Abstract
The life cycles of many organisms are constrained by the seasonality of resources. This is particularly true for leaf-mining herbivorous insects that use deciduous leaves to fuel growth and reproduction even beyond leaf fall. Our results suggest that an intimate association with bacterial endosymbionts might be their way of coping with nutritional constraints to ensure successful development in an otherwise senescent environment. We show that the phytophagous leaf-mining moth Phyllonorycter blancardella (Lepidoptera) relies on bacterial endosymbionts, most likely Wolbachia, to manipulate the physiology of its host plant resulting in the ‘green-island’ phenotype—photosynthetically active green patches in otherwise senescent leaves—and to increase its fitness. Curing leaf-miners of their symbiotic partner resulted in the absence of green-island formation on leaves, increased compensatory larval feeding and higher insect mortality. Our results suggest that bacteria impact green-island induction through manipulation of cytokinin levels. This is the first time, to our knowledge, that insect bacterial endosymbionts have been associated with plant physiology.
Keywords: plant–insect interaction, leaf-miner, green-island, endosymbiont, Wolbachia, extended phenotype
1. Introduction
Endophagous herbivorous insects live within plants resulting, at times, in the formation of remarkable new plant architectures such as galls and mines. These insect-derived shelters presumably offer protection from natural enemies and hostile environmental conditions, but they are also thought to enable the resident to feed selectively on tissues with high nutrient content, and low defence responses (Connor & Taverner 1997; Stone & Schönrogge 2003). Phytophagous organisms such as insect gallers, for instance, are known to alter plant primary and/or secondary metabolisms, suggesting that the insects not only determine the location, size and shape of the galls, but that they also control their chemical properties (Nyman & Julkunen-Tiitto 2000; Stone & Schönrogge 2003). Indeed, certain insect gallers are actively involved in the differentiation and growth of nutrient-rich plant tissues, in the upregulation of protein and/or sugar synthesis in situ, or in the modification of source–sink relationships leading to nutrient translocation towards the insect's feeding site (Larson & Whitham 1991; Wool et al. 1999; Stone & Schönrogge 2003; Giron et al. 2007; Schwachtje & Baldwin 2008). However, the molecules and mechanisms involved in insect gall formation remain totally unknown (Stone et al. 2002; Stone & Schönrogge 2003).
Interestingly, insect leaf-miners also appear to be playing an active part in the molecular cross-talk with their host plant. Indeed, certain leaf-miners are known to induce spectacular ‘green-islands’ on yellow leaves in autumn. These green-islands are characterized by photosynthetically active green patches in otherwise senescing leaves, and correspond to regions with an increased concentration in cytokinins and an enriched nutritional environment (Giron et al. 2007). Indeed, cytokinins are plant hormones involved in a variety of biological processes and notably in the inhibition of senescence, in the maintenance of chlorophyll and the control of source–sink relationships causing nutrient mobilization (Gan & Amasino 1995; Balibrea Lara et al. 2004; Walters & McRoberts 2006). Green-island formation is likely to have a strong influence on the insect leaf-miner life-history traits since plant levels of available energy in mined tissues exceed that of uninfected green or yellow plant tissue (Giron et al. 2007; D. Giron 2009, unpublished data). This might also explain the limited consumption of tissues within the mine—indeed larvae usually spare a central area of uneaten tissue that plays a role in thermal regulation and parasitoid avoidance (Djemaï et al. 2000). Furthermore, green-islands induced by leaf-miners on yellow leaves in autumn enable the insect to win a race against time and hence potentially enable the completion of a supplementary generation (Engelbrecht et al. 1969; Giron et al. 2007).
This green-island phenomenon is not restricted to plant–insect interactions and has also been described in plant–fungi and plant–bacteria systems (Engelbrecht et al. 1969; Walters & McRoberts 2006; Giron et al. 2007; Walters et al. 2008). Cytokinins have also already been implicated in a variety of plant–pest and plant–pathogen interactions (Jameson 2000; Farnsworth 2004; Walters & McRoberts 2006; Giron et al. 2007; Robert-Seilaniantz et al. 2007; Walters et al. 2008). Mechanisms employed by endophagous insects to manipulate the host plant physiology are however unknown. In leaf-miner systems, the origin of cytokinins has not yet been elucidated. Several lines of evidence suggest that cytokinins actually derive from the leaf-miner. First, initial results obtained by Engelbrecht et al. (1969) showed that large quantities of cytokinins are present in the labial glands of a birch leaf-miner. Second, quantification of cytokinins in apple leaf-miner larvae, which had been purged of plant remains revealed that not only did larvae exhibit significant amounts of cytokinins, but the relative proportion of cytokinins within the insects perfectly matched those found within the mines (D. Giron 2009, personal observation). Additionally, cytokinins have been found in insect secretions or glands associated with oviposition and initiation of galls in a wide range of galling insect species (Elzen 1983).
Interestingly, many invertebrates are known to have intimate relationships with bacterial symbionts and it is also well documented that key genes in cytokinin synthesis can be of bacterial origin—as in the case of Agrobacterium—which induces spectacular galls by the production of cytokinins (Robert-Seilaniantz et al. 2007). Molecular studies on the evolution and function of these symbionts are revealing diverse ecological and evolutionary effects on insect hosts (Moran et al. 2008). As research progresses, it has become apparent that many phenotypes associated with insects are now being reattributed to symbionts living in tight association. This might explain why insects represent over 75 per cent of animal species and have successfully invaded very diverse habitats illustrating their remarkable adaptive capacities. These insect symbionts have contributed to insect adaptation mainly by providing novel metabolic capacity enabling better exploitation of nutritional resources, but also by providing novel defence mechanisms and also by affecting reproductive strategies (Moran et al. 2008). The provision of nutrients or novel metabolic pathways to hosts has been proposed to be one of the major routes leading micro-organisms to symbiosis with many insect symbionts, enabling their hosts to develop on limited nutrient sources, such as blood in the case of blood-feeding insects, or plant sap and wood in the case of herbivore–plant interactions (Moran 2007; Hosokawa et al. 2010).
