecology 77, 1321–1328 (doi:10.2307/2265529) [Google Scholar]
- Abrams P. A.2000The evolution of predator–prey interactions: theory and evidence. Annu. Rev. Ecol. Syst. 31, 79–105 (doi:10.1146/annurev.ecolsys.31.1.79) [Google Scholar]
- Abrams P. A., Matsuda H.1997aPrey evolution as a cause of predator–prey cycles. Evolution 51, 1742–1750 (doi:10.2307/2410997) [DOI] [PubMed] [Google Scholar]
- Abrams P. A., Matsuda H.1997bFitness minimization and dynamic instability as a consequence of predator–prey coevolution. Evol. Ecol. 11, 1–20 (doi:10.1023/A:1018445517101) [Google Scholar]
- Allen J. C., Schaffer W. M., Rosko D.1993Chaos reduces species extinction by amplifying local population noise. Nature 364, 229–232 (doi:10.1038/364229a0) [DOI] [PubMed] [Google Scholar]
- Barnosky A.2001Distinguishing the effects of the Red Queen and Court Jester on Meiocene mammal evolution in the northern Rocky Mountains. J. Vertebr. Paleontol. 21, 172–185 (doi:10.1671/0272-4634(2001)021[0172:DTEOTR]2.0.CO;2) [Google Scholar]
- Beatty J.2006Replaying life's tape. J. Phil. 103, 336–362 [Google Scholar]
- Bell G.2007The evolution of trophic structure. Heredity 99, 494–505 (doi:10.1038/sj.hdy.6801032) [DOI] [PubMed] [Google Scholar]
- Benkman C. W.1999The selection mosaic and diversifying coevolution between crossbills and lodgepole pine. Am. Nat. 154, S75–S91 (doi:10.1086/303213) [DOI] [PubMed] [Google Scholar]
- Berenbaum M. R., Zangerl A. R.1992Genetics of physiological and behavioral resistance to host furanocoumarins in the parsnip webworm. Evolution 46, 1373–1384 (doi:10.2307/2409943) [DOI] [PubMed] [Google Scholar]
- Berenbaum M. R., Zangerl A. R., Nitao J. K.1986Constraints on chemical coevolution—wild parsnips and the parsnip webworm. Evolution 40, 1215–1228 (doi:10.2307/2408949) [DOI] [PubMed] [Google Scholar]
- Bohannan B. J. M., Lenski R. E.2000Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 3, 362–377 (doi:10.1046/j.1461-0248.2000.00161.x) [Google Scholar]
- Brodie E. D., Jr, Ridenhour B. J., Brodie E. D., III2002The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution 56, 2067–2082 [DOI] [PubMed] [Google Scholar]
- Buckling A., Rainey P. B.2002Antagonistic coevolution between a bacterium and a bacteriophage. Proc. R. Soc. Lond. B 269, 931–936 (doi:10.1098/rspb.2001.1945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarelli G., Higgs P. G., McKane A. J.1998Modeling coevolution in multispecies communities. J. Theor. Biol. 193, 345–358 (doi:10.1006/jtbi.1998.0706) [DOI] [PubMed] [Google Scholar]
- Champagnat N., Ferrière R., Méléard S.2006Unifying evolutionary dynamics: from individual stochastic processes to macroscopic models. Theor. Popul. Biol. 69, 297–321 (doi:10.1016/j.tpb.2005.10.004) [DOI] [PubMed] [Google Scholar]
- Clayton D. H., Bush S. E., Goates B. M., Johnson K. P.2003Host defenses reinforce host–parasite cospeciation. Proc. Natl Acad. Sci. USA 100, 15 694–15 699 (doi:10.1073/pnas.2533751100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. E., Pimm S. L., Yodzis P., Saldan J.1993Body sizes of animal predator and animal prey in food webs. J. Anim. Ecol. 62, 67–78 (doi:10.2307/5483) [Google Scholar]
- Cohen J. E., Schittler D. N., Raffaelli D. G., Reuman D. C.2009Food webs are more than the sum of their tritrophic parts. Proc. Natl Acad. Sci. USA 106, 2335–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A., Dercole F., Rinaldi S.2008Remarks on metacommunity synchronization with application to prey–predator systems. Am. Nat. 171, 430–442 (doi:10.1086/528959) [DOI] [PubMed] [Google Scholar]
- Currie C. R., Wong B., Stuart A. E., Schultz T. R., Rehner S. A., Mueller U. G., Sung G. H., Spatafora J. W., Straus N. A.2003Ancient tripartite coevolution in the attine ant–microbe symbiosis. Science 299, 386–388 (doi:10.1126/science.1078155) [DOI] [PubMed] [Google Scholar]
- Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L.2007Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870–873 (doi:10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- Dercole F., Ferrière R., Rinaldi S.2002Ecological bistability and evolutionary reversals under asymmetrical competition. Evolution 56, 1081–1090 [DOI] [PubMed] [Google Scholar]
- Dercole F., Irisson J.-O., Rinaldi S.2003Bifurcation analysis of a prey–predator coevolution model. SIAM J. Appl. Math. 63, 1378–1391 (doi:10.1137/S0036139902411612) [Google Scholar]
- Dercole F., Gragnani A., Ferrière R., Rinaldi S.2006Coevolution of slow–fast populations: evolutionary sliding, evolutionary pseudo-equilibria and complex Red Queen dynamics. Proc. R. Soc. B 273, 983–990 (doi:10.1098/rspb.2005.3398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann U., Law R.1996The dynamical theory of coevolution: a derivation from stochastic ecological processes. J. Math. Biol. 34, 579–612 (doi:10.1007/BF02409751) [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Marrow U., Law R.1995Evolutionary cycling in predator–prey interactions: population dynamics and the Red Queen. J. Theor. Biol. 176, 91–102 (doi:10.1006/jtbi.1995.0179) [DOI] [PubMed] [Google Scholar]
- Dixon P. A., Milicich M. J., Sugihara G.1999Episodic fluctuations in larval supply. Science 283, 1528–1530 (doi:10.1126/science.283.5407.1528) [DOI] [PubMed] [Google Scholar]
- Doebeli M.1997Genetic variation and the persistence of predator–prey interactions in the Nicholson–Bailey model. J. Theor. Biol. 188, 109–120 (doi:10.1006/jtbi.1997.0454) [Google Scholar]
- Doebeli M., Dieckmann U.2003Speciation along environmental gradients. Nature 421, 259–264 (doi:10.1038/nature01274) [DOI] [PubMed] [Google Scholar]
- Ebert D.2008Host–parasite coevolution: insights from the Daphnia–parasite model system. Curr. Opin. Microbiol. 11, 290–301 (doi:10.1016/j.mib.2008.05.012) [DOI] [PubMed] [Google Scholar]
- Elena S. F., Lenski R. E.2003Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4, 457–469 (doi:10.1038/nrg1088) [DOI] [PubMed] [Google Scholar]
- Elena S. F., Cooper V. S., Lenski R. E.1996Punctuated evolution caused by selection of rare beneficial mutations. Science 272, 1802–1804 (doi:10.1126/science.272.5269.1802) [DOI] [PubMed] [Google Scholar]
- Ellner S. P., Turchin P.1995Chaos in a noisy world: new methods and evidence from time series analysis. Am. Nat. 145, 343–375 (doi:10.1086/285744) [Google Scholar]
- Ellner S. P., Turchin P.2005When can noise induce chaos and why does it matter: a critique. Oikos 111, 620–631 (doi:10.1111/j.1600-0706.2005.14129.x) [Google Scholar]
- Feldman C. R., Brodie E. D., Jr, Brodie E. D., III, Pfrender M. E.2009The evolutionary origins of beneficial alleles during the repeated adaptation of garter snakes to deadly prey. Proc. Natl Acad. Sci. USA 106, 13 415–13 420 (doi:10.1073/pnas.0901224106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrière R.2000Adaptive responses to environmental threats: evolutionary suicide, insurance and rescue. Options Spring 2000, 12–16 [Google Scholar]
- Ferrière R., Fox G. A.1995Chaos and evolution. Trends Ecol. Evol. 10, 480–485 (doi:10.1016/S0169-5347(00)89194-6) [DOI] [PubMed] [Google Scholar]
- Ferrière R., Gatto M.1995Lyapunov exponents and the mathematics of invasion in oscillatory or chaotic populations. Theor. Popul. Biol. 48, 126–171 (doi:10.1006/tpbi.1995.1024) [Google Scholar]
- Ferrière R., Gauduchon M., Bronstein J. L.2007Evolution and persistence of obligate mutualists and exploiters: competition for partners and evolutionary immunization. Ecol. Lett. 10, 115–126 (doi:10.1111/j.1461-0248.2006.01008.x) [DOI] [PubMed] [Google Scholar]
