Abstract
Most species of the modern family Isoëtaceae (Quillworts) some other modern hydrophytes, use a metabolic pathway for carbon fixation that involves uptake of sedimentary carbon and enrichment of CO2 in internal gas spaces as a carbon-concentrating mechanism. This metabolism, which is related to ‘aquatic CAM’, is characterized by morphological, physiological and biochemical adaptations for decreasing photorespirative loss, aerating roots and maintaining high growth rates in anoxic, oligotrophic, stressed environments. Some of the closest relatives of the Isoëtaceae were the ‘arborescent lycopsids’, which were among the dominant taxa in the coal swamps found in lowland ecosystems during the Carboniferous and Permian periods (approx. 300 Ma). Morphological, ecological and geochemical evidence supports the hypothesis that the arborescent lycopsids had an unusual metabolism similar to that of modern Isoëtaceae and processed a biogeochemically significant proportion of organically fixed carbon over a period of about 100 million years in the late Palaeozoic. The temporal coincidence between the dominance of plants with this metabolism and an anomalous global atmosphere (high O2; low CO2) supports the idea that biosphere feedbacks are important in regulating global climatic homeostasis. The potential influence of this metabolism on the global carbon cycle and its specific adaptive function suggest that it should perhaps be considered a fourth major photosynthetic pathway.
Keywords: aerenchyma, aquatic CAM, arborescent lycopsids, metabolic pathways, parichnos
1. Introduction
Three routes through which plants fix carbon from atmospheric CO2 to carbohydrates are generally accepted as major photosynthetic pathways: C3, C4 and CAM (Crassulacean acid metabolism). Since the 1980s, however, evidence from physiological experiments has been accumulating that there are both intermediates between these categories and variations upon them (Holaday & Bowes 1980; Koch & Kennedy 1980; Keeley 1981; Cockburn 1985; Brown & Hattersley 1989; Ehleringer & Monson 1993; Li & Jones 1995; Lüttge 1996; Mazen 1996; Smirnoff 1996; Winter & Smith 1996; Ehleringer et al. 1997; Keeley 1998; Lambers et al. 1998; Voznesenskaya et al. 2001; Hibberd & Quick 2002). Three major pathways are nevertheless distinguished from many intermediates; they dominate habitats or higher taxonomic groups and have been evolutionarily important in the development of terrestrial ecosystems or in the production and regulation of the Earth's atmosphere. The evolutionary role and importance of variant pathways is less clear.
One particular variant metabolism has been called ‘diurnal/diel acid metabolism’ (Keeley 1982; Aulio 1985), ‘aquatic acid metabolism’ (Cockburn 1985), ‘CAM-like metabolism’ (Raven et al. 1988), ‘specialized mechanisms associated with photosynthetic carbon acquisition in aquatic plants’ (Lambers et al. 1998, p. 80), and, most frequently, ‘aquatic CAM photosynthesis’ (Keeley 1998, etc.). It is considered a CAM variant because plants that display it sometimes have diurnal cycles of acidification due to the nocturnal accumulation of 4-carbon acids, one of the defining characteristics of ordinary CAM. More importantly, however, many aquatic plants that have been described as demonstrating aquatic CAM photosynthesis have organized aerenchyma (internal gas spaces) connecting buried and photosynthetic organs, and transport of O2 downward and CO2 upward in these spaces. Stomates may be present (frequently deeply sunken, occluded by cuticular wax, or in low numbers) or entirely lacking. The habitat of these species is predominantly aquatic, hypoxic or anoxic and oligotrophic. Fixed carbon may be obtained from the atmosphere, from the sediment, or from respiration, usually with a substantial component from the latter two sources.
In this paper, I offer morphological, ecological and biogeochemical evidence that a variant metabolism similar to aquatic CAM was much more prevalent in the late Palaeozoic than it is now. This metabolism is accompanied by recognizable morphological adaptations; it shows ecological and phylogenetic specificity; it has evolved more than once in response to the same ecological conditions; its ability to recycle sedimentary carbon could have potential long-term effects on the global carbon cycle. I argue that these characteristics constitute an evolutionarily important adaptive strategy (in which diurnal acidity cycles are incidental). Therefore it should perhaps be considered an independent metabolic pathway rather than a variant of CAM. More importantly, the realization that the late Palaeozoic ecosystems were populated by plants with a radically different metabolism from those dominating modern floras suggests that uniformitarian assumptions about flora–atmosphere feedbacks should be re-examined.
2. The role of aerenchyma in gas-phase carbon concentration: details of the unusual pathway
Until the 1980s, CAM was considered primarily an adaptation to physiologic drought: it allows plants to open their stomata during the night, when water loss is minimized, and accumulate atmospheric carbon in 4-carbon acids (malate or aspartate) that are then converted to carbohydrates during the day with less water loss (Kluge & Ting 1978; Winter & Smith 1996). The water stress that makes this strategy advantageous does not affect plants living submerged or in waterlogged soils. As Keeley (1981) and many subsequent authors have noted, some aquatic plants do store up carbon fixed from dissolved CO2 as 4-carbon acids during the night; they do so not in order to prevent transpirative water loss during the day but because dissolved CO2 levels in water usually rise at night above their daytime values. Expanding the term CAM to describe aquatic plants blurs its ecological specificity and obscures its function as an adaptation to specific environmental conditions.
The term ‘aquatic CAM’ is used because the diurnal acidity cycles of terrestrial and aquatic CAM plants are biochemically identical. Not all aquatic plants, however, show diurnal acidity cycles, and tying the definition of CAM to a single measurement (the presence of diurnal acidity cycles) seems arbitrary when individuals of the same species of aquatic plants and even organs of the same plant can show acidity cycles when submerged but not when emergent (Keeley 1998).
Aquatic CAM does not satisfactorily describe a plant that has absent or weak diurnal acidity cycles but does have an aerenchyma system in which CO2 is either accumulated during the night for daytime fixation or merely concentrated above ambient atmospheric levels to reduce photorespiration without significant nocturnal storage. Since there seems to be no convenient term to describe this metabolic pathway, for the purposes of this paper I refer to it as the ‘lycopsid photosynthetic pathway’ (LPP). The term ‘photosynthetic pathway’ rather than ‘metabolism’ underlines the route taken by carbon. The enzymes necessary for any metabolic pathway have been found in virtually all plant groups (albeit in different concentrations), so it is the pathway and timing of carbon movement between atmosphere or sediment and carbohydrate, rather than the biochemistry, that distinguishes a photosynthetic pathway.
LPP metabolism is defined by the presence of internal gas spaces showing CO2 enrichment above ambient levels in subaerial portions and O2 in buried portions, with a substantial proportion of net carbon fixation coming from respired or sedimentary carbon. The term ‘aquatic CAM’ as currently used corresponds to a CAM-LPP intermediate: an aquatic or wetland plant that shows CAM-like biochemistry (diurnal acidity cycles and fixation of a large proportion of its acquired carbon via phosphoenolpyruvate carboxylase and the Hatch/Slack/Korshak cycle) as well as an aerenchyma system used for gas-phase CO2 concentration and transport (figure 1; see the electronic supplementary material, table S1).
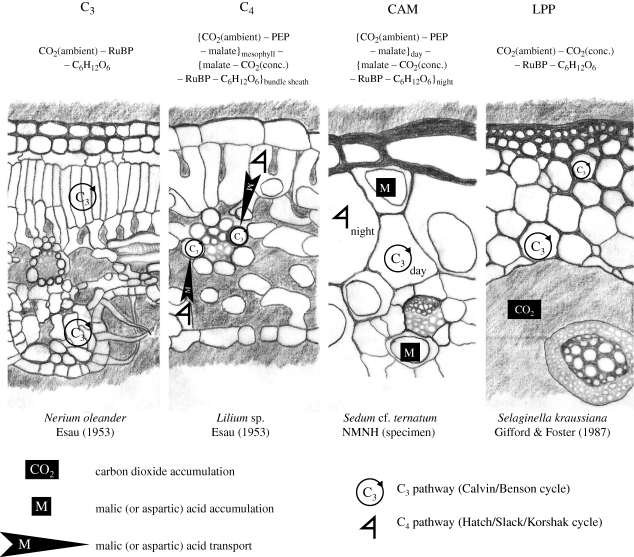
Figure 1.
