Abstract
Host-associated microbial interactions may involve genome complementation, driving-enhanced communal efficiency and stability. The tsetse fly (Diptera: Glossinidae), the obligate vector of African trypanosomes (Trypanosoma brucei subspp.), harbours two enteric Gammaproteobacteria symbionts: Wigglesworthia glossinidia and Sodalis glossinidius. Host coevolution has streamlined the Wigglesworthia genome to complement the exclusively sanguivorous tsetse lifestyle. Comparative genomics reveal that the Sodalis genome contains the majority of Wigglesworthia genes. This significant genomic overlap calls into question why tsetse maintains the coresidence of both symbionts and, furthermore, how symbiont homeostasis is maintained. One of the few distinctions between the Wigglesworthia and Sodalis genomes lies in thiamine biosynthesis. While Wigglesworthia can synthesize thiamine, Sodalis lacks this capability but retains a thiamine ABC transporter (tbpAthiPQ) believed to salvage thiamine. This genetic complementation may represent the early convergence of metabolic pathways that may act to retain Wigglesworthia and evade species antagonism. We show that thiamine monophosphate, the specific thiamine derivative putatively synthesized by Wigglesworthia, impacts Sodalis thiamine transporter expression, proliferation and intracellular localization. A greater understanding of tsetse symbiont interactions may generate alternative control strategies for this significant medical and agricultural pest, while also providing insight into the evolution of microbial associations within hosts.
Keywords: symbiosis, tsetse fly, homeostasis, comparative genomics
1. Introduction
Microbial associations are significant drivers of evolution (Margulis & Fester 1991). Since most microbes are localized within a complex consortium, little is known regarding how species interact, and even less is known about mechanisms that prevent species antagonism, which can ultimately compromise the integrity of the biological system. Elucidating these complex microbe–microbe interactions can be enabled through the use of host model systems that harbour naturally simple microbial communities.
The haematophagous tsetse fly (Diptera: Glossinidae) is the sole vector of African trypanosomes (Trypanosoma brucei subspp.), the causative agents of the fatal African trypanosomiasis (commonly known as sleeping sickness) in humans and nagana in other animals. In addition to serving as a vector for African trypanosomes, the tsetse fly also harbours two enteric gamma-proteobacterial symbionts: the obligate mutualist Wigglesworthia glossinidia (Aksoy 1995) and a secondary symbiont, Sodalis glossinidius (Dale & Maudlin 1999). These symbionts are necessary for tsetse's survival, as they are believed to supplement nutrients that the host is incapable of producing or obtaining from its restricted blood diet. The loss of Wigglesworthia and Sodalis associations results in significant detriment to tsetse, including reduction in reproductive output and shortened lifespan, respectively (Nogge 1976, 1981; Dale & Welburn 2001; Pais et al. 2008). Tsetse may also harbour Wolbachia, which is typically found in reproductive tissues (O'Neill et al. 1993) and to date has an unknown functional role. Although examples of fecundity enhancement and greater competitive efficiency have been described (Wade & Chang 1995; Dedeine et al. 2001; Hosokawa et al. 2010), Wolbachia associations are generally construed as a form of facultative parasitism within insects (reviewed in Dobson 2003).
In contrast to the majority of insects, tsetse flies have a unique reproductive strategy known as adenotrophic viviparity (i.e. live birth). Progeny develop through three larval instars in utero where they are provided with protein- and lipid-rich nutrients, and also inoculated with Wigglesworthia and Sodalis via maternal milk gland secretions (Ma & Denlinger 1974; Attardo et al. 2008). The maternal transmission of tsetse symbionts is associated with significant population bottlenecks at each generation (Rio et al. 2006). Consequently, stability between the different microbial symbiont species is especially critical towards maintaining the cohesiveness and evolutionary success of the biological system.
Molecular phylogenetic analysis of the association between Wigglesworthia and tsetse supports an ancient establishment, dating back 50–80 Myr with a high degree of concordance (Chen et al. 1999). Extensive host coevolution has streamlined Wigglesworthia's genome to complement the exclusively sanguivorous tsetse lifestyle (Akman et al. 2002). In comparison to the Wigglesworthia symbiosis, molecular phylogenetic analyses date the Sodalis–tsetse association to be of recent origin (Aksoy et al. 1997; Weiss et al. 2006). Also supporting its recent transition into symbiosis, Sodalis remains one of the few insect symbionts that can still be maintained in culture outside of its host (Welburn et al. 1987).