Since senescent autumnal leaves also represent a poor and declining source of nutrients for phytophagous insects, we investigated whether symbionts could be implicated in a host plant leaf-miner interaction, involving apple trees, Malus domestica, and the lepidopteran leaf-miner, Phyllonorycter blancardella. We show that curing P. blancardella of its endosymbiotic bacteria results in the loss of the green-island phenotype, indicating that symbionts can play an active role in the manipulation of plant physiology during plant–herbivore interactions.
2. Material and methods
(a). Biological model
Phyllonorycter blancardella F. (Lepidoptera: Gracillariidae) is a multivoltine leaf-mining microlepidopteran. The larval development is divided into five instars (Pottinger & Leroux 1971). All instars can be clearly separated from each other, especially first-to-third (L1–L2–L3) and fourth-to-fifth (L4–L5) instars which are morphologically distinct because of the hypermetamorphosis occurring between L3 and L4 instars (Pottinger & Leroux 1971).
During the first three sap-feeding instars, larvae define the outline of their mine by separating the two leaf integuments. The outline of the mine corresponds to the total surface available to later stages and following instars do not have the capacity to expand this feeding area. During the fourth and fifth instars, larvae are tissue-feeders and this feeding behaviour results in the formation of feeding windows (Djemaï et al. 2000). Feeding windows are translucent patches, where all tissues but the epidermis has been consumed by the caterpillar. A young fourth instar larva creates a new feeding window every time it eats. Later, enlarged windows are created as a result of the superposition of feeding events. The percentage of tissue eaten (total surface of feeding windows) is a convenient measure of the feeding activity of the leaf-miner, as the total area of a mine fluctuates from leaf-to-leaf and mine-to-mine (Djemaï et al. 2000).
(b). Detection of endosymbionts using universal bacterial primers
Detection of bacteria was performed using polymerase chain reaction (PCR) amplification on DNA extracted from P. blancardella larvae with rRNA 16S universal primers (electronic supplementary material, table S1). Two pools of three larvae were exposed to three successive baths of 3 or 6 per cent sodium hypochlorite, respectively, followed by a water rinse prior to DNA extraction, to avoid contamination by bacteria which could be present at the surface of the larvae. Highly pure water (Qiagen, France) was used and was exposed to a 254 nm ultraviolet lamp along with PCR tubes, MgCl2 and PCR buffer to avoid contamination from the bacteria in the environment. All experiments were performed under a flow-hood. DNA was extracted using the Puregene Kit (Gentra Systems, MN, USA). PCR reactions were performed on 2 µl of DNA using 0.1 units of Goldstar polymerase (Eurogentec, France), 0.2 mM dNTP, 1.5 mM MgCl2 and 50 pmole of each primer. DNA was initially denatured for 4 min at 95°C, followed by 40 cycles of 1 min denaturation at 95°C, 1 min hybridization at 52°C and 1 min elongation at 72°C, followed by a final elongation step at 72°C for 10 min. PCR products from the two pools of larvae were purified using the Nucleospin Extract II Kit (Macherey-Nagel, France) and cloned into the pDrive vector using the Qiagen PCR Cloning Kit. Twelve independent clones were sequenced from each pool. All 24 clones were sequenced in both directions with a capillary DNA sequencer (ABI PRISM 3100, Applied Biosystems, France; table 1). All 16S rDNA sequences were analysed by sequence alignment and BLAST analysis (Zhang et al. 2000).
Table 1.
Detection of bacteria associated with P. blancardella.
| bacteria | gene (type of primer) | number of clones sequenced | number of individuals tested |
|---|---|---|---|
| Wolbachia | 16S rRNA (universal primers) | 24 | 6 (2 pools of 3) |
| wsp (specific primers) | 48 | 5 | |
| ftsZ (specific primers) | 12 | 5 | |
| Arsenophonus | 16S rRNA (specific primers) | no amplification | 2 |
| Cardinium | 16S rRNA (specific primers) | no amplification | 2 |
| Flavobacteria | 16S rRNA (specific primers) | no amplification | 2 |
| Rickettsia | 16S rRNA (specific primers) | no amplification | 2 |
| Spiroplasma | 16S rRNA (specific primers) | no amplification | 2 |
(c). Detection of endosymbionts using group-specific bacterial primers
DNA was extracted using the Puregene Kit (Gentra Systems). Each DNA sample was used for different PCR reactions using specific primers for the following symbiotic bacteria: Wolbachia, Cardinium, Rickettsia, Flavobacteria, Spiroplasma and Arsenophonus (see electronic supplementary material, table S1 and appendix S2 for primers and exact PCR conditions; see table 1 for the number of individuals and clones tested). As a positive control, PCR amplification of an insect gene (EF1-α) was always performed to ensure the quality of DNA and that lack of amplification of bacterial genes was not owing to manipulation errors. As positive controls, PCR amplifications of Wolbachia, Spiroplasma, Ricketssia, Cardinium and Flavobacteria genes from infected insects were performed at the same time to ensure the efficiency of the different primers used for detection.
Amplified products were cloned into the pDrive vector (Qiagen, France) and then sequenced in both directions. All sequences were analysed by sequence alignment and BLAST analysis (Zhang et al. 2000).