Comparison of photosynthetic pathways. Transverse sections showing the different anatomies characteristic of the pathways and schematic descriptions of the route taken by carbon during photosynthesis. The path taken by a carbon molecule during fixation is given at the top: C6H12O6 (glucose) is used as a proxy for more complex carbohydrates; RuBP, ribulose-1,5-bisphosphate; PEP, phosphoenolpyruvate; conc., concentrated. Sections are modified from illustrations in Esau (1953); Gifford & Foster (1987), and an unaccessioned anatomical slide from the botany collections of the National Museum of Natural History. Light grey shading shows gas spaces and the icons show the different locations of carbon storage, fixation, and transport via the C3 and C4 cycles in each metabolic pathway.
The largest group of plants that show aquatic CAM and LPP (extensively documented by Keeley 1981, 1982; Keeley & Busch 1984; Keeley et al. 1984; Keeley 1998) are species of the genus Isoëtes (Isoëtaceae), which includes the nearest living relatives of the arborescent lycopsids of the Palaeozoic. LPP is not restricted to the isoëtaleans. In his 1998 review of CAM-like photosynthesis in aquatic plants, Keeley listed 180 submerged aquatics whose metabolisms have been described since the 1980s, of which 69 have CAM-like diurnal malate cycles. Other examples include Cyperus papyrus (Cyperaceae), studied by Li & Jones (1995), who found that the O2 in submerged (hypoxic) rhizomes rose from 10.3 per cent at night to 15.1 per cent during the day, while the CO2 remained stable at 4.5 per cent. The maximum concentration of CO2 in the culm rose to about 74 times its ambient atmospheric level (2.6%), averaging about 0.07 per cent during the day and 0.16 per cent at night. About half (35–57%) of the respired CO2 was refixed. Measurements of the internal atmosphere of Lobelia dortmanna (Campanulaceae) by Pedersen & Sand-Jensen (1992) show that the concentration of CO2 in its lacunae rose to 23 times its ambient atmospheric level, ranging from about 0.3 per cent during the day to about 0.7 per cent at night while the sediment was heavily enriched in O2 in the vicinity of the roots. Similar results were obtained from Typha latifolia (Typhaceae) by Constable et al. (1992) in which the gas in the leaf aerenchyma ranged from ambient CO2 levels around noon to about 0.6 per cent CO2 (18 times ambient) in the early morning, and a dye tracer showed interconnection of the aerenchyma system from rhizomes to leaves. This pathway also appears to be inducible: Nielsen et al. (1991) measured lacunar CO2 from 1.1 to 1.3 per cent (about 40 times ambient) in Littorella uniflora (Plantaginaceae) which has a diurnal malate cycle if grown submerged but not when emergent (Aulio 1985). Experiments on Phaseolus vulgaris (Fabaceae) using a 14C tracer to measure root uptake of carbon showed carbon uptake rates in aerated conditions that did not exceed passive transpiration rates. When the nutrient solution was not aerated, however, facultative aerenchyma formed in the stem and a higher rate of root uptake of carbon was measured, due at least in part to mass flow as the observed rate was too high for passive uptake or diffusion alone (Amiro & Ewing 1992). Root aeration via mass flow has been documented in Nuphar as a result of heat pressurization (Dacey 1980), and in Phragmites and Equisetum caused by a combination of humidity-driven convection and the Venturi effect (Armstrong & Armstrong 1988, 2009; Armstrong et al. 1992).
These examples are selected from a much larger body of existing literature to illustrate the variety of aquatic plants that show diurnal cycles of gas phase accumulation and transport (CO2 up, O2 down), and fixation of sedimentary and respired carbon. If this metabolic pathway only appeared in a few modern plants, it might be reasonable to continue treating it as a relatively rare and evolutionarily insignificant oddity. Instead, however, evidence seems to suggest that it was much more prevalent during the late Palaeozoic.
3. Interpretation of the parichnos system: morphological evidence
The arborescent lycopsids1 constitute a group of extinct plants most of which are thought to be phylogenetically bracketed (Witmer 1995) by the extant genera Selaginella and Isoëtes (Bateman et al. 1992; Pigg 1992; Kenrick & Crane 1997; Korall & Kenrick 2002; figure 2). They were among the taxa that dominated biomass production in the coal-swamp ecosystems during the Late Carboniferous (Pennsylvanian) in Euramerica (Phillips et al. 1985; Phillips & DiMichele 1992) and the Early Permian in China (Hilton & Cleal 2007). Arborescent lycopsids are preserved as fossil casts and moulds as well as in carbonate concretions known as coal balls, which preserve internal anatomical details at a cellular level; their physiology, of course, cannot be directly observed.
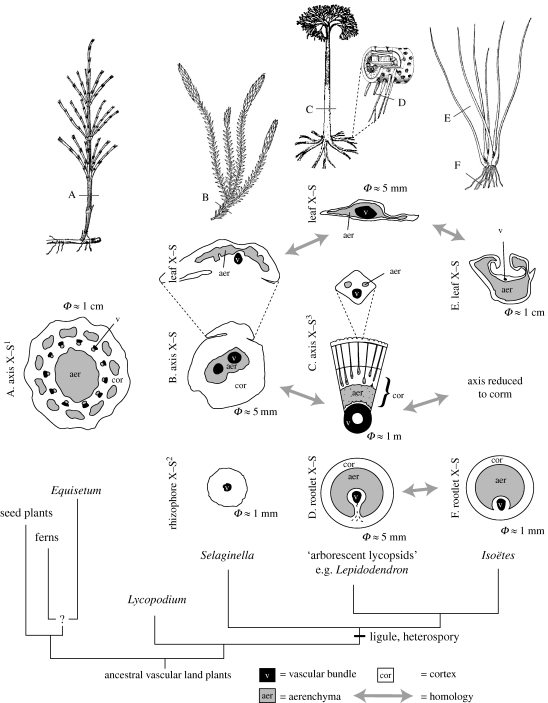
Figure 2.
The parichnos system. Schematics showing aerenchyma system connecting buried and subaerial organs in Equisetum, Selaginella, the arborescent lycopsid Lepidodendron and Isoëtes. Note positional homology of the aerenchyma across the lycopsids. The phylogenetic relationships are shown by a schematic tree in which the branch lengths are not significant and many taxa are omitted for clarity. 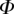 indicates approximate diameters of organs. Parts of the illustration are adapted from Britton & Brown (1913), Stewart (1947), Gifford & Foster (1987), Pigg (1992) and Stewart & Rothwell (1993). Note 1. At internode; rhizome is similar. 2. Rhizophore is shown, though the species of Selaginella pictured at the top is S. selaginoides, which lacks a rhizophore. Some species of Selaginella show aerenchyma developement in the rhizophore, but it is probably not homologous with the aerenchyma in the rooting organ of the arborescent lycopsids, Stigmaria (Karrfalt 1981). 3. With expanded paradermal section of the leaf base.
indicates approximate diameters of organs. Parts of the illustration are adapted from Britton & Brown (1913), Stewart (1947), Gifford & Foster (1987), Pigg (1992) and Stewart & Rothwell (1993). Note 1. At internode; rhizome is similar. 2. Rhizophore is shown, though the species of Selaginella pictured at the top is S. selaginoides, which lacks a rhizophore. Some species of Selaginella show aerenchyma developement in the rhizophore, but it is probably not homologous with the aerenchyma in the rooting organ of the arborescent lycopsids, Stigmaria (Karrfalt 1981). 3. With expanded paradermal section of the leaf base.