Large-scale sequencing and annotation has begun to shed light on the functional capabilities of host-associated microbes and their potential roles towards host biology and development (Moran et al. 2008). The comparative analyses of the annotated Sodalis (Toh et al. 2006) and Wigglesworthia (Akman et al. 2002) genomes enable the identification of complementary pathways of potential metabolic integration. Additionally, during intensive periods of host development, the growth dynamics of Wigglesworthia and Sodalis mirror one another's, suggestive of intertwined metabolic pathways (Rio et al. 2006). Interestingly, the Sodalis proteome contains most of the putative Wigglesworthia products (i.e. greater than 90% of Wigglesworthia coding sequences are orthologues within the Sodalis genome). This significant genomic overlap calls into question why tsetse maintains the energetically expensive coresidence of both symbiont species and how symbiont homeostasis is maintained.
The deficiency of B vitamins in blood (Edwards et al. 1957) coupled with the inability of insects to synthesize these essential nutrients (Sweetman & Palmer 1928; Craig & Hoskins 1940) suggest their provisioning to tsetse through microbial interactions. One of the few distinctions between the Sodalis and Wigglesworthia genomes lies in thiamine (vitamin B1) biosynthesis and transport. Although Wigglesworthia retains de novo thiamine biosynthetic capabilities, Sodalis is incapable of its production. To complement its thiamine biosynthetic deficiency, the Sodalis genome contains a putative thiamine ABC transport system (TbpAThiPQ), which in other closely related prokaryotes is used to salvage exogenous thiamine (Webb et al. 1998). We believe that this complementation of genetic inventory between Wigglesworthia and Sodalis may represent the early convergence of metabolic pathways that may act to ensure the maintenance of the Wigglesworthia association while also evading antagonism between the symbiont species.
Here, we examine one aspect of possible interplay between tsetse symbionts: the dependence of Sodalis on the provisioning of thiamine by Wigglesworthia. We investigate the effect of thiamine and its derivatives towards Sodalis proliferation and intracellular localization, a lifestyle feature that is associated with enhanced replication for this microbial symbiont (Dale et al. 2001). Functional assays characterizing the expression and regulatory patterns of the Sodalis thiamine ABC transporter were performed. We present evidence for the necessity of exogenous thiamine towards Sodalis fitness, both in vitro and within the tsetse fly. The biosynthesis and utilization of thiamine by Wigglesworthia and Sodalis, respectively, may be pivotal not only towards the retention of the tsetse–Wigglesworthia association, but also to preserve homeostasis of the microbial community within the host. Understanding the metabolic interactions of tsetse symbionts can lead to the identification of novel control strategies towards combating trypanosomiasis prevalence, while also providing insight towards the evolution of microbial associations within hosts.
2. Material and methods
(a). Insects
Tsetse flies, Glossina morsitans morsitans, were maintained at West Virginia University within the Department of Biology insectary at 24 ± 1°C with 50 to 55 per cent relative humidity on a 12/12 h light/dark schedule. Tsetse flies received defibrinated bovine blood (Haemostat, Dixon, CA, USA) every 48 h through an artificial membrane feeding system (Moloo 1971).
(b). Cell cultures
Sodalis were isolated from surface-sterilized G. m. morsitans pupae and cultured on Aedes albopictus C6/36 cells as described previously (Dale & Maudlin 1999). Sodalis were subsequently maintained cell-free in vitro at 28°C in Mitsuhashi–Maramorosch (MM) medium (Weiss et al. 2006) supplemented with 5 per cent heat-inactivated foetal bovine serum (FBS). C6/36 cells were maintained in MM medium supplemented with 15 per cent FBS at 28°C.
(c). Growth assays
Sodalis growth was compared upon inoculation into six different M9 minimal glucose media types (Sambrook & Russell 2001; plus additional supplements as indicated in figure 1a). Log-phase Sodalis was diluted to an initial OD600 of 0.01. Subsequently, 1 ml of diluted culture was inoculated into 4 ml of each of the various media types and grown at 28°C without shaking. OD600 readings were taken every 24 h for 5 days, with three independent trials performed.
Figure 1.
Sodalis growth and thiamine ABC transporter expression in vitro within TMP supplemented minimal media. (a) Sodalis growth in M9 minimal media ± glucose ± vitamins ± various thiamine derivatives (100 µM) at 120 h post-inoculation (vitamins contain a negligible amount of thiamine). (b) Mean Sodalis growth through 168 h in Media 1 and 0, 50 or 500 µM TMP. Letters designate treatments that are significantly different from others (ANOVA, p < 0.0001). (c) qRT–PCR analysis of tbpA expression from Sodalis grown in Media 1 ± 50 µM TMP. * and ** denote statistically significant differences, ANOVA p < 0.05 and p < 0.01, respectively, within each time point. Error bars signify ±1 standard error of the mean (s.e.m.). (n ≥ 6 samples at each time point). Black bars, 0 µM TMP; white bars, 50 µM TMP.