- Futuyma D. J.1983Evolutionary interactions among herbivorous insects and plants. In Coevolution (eds Futuyma D. J., Slatkin M.), pp. 207–231 Sunderland, MA: Sinauer Associates [Google Scholar]
- Gaba S., Ebert D.2009Time-shift experiments as a tool to study antagonistic coevolution. Trends Ecol. Evol. 24, 226–232 (doi:10.1016/j.tree.2008.11.005) [DOI] [PubMed] [Google Scholar]
- Gallet R., Tully T., Evans M. E. K.2009Ecological conditions affect evolutionary trajectory in a predator–prey system. Evolution 63, 641–651 (doi:10.1111/j.1558-5646.2008.00559.x) [DOI] [PubMed] [Google Scholar]
- Gandon S.2002Local adaptation and the geometry of host–parasite coevolution. Ecol. Lett. 5, 246–256 (doi:10.1046/j.1461-0248.2002.00305.x) [Google Scholar]
- Gandon S.2004Evolution of multihost parasites. Evolution 58, 455–469 [PubMed] [Google Scholar]
- Gavrilets S.1997Coevolutionary chase in exploiter–victim systems with polygemic characters. J. Theor. Biol. 186, 527–534 (doi:10.1006/jtbi.1997.0426) [DOI] [PubMed] [Google Scholar]
- Gross T., Ebenhoh W., Feudel U.2005Long food chains are in general chaotic. Oikos 109, 135–144 (doi:10.1111/j.0030-1299.2005.13573.x) [Google Scholar]
- Hamilton W. D., Axelrod R., Tanese R.1990Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 35, 3566–3573 (doi:10.1073/pnas.87.9.3566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin C. T., Brodie E. D., Jr, Brodie E. D., III2008Phenotypic mismatches reveal escape from arms-race coevolution. PLoS Biol. 6, e60 (doi:10.1371/journal.pbio.0060060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings A.2004Transients: the key to long-term ecological understanding? Trends Ecol. Evol. 19, 39–45 (doi:10.1016/j.tree.2003.09.007) [DOI] [PubMed] [Google Scholar]
- Hastings A., Powell T.1991Chaos in a three species food chain. Ecology 72, 896–903 (doi:10.2307/1940591) [Google Scholar]
- Hofbauer J., Sigmund K.1998Evolutionary games and population dynamics Cambridge, UK: Cambridge University Press [Google Scholar]
- Jones E. I., Ferrière R., Bronstein J. L.2009Eco-evolutionary dynamics of mutualists and exploiters. Am. Nat. 174, 780–794 (doi:10.1086/647971) [DOI] [PubMed] [Google Scholar]
- Kelley P. H., Kowalewski M., Hansen T. A. (eds) 2003Predator–prey interaction in the fossil record. New York, NY: Plenum [Google Scholar]
- Khibnik A. I., Kondrashov A. S.1997Three mechanisms of Red Queen dynamics. Proc. R. Soc. Lond. B 264, 1049–1056 (doi:10.1098/rspb.1997.0145) [Google Scholar]
- Kirkwood T. B. L. M., Bangham C. R. M.1994Cycles, chaos, and evolution in virus cultures: a model of defective interfering particles. Proc. Natl Acad. Sci. USA 91, 8685–8689 (doi:10.1073/pnas.91.18.8685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisdi E., Liu S.2006Evolution of handling time can destroy the coexistence of cycling predators. J. Evol. Biol. 19, 49–58 (doi:10.1111/j.1420-9101.2005.00993.x) [DOI] [PubMed] [Google Scholar]
- Koskella B., Lively C. M.2009Evidence for negative frequency-dependent selection during experimental coevolution of a freshwater snail and a sterilizing trematode. Evolution 63, 2213–2221 (doi:10.1111/j.1558-5646.2009.00711.x) [DOI] [PubMed] [Google Scholar]
- Lai Y. C., Liu Z., Billings L.2003Noise-induced unstable dimension variability and transition to chaos in random dynamical systems. Phys. Rev. E 67, 026210 (doi:10.1103/PhysRevE.67.026210) [DOI] [PubMed] [Google Scholar]
- Lenormand T., Roze D., Rousset F.2008Stochasticity in evolution. Trends Ecol. Evol. 24, 157–165 [DOI] [PubMed] [Google Scholar]
- Lenski R. E., Levin B. R.1985Constraints on the coevolution of bacteria and virulent phage: a model, some experiments, and predictions for natural communities. Am. Nat. 125, 585–602 (doi:10.1086/284364) [Google Scholar]