According to Hill (1906), the term ‘parichnos’ (from Greek 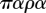 , beside + '
, beside + '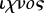 , trace) was coined by Bertrand (1891) to describe what he took to be mucilege ducts surrounding the leaf trace in the axis of the arborescent lycopsid Lepidodendron harcourtii. The term has since come to be used to describe the pits initially described as ‘glanduoli’ (little glands) by Sternberg (1820–1838) that are visible in the fossilized ‘bark’ of arborescent lycopsids, where the parichnos system leaves the axis. The anatomical studies by DiMichele (e.g. DiMichele 1979, 1981, 1985) show how a single parichnos strand splits either once or twice under the surface of the leaf cushion to form two pits in the leaf scar (representing canals feeding the leaf before abscission). In some cases, two additional pits below the leaf scar are present, representing connections to the atmosphere. From the leaf cushion, the parichnos system can be traced back into the ‘middle cortex’—the aerenchymatous middle of the primary cortex—which is composed of thin-walled parenchyma cells separated by large intercellular spaces. The middle cortex is continuous down into the rooting organ associated with the arborescent lycopsids (the form-genus Stigmaria, sometimes called a ‘rhizomorph’ or ‘rhizophore’) and connects with an air canal in the stigmarian rootlets (DiMichele 1979, 1981, 1985; Gifford & Foster 1987; Stewart & Rothwell 1993; figure 2). To avoid ambiguity, I use the terms ‘(stigmarian) root’ to describe the radially symmetric, branched, rooting axis with secondary growth and ‘(stigmarian) rootlet’ to describe the bilaterally symmetric appendages radiating from it. I also extend the term ‘parichnos’ to describe any continuous aerenchymatous tissue system connecting buried or submerged and subaerial organs of a plant.
, trace) was coined by Bertrand (1891) to describe what he took to be mucilege ducts surrounding the leaf trace in the axis of the arborescent lycopsid Lepidodendron harcourtii. The term has since come to be used to describe the pits initially described as ‘glanduoli’ (little glands) by Sternberg (1820–1838) that are visible in the fossilized ‘bark’ of arborescent lycopsids, where the parichnos system leaves the axis. The anatomical studies by DiMichele (e.g. DiMichele 1979, 1981, 1985) show how a single parichnos strand splits either once or twice under the surface of the leaf cushion to form two pits in the leaf scar (representing canals feeding the leaf before abscission). In some cases, two additional pits below the leaf scar are present, representing connections to the atmosphere. From the leaf cushion, the parichnos system can be traced back into the ‘middle cortex’—the aerenchymatous middle of the primary cortex—which is composed of thin-walled parenchyma cells separated by large intercellular spaces. The middle cortex is continuous down into the rooting organ associated with the arborescent lycopsids (the form-genus Stigmaria, sometimes called a ‘rhizomorph’ or ‘rhizophore’) and connects with an air canal in the stigmarian rootlets (DiMichele 1979, 1981, 1985; Gifford & Foster 1987; Stewart & Rothwell 1993; figure 2). To avoid ambiguity, I use the terms ‘(stigmarian) root’ to describe the radially symmetric, branched, rooting axis with secondary growth and ‘(stigmarian) rootlet’ to describe the bilaterally symmetric appendages radiating from it. I also extend the term ‘parichnos’ to describe any continuous aerenchymatous tissue system connecting buried or submerged and subaerial organs of a plant.
As figure 2 illustrates, transverse sections of Selaginella, Lepidodendron, and Isoëtes all show an organized parichnos system. The middle cortex in the axis of arborescent lycopsids occupies the same relative position as the trabecular aerenchyma in the axis of Selaginella, and the air space in the stigmarian rootlet is homologous with that in the ‘rootlet’ of modern Isoëtes (Stewart 1947; Rothwell & Erwin 1985). Therefore, making allowances for the reduction of the stem in Isoëtes and the absence of a clearly homologous rooting organ in Selaginella,2 the parichnos system is positionally homologous across the three groups.
The identification of the parichnos system with aerenchyma in modern plants is not a new idea: as early as 1893, Bower suggested that ‘the trabecular [aerenchyma] development in Selaginella is a specialized and more definite example of that lacunar development which appears in such various forms and positions in cortical tissues of various other Lycopodinous plants’ (Bower 1893, p. 349). A few years later, it was suggested that the leaf traces in the woody base of the genus Isoëtes are surrounded by ‘strands of degenerating [lysigenous] tissue … representing the parichnos occurring in Lepidodendron, Sigillaria, Lepidocarpon, &c.’ (Hill 1904, p. 654).
Figure 2 shows other strong similarities between the rootlets of the arborescent lycopsids and the rootlets of modern Isoëtes. As has been recognized for some time, both stigmarian appendages and Isoëtes rootlets are spirally arranged on an axis and bear a leaf-trace-like single monarch xylem strand suspended in a gas-filled lacuna on parenchymatous trabeculae or bars. Stewart (1947) concluded that stigmarian and Isoëtes rootlets are indeed homologous. More recent work (Raven & Edwards 2001) supports this homology and convincingly identifies Isoëtes rootlets as leaf homologues, which lack root hairs and have a documented role in CO2 uptake.
The functional significance of the parichnos system has also been suggested: Hill (1906) argued that the parichnos found in extant Isoëtes had a secretory function, but did not rule out its use in aeration or respiration, as suggested by Scott (1900) and Weiss (1903). The interpretation of the parichnos system as having a poorly defined role in gas transport seems to be the most current view (Stewart & Rothwell 1993). Both CO2 uptake and root aeration have been specifically mentioned: ‘the early colonists (of the land) were … able to take up CO2 through their roots’ (Moore 1984, p. 633); ‘the parichnos would appear to be an internal system of gas exchange associated with photosynthesis, corresponding more to recycling of CO2 and O2 than to external diffusion balances’ (Phillips & DiMichele 1992, p. 568).
Barclay (1931) and Jagels (1970a,b) have also observed that chloroplast densities in some modern species of Selaginella are higher in the cells bounding internal gas spaces than in subdermal parenchyma cells. Keeley (1998) reports the same distribution in species of Isoëtes as well as in Littorella, another aquatic of ‘isoëtid’ life form. This supports the conclusion that carbon fixation in these plants is primarily from internal gases rather than from direct communication with the atmosphere via stomata. Cell contents are not preserved in permineralized arborescent lycopsids, so chloroplast densities cannot be directly measured, but the structural and positional homologies between the preserved parichnos system and aerenchyma in modern LPP plants suggest that the arborescent lycopsids also probably relied more heavily on internal than on external atmosphere to supply carbon for photosynthesis.
The results reviewed in this section are intended to document the link between the parichnos system in the arborescent lycopsids and aerenchyma found in their modern relatives. The previous section covered the functional role of aerenchyma in modern plants. It seems reasonable, therefore, to suggest on grounds of functional morphology alone that the arborescent lycopsids showed the upward CO2 transport and gas-phase carbon concentration that is characteristic of modern LPP plants. Carbon concentration to reduce photorespirative loss is only one of the adaptive advantages provided by LPP metabolism. As discussed below, this would have been particularly important in the high O2 to CO2 ratio atmosphere of the late Palaeozoic. Another major function performed by LPP metabolism, which will be discussed in the following section, is to supply O2 to roots via the parichnos system as an adaptation to growth submerged or in waterlogged soils.
4. Ecological evidence
In addition to documenting an enrichment of CO2 in internal gas spaces, recent research on aerenchyma has firmly associated it with a need for root aeration. Aerenchyma (sensu lato) is simply parenchymatous tissue with a large volume of intercellular space. The amount of organization implied by the term varies, as it can be applied both to the slightly porous parenchyma found in the root cortices of many wetland plants and to the well-defined longitudinal canals found in the stems and rhizomes of Equisetum. The spongy mesophyll in dicot leaves is also referred to as aerenchyma. In some species (e.g. Zea mays, Spartina patens), aerenchyma forms when the plant is grown in oxygen-depleted soils (this is called ‘induced’ or ‘facultative’ aerenchyma); in other species (e.g. Tripsicum dactyloides, Zea luxurians, Sagittaria lancifolia), it is formed regardless of the environmental conditions in which the plant is grown (‘constitutive’ aerenchyma; Drew et al. 2000).