(d). Impact of thiamine monophosphate on Sodalis fitness
Log-phase Sodalis was inoculated at an OD600 of 0.01 into Media 1 (M9 minimal glucose media +50 µg ml−1 Bacto Vitamin Assay Casamino Acids; BD, Franklin Lakes, NJ, USA) with the addition of 0, 50 or 500 µM thiamine monophosphate (TMP; Sigma-Aldrich, St Louis, MO, USA). Every 24 h for 7 days, OD600 readings were obtained to measure growth. Three independent trials were performed.
(e). Analysis of symbiont gene expression in vitro
To examine the transcription of the Sodalis thiamine ABC transporter relative to TMP concentration, we chose to analyse the expression of the tbpA gene that encodes the thiamine transporter substrate-binding subunit. RNA was isolated during in vitro growth in Media 1 ± 50 µM TMP using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The absence of DNA contamination was verified through PCR using an RNA template lacking a reverse-transcription step. First-strand cDNA synthesis was performed with Superscript III Reverse Transcriptase (Invitrogen), 25 ng random hexamer primers and 200 ng RNA. Real-time quantitative PCR (qPCR) was performed in an iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using Bio-Rad iQ SYBR Green Supermix, 10 mM of primers (tbpAQTfor and tbpAQTrev; electronic supplementary material, table S1) and 2 µl cDNA template. The amplification settings were an initial 3 min denaturation step at 95.0°C, followed by 40 cycles of 10 s at 95.0°C and 30 s at 54.1°C. Internal standard curves were developed by cloning tbpA into the pGEM-T vector (Promega, Madison, WI, USA) using tbpAlongF and tbpAlongR primers (electronic supplementary material, table S1). Quantification of the amplicons relative to the standard curves was performed using Bio-Rad iCycler iQ multi-colour real-time PCR optical system software v. 2.0. The respective OD600 readings of each time point were used for the normalization of tbpA expression. All assays were performed in triplicate and replicates were averaged for each sample.
(f). Regulation of Sodalis thiamine transporter
The control of the Sodalis thiamine ABC transporter by a thi box regulatory region was examined using the plasmid-borne tbpA–gfp fusions pRJ12, pRJ13 and pRJ14 (figure 3b) in wild-type Escherichia coli MG1655. To construct the tbpA–gfp reporter fusions, primers (UR281 and UR282, UR283 or UR284) were used to amplify promoter DNA from three different tbpA regions of the Sodalis chromosome (figure 3b). The PCR products were digested with BamHI and XbaI and cloned into the promoterless gfp vector pLR29 (Runyen-Janecky & Payne 2002) to generate pRJ12, pRJ13 and pRJ14, respectively. Overnight cultures of MG1655 containing each respective plasmid were started from freezer stocks inoculated into M9 minimal glucose media and 125 µg ml−1 carbenicillin. Cultures were grown overnight at 37°C with shaking. Following overnight growth, each culture was pelleted and resuspended in the original volume of M9 media and carbenicillin. Resuspended cultures (20 µl) were inoculated into 2 ml of M9 media and 125 µg ml−1 of carbenicillin ± 50 µM TMP. Cultures were grown at 37°C with shaking. At 24 h, 500 µl of each sample was fixed in 2 per cent paraformaldehyde and green fluorescence was quantified using an FACSCaliber (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) fluorescence-activated cell sorter with an excitation at 488 nm to measure single-cell fluorescence. FACSCaliber settings were forward scatter = E01, side scatter = 505 and relative fluorescence between 515 and 545 nm = 798. Three independent trials were performed, with 10 000 cells analysed per sample.
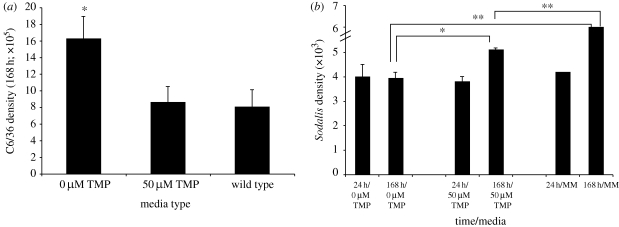
Figure 3.