(d). Curing experiments using antibiotic treatments
(i). Antibiotic feeding
Emerging females were kept with males for 3 days in Petri dishes containing either 40 per cent glucose solution, or 1 per cent antibiotic and 40 per cent glucose solution. To control for antibiotic side effects, two antibiotics differing in their structure and mode of action were used. Rifampicin inhibits prokaryotic DNA-dependent RNA polymerase, whereas tetracycline affects protein synthesis on bacterial ribosomes. These antibiotics have been used in a large number of experiments in various insect species and have been proven to have no toxic effects in all of these systems (e.g. Zchori-Fein & Perlman 2004; Werren et al. 2008; Hosokawa et al. 2010). The initial generation (G0) consumed the antibiotics, and females were individually placed on small 3-year-old apple (M. domestica B.) seedlings grown in a greenhouse for oviposition. Only females that had truly fed on antibiotic solutions and for which wsp gene amplification was negative were used in the experiment. Untreated females (fed on 40% glucose solution) were used as controls.
After oviposition of G0 females, apple seedlings were kept under temperate conditions (mean air temperature 22°C, mean relative humidity 59.5%, irradiance up to 718 W m−2, and natural light/dark cycle 11 : 13) and watered daily in the greenhouse until larval emergence (egg stage: 9.5 ± 0.8 days). After 10 days, corresponding to the beginning of the L1 larval stage, a subset of individual trees was placed outside, under natural autumnal conditions to allow natural senescence of leaves on trees without chemical interference (experiments were conducted during October–November 2007, 2008 and 2009). Remaining trees were kept in the greenhouse under the abovementioned summer conditions as a control. During the 15 following days (L1–L3 larval stage: 15.2 ± 2.6 days), a natural progressive yellowing of leaves occurred for apple seedlings placed under autumnal conditions. At the onset of the fourth larval instar, only seedlings with mines developing on fully yellow senescent leaves were kept for further analyses (average hue value for yellow leaves = 43 ± 0.65, min = 37, max = 47; Stevens et al. 2007).
These individuals from the first generation (G1 = offspring of G0 untreated or treated insects) were allowed to complete their development and were checked for green-island formation, adult emergence success, endosymbiotic bacterial composition (by 16S rRNA gene amplification) and Wolbachia infection status (by wsp gene amplification).
Offspring of treated (n = 15) and untreated (n = 15) individuals were followed over three generations (G1, G2 and G3) on green leaves (average hue value for green leaves = 77.9 ± 1.30, min = 68, max = 88; Stevens et al. 2007). Owing to the strong experimental constraints of synchronizing insect development and natural yellowing of leaves, treated individuals were only followed over two generations (G1 and G2) on yellow leaves. In this last case, insects were reared on green leaves as previously described and transferred under natural autumnal conditions during the first or the second generation. All successive generations were checked for survival and Wolbachia infection status by wsp amplification (table 2). It should be noted that only G0 insects fed on antibiotic solution and that G1, G2 and G3 individuals did not directly feed on antibiotics.
Table 2.
Phenotypes and Wolbachia detection associated with P. blancardella before and after antibiotic treatment. (G, generation; n, number of individuals tested in each experiment.)
| untreated insects | treated insects G0 | treated insects G1 | treated insects G2 | treated insects G3 | |
|---|---|---|---|---|---|
| wsp gene amplification (Wolbachia-specific primers) | strong signal (Wolbachia group A) (n = 15) | no amplification (n = 15) | no amplification (n = 15) | no amplification (n = 15) | no amplification (n = 15) |
| phenotype on green leaves | normal survival, reproduction and development (n = 15) | not relevant | successful survival, reproduction and development (n = 15) | successful survival, reproduction and development (n = 15) | successful survival, reproduction and development (n = 15) |
| phenotype on yellow leaves | green-islands; normal survival, reproduction and development (n = 20) (18 emerged) | not relevant | no green-islands; high mortality rate; modified feeding behaviour; (n = 22) (3 emerged) | no green-islands; high mortality rate; modified feeding behaviour; (n = 12) (1 emerged) | not done |
As a positive control for PCR reactions, PCR amplification of an insect gene (EF1-α) was always performed on DNA from non-treated and antibiotic-treated individuals. A signal of similar intensity corresponding to EF1-α was obtained for all individuals.
(ii). Physiological conditions of yellow leaves
To control for possible effects of the physiological status of individual yellow leaves on insect survival, experiments were performed where treated and untreated females oviposited on the same leaf simultaneously. Different concentrations of antibiotics (0%, 0.25%, 0.5% and 1% in 40% glucose solution) were provided to G0 females by the abovementioned method. During the second generation (G2), the proportion of eaten tissues and the relative area of green and yellow tissues within the mine were measured 7 days after hypermetamorphosis occurred (i.e. 22 days post-emergence) corresponding to the end of the fourth instar (L4 larval stage: 6.9 ± 0.9 days). Measures were conducted on mines from L4 instars in order to ensure that a sufficient amount of uneaten tissue remained within the mine and in order to make sure that all larvae (treated and untreated) were still alive. Indeed, by the end of the fifth instar (17 days post-hypermetamorphosis; L4–L5 larval stages: 17 ± 2.5 days), on yellow leaves most of the 1 per cent antibiotic-treated larvae were dead (see §3). Concentration of cytokinins in the corresponding mined leaf tissues were also measured for untreated (n = 9) and 1 per cent antibiotic solution-treated insects (n = 7). A subset of individuals was also allowed to pursue its development after the L4 larval stage and to complete the entire life cycle. Larval survival of untreated (n = 10) and 1 per cent antibiotic solution-treated insects (n = 12) was recorded.
Feeding patterns were captured using a digital camera (Nikon D70s) and pictures were analysed using the Matlab environment to convert images to binary images based on thresholds (image processing toolbox) (Djemaï et al. 2000; Stevens et al. 2007). Each image was partitioned into mine and background. Grey-scale images were then converted to binary ones using thresholding. A binary image allowed us to calculate the surface of both intact tissues and eaten areas. The total area of the mine is the sum of both surfaces. The proportion of green and yellow uneaten tissues was calculated using the same procedure with different thresholds.