In developmental terms, aerenchyma can be formed by the physical separation of cells at the middle lamella during ontogeny (schizogeny), by the death and lysis of cells (lysigeny), by the physical rupture of cells (rexigeny) or by some combination of these modes. Justin & Armstrong (1987), who examined aerenchyma formation in 91 angiosperms, and Drew (1997) document some patterns: the propensity of wetland species to form constitutive aerenchyma in any environmental conditions and to form induced aerenchyma when subjected to a given level of oxygen depletion. They also point out a connection between the cubic packing of parenchyma cells (as opposed to hexagonal close packing) and the formation of aerenchyma. Despite recent studies that have examined the details of aerenchyma formation in certain species (Drew et al. 2000; Longstreth & Borkhsenious 2000), there seems to be no simple link between the mode of formation or degree of organization of the aerenchyma and whether it is constitutive or induced. On one hand, Drew et al. (2000) conclude that hypoxia-induced ethylene promotes lysigenous productions of induced aerenchyma in maize roots; but ‘gas space formation does not require lysis and cell death in at least three wetland species’ (Longstreth & Borkhsenious 2000, p. 642). So, although there is considerable variation in the conditions and cellular processes that give rise to aerenchyma in different plants, several studies of angiosperm roots have clearly established that induced aerenchyma forms as an adaptation to low O2 in the rhizosphere.
General morphological studies of the genus Selaginella (Harvey Gibson 1894; Browne 1908; Cusick 1953; Rosello 1966; Buck & Lucansky 1976) deal with the air spaces in the cortex merely in passing; Jagels (1970a,b) concentrates exclusively on photosynthetic apparatus; Uphof (1920) briefly discusses aerenchyma in xerophytic species; and recent developmental studies (Webster & Steeves 1964, 1967; Webster & Jagels 1977; Karrfalt 1981; Imaichi & Kato 1989; Webster 1992) are largely focused on identifying the the origin of the rhizophore, or have been concerned with other anatomical features such as the ligule. No recent study seems to have concentrated on aerenchyma formation or function, with the result that the best obtainable description of lycopsid aerenchyma formation comes from 1931: ‘The rate of growth of the endodermis mother cells does not keep up with the differential increase in diameter and length of the cortical cylinder and central bundle. As a result, the endodermis mother cells become stretched radially between the pericycle and inner cortex …. An air cavity is thus formed … bridged by the endodermis mother cells …. This is the ‘trabecular cortex’ (Barclay 1931, p. 458). In modern terminology, this would be considered schizogenous and therefore developmentally unrelated to the lysigenous aerenchyma appearing in Arabidopsis (Mühlenbock et al. 2007). The relationship between rhizosphere anoxia and aerenchyma formation in extant lycopsids has not been studied in detail, but in many of the environments in which they grow, root respiration would not be possible without some oxygen transport downward through an aerenchyma system. Therefore, like the other three major photosynthetic pathways, LPP metabolism has an element of habitat specificity; it is at least partially an adaptation to low oxygen levels in the rhizosphere.
From the sedimentology of the carbonaceous shales and coals in which they are typically found, it is clear that the arborescent lycopsids grew predominantly in backswamp floodplain communities with permanently inundated soils (DiMichele & Phillips 1994). The plant fossil record is generally considered to be biased towards such lowland, anoxic settings, so upland settings dominated by ferns or pteridosperms may be under-represented. Even allowing for this bias, however, it seems reasonable to assume that (as is the case today) the greatest accumulation of biomass was in lowland communities, which contained a large proportion of arborescent lycopsids.
Other important components of these communities were horsetail relatives (Sphenophyta), tree-ferns (Marattiales) and presumed conifer relatives (Cordaitales). Most Palaeozoic sphenophytes, tree-ferns like Psaronius, and Cordaites roots (the form-genus Amyelon) show aerenchyma development, so it is important to distinguish between LPP metabolism (connected aerenchyma, CO2 concentration in internal gas spaces, use of sediment-derived carbon, root aeration) and the aerenchyma formation simply to provide root aeration found, for instance, in modern bald cypress (Taxodium), which relies on pneumatophores for root respiration when growing in standing water. Like succulence in CAM plants; a parichnos system is a necessary but insufficient condition for diagnosing LPP metabolism. With that important proviso, LPP metabolism may indeed have been more common across taxonomic groups throughout lowland, backswamp communities in the late Palaeozoic. In some plants, there is also evidence for axial carbon transport in the xylem (Zelawski et al. 1970; Martin et al. 1994; Hibberd & Quick 2002), so there may be intermediate metabolic pathways between LPP and C3, represented by taxa with weak aerenchyma formation and some recycling of respired CO2 via the transpiration stream instead of as gas-phase CO2. As discussed above, LPP–CAM intermediates have been well characterized as aquatic CAM plants. Further investigation will help to characterize variants and details of LPP; this paper is primarily concerned with defining it as a metabolic pathway and documenting its importance in the late Palaeozoic.
In addition to CO2 concentration and root oxygenation, LPP would have enabled Palaeozoic arborescent lycopsids to sustain a higher rate of growth than would otherwise have been possible. Phillips & DiMichele (1992) suggested that the arborescent phase of the arborescent lycopsid life history was much more transient than previously assumed. Instead of spending most of their lives as 20–30 m trees, they may have shot up from low rhizomatous structures in the last few years before reproduction. It seems probable that rapid accumulation of biomass would have been limited by the amount of carbon that could be obtained from a low CO2 atmosphere via stomata. If carbon was obtained from the sediment, however, growth rate would not be limited by carbon availability.
According to the species diversity curves of Nikas et al. (1985), the arborescent lycopsids increased in diversity beginning in the Upper Devonian, reaching a maximum that may have been as high as 100 described species at the end of the Carboniferous (figure 3c). Most of the taxa with large adult stature went extinct by the Middle Triassic, leaving only diminutive or herbaceous taxa extant (Pigg 1992). Note that modern Isoëtes is considered non-arborescent in the compilation by Nikas et al. (1985).
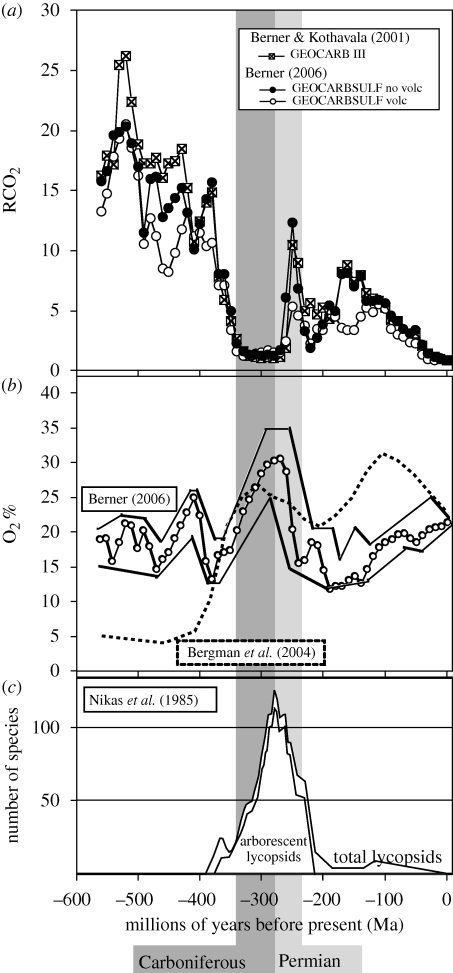
Figure 3.