Sodalis intracellular invasion and replication is significantly lower in the absence of TMP. (a) C6/36 density at 168 h post-inoculation with Sodalis grown in Media 1 (±50 µM TMP) or wild-type media (MM media + 5% FBS). Mean C6/36 density values are represented and errors bars signify 1 s.e.m. Asterisk denotes significant difference (ANOVA, p < 0.0001). (b) Sodalis density at 24 and 168 h post-inoculation of C6/36 cells. Mean Sodalis density values are represented and errors bars signify 1 s.e.m. * and ** denote significant difference, ANOVA, p < 0.05 and p < 0.01, respectively (n ≥ 6 samples per treatment).
(g). Examining the effect of thiamine on Sodalis intracellular replication
Intracellular localization and proliferation, followed by host cell lysis, is a process associated with Sodalis replication both in culture and within the tsetse host (Welburn et al. 1987; Dale & Maudlin 1999; Dale et al. 2001). To examine the influence of TMP towards intracellular infection and replication by Sodalis, C6/36 cells were split into six-well culture plates with MM media + 15 per cent FBS. Log-phase Sodalis grown in various media types (Media 1 ± 50 µM TMP or MM media + 5% FBS) were inoculated into a confluent lawn of C6/36 cells at an OD600 of 0.01. Prior to inoculation, the supernatant from the wells was replaced with the media used to grow the respective Sodalis. To account for any potential effects of the various media types towards C6/36 viability, a replicate of the experiment was performed that lacked Sodalis inoculation. At 24 and 168 h post-inoculation, the total well contents (including any adhered C6/36 cells) were aspirated and total DNA isolation was performed using the Holmes–Bonner method (Holmes & Bonner 1973). The quantification of C6/36 cells was determined through qPCR using the rpL8QTfor and rpL8QTrev primers (electronic supplementary material, table S1), which amplify the A. albopictus ribosomal protein (rpL8) gene (GenBank accession no. M99055). The quantification of Sodalis density was also determined through qPCR, with corresponding SgexochiQTfor and SgexochiQTrev oligonucleotides (electronic supplementary material, table S1), which amplify the single-copy exochitinase gene (chi; GenBank accession no. BSPY11391; Rio et al. 2006). Internal standard curves were developed by cloning amplicons for A. albopictus rpl8, using rpl8for and rpl8rev primers (electronic supplementary material, table S1), and Sodalis chi was produced with Sg exochifor and Sgexochirev (electronic supplementary material, table S1) into the pGEM-T vector (Promega, Madison, WI, USA), as described previously (Rio et al. 2006). Quantification of the amplicons relative to the standard curves was performed using SYBR Green I Dye (Bio-Rad) and Bio-Rad iCycler iQ multi-colour real-time PCR optical system software v. 2.0. The experiment was performed twice with multiple replicates within each trial.
(h). Expression of Sodalis thiamine ABC transporter through tsetse fly development
Tsetse flies, G. m. morsitans, were sacrificed at distinct developmental stages (i.e. late larval, dissected approx. 6–9 days in utero; early pupal, less than 48 h post-maternal deposition; late pupal, approx. 28–30 days post-maternal deposition; teneral, newly eclosed adults prior to first blood meal; and two-week-old adults. Whole-fly RNA was isolated from single tsetse fly individuals using TRIzol (Invitrogen, Carlsbad, CA, USA) and treated with RNase free–DNase I (Invitrogen). The absence of DNA contamination was verified using PCR. First-strand cDNA synthesis was performed with 200 ng RNA, a 2 µM primer cocktail of tbpArev and gapDHrev (table S1), and Invitrogen Superscript II Reverse Transcriptase. Second-strand synthesis was performed with the addition of complementary 5′ end gene primers (electronic supplementary material, table S1) at 55°C for 35 cycles. The amplification products were analysed by agarose gel electrophoresis and visualized with Kodak one-dimensional image analysis software. The expression level of endogenous Sodalis glyceraldehyde-3-phosphate dehydrogenase (gapDH) within respective time points was used as a loading control.
(i). The effect of TMP-supplemented blood meals towards Sodalis thiamine ABC transporter expression within tsetse
Teneral tsetse were maintained on blood meals supplemented with 50 or 500 µM TMP for two weeks. Whole-fly RNA was isolated from single tsetse individuals using TRIzol, and tbpA and gapDH reverse-transcriptional analyses were performed as described above.
(j). The impact of TMP-supplemented blood meals towards tsetse biology
Teneral tsetse were maintained on blood meals supplemented with TMP as described previously. Tsetse flies were sacrificed at two weeks of age and DNA isolation performed using the Holmes–Bonner protocol (Holmes & Bonner 1973). DNA from each experimental sample was analysed to quantify the density of Wigglesworthia, Sodalis and Wolbachia symbionts as described previously (Rio et al. 2006).