Cytokinin concentrations in leaf tissues were measured as described previously (Giron et al. 2007). Briefly, leaf samples were lyophilized, pulverized and extracted overnight in aqueous methanol containing butylated-hydroxytoluene as an antioxidant. Purification was performed using a nitrocellulose prefilter (4.5 µm, Sartorius, Germany) connected to a Oasis cartridge (Waters, USA) with a Teflon filter (0.2 µm, Sartorius, Germany) at the outlet. Cytokinins were then measured using an enzyme-linked immunosorbent assay (ELISA). Isopentenyladenine (iP) and isopentenyl adenosine (iPA) were measured using anti-iPA polyclonal antibodies. Zeatin riboside (ZR) and Zeatin (Z) were measured using anti-ZR polyclonal antibodies. ELISA was performed with microtitration plates (Nunc, Germany) and optical densities were measured at 405 nm. Values obtained from ELISAs were corrected for extraction and purification losses according to the percentage recovery of the tritiated-adenine included in the sample extraction procedure. Amounts of cytokinins obtained were expressed as fmole mg−1 of plant material (dry weight).
(e). Prevalence of Wolbachia in natural populations
Field-collected P. blancardella were controlled for the presence of Wolbachia using wsp gene amplification as previously described. Insects were collected as larvae or pupae within mined leaves. Mined leaves were collected on sites and individually placed in Petri dishes under room temperature. Petri dishes were checked daily for adult insect emergence and newly emerged adults were frozen at −80°C for further analyses. Sixty newly emerged P. blancardella adults from field-collected samples on three different locations in the Loire Valley area (average distance between sites = 13 km; maximum distance = 17 km) were controlled for the presence of Wolbachia.
(f). Accession numbers
Sequences have been deposited in the EMBL Nucleotide Sequence Database under the following accession numbers: P. blancardella partial EF1-α gene (accession no. FM883703); Wolbachia associated with P. blancardella partial wsp gene (accession no. FM883704); Wolbachia associated with P. blancardella partial ftsZ gene (accession no. FM883705) and Wolbachia associated with P. blancardella partial 16S rRNA gene (accession no. FM883706).
(g). Statistical analyses
Statistical analyses were performed using R v. 2.3.0 software. Data were analysed by non-parametric Kruskal–Wallis tests followed by Behrens–Fisher post hoc tests. Percentages of survival were analysed by χ2 tests.
3. Results
(a). Detection and identification of bacterial symbionts in P. blancardella: Wolbachia is the only symbiont detected in leaf-miner larvae
We first investigated the presence of potential bacterial symbionts associated with the larvae of the leaf-mining moth P. blancardella by performing 16S rRNA gene amplification using universal bacterial primers. Cloning and sequencing of the corresponding products revealed that only one group of bacteria, Wolbachia, could be detected in larvae, indicating that this is the most highly abundant bacteria in this insect at this stage. Highest BLAST scores were obtained with Wolbachia pipientis 16S rRNA. In addition, attempts to amplify specific genes corresponding to symbionts that can be associated to arthropods (Duron et al. 2008), namely Cardinium, Rickettsia, Flavobacteria, Spiroplasma and Arsenophonus were unsuccessful and only specific Wolbachia genes (wsp, ftsZ) were amplified successfully (table 1).
(i). All individuals were found to be infected by group A Wolbachia
A previous study had also reported the presence of supergroup A Wolbachia in P. blancardella (West et al. 1998). To determine whether this leaf-miner was subject to multiple Wolbachia infections, we amplified and sequenced the wsp and ftsZ genes that enable discrimination between different Wolbachia supergroups (Zhou et al. 1998). All individuals tested harboured the same wsp and ftsZ gene sequences, corresponding to group A Wolbachia, indicating that P. blancardella is infected with a single Wolbachia variant belonging to this group only. For ftsZ, highest BLAST scores were obtained with ftsZ sequences from Wolbachia associated with Drosophila simulans and Asobara tabida, whereas wsp showed higher similarity with Wolbachia associated with a Cynipid gall wasp and the Lepidoptera Ephestia kuehniella.
The prevalence of Wolbachia in P. blancardella was estimated in three different locations in the Loire Valley area and was found to be 100 per cent.
(b). Effect of endosymbiont curing on the green-island phenotype
We assessed the effects of endosymbionts on green-island formation by treating P. blancardella with antibiotics. Two independent curing experiments using two unrelated antibiotics with two different modes of action at a concentration of 1 per cent were performed. The following three generations (G1, G2, G3) of insects that had not directly consumed the antibiotics were analysed.
On green apple trees, no wsp amplification could be detected in G1–G3 individuals originating from G0-treated individuals (n = 15 for each generation) and these offspring did not differ from controls (untreated individuals; n = 15 for each generation) in their capacity to successfully reproduce, survive and develop (data not shown) (table 2).
(i). Absence of symbionts leads to a loss of the green-island phenotype
On yellow leaves, all individuals originating from antibiotic-fed females (G1) were checked for bacterial presence by 16S rRNA and wsp gene amplification (n = 15 for each generation and for each antibiotic). 16S rRNA gene amplification on G1-cured individuals did not reveal the presence of other bacteria that might have thrived on antibiotic-fed individuals, but did reveal a very faint level of Wolbachia, although at considerably lower densities when compared with non-treated controls (figure 1). In accordance, no wsp gene amplification could be detected in these individuals (figure 1). We therefore obtained from antibiotic-fed G0 females, G1 offspring adult moths with barely detectable levels of Wolbachia and no other detectable bacterial symbionts (figure 1). In a spectacular way, G1 larvae originating from G0 1 per cent antibiotic-fed adults were no longer capable of inducing green-islands on yellow leaves (figure 1). These G1 leaf-miners also experienced significantly higher mortality rates in the absence of green-island formation. Indeed, 90 per cent of control untreated larvae (n = 20) induced green-islands and these adult moths emerged successfully (n = 18), whereas G1 larvae originating from antibiotic-fed adult moths (n = 22) failed to induce green-islands and massively died before adult emergence (only 13% successfully emerged: n = 2 for tetracycline, n = 1 for rifampicin; χ2 = 24.436, d.f. = 1, p < 0.01) (table 2).