(a) CO2, (b) O2 and (c) numbers of described species of lycopsids through time. Note that arborescent lycopsids were diverse only in the high O2, low CO2 atmosphere of the Carboniferous and Permian. In (a), CO2 values are given as RCO2; relative enrichment over modern (preindustrial) level. Redrawn from figures in Berner (2006), Bergman et al. (2004), Berner & Kothavala (2001) and Nikas et al. (1985). The curves in (c) are traced compilations of several figures and therefore represent approximate rather than exact values.
From the mid-Carboniferous to the mid-Permian period, the arborescent lycopsids probably constituted a substantial proportion of terrestrial vegetation, but they have slowly declined in ecological importance since then and now are represented only by a few genera of widely distributed herbaceous and shrubby plants. Many species of modern isoëtaleans are found in oligotrophic lakes and bogs, which, like backswamps, are considered stressed environments because of their high acidity and low nutrient availability. This implies that the isoëtalian lycopsid lineage has maintained a degree of habitat specificity since the Palaeozoic (DiMichele et al. 2001). Nowhere do modern lycopsids provide more than a small fraction of the biomass of an ecosystem. Nevertheless, the remaining isoëtaleans remain of particular interest because we can infer that their LPP metabolism was inherited from their extinct relatives, the arborescent lycopsids. These flourished in the late Palaeozoic, when LPP would have been far more evolutionarily advantageous than subsequently.
5. Biogeochemical evidence
Computer modelling based in part on the amount of organic carbon and pyrite in sedimentary rocks suggests that the mid-Carboniferous through mid-Permian was a period of extremely low CO2 (figure 3a) and high O2 (figure 3b) levels in the atmosphere (Berner & Canfield 1989; Berner 2001, 2006; Berner & Kothavala 2001; Bergman et al. 2004). These conditions would have been particularly favourable for a metabolic pathway such as LPP, which allowed utilization of sedimentary carbon and reduced the apparent atmospheric ratio of partial pressures of O2 to CO2 by concentrating CO2 in internal spaces. This is metabolically important because the oxygenase versus carboxylase activity of rubisco is dependent on the O2 to CO2 ratio in the gas available for carbon fixation. So photorespiration is greatly increased (leading to a loss of net photosynthetic efficiency) in a high O2, low CO2 atmosphere.
The high O2, low CO2 excursion coincides both with the greatest diversity of arborescent lycopsids (figure 3c) and with the stratigraphic distribution of coal balls in the fossil record. Coal balls were formed by carbonate precipitation in coal swamp environments between about 290 and 320 Ma (latest Mississippian through earliest Permian; Phillips et al. 1985; Hilton et al. 2001). The large-scale local removal of carbon dioxide from groundwater would drive to the left the equilibrium CO2 + CaCO3 + H2O ⇌ 2HCO3− + Ca2+, and may have helped to precipitate coal balls where they are found, in an anoxic rhizosphere.
In modern floras, measurement of carbon isotope ratios allows the statistical separation of C3 from C4 plants, although intermediate carbon fractionation shown by CAM plants can blur the picture. It is possible that isotopic data on fossil plants would allow identification of LPP metabolism in fossil material. Since the arborescent lycopsids may have extracted a substantial proportion of their carbon from the sediments, they are likely to reflect the isotopic ratios of their substrates, a hypothesis supported by the measurements made of Stylites (a segregate of Isoëtes lacking stomata; −25.7‰), the peat in which it was growing (−26.6‰) and groundwater carbonates (−25.0‰) by Keeley et al. (1984).
The lack of fractionation in Stylites indicates that essentially all of the carbon taken up by the plant from the sediments was eventually absorbed. In the case of plants with functional stomata, fractionation may show the use of some atmospheric oxygen or loss of sedimentary carbon, but there is clearly no dramatic signal like that shown by C4 plants (Lloyd & Farquhar 1994). Because of the overlap between the expected carbon isotope ratios in C3 and LPP plants, available data on the isotope ratios of fossil lycopsids (Raven & Spicer 1996; Beerling et al. 2002; Osborne & Beerling 2006) is not sufficient to identify LPP metabolism. Measurements of sedimentary organic carbon in coal swamp environments seldom differ greatly from −25‰, so isotopic discrimination of LPP would require many paired measurements of plant tissues along with the organic carbon in the sediments surrounding them. Finding these paired measurements significantly correlated would suggest sedimentary carbon utilization and LPP metabolism. Unlike modern C4 grassland ecosystems, which can show substantially higher δ13C fractionations, the majority of buried carbon in Carboniferous coal swamps must ultimately have been obtained from the atmosphere via the rubisco-mediated Calvin/Benson (C3) cycle and then reprocessed by LPP, providing a selective advantage for the arborescent lycopsids, but not dramatically altering the ecosystem-scale C3 isotopic signal.
6. Broader significance
Study of forest ecosystems—ecosystems with complex and dense photosynthetic canopies, generally found in regions where precipitation exceeds evaporation—has a long, productive history in palaeontology because trees provide a comparatively good fossil record compared with that of smaller plants (e.g. Behrensmeyer et al. 1992). Forests have also been a particular concern of the environmental movement because loss of diversity in tropical rainforests, massive deforestation for agricultural purposes with the consequent loss of biomass to greenhouse CO2, and the growing problem of forest fires in residential areas are well-known social and economic problems.
In the past (Green & Hickey 2005), I have argued that if leaf architecture is used as a proxy for ecosystem structure, the available data show structural continuity in forest ecology since before the Cretaceous/Tertiary boundary. Many species of plants have been replaced, but the basic architecture of a late Cretaceous forest would not seem out of place in the modern world. Palaeozoic ecosystems with complex, differentiated photosynthetic canopies, on the other hand, were dominated by plants such as the arborescent lycopsids, which were only distantly related to the angiosperms and may have functioned very differently. These ecosystems may have been so architecturally and physiologically different from modern forests that they should probably not be referred to as ‘forests’ at all. In order to appreciate the global environmental significance of plant ecosystems in the Palaeozoic, it is therefore necessary to examine carefully any uniformitarian assumptions about plant metabolism.
Although details of the metabolism of the arborescent lycopsids remain to be determined, available evidence seems sufficient to demonstrate that (i) the arborescent lycopsids relied heavily on sedimentary/respired carbon concentrated in internal gas spaces for photosynthesis; (ii) the internal gas spaces also had a function in oxygen transport downward for root respiration; (iii) the metabolism associated with these two functions is usually identifiable from the presence of an organized aerenchyma/parichnos system connecting buried and photosynthetic organs; (iv) the temporal co-occurrence of this metabolic pathway with the high O2, low CO2 late Palaeozoic atmosphere is strong if not conclusive evidence for its adaptive significance in a major clade (lycopsids) and an important biome (lowland coal-forming swamps). I would argue that the importance of the LPP in the late Palaeozoic and its ecological specificity warrants consideration of it as a fourth major photosynthetic pathway.
There remain many details to be ascertained: (i) whether the arborescent lycopsids relied primarily on dissolved CO2 or also on HCO3−; (ii) how prevalent LPP or intermediate metabolic pathways were among other Palaeozoic taxa with organized aerenchyma, such as the sphenophytes and marattialean tree ferns; (iii) what percentage of fixed carbon in a Palaeozoic ecosystem was atmospheric as opposed to sedimentary; and (iv) how often LPP evolved. In addition to the lycopsids, it has clearly appeared in both dicots and monocots and possibly in the sphenophytes and ferns; physiological study of more extinct taxa and phylogenetic analysis will be necessary to ascertain the number of independent origins.