(k). Statistical analysis
The data were analysed using JMP 7.0 software (SAS Institute, Cary, NC, USA). A one-way analysis of variance (ANOVA) and Tukey–Kramer post hoc pairwise comparison of the mean were performed where appropriate to determine whether symbiont density, thiamine ABC transporter expression or C6/36 density differed between the various treatments. Student's t-tests were employed to assess the differences in gfp fluorescence of the plasmid constructs. F-tests were applied to assess the homogeneity of variances. The normality of density distributions was determined with a goodness-of-fit test. Wolbachia densities were square-root-transformed to satisfy normality. Significant differences (p ≤ 0.05) are reported.
3. Results
(a). The role of thiamine in Sodalis fitness
The growth of Sodalis in media supplemented with glucose, vitamins and various thiamine derivatives including thiamine–HCl, thiamine pyrophosphate (TPP) or TMP was observed over 120 h. An increase in the Sodalis growth yield was observed with the incremental supplementation of various nutrients, such as glucose and vitamins, to an M9 minimal media base (Media 1). Sodalis proliferation increased significantly in cultures supplemented with TMP (figure 1a); however, a similar enhancement was not observed upon the addition of other thiamine derivatives (i.e. TPP or thiamine–HCl) to Media 1. These results indicate that Sodalis requires an exogenous thiamine source, preferably in the form of TMP, for optimal growth. Furthermore, Sodalis growth is impacted through time, not only by the presence of TMP, but also by different concentrations of this nutrient. A significantly higher mean Sodalis density was realized in Media 1 supplemented with 50 µM TMP (ANOVA, p < 0.0001; figure 1b) in comparison to 500 µM TMP. Moreover, a detrimental growth effect was observed when Sodalis was inoculated into Media 1 containing 500 µM TMP. These results demonstrate that Sodalis requires exogenous nutrients including TMP for its cultivation outside of the tsetse host.
(b). Impact of exogenous TMP towards Sodalis thiamine ABC transporter expression in vitro
In free-living bacterial species, exogenous thiamine and its derivatives can be imported into the cell through an ATP-driven thiamine ABC transporter localized to the cell wall (Webb et al. 1998; Rodionov et al. 2002). At sufficient levels, thiamine and its derivatives can transcriptionally repress further TMP uptake by binding to a riboswitch localized upstream of the thiamine ABC transporter operon known as the thi box (Winkler et al. 2002).
To determine whether a similar expression pattern occurs with the Sodalis thiamine ABC transporter, we analysed the expression of tbpA in media containing or lacking TMP using qPCR. Sodalis grown in media lacking TMP exhibited significantly higher tbpA expression than cultures grown in the presence of TMP (ANOVA, p < 0.001; figure 1c). The significantly higher expression of tbpA in cells lacking exogenous TMP through time suggests that these Sodalis are attempting to import a vital nutrient for growth via its transporter, and that its functional regulation is intact and similar to that exhibited by free-living bacteria. Interestingly, a similar relationship of decreased tbpA expression through time was observed for Sodalis in both media types, suggesting the significance of TMP early in growth.
(c). Regulation of Sodalis thiamine ABC transporter
The Sodalis tbpA promoter has a putative thi box (Miranda-Ríos et al. 1997) at nucleotides 58–97 5′ of the transcriptional start site. Thus, based on the high conservation of the thi box region upstream of tbpA (figure 2a), we hypothesized that the thi box still mediates thiamine repression of Sodalis's thiamine ABC transporter. To test this hypothesis, we constructed tbpA–gfp fusions (± thi box, figure 2b) and examined GFP expression in E. coli containing these fusions grown with and without TMP. Escherichia coli containing the two tbpA–gfp fusions containing the thi box (pRJ12 and pRJ13) showed significant reductions (Student's t-test, p<0.05)–specifically, decreases of 42 and 37 per cent, respectively, in GFP levels when grown in media containing TMP when compared with media lacking TMP (figure 2c). In contrast, there was no statistically significant change in the GFP level with E. coli containing the pRJ14 fusion (Student's t-test, p > 0.05), which lacks the thi box, in either media type. These data suggest that the thi box remains functionally relevant for TMP regulation of tpbA expression by Sodalis.
Figure 2.