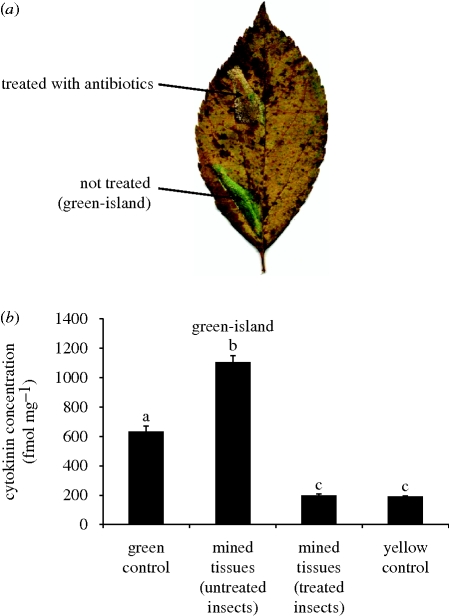
Figure 1.
Influence of Wolbachia on green-island formation. (a) Apple tree leaves infected with the tentiform leaf-miner Phyllonorycter blancardella in autumn. Offspring (G1) obtained from untreated controls and antibiotic-treated adults show distinct feeding behaviours and emergence success in autumnal conditions. On the left panel, untreated individuals induce green-islands on host plants. The feeding area exhibits intact green chlorophyll-containing tissues, while the remaining leaf tissues undergo leaf senescence. The white spots on the mine are feeding windows, where all but the epidermis has been consumed by the caterpillar. On the right panel, larvae from antibiotic-treated individuals are unable to induce green-islands and consume almost the entire amount of tissues present within the mine leaving mostly non-nutritional epidermal cells only. (b) Representative PCR amplifications from offspring obtained from non-treated (left panel) and antibiotic-treated individuals (right panel) with the insect EF1-α primers, universal bacterial 16S rRNA primers and Wolbachia-specific wsp primers. 16S rRNA-amplified products correspond to Wolbachia, only a very faint signal could be detected in antibiotic-treated individuals. No wsp amplification was detected in treated individuals.
(ii). Loss of the green-island phenotype persists over successive generations
Analysis of successive generations after antibiotic treatments allowed us to confirm these results. In the second generation (G2), the presence or absence of green-islands in untreated and 1 per cent antibiotic-treated insects, respectively, and the corresponding insect survival followed the same pattern than for the first generation (G1) (table 2 and figure 2). Indeed, 100 per cent of control untreated larvae (n = 10) successfully induced green-islands and survived to the adult stage, while all G2 larvae originating from 1 per cent antibiotic-fed adult moths (n = 12) failed to induce green-islands and mostly died before adult emergence (only one individual successfully emerged) (table 2). As treated and untreated larvae developed simultaneously on the same leaf, differences in insect survival (χ2 = 18.333, d.f. = 1, p < 0.01) are unlikely to be caused by specific physiological conditions of yellow leaves and can only be explained by presence or absence of green-islands (figure 2a).
Figure 2.
Influence of the presence of endosymbionts on green-island formation. (a) Representative leaf showing the impact of symbionts on green-island induction. Second generation-cured individuals failed to produce green-islands (upper mine), while untreated insects (control) successfully maintained intact green chlorophyll-containing tissues within the mine (lower mine). (b) Cytokinin concentration in control and mined leaf tissues. Green-islands (untreated) contained high levels of cytokinins, while tissues mined by antibiotic-treated insects had very low levels of phytohormones. Homologous groups are indicated by identical letters (Kruskal–Wallis test: p < 0.01). Means ± s.e.
(iii). Loss of the green-island phenotype is associated with reduced cytokinin levels in the mine
We had previously shown that green-island induction was related to the amount of cytokinin production (Giron et al. 2007). In this experiment, as expected, cytokinin levels in green-islands induced by untreated insects were found to be higher than cytokinin levels in uninfected green tissues. By contrast, cytokinin levels in mines induced by antibiotic-treated insects (G2 generation) that failed to produce green-islands were low and similar to levels found in yellow leaves (Kruskal–Wallis test: p < 0.01; figure 2b). These results show that curing leaf-miners of their endosymbiotic community impacts green-island induction via cytokinin contents.
(iv). Non-senescing effects and insect feeding behaviour are correlated to Wolbachia levels
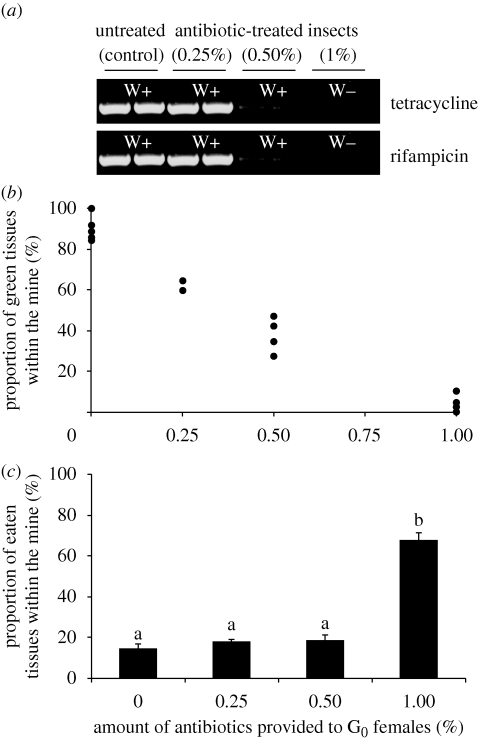
Applying increasing concentrations of antibiotics (0–1%) led to a corresponding decrease of the level of Wolbachia infection (figure 3a). Mines harbouring insects submitted to increasing levels of antibiotics displayed a drop in the proportion of green tissues from 94.6 ± 2.25 per cent in control to 4.58 ± 1.6 per cent in 1 per cent antibiotic-treated insects (logistic regression, generalized linear model, p < 0.001) (tetracycline treatment, figure 3b). Furthermore, treated larvae also displayed a modified feeding behaviour. Indeed, whereas insects originating from untreated, 0.25 or 0.50 per cent antibiotic-fed females consumed on an average 15–19% of tissues within the mine by the end of the fourth instar (L4), larvae from 1 per cent antibiotic-fed females consumed about 70 per cent of leaf tissues within the mine during the same amount of time (Kruskal–Wallis test: p < 0.05) (tetracycline treatment, figure 3c). These results suggest that Wolbachia is a key player in the origin of the green-island phenotype.