Even without these details, the evidence offered here seems to suggest that the arborescent lycopsids demonstrated an aberrant metabolism that may not be well described by, for instance, isotopic fractionation models intended for C3 or C4 plants (Lloyd & Farquhar 1994). Similarly, stomatal atmospheric proxies are sensitive even to species-level differences (Royer 2003) and therefore application of them to arborescent lycopsids in extinct families (such as Lepidodendraceae) requires phylogenetic bracketing (Witmer 1995) and conservative error calculations. Both analytic models of photosynthesis and statistical proxies based on extant C3 plants may need modifications to describe Palaeozoic plants with LPP metabolism accurately; discussing these in detail is beyond the scope of this review, but may form the basis of future work.
In the last half century, both scientific research programs and political and social movements have become deeply concerned with the rate and significance of change in the global biosphere and atmosphere. It has frequently been pointed out, perhaps most influentially by Lovelock (1979), that the biosphere—in particular the dominant primary producers—has played an active role in the maintenance of conditions suitable for life. Other things being equal, organic carbon burial is dependent on the balance between net photosynthesis and respiration, so relatively small changes in the residence times or annual fluxes of carbon can cumulatively affect the balance between buried organic carbon and atmospheric CO2. The success of the arborescent lycopsids is temporally associated with an anomalously low CO2 global atmosphere and the deposition and burial of large coal beds. A metabolism such as LPP may have created a positive feedback, increasing net photosynthesis, sequestrating more atmospheric carbon in coal swamps, and thus promoting conditions favourable for the arborescent lycopsids. Currently available evidence only allows identification and recognition of the aberrant metabolism; additional investigation may allow further description of the LPP and elucidation of its links to change in the global atmosphere and long-term climate change.
Acknowledgments
Thanks to Erika Edwards and Leo Hickey for suggesting the original idea and to Rich Barclay, Richard Bateman, Graeme Berlyn, Katy Black, Kevin Boyce, Bill DiMichele, Nancy Green, Missy Holbrook, Jon Keeley, Andy Knoll, Cindy Looy, Jenny McElwain, John Raven, Gar Rothwell, Dana Royer, Heather Sanders, Terry Webster, Jon Wilson, Klaus Winter, Elizabeth Zacharias and several anonymous reviewers for discussions about and comments on earlier versions of the manuscript. Scott Wing provided the composite species-through-time schematic used in figure 3c. This is publication number 227 of the Evolution of Terrestrial Ecosystems program at the Smithsonian Institution.
Endnotes
This term is used throughout in its loose traditional sense to describe extinct tree-like lycopsids, excluding modern Isoëtes. Bateman et al. (1992) has pointed out that ‘arboreous’ (having tree-like adult stature) would be a more accurate term for this group than ‘arborescent’ (having extensive secondary growth) because Isoëtes has secondary growth in its corm and therefore is also technically an arborescent lycopsid. For the purposes of this paper, however, I need to refer to the group of fossil taxa that are phylogenetically bracketed between extant Selaginella and Isoëtes. This paraphyletic group is probably roughly coextensive with the group of taxa that have traditionally been called arborescent lycopsids, so I employ the term loosely to avoid the precise phylogenetic hypothesis that would be implied by the use of ‘Lepidodendrales’. See Bateman et al. (1992), DiMichele & Bateman (1996) and Kenrick & Crane (1997) for further discussion of the taxonomy and systematics of the lycopsids.
Note that many derived species of Selaginella have an organ called a rhizophore that serves the same functional role as aerial roots in angiosperms but is not homologous either to true angiosperm roots or to the isoëtalian/stigmarian root or rootlet.
References
- Amiro B. D., Ewing L. L.1992Physiological conditions and uptake of inorganic C14 by plant roots. Environm. Exp. Botany 32, 203–211 (doi:10.1016/0098-8472(92)90003-K) [Google Scholar]
- Armstrong W., Armstrong J.1988Phragmites australis—a preliminary study of soil-oxidizing sites and internal gas transport pathways. New Phytol. 108, 373–382 (doi:10.1111/j.1469-8137.1988.tb04177.x) [Google Scholar]
- Armstrong W., Armstrong J.2009Record rates of pressurized gas-flow in the great horsetail, Equisetum telmateia. Were Carboniferous Calamites similarly aerated? New Phytol. 184, 202–215 (doi:10.1111/j.1469-8137.2009.02907.x) [DOI] [PubMed] [Google Scholar]
- Armstrong J., Armstrong W., Beckett P. M.1992Phragmites australis—venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol. 120, 197–207 (doi:10.1111/j.1469-8137.1992.tb05655.x) [Google Scholar]
- Aulio K.1985Differential expression of diel acid metabolism in two life forms of Littorella uniflora (L.) Aschers. New Phytol. 100, 533–536 (doi:10.1111/j.1469-8137.1985.tb02799.x) [Google Scholar]
- Barclay B. D.1931Origin and development of tissues in stem of Selaginella wildenovii. Botanical Gazette 91, 452–461 (doi:10.1086/334168) [Google Scholar]
- Bateman R. M., DiMichele W. A., Willard D. A.1992Experimental cladistic analysis of anatomically preserved arborescent lycopsids from the Carboniferous of Euramerica: an essay on paleobotanical phylogenetics. Ann. Mo Botanical Garden 79, 500–559 (doi:10.2307/2399752) [Google Scholar]
- Beerling D. J., Lake J. A., Berner R. A., Hickey L. J., Taylor D. W., Royer D. L.2002Carbon isotope evidence implying high O2/CO2 ratios in the Permo-Carboniferous atmosphere. Geochem. Cosmochem. Acta 66, 3757–3767 (doi:10.1016/S0016-7037(02)00901-8) [Google Scholar]
- Behrensmeyer A. K., Damuth J. D., DiMichele W. A., Potts R., Sues H., Wing S. L. (eds) 1992Terrestrial ecosystems through time. Chicago, IL: University of Chicago Press [Google Scholar]
- Bergman N. M., Lenton T. M., Watson A. J.2004COPSE: a new model of biogeochemical cycling over Phanerozoic time. Am. J. Sci. 304, 397–437 (doi:10.2475/ajs.304.5.397) [Google Scholar]
- Berner R. A.2001Modeling atmospheric O2 over Phanerozoic time. Geochem. Cosmochem. Acta 65, 685–694 (doi:10.1016/S0016-7037(00)00572-X) [Google Scholar]
- Berner R. A.2006GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta 70, 5653–5664 (doi:10.1016/j.gca.2005.11.032) [Google Scholar]
- Berner R. A., Canfield D. E.1989A new model for atmospheric oxygen over Phanerozoic time. Am. J. Sci. 289, 59–91 [DOI] [PubMed] [Google Scholar]
- Berner R. A., Kothavala Z.2001GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. Am. J. Sci. 301, 182–204 (doi:10.2475/ajs.301.2.182) [Google Scholar]
- Bertrand C. E.1891Remarques sur le Lepididendron harcourtii de Witham. Travaux et Mémoires des Facultés de Lille, vol. 2. [Google Scholar]
- Bower F. O.1893On the structure of the axis of Lepidostrobus brownii Schpr. Ann. Bot. 7, 329–354 [Google Scholar]