- Lively C. M.1999Migration, virulence, and the geographic mosaic of adaptation by parasites. Am. Nat. 153, S34–S47 (doi:10.1086/303210) [DOI] [PubMed] [Google Scholar]
- Loeuille N., Loreau M.2005Evolutionary emergence of size-structured food webs. Proc. Natl Acad. Sci. USA 102, 5761–5766 (doi:10.1073/pnas.0408424102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuille N., Loreau M., Ferrière R.2002Consequences of plant–herbivore coevolution on the dynamics and functioning of ecosystems. J. Theor. Biol. 217, 369–381 (doi:10.1006/jtbi.2002.3032) [DOI] [PubMed] [Google Scholar]
- Lythgoe K. A., Read A. F.1998Catching the Red Queen? The advice of the Rose. Trends Ecol. Evol. 13, 473–474 (doi:10.1016/S0169-5347(98)01486-4) [DOI] [PubMed] [Google Scholar]
- Marrow P., Law R., Cannings C.1992The coevolution of predator–prey interactions: ESSs and Red Queen dynamics. Proc. R. Soc. Lond. B 250, 133–141 (doi:10.1098/rspb.1992.0141) [Google Scholar]
- Marrow P., Dieckmann U., Law R.1996Evolutionary dynamics of predator–prey systems: an ecological perspective. J. Math. Biol. 13, 556–578 (doi:10.1007/BF02409750) [DOI] [PubMed] [Google Scholar]
- Martin A. P., Palumbi S. R.1993Body size, metabolic rate, generation time, and the molecular clock. Proc. Natl Acad. Sci. USA 90, 4087–4091 (doi:10.1073/pnas.90.9.4087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Abrams P. A.1994Runaway evolution to self-extinction under asymmetrical competition. Evolution 48, 1764–1772 (doi:10.2307/2410506) [DOI] [PubMed] [Google Scholar]
- May R. M., Anderson R. M.1983Epidemiology and genetics in the coevolution of parasites and hosts. Proc. R. Soc. Lond. B 219, 281–313 (doi:10.1098/rspb.1983.0075) [DOI] [PubMed] [Google Scholar]
- Metz J. A. J., Nisbet R. M., Geritz S. A. H.1992How should we define fitness for general ecological scenarios? Trends Ecol. Evol. 7, 198–202 (doi:10.1016/0169-5347(92)90073-K) [DOI] [PubMed] [Google Scholar]
- Metz J. A. J., Geritz S. A. H., Meszéna G., Jacobs F. J. A., van Heerwaarden J. S.1996Adaptive dynamics: a geometrical study of the consequences of nearly faithful reproduction. In Stochastic and spatial structures of dynamical systems (eds van Strien S. J., Verduyn Lunel S. M.), pp. 183–231 Burlington, MA: Elsevier Science [Google Scholar]
- Moya A., Holmes E. C., Gonzalez-Candelas F.2004The population genetics and evolutionary epidemiology of RNA viruses. Nat. Rev. Microbiol. 2, 279–288 (doi:10.1038/nrmicro863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Sigmund K.2003Chaos and the evolution of cooperation. Proc. Natl Acad. Sci. USA 90, 5091–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuismer S. L., Doebeli M.2004Genetic correlations and the coevolutionary dynamics of three-species systems. Evolution 58, 1165–1177 [DOI] [PubMed] [Google Scholar]
- Nuismer S. L., Ridenhour B. J., Oswald B. P.2007Antagonistic coevolution mediated by phenotypic differences between quantitative traits. Evolution 61, 1823–1834 (doi:10.1111/j.1558-5646.2007.00158.x) [DOI] [PubMed] [Google Scholar]
- Olden J. D., LeRoy Poff N., Douglas M. R., Douglas M. E., Faush K. D.2004Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24 (doi:10.1016/j.tree.2003.09.010) [DOI] [PubMed] [Google Scholar]
- Palumbi S. R.1994Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Syst. 25, 547–572 (doi:10.1146/annurev.es.25.110194.002555) [Google Scholar]
- Rand D. A., Wilson H. B.1991Chaotic stochasticity—a ubiquitous source of unpredictability in epidemics. Proc. R. Soc. Lond. B 246, 179–184 (doi:10.1098/rspb.1991.0142) [DOI] [PubMed] [Google Scholar]