Figure 3.
Various effects of antibiotic treatments. (a) Impact of antibiotics on the Wolbachia hosted by leaf-miner larvae. Increased levels of antibiotics provided to G0 females lead to a strong decrease of the wsp signal in G2 offspring. A Wolbachia-specific signal can be detected (W+) up to a level of 0.50% of antibiotics in insect diet. When 1% antibiotic diet is provided to insects, the Wolbachia-specific signal cannot be detected anymore (W−). (b) Relative proportion of green tissues within the mine. For control insects, most tissues within the mine remain green on yellow leaves. At high antibiotic concentrations, most tissues enter senescence and turn yellow. Results shown for the tetracycline treatment. (c) Impact of antibiotics on the insect feeding behaviour. For levels of up to 0.50% of antibiotics in the parent (G0) insect diet, less than 20% of tissues are consumed by the second generation (G2) larva by the end of its fourth instar. One per cent of antibiotics leads to a significant increase of the feeding activity of G2 larvae, inducing the consumption of about 70 per cent of tissues within the mine by the end of the fourth instar. Homologous groups are indicated by identical letters (Kruskal–Wallis test: p < 0.01). Means ± s.e. Results shown for the tetracycline treatment.
4. Discussion
Maternally heritable insect symbionts are revealed to be widespread, and include obligate nutritional mutualists that are required to support host development; facultative mutualists that confer fitness benefits by protection against natural enemies and stress and reproductive manipulators that enhance the frequency of infected daughters (Moran et al. 2008). Nutritional benefits involve the symbiont providing the host with essential metabolites that are absent from the host diet (i.e. plant sap or vertebrate blood), thereby enabling specialization on restricted diets, leading to important impacts on insect host ecology (Moran & Degnan 2006). The best-characterized examples involve obligate Buchnera symbionts that possess genes for biosynthesis of rare or absent amino acids from the aphid host diet, thereby enabling aphids to feed on phloem sap (Moran & Degnan 2006).
Here, we present evidence that insect bacterial symbionts can be involved in insect herbivore–plant interactions by a different mechanism involving manipulation of host plant physiology. Green-islands are believed to procure important fitness benefits to herbivorous insects via elevated levels of cytokinins that are known to play an important role in nutrient mobilization (Giron et al. 2007). Furthermore, green-islands potentially allow for an additional generation of insects in an otherwise senescent autumnal environment. We show that treating leaf-miner insects with antibiotics resulted in a strong decrease and almost complete loss of their symbiotic association with Wolbachia and in the loss of the green-island phenotype on yellow leaves. We conclude that antibiotic toxicity is unlikely given that: (i) G1–G3 individuals from treated G0 females did not directly consume the antibiotics and were able to successfully develop and survive on green leaves; (ii) G2 individuals originating from G0 antibiotic-fed females also failed to produce green-islands on yellow leaves; (iii) on senescing tissues, offspring from treated individuals kept on eating leaf tissues until the final instars; and (iv) similar results were obtained with two antibiotics differing in their structures and mode of action.
Treated insects also displayed increased compensatory larval feeding, and higher insect mortality on yellow leaves. Up to a level of 0.50 per cent of antibiotics, insects maintained a similar feeding behaviour but strongly increased their feeding activity when treated with 1 per cent antibiotic solution (figure 3). In parallel, increasing levels of antibiotics led to a progressive loss of the green-island phenotype, inducing a decrease in the nutritional quality of leaf tissues (figure 3) (Giron et al. 2007; D. Giron 2009, unpublished data) These results most probably illustrate the evolutionary trade-off that leaf-miner larvae have to face between nutrient acquisition on the one hand and limited consumption of tissues within the mine to avoid parasitoids and excessive radiations on the other hand (Djemaï et al. 2000; Pincebourde & Casas 2006). Precise quantification of the impact of green-islands on insect life-history traits will need further investigation, both on green and yellow leaves.
The molecular mechanisms at the basis of green-island induction and in particular, the origin of cytokinins within the green mine are still unknown but several hypotheses can be advanced to explain the implication of symbiotic bacteria in the green-island phenotype. Symbiotic bacteria might (i) be directly synthesizing cytokinins, or (ii) enable the insect to synthesize/secrete cytokinins, or even (iii) produce regulators of plant cytokinin gene expression. The first two hypotheses are backed-up by initial results obtained by Engelbrecht et al. (1969) who showed that large quantities of cytokinins are present in the labial glands of a birch leaf-miner. Furthermore, cytokinins have already been implicated in a variety of plant–pest and plant–pathogen interactions involving galling insects and bacterial pathogens such as Agrobacterium (Elzen 1983; Walters & McRoberts 2006; Robert-Seilaniantz et al. 2007). In symbiotic plant bacterial interactions, which lead to nodulation, cytokinins are also important in mediating signalling for nodule formation. In this system, cytokinins are suspected to have a dual origin: via expression of bacterial genes and induction of plant genes (Frugier et al. 2008). We cannot exclude at this stage that, in our system, the symbiotic community associated with the leaf-miner insects may be indirectly affecting other aspects of plant physiology, which impact on cytokinin levels as a side-effect. Indeed, other aspects of apple tree physiology have been shown to be altered by bacteria resulting in modified plant–phytophagous insect interactions (Mayer et al. 2008a,b).