- Britton N. L., Brown A.1913An illustrated flora of the northern United States, Canada, and the British possessions, 2nd edn. New York, NY: Charles Scribner's Sons [Google Scholar]
- Brown R. H., Hattersley P. W.1989Leaf anatomy of C3-C4 species as related to evolution of C4 Photosynthesis. Plant Physiol. 91, 1543–1550 (doi:10.1104/pp.91.4.1543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne I.1908The phylogeny and inter-relationships of the Pteridophyta. IV. The Lycopodiales (continued). New Phytol. 7, 181–197 (doi:10.1111/j.1469-8137.1908.tb06086.x) [Google Scholar]
- Buck W. R., Lucansky T. W.1976An anatomical and morphological comparison of Selaginella apoda and Selaginella ludoviciana. Bull. Torrey Bot. Club 103, 9–16 (doi:10.2307/2484743) [Google Scholar]
- Cockburn W.1985Variation in photosynthetic acid metabolism in vascular plants: CAM and related phenomena. New Phytol. 101, 3–24 (doi:10.1111/j.1469-8137.1985.tb02815.x) [DOI] [PubMed] [Google Scholar]
- Constable J. V. H., Grace J. B., Longstreth D. J.1992High carbon dioxide concentrations in aerenchyma of Typha latifolia. Am. J. Bot. 79, 415–418 (doi:10.2307/2445153) [Google Scholar]
- Cusick F.1953Experimental and analytical studies of Pteridophytes. XXII. Morphogenesis in Selaginella willdenovii Baker. Ann. Bot. 17, 369–383 [Google Scholar]
- Dacey J. W. H.1980Internal winds in water lilies: an adaptation for life in anaerobic sediments. Science 210, 1017–1019 (doi:10.1126/science.210.4473.1017) [DOI] [PubMed] [Google Scholar]
- DiMichele W. A.1979Arborescent lycopods of Pennsylvanian age coals; Lepidophloios. Palaeontographica B 171, 57–77 [Google Scholar]
- DiMichele W. A.1981Arborescent lycopods of Pennsylvanian age coals; Lepidodendron, with description of a new species. Palaeontographica B 175, 85–125 [Google Scholar]
- DiMichele W. A.1985Diaphorodendron, gen. nov., a segregate from Lepidodendron (Pennsylvanian age). Syst. Bot. 10, 453–458 (doi:10.2307/2419138) [Google Scholar]
- DiMichele W. A., Bateman R. M.1996Plant paleoecology and evolutionary inference: two examples from the Paleozoic. Rev Palaeobot. Palynol. 90, 223–247 [Google Scholar]
- DiMichele W. A., Phillips T. L.1994Paleobotanical and paleoecological constraints on models of peat formation in the Late Carboniferous of Euramerica. Palaeogeogr. Palaeoclimatol. Palaeoecol. 106, 39–90 (doi:10.1016/0031-0182(94)90004-3) [Google Scholar]
- DiMichele W. A., Stein W. E., Bateman R. M.2001. In Evolutionary paleoecology (eds Allmon W. D., Bottjer D. J.), pp. 285–335 New York, NY: Columbia University Press [Google Scholar]
- Drew M. C.1997Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 223–250 (doi:10.1146/annurev.arplant.48.1.223) [DOI] [PubMed] [Google Scholar]
- Drew M. C., He C.-J., Morgan P. W.2000Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 5, 123–127 (doi:10.1016/S1360-1385(00)01570-3) [DOI] [PubMed] [Google Scholar]
- Ehleringer J. R., Monson R. K.1993Evolutionary and ecological aspects of photosynthetic pathway variation. Annu. Rev. Ecol. Syst. 24, 411–439 (doi:10.1146/annurev.es.24.110193.002211) [Google Scholar]
- Ehleringer J. R., Cerling T. E., Helliker B. R.1997C4 photosynthesis, atmospheric CO2, and climate. Oecologia 12, 285–299 (doi:10.1007/s004420050311) [DOI] [PubMed] [Google Scholar]
- Esau K.1953Plant anatomy. New York, NY: Wiley [Google Scholar]
- Gifford E. M., Foster A. S.1987Morphology and evolution of vascular plants, 3rd edn. New York, NY: W. H. Freeman and Company [Google Scholar]
- Green W. A., Hickey L. J.2005Leaf architectural profiles of angiosperm floras across the Cretaceous/Tertiary boundary. Am. J. Sci. 305, 983–1013 (doi:10.2475/ajs.305.10.983) [Google Scholar]
- Harvey Gibson R. J.1894Contributions towards a knowledge of the anatomy of the genus Selaginella Spr. Ann. Bot. 8, 133–206 [Google Scholar]
- Hibberd J. M., Quick W. P.2002Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415, 451–454 (doi:10.1038/415451a) [DOI] [PubMed] [Google Scholar]
- Hill T. G.1904On the presence of a parichnos in recent plants. Ann. Bot. 8, 654 [Google Scholar]
- Hill T. G.1906On the presence of a parichnos in recent plants. Ann. Bot. 20, 267–273 [Google Scholar]
- Hilton J., Cleal C. J.2007The relationship between Euramerican and Cathaysian tropical floras in the Late Palaeozoic: palaeobiogeographical and palaeogeographical implications. Earth Sci. Rev. 85, 85–116 [Google Scholar]
- Hilton J., Wang S.-J., Galtier J., Li C.-S.2001An Early Permian plant assemblage from the Taiyuan Formation of northern China with compression/impression and permineralized preservation. Rev. Palaeobot. Palynol. 114, 175–189 (doi:10.1016/S0034-6667(01)00045-8) [DOI] [PubMed] [Google Scholar]
- Holaday A. S., Bowes G.1980C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiol. 65, 331–335 (doi:10.1104/pp.65.2.331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaichi R., Kato M.1989Developmental anatomy of the shoot, apical cell, rhizophore and root of Selaginella uncinata. Bot. Mag. Tokyo 102, 369–380 (doi:10.1007/BF02488120) [Google Scholar]
- Jagels R.1970aPhotosynthetic apparatus in Selaginella. I. Morphology and photosynthesis under different light and temperature regimes. Can. J. Bot. 48, 1843–1852 (doi:10.1139/b70-270) [Google Scholar]
- Jagels R.1970bPhotosynthetic apparatus in Selaginella. II. Changes in plastid ultrastructure and pigment content under different light and temperature regimes. Can. J. Bot. 48, 1853–1860 (doi:10.1139/b70-271) [Google Scholar]
- Justin S. H. F. W., Armstrong W.1987The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 106, 465–495 (doi:10.1111/j.1469-8137.1987.tb00153.x) [Google Scholar]
- Karrfalt E. E.1981The comparative and developmental morphology of the root system of Selaginella selaginoides (L.) Link. Am. J. Bot. 68, 244–253 (doi:10.2307/2442856) [Google Scholar]
- Keeley J. E.1981Isoëtes howellii: a submerged CAM plant. Am. J. Bot. 68, 420–424 (doi:10.2307/2442779) [Google Scholar]
- Keeley J. E.1982Distribution of diurnal acid metabolism in the genus Isoëtes. Am. J. Bot. 69, 254–257 (doi:10.2307/2443012) [Google Scholar]
- Keeley J. E.1998CAM photosynthesis in submerged aquatic plants. Botanical Rev. 64, 121–175 (doi:10.1007/BF02856581) [Google Scholar]
- Keeley J. E., Busch G.1984Carbon assimilation characteristics of the aquatic CAM plant Isoëtes howellii. Plant Physiol. 76, 525–530 (doi:10.1104/pp.76.2.525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley J. E., Osmond C. B., Raven J. A.1984Stylites, a vascular land plant without stomata absorbs CO2 via its roots. Nature 310, 694–695 (doi:10.1038/310694a0) [Google Scholar]
- Kenrick P., Crane P. R.1997The origin and early diversification of land plants: a cladistic study. Washington, DC: Smithsonian Institution [Google Scholar]
- Kluge M., Ting I. P.1978Crassulacean acid metabolism: analysis of an ecological adaptation. Berlin, Germany: Springer [Google Scholar]