Conservation of thi box and regulatory regions of Sodalis thiamine ABC transporter. (a) Graphical representation of thi box (Miranda-Ríos et al. 1997) nucleotide sequence alignment of Sodalis, E. coli and Salmonella typhimurium. Image generated through the WebLogo website (http://weblogo.berkeley.edu/). (b) tbpA–gfp fusions are depicted. The thi box and tbpA start codon are represented by a black box and an asterisk, respectively. The gfp sequences are represented by arrows. (c) Escherichia coli MG1655 carrying the tbpA–gfp fusions were grown for 24 h in Media 1 and carbenicillin in the absence (grey bars) or presence (black bars) of 50 µM TMP, and the fluorescence quantified with FACS. Asterisks denote significant differences, Student's t-test, p < 0.05, within each construct. The data represent the mean fluorescence of at least three independent trials, with 10 000 cells analysed per sample. Standard deviations are indicated.
(d). The effect of TMP towards Sodalis intracellular localization and replication
To determine whether Sodalis intracellular replication is compromised when grown in the absence of TMP, a monolayer of A. albopictus C6/36 cells was inoculated with Sodalis grown in the presence or absence of TMP. This particular cell line has previously been demonstrated to support intracellular localization and subsequent increases in Sodalis density (Welburn et al. 1987; Dale & Maudlin 1999; Dale et al. 2001). To ensure that any changes in the C6/36 density were due solely to Sodalis infection and not respective media types, replicate assays lacking Sodalis were performed and no effects on C6/36 density were found (data not shown). At 24 h post-inoculation, no significant differences were observed in either C6/36 or Sodalis density between the various treatments (data not shown and figure 3b, respectively). As incubation progressed to 168 h, C6/36 density was significantly lower upon inoculation with Sodalis grown in TMP-supplemented media and comparable to when the bacteria are cultured in a rich media base (ANOVA, p < 0.0001; figure 3a). Moreover, at the 168 h time point, Sodalis density was significantly higher with TMP supplementation than with cells cultured in media lacking this nutrient, supporting an increase in replication rate (ANOVA, *p < 0.05; figure 3b). The highest Sodalis density was supported with nutrient-rich MM media. This suggests that although TMP is critical for its proliferation, this metabolite is not the sole dietary necessity as additional nutrients further enhance replication (ANOVA, **p < 0.01; figure 3b). These results demonstrate that the intracellular infection and subsequent replication of Sodalis, typical of its lifestyle within the tsetse fly, is compromised when TMP is lacking.
(e). Sodalis thiamine ABC transporter expression through tsetse development and upon TMP supplementation of host blood meals
Semiquantitative reverse-transcriptional analyses of whole tsetse fly RNA reveals that thiamine transport by Sodalis is dynamic through host development (figure 4a). Expression levels of Sodalis tbpA were highest in the late pupal and teneral adult life stages in both males and females and lowest during the larval and early pupal time points.
Figure 4.
Sodalis thiamine ABC transporter expression and tsetse symbiont density through host development and with supplementation of blood meal. Semiquantitative RT–PCR analysis of Sodalis tbpA expression (a) through host development and (b) following two-week TMP supplementation of blood meals. TM, teneral male; 2 wk M, two-week-old male; TF, teneral female; 2 wk F, two-week-old female. Sodalis gapDH expression served as a loading control. (c) Wigglesworthia and (d) Sodalis density were compared in two-week-old tsetse fed blood-only and TMP-supplemented meals. Mean density values are represented and error bars signify 1 s.e.m. Letters depict significant differences (ANOVA, p < 0.05) between treatments (n ≥ 3 samples at each time point).
Expression levels of Sodalis tbpA also demonstrated variability between two-week-old female and male flies, with higher transcriptional activity demonstrated within females (figure 4a,b). We also examined Sodalis tbpA expression in tsetse adults maintained on various TMP-supplemented blood meals (figure 4b). The expression of Sodalis tbpA decreased in females with greater levels of TMP supplementation in blood meals, while this pattern was not observed within males as augmenting TMP had no effect on transcriptional profiles.
(f). The impact of TMP-supplemented blood meals towards symbiont density
Like other obligate insect mutualists, Wigglesworthia is unable to be cultured using in vitro methods in the laboratory. Consequently, genetic manipulation is not feasible. We bypassed the inability to mutate Wigglesworthia to produce increased levels of TMP by supplementing tsetse blood meals with this vitamin derivative and examining the effects on symbiont density. Because symbionts may contain multiple genomes per cell (Komaki & Ishikawa 2000), qPCR was used to determine bacterial genome number by using single copy genes normalized to host single copy genes. In support of previous descriptions (Rio et al. 2006), Wigglesworthia abundance was significantly greater within females than males across all treatment groups (ANOVA, p = 0.01; figure 4c). Within female tsetse, a higher Wigglesworthia density was evident within tsetse maintained on blood only in comparison with TMP-supplemented meals, although statistical significance was lacking (ANOVA, p = 0.71). Within males, no differences in Wigglesworthia density were found between the various treatments (ANOVA, p = 0.74). Interestingly, Sodalis was more copious within female tsetse fed blood meals supplemented with 50 µM TMP in comparison with those fed blood only or 500 µM TMP-supplemented blood meals (ANOVA, p = 0.002; figure 4d). A similar reduction in Sodalis density was observed when tsetse females were fed a higher TMP concentration (i.e. 500 µM TMP; this finding is similar to what we observed with Sodalis in culture). No significant differences in Sodalis density were observed among the male treatment groups (ANOVA, p = 0.96). In addition, Wolbachia density did not significantly differ (ANOVA, p = 0.6; data not shown) between the various treatment groups within each sex.