Green-islands in apple leaves can be simulated by external application of cytokinin (Shantz et al. 1988) and delay in leaf senescence observed here is associated with both cytokinin levels in the mine (figure 2) and levels of Wolbachia in insects (figure 3). These results strongly suggest that Wolbachia is a good candidate to be involved in the green-island phenotype through manipulation of cytokinin levels. Wolbachia are usually considered as manipulators of reproduction in insects (Werren et al. 2008). However, Wolbachia has recently been suggested to play the role of a nutritional mutualist for bedbugs, highlighting that this most prevalent endosymbiotic group may be involved in a higher diversity of phenotypes than so far described (Hedges et al. 2008; Hosokawa et al. 2010). It is also worth noting that a key gene involved in cytokinin biosynthesis (tRNA-IPT) is present in all available Wolbachia genomes.
The only detectable bacteria associated with P. blancardella larvae is Wolbachia, and antibiotic curing leads to considerable reduction in bacterial density. Although sequencing of a high number of clones (n = 24 clones) strongly suggests that Wolbachia is indeed the only bacterial symbiont present in apple leaf-miners, it will now be essential to determine in depth whether other bacteria could be involved in this new phenotype. In particular, the localization and the relative density of the different microbial communities will have to be assessed by in situ hybridization and qRT–PCR experiments in different tissues, respectively. Given that cytokinin accumulation has been observed in P. blancardella salivary glands and another leaf-miner species (Engelbrecht et al. 1969), it will be of particular interest to determine the nature of the microbial community in this tissue.
5. Conclusion
Many leaf-miners are condemned to make the best of their mother's choice; enclosed in the two epidermis layers of a single leaf for their entire larval life, they have a restricted choice of food, minimal locomotion abilities and suffer high parasitism rate (Connor & Taverner 1997). Despite these apparent handicaps, this group is highly diverse (Lopez-Vaamonde et al. 2003). This evolutionary success could be explained in part by the intimate association with bacterial endosymbionts that might be their way of coping with additional nutritional constraints to ensure successful development and adequate food supply in an otherwise senescent environment. Indeed, the phytophagous leaf-mining moth P. blancardella relies on bacterial endosymbionts (possibly Wolbachia) to manipulate the physiology of its host plant, resulting in the green-island phenotype. This is the first time, to out knowledge, that insect bacterial endosymbionts have been associated with plant physiology manipulation. The challenge will now be to determine exactly how these bacteria act and how widespread is this phenomenon among endophagous insects.
Acknowledgements
This work was supported by the ANR grant no. ANR-05-JCJC-0203-01 to D.G. We thank L. Ardouin for full access to his orchard, D. Bouchon and his group for helpful discussions. We thank J. M. Drezen for providing us with a full access to his genomic platform, but also A. Bezier, A. Pichon, C. Serbielle, S. Dupont and T. Steinmann for their technical help and E. Desouhant for statistical advice. Positive controls for endosymbionts were provided by D. Bouchon and A. Aebi. We are grateful to C. Lopez-Vaamonde, F. Dedeine, E. McCauley, E. E. Farmer, M. R. Strand and anonymous referees for helpful comments on the manuscript.
References
- Balibrea Lara M. E., Gonzalez Garcia M. C., Fatima T., Ehness R., Lee T. K., Proels R., Tanner W., Roitsch T.2004Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16, 1276–1287 (doi:10.1105/tpc.018929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. F., Taverner M. P.1997The evolution and adaptive significance of the leaf-mining habit. Oikos 79, 6–25 (doi:10.2307/3546085) [Google Scholar]
- Djemaï I., Meyhöfer R., Casas J.2000Geometrical games between a host and a parasitoid. Am. Nat. 156, 257–265 (doi:10.1086/303388) [DOI] [PubMed] [Google Scholar]
- Duron O., Bouchon D., Boutin S., Bellamy L., Zhou L., Engelstadter J., Hurst G. D.2008The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol. 6, 27 (doi:10.1186/1741-7007-6-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzen G. W.1983Cytokinins and insect galls. Comp. Biochem. Physiol. 76, 17–19 (doi:10.1016/0300-9629(83)90286-4) [Google Scholar]
- Engelbrecht L., Orban U., Heese W.1969Leaf-miner caterpillars and cytokinins in the green islands of autumn leaves. Nature 223, 319–321 (doi:10.1038/223319a0) [Google Scholar]
- Farnsworth E.2004Hormones and shifting ecology throughout plant development. Ecology 85, 5–15 (doi:10.1890/02-655) [Google Scholar]
- Frugier F., Kosuta S., Murray J. D., Crespi M., Szczyglowski K.2008Cytokinin: secret agent of symbiosis. Trends Plant Sci. 13, 115–120 (doi:10.1016/j.tplants.2008.01.003) [DOI] [PubMed] [Google Scholar]
- Gan S., Amasino R. M.1995Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270, 1986–1988 (doi:10.1126/science.270.5244.1986) [DOI] [PubMed] [Google Scholar]