- Robert M., Sorci G.1999Rapid increase of host defence against brood parasites in a recently parasitized area: the case of village weavers in Hispaniola. Proc. R. Soc. Lond. B 266, 941–946 (doi:10.1098/rspb.1999.0727) [Google Scholar]
- Rosenzweig M. L., Brown J. S., Vincent T. L.1987Red Queen and ESS: the coevolution of evolutionary rates. Evol. Ecol. 1, 59–94 (doi:10.1007/BF02067269) [Google Scholar]
- Royama T.1992Analytical population dynamics London, UK: Chapman & Hall [Google Scholar]
- Salathe M., Kouyos R. D., Bonhoeffer S.2008The state of affairs in the kingdom of the Red Queen. Trends Ecol. Evol. 23, 439–445 (doi:10.1016/j.tree.2008.04.010) [DOI] [PubMed] [Google Scholar]
- Seger J.1992Evolution of exploiter–victim relationships. In Natural enemies: the population biology of predators, parasites and diseases (ed. Crawley M. J.), pp. 3–25 Oxford, UK: Blackwell Scientific [Google Scholar]
- Shoresh N., Hegreness M., Kishony R.2008Evolution exacerbates the paradox of the plankton. Proc. Natl Acad. Sci. USA 105, 12 365–12 369 (doi:10.1073/pnas.0803032105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé R. V., Sardanyés J.2007Red Queen strange attractors in host–parasite replicator gene-for-gene coevolution. Chaos Soliton. Fract. 32, 1666–1678 [Google Scholar]
- Stegen J. C., Enquist B. J., Ferrière R.2009Advancing the metabolic theory of biodiversity. Ecol. Lett. 12, 1001–1015 (doi:10.1111/j.1461-0248.2009.01358.x) [DOI] [PubMed] [Google Scholar]
- Stenseth N. C., Maynard Smith J.1984Coevolution in ecosystems: Red Queen evolution or stasis. Evolution 38, 870–880 (doi:10.2307/2408397) [DOI] [PubMed] [Google Scholar]
- Strauss S. Y., Sahli H., Conner J. K.2005Toward a more trait-centered approach to diffuse (co)evolution. New Phytol. 165, 81–89 (doi:10.1111/j.1469-8137.2004.01228.x) [DOI] [PubMed] [Google Scholar]
- Thompson J. N.1994The coevolutionary process Chicago, IL: Chicago University Press [Google Scholar]
- Thompson J. N.2005The geographic mosaic of coevolution Chicago, IL: Chicago University Press [Google Scholar]
- Thrall P. H., Hochberg M. E., Burdon J. J., Bever J. D.2007Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol. Evol. 22, 120–126 (doi:10.1016/j.tree.2006.11.007) [DOI] [PubMed] [Google Scholar]
- Toju H.2009Natural selection drives the fine-scale divergence of a coevolutionary arms race involving a longmouthed weevil and its obligate host plant. BMC Evol. Biol. 9, 273–292 (doi:10.1186/1471-2148-9-273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toju H., Sota T.2006Imbalance of predator and prey armament: geographic clines in phenotypic interface and natural selection. Am. Nat. 167, 105–117 (doi:10.1086/498277) [DOI] [PubMed] [Google Scholar]
- Toju H., Sota T.2009Do arms races punctuate evolutionary stasis? Unified insights from phylogeny, phylogeography and microevolutionary processes. Mol. Ecol. 18, 3940–3954 (doi:10.1111/j.1365-294X.2009.04340.x) [DOI] [PubMed] [Google Scholar]
- Travisano M., Mongold J. A., Bennett A. F., Lenski R. E.1995Experimental tests of the role of adaptation, chance, and history in evolution. Science 267, 87–90 (doi:10.1126/science.7809610) [DOI] [PubMed] [Google Scholar]
- Turchin P.2003Complex population dynamics: a theoretical/empirical synthesis Princeton, NJ: Princeton University Press [Google Scholar]
- Van Valen L.1973A new evolutionary law. Evol. Theory 1, 1–30 [Google Scholar]
- Vermeij G. J.1982Unsuccessful predation and evolution. Am. Nat. 120, 701–720 (doi:10.1086/284025) [Google Scholar]
- Vermeij G. J.1994The evolutionary interaction among species—selection, escalation, and coevolution. Annu. Rev. Ecol. Syst. 25, 219–236 (doi:10.1146/annurev.es.25.110194.001251) [Google Scholar]
- Williams R. J., Martinez N. D.2000Simple rules yield complex food webs. Nature 404, 180–183 (doi:10.1038/35004572) [DOI] [PubMed] [Google Scholar]