4. Discussion
The significance of microbial interactions within hosts is gaining steadfast recognition (Dethlefsen et al. 2007). Recent studies have demonstrated that symbionts of ancient origin are associated with genomic complementation, enabling microbial species to reach a synergistic equilibrium that cultivates a highly complex interdependence (Wu et al. 2006). In contrast to insect associations where symbionts are of ancient origins (Moran et al. 2005; Takiya et al. 2006), the tsetse enteric partners have vastly different acquisition times (Aksoy et al. 1997; Chen et al. 1999), providing a unique opportunity for insight into the adaptation processes associated with early coresidence of microbes within a symbiotic system.
Despite a severely reduced genome (Toh et al. 2006), Wigglesworthia significantly impacts several aspects of tsetse fly biology including reproduction, blood-meal digestion, temperature sensitivity, immunological processing and vector competence (Pais et al. 2008; Wang et al. 2009). Although Sodalis has a relatively large (4.2 Mb) chromosome, a significant degree of genomic decay is apparent, mostly represented in the plethora of pseudogenes. This abundance of pseudogenes results in a diminished coding capacity of only 51 per cent, making the Sodalis genome one of the least coding bacterial genomes known to date (Toh et al. 2006). The majority of pseudogenes are homologues of proteins that have functions related to immunological defence or transport and metabolism of carbohydrates and inorganic ions in free-living bacteria. These functions are probably no longer necessary, given the fidelity of vertical transmission through successive tsetse generations (Rio et al. 2006).
One of the few distinctions between the Wigglesworthia and Sodalis genomes lies in thiamine biosynthesis. While Wigglesworthia is capable of synthesizing thiamine (electronic supplementary material, figure S1), Sodalis lacks this capability. While the genes necessary for thiamine biosynthesis have clearly been eroded within the Sodalis genome (Toh et al. 2006), this biosynthetic inability appears to be circumvented through the retention of genes that encode a thiamine ABC transporter (tbpAthiPQ). Other Sodalis genome tailoring events have occurred following its transition to a host-associated lifestyle. Such events include the alteration of immunogenic components of its cell membrane—notably a truncated lipopolysaccharide, an absent O antigen and modified outer membrane protein A (i.e. ompA)—which are believed to protect against a systemic host immune response and enable tsetse establishment (Weiss et al. 2008). Additionally, extensive genome divergence between Sodalis and closely related Sitophilus oryzae primary endosymbiont appears tailored towards acquiring metabolites absent from the restricted diets of their specific hosts (Rio et al. 2003). It is tempting to postulate that the evolutionary pressures, resulting in the maintenance of the Sodalis thiamine ABC transporter over thiamine biosynthesis capability, may be indicative of selection at the host (Wernegreen & Moran 2000) rather than the individual symbiont level, acting to promote microbial homeostasis and ultimately tsetse fitness. Recognizing mechanisms that drive homeostasis between microbial species provides a basis of understanding fundamental molecular processes associated with the selection, regulation and evolution of symbiotic communities.
Many vitamins must be obtained either through diet or microbial interactions. Thiamine, an important cofactor in carbohydrate and amino acid metabolism, is essential for cellular physiology and growth (Schowen 1998). Within various insect groups, thiamine deficiency results in the degeneration of the fat body, stunted larval growth and reduced fertility (Sweetman & Palmer 1928; Craig & Hoskins 1940). The exclusive blood diet of tsetse, lacking in B-complex vitamins (particularly thiamine; Edwards et al. 1957), coupled with the inability of Sodalis, Wolbachia and tsetse to synthesize thiamine, supports the provisioning of this essential cofactor exclusively by Wigglesworthia. With thiamine biosynthesis being a unique Wigglesworthia role, provisioning of this vitamin may be essential for both preventing antagonism between tsetse's microbial symbionts and ensuring the maintenance of this obligate mutualist through time.