- Giron D., Kaiser W., Imbault N., Casas J.2007Cytokinin-mediated leaf manipulation by a leaf-miner caterpillar. Biol. Lett. 3, 340–343 (doi:10.1098/rsbl.2007.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges L. M., Brownlie J. C., O'Neill S. L., Johnson K. N.2008Wolbachia and virus protection in insects. Science 322, 702 (doi:10.1126/science.1162418) [DOI] [PubMed] [Google Scholar]
- Hosokawa T., Koga R., Kikuchi Y., Meng X. Y., Fukatsu T.2010Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107, 769–774 (doi:10.1073/pnas.0911476107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson P. E.2000Cytokinins and auxins in plant-pathogen interactions: an overview. Plant Growth Regul. 32, 369–380 (doi:10.1023/A:1010733617543) [Google Scholar]
- Larson K. C., Whitham T. G.1991Manipulation of food resources by a gall-forming aphid: the physiology of sink–source interactions. Oecologia 88, 15–21 (doi:10.1007/BF00328398) [DOI] [PubMed] [Google Scholar]
- Lopez-Vaamonde C., Godfray H. C., Cook J. M.2003Evolutionary dynamics of host-plant use in a genus of leaf-mining moths. Evolution 57, 1804–1821 [DOI] [PubMed] [Google Scholar]
- Mayer C. J., Vilcinskas A., Gross J.2008aPhytopathogen lures its insect vector by altering host plant odor. J. Chem. Ecol. 34, 1045–1049 (doi:10.1007/s10886-008-9516-1) [DOI] [PubMed] [Google Scholar]
- Mayer C. J., Vilcinskas A., Gross J.2008bPathogen-induced release of plant allomone manipulates vector insect behavior. J. Chem. Ecol. 34, 1518–1522 (doi:10.1007/s10886-008-9564-6) [DOI] [PubMed] [Google Scholar]
- Moran N. A.2007Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA 104(Suppl. 1), 8627–8633 (doi:10.1073/pnas.0611659104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A., Degnan P. H.2006Functional genomics of Buchnera and the ecology of aphid hosts. Mol. Ecol. 15, 1251–1261 (doi:10.1111/j.1365-294X.2005.02744.x) [DOI] [PubMed] [Google Scholar]
- Moran N. A., McCutcheon J. P., Nakabachi A.2008Genomics and evolution of heritable bacterial symbionts. Ann. Rev. Genet. 42, 165–190 (doi:10.1146/annurev.genet.41.110306.130119) [DOI] [PubMed] [Google Scholar]
- Nyman T., Julkunen-Tiitto R.2000Manipulation of the phenolic chemistry of willows by gall-inducing sawflies. Proc. Natl Acad. Sci. USA 97, 13 184–13 187 (doi:10.1073/pnas.230294097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincebourde S., Casas J.2006Leaf miner-induced changes in leaf transmittance cause variations in insect respiration rates. J. Insect Physiol. 52, 194–201 (doi:10.1016/j.jinsphys.2005.10.004) [DOI] [PubMed] [Google Scholar]
- Pottinger R. P., LeRoux E. J. The biology and the dynamics of Lithocolletis blancardella(Lepidoptera: Gracillariidae) on apple in Quebec. 1971. Memoirs of the Entomological Society of Canada 77. [Google Scholar]
- Robert-Seilaniantz A., Navarro L., Bari R., Jones J. D.2007Pathological hormone imbalances. Curr. Opin. Plant Biol. 10, 372–379 (doi:10.1016/j.pbi.2007.06.003) [DOI] [PubMed] [Google Scholar]
- Shantz G. M., Proctor J. T. A., Chiba M.1988Cytokinins in apple leaves and their relationship to spotted tentiform leafminer injury. Hortscience 23, 878–879 [Google Scholar]
- Schwachtje J., Baldwin I. T.2008Why does herbivore attack reconfigure primary metabolism? Plant Physiol. 146, 845–851 (doi:10.1104/pp.107.112490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M., Parraga C. A., Cuthill I. C., Partridge J. C., Troscianko T. S.2007Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237 (doi:10.1111/j.1095-8312.2007.00725.x) [Google Scholar]
- Stone G. N., Schönrogge K.2003The adaptive significance of insect gall morphology. Trends Ecol. Evol. 18, 512–522 (doi:10.1016/S0169-5347(03)00247-7) [Google Scholar]
- Stone G. N., Schonrogge K., Atkinson R. J., Bellido D., Pujade-Villar J.2002The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 47, 633–668 (doi:10.1146/annurev.ento.47.091201.145247) [DOI] [PubMed] [Google Scholar]
- Walters D. R., McRoberts N.2006Plants and biotrophs: a pivotal role for cytokinins? Trends Plant Sci. 11, 581–586 (doi:10.1016/j.tplants.2006.10.003) [DOI] [PubMed] [Google Scholar]
- Walters D. R., McRoberts N., Fitt B. D.2008Are green-islands red herrings? Significance of green-islands in plant interactions with pathogens and pests. Biol. Rev. Camb. Phil. Soc. 83, 79–102 [DOI] [PubMed] [Google Scholar]
- Werren J. H., Baldo L., Clark M. E.2008Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751 (doi:10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- West S. A., Cook J. M., Werren J. H., Godfray H. C.1998Wolbachia in two insect host-parasitoid communities. Mol. Ecol. 7, 1457–1465 (doi:10.1046/j.1365-294x.1998.00467.x) [DOI] [PubMed] [Google Scholar]
- Wool D., Aloni R., Ben-Zvi O., Wollberg M.1999A galling aphid furnishes its home with a built-in pipeline to the host food supply. Entomol. Experiment. Applicata 91, 183–186 (doi:10.1046/j.1570-7458.1999.00482.x) [Google Scholar]
- Zchori-Fein E., Perlman S. J.2004Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 13, 2009–2016 (doi:10.1111/j.1365-294X.2004.02203.x) [DOI] [PubMed] [Google Scholar]
- Zhang Z., Schwartz S., Wagner L., Miller W.2000A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7, 203–214 (doi:10.1089/10665270050081478) [DOI] [PubMed] [Google Scholar]
- Zhou W., Rousset F., O'Neil S.1998Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265, 509–515 (doi:10.1098/rspb.1998.0324) [DOI] [PMC free article] [PubMed] [Google Scholar]