- Koch K., Kennedy R. A.1980Characteristics of Crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiol. 65, 193–197 (doi:10.1104/pp.65.2.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korall P., Kenrick P.2002Phylogenetic relationships in Selaginellaceae based on rbcL sequences. Am. J. Bot. 89, 506–517 (doi:10.3732/ajb.89.3.506) [DOI] [PubMed] [Google Scholar]
- Lambers H., Chapin F. S., III, Pons T. L.1998Plant physiological ecology Berlin, Germany: Springer. [Google Scholar]
- Li M., Jones M. B.1995CO2 and O2 transport in the aerenchyma of Cyperus papyrus L. Aquat. Bot. 52, 93–106 (doi:10.1016/0304-3770(95)00484-H) [Google Scholar]
- Lloyd J., Farquhar G. D.199413C discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia 99, 201–215 (doi:10.1007/BF00627732) [DOI] [PubMed] [Google Scholar]
- Longstreth D. J., Borkhsenious O. N.2000Root cell ultrastructure in developing aerenchyma tissue of three wetland species. Ann. Bot. 86, 641–646 (doi:10.1006/anbo.2000.1151) [Google Scholar]
- Lovelock J.1979Gaia: a new look at life on Earth. Oxford, UK: Oxford University Press [Google Scholar]
- Lüttge U.1996Clusia: plasticity and diversity in a genus of C3/CAM intermediate tropical trees. In Crassulacean acid metabolism (eds Winter K., Smith J. A. C.), pp. 296–311 Berlin, Germany: Springer. [Google Scholar]
- Martin T. A., Teskey R. O., Dougherty P. M.1994Movement of respiratory CO2 in stems of loblolly pine (Pinus taeda L.) seedlings. Tree Physiol. 14, 481–495 [DOI] [PubMed] [Google Scholar]
- Mazen A. M. A.1996Changes in levels of phosphoenolpyruvate carboxylase with induction of Crassulacean acid metabolism (CAM)-like behavior in the C4 plant Portulaca oleracea. Plant Physiol. 98, 111–116 (doi:10.1111/j.1399-3054.1996.tb00681.x) [Google Scholar]
- Moore P. D.1984Novel carbon supply on land. Nature 310, 633 (doi:10.1038/310633a0) [Google Scholar]
- Mühlenbock P., Plaszczyca M., Plaszczyca M., Mellerowicz E., Karpinski S.2007Lysigenous aerenchyma formation in Arabidopsis is controlled by Lesion Stimulating Disease 1. The Plant Cell 19, 3819–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. L., Gacia E., Sand-Jensen K.1991Land plants of amphibious Littorella uniflora (L.) Aschers. maintain utilization of CO2 from the sediment. Oecologia 88, 258–262 (doi:10.1007/BF00320820) [DOI] [PubMed] [Google Scholar]
- Nikas K. J., Tiffney B. H., Knoll A. H.1985Patterns in vascular land plant diversification: an analysis at the species level. In Phanerozoic diversity patterns (ed. Valentine J.), pp. 97–127 Princeton, NJ: Princetion University Press [Google Scholar]
- Osborne C. P., Beerling D. J.2006Nature's green revolution: the remarkable evolutionary rise of C4 plants. Phil. Trans. R. Soc. B 361, 173–194 (doi:10.1098/rstb.2005.1737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen O., Sand-Jensen K.1992Adaptations of submerged Lobelia dortmanna to aerial life form: morphology, carbon sources, and oxygen dynamics. Oikos 65, 89–96 (doi:10.2307/3544890) [Google Scholar]
- Phillips T. L., DiMichele W. A.1992Comparative ecology and life-history biology of arborescent lycopsids in Late Carboniferous swamps of Euramerica. Ann. Mo Botanical Garden 79, 560–588 (doi:10.2307/2399753) [Google Scholar]
- Phillips T. L., Peppers R. A., DiMichele W. A.1985Stratigraphic and interregional changes in Pennsylvanian coal-swamp vegetation: environmental inferences. Int. J. Coal Geol. 5, 43–109 (doi:10.1016/0166-5162(85)90010-2) [Google Scholar]
- Pigg K. B.1992Evolution of isoetalean lycopsids. Ann. Mo Botanical Garden 79, 589–612 (doi:10.2307/2399754) [Google Scholar]
- Raven J. A., Edwards D.2001Roots: evolutionary origins and biogeochemical significance. J. Exp. Bot. 52, 381–401 [DOI] [PubMed] [Google Scholar]
- Raven J. A., Spicer R. A.1996The evolution of Crassulacean acid metabolism. In Crassulacean acid metabolism (eds Winter K., Smith J. A. C.), pp. 360–385 Berlin, Germany: Springer. [Google Scholar]
- Raven J. A., Handley L. L., MacFarlane J. J., McInroy S., McKenzie L., Richards J. H., Samuelsson G.1988The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review, with new data on Eriocaulon decangulare L. New Phytol. 108, 125–148 (doi:10.1111/j.1469-8137.1988.tb03690.x) [DOI] [PubMed] [Google Scholar]
- Rosello S.1966L'anatomie de Selaginella willdenowii Baker et la notion de polystélie. Naturalia Monspeliensia, série Botanique 17, 189–207 [Google Scholar]
- Rothwell G. W., Erwin D. M.1985The rhizomorph apex of Paurodendron; implications for homologies among the rooting organs of Lycopsida. Am. J. Bot. 72, 86–98 (doi:10.2307/2443571) [Google Scholar]
- Royer D. L.2003Estimating latest Cretaceous and Tertiary atmopheric CO2 from stomatal indices. Geol. Soc. Am. Special Papers 369, 79–93 [Google Scholar]
- Scott H. D.1900Studies in fossil botany London, UK: Adam and Charles Black [Google Scholar]
- Smirnoff N.1996Regulation of Crassulacean acid metabolism by water status in the C3/CAM intermediate Sedum telphium. In Crassulacean acid metabolism (eds Winter K., Smith J. A. C.), pp. 176–191 Berlin, Germany: Springer. [Google Scholar]
- Sternberg K.1820–1838Versuch einer geognostisch-botanischen Darstellung der Flora der Vorwelt. Leipzig and Prague: Fleischer [Google Scholar]
- Stewart W. N.1947A comparative study of stigmarian appendages and Isoëtes roots. Am. J. Bot. 34, 315–324 (doi:10.2307/2437143) [Google Scholar]
- Stewart W. N., Rothwell G. W.1993Paleobotany and the evolution of plants, 2nd edn. Cambridge, MA: Cambridge University Press [Google Scholar]
- Uphof J. C. Th.1920Physiological anatomy of xerophytic Selaginellas. New Phytol. 19, 101–131 (doi:10.1111/j.1469-8137.1920.tb07321.x) [Google Scholar]
- Voznesenskaya E. V., Franceschi V. R., Kiirats O., Freitag H., Edwards G. E.2001Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414, 543–546 (doi:10.1038/35107073) [DOI] [PubMed] [Google Scholar]
- Webster T. R.1992Developmental problems in Selaginella (Selaginellaceae) in an evolutionary context. Ann. Mo Botanical Garden 79, 632–647 (doi:10.2307/2399757) [Google Scholar]
- Webster T. R., Jagels R.1977Morphology and development of aerial roots of Selaginella martensii grown in moist containers. Can. J. Bot. 55, 2149–2158 (doi:10.1139/b77-243) [Google Scholar]
- Webster T. R., Steeves T. A.1964Developmental morphology of the root of Selaginella kraussiana A. Br. and Selaginella wallacei Hieron. Can. J. Bot. 42, 1665–1676 (doi:10.1139/b64-165) [Google Scholar]
- Webster T. R., Steeves T. A.1967Developmental morphology of the root of Selaginella martensii Spr. Can. J. Bot. 45, 395–404 (doi:10.1139/b67-039) [Google Scholar]
- Weiss F. E.1903A biseriate halonial branch of Lepidophloios fuliginosus. Trans. Linn. Soc. Lond. Ser. 2, Bot. 6, 217–235 [Google Scholar]
- Winter K., Smith J. A. C. (eds) 1996Crassulacean acid metabolism Berlin, Germany: Springer [Google Scholar]
- Witmer L. M.1995The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional morphology in vertebrate paleontology (ed. Thompson J. J.), pp. 19–33 New York, NY: Cambridge University Press [Google Scholar]
- Zelawski W., Riech E. P., Stanley R. G.1970Assimilation and release of internal carbon dioxide by woody plant shoots. Can. J. Bot. 48, 1351–1354 (doi:10.1139/b70-204) [Google Scholar]