We demonstrate that Sodalis proliferation, both extra- and intracellular, is nutrient-limited, specifically by TMP. In essence, Sodalis population dynamics may be regulated not only by presence or absence of TMP but specifically by varying concentrations of this vitamin supplied by Wigglesworthia. Nutritional interactions, such as the metabolic interplay of thiamine biosynthesis and transport between the tsetse symbionts, may act to stabilize bacterial cohabitation within a host. The expression of the Sodalis thiamine ABC transporter, regulated by TMP through a functionally conserved thi box, appears to be reflective of host nutritional status. We observed higher expression of the Sodalis thiamine ABC transporter in tsetse's late pupal and teneral life stages. These particular developmental stages, demarcated by only 48 h, culminate a long quiescent developmental period consisting of approximately 30 days in the soil during which nutrient supplies have been vastly reduced (Leak 1999).
Tsetse fly fitness has been shown to influence the susceptibility towards trypanosome infection. Specifically, starvation periods greatly increase the probability of parasite establishment within tsetse (Kubi et al. 2006), with the teneral stage being of highest vector competence (Welburn & Maudlin 1992). The decrease in Sodalis thiamine ABC transporter expression in two-week-old adults probably reflects an increase in the Wigglesworthia population (Rio et al. 2006) and, correspondingly, the ability to synthesize TMP at higher levels. Additionally, Sodalis transporter expression was both higher and most affected by TMP supplementation of tsetse blood meals within teneral females in comparison with similarly aged males. This phenomenon is probably due to additional female-specific roles such as reproduction and nourishment of intrauterine progeny, both processes that will result in greater demands and competition for available nutrients.
These studies provide insight into a metabolic factor: the provisioning of TMP by the obligate mutualist Wigglesworthia, which may aid the maintenance of microbial homeostasis within tsetse. Future studies will focus on identifying Sodalis compensatory roles towards tsetse symbiosis and whether these also act to stabilize the symbiont community. Given the critical role of tsetse symbiosis on host physiology and ecology, these associations provide a weak link in tsetse's biology. A greater understanding of tsetse symbiont interactions may generate alternative biological control methods for use in decreasing the prevalence of African trypanosomiasis.
Acknowledgements
We thank Alexandria Brown and Lindsay Zweibel for technical assistance. We also thank Dr Yoshitomo Kikuchi, Dr Adam Silver and Dr Brian Weiss for providing comments on the manuscript. This work was supported through the Oak Ridge Associated University's Ralph E. Powe Junior Faculty Enhancement Award and the West Virginia University Research Corporation. This research was supported by NIH R03AI081701-01A2.
References
- Akman L., Yamashita A., Watanabe H., Oshima K., Shiba T., Hattori M., Aksoy S.2002Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Gen. 32, 402–407 (doi:10.1038/ng986) [DOI] [PubMed] [Google Scholar]
- Aksoy S.1995Wigglesworthia gen nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbiont of tsetse flies. Int. J. Syst. Bacteriol. 45, 848–851 [DOI] [PubMed] [Google Scholar]
- Aksoy S., Chen X., Hyspa V.1997Phylogeny and potential transmission routes of midgut associated endosymbionts of tsetse (Diptera: Glossinidae). Insect Mol. Biol. 6, 183–190 (doi:10.1111/j.1365-2583.1997.tb00086.x) [DOI] [PubMed] [Google Scholar]
- Attardo G. M., Lohs C., Heddi A., Alam U. H., Yildirim S., Aksoy S.2008Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect Physiol. 51, 1236–1442 (doi:10.1016/j.jinsphys.2008.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. A., Song L., Aksoy S.1999Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont Wigglesworthia glossinidia. J. Mol. Evol. 48, 49–58 (doi:10.1007/PL00006444) [DOI] [PubMed] [Google Scholar]
- Craig R., Hoskins W. M.1940Insect biochemistry. Annu. Rev. Biochem. 9, 617–640 (doi:10.1146/annurev.bi.09.070140.003153) [Google Scholar]
- Dale C., Maudlin I.1999Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int. J. Syst. Bacteriol. 49, 267–275 (doi:10.1016/S0020-7519(01)00151-5) [DOI] [PubMed] [Google Scholar]
- Dale C., Welburn S. C.2001The endosymbionts of tsetse flies—manipulating host–parasite interactions. Int. J. Parasitol. 31, 628–631 (doi:10.1016/S0020-7519(01)00151-5) [DOI] [PubMed] [Google Scholar]
- Dale C., Young S. A., Haydon D. T., Welburn S. C.2001The insect endosymbiont Sodalis glossinidius utilizes a type III secretion system for cell invasion. Proc. Natl Acad. Sci. USA 98, 1883–1888 (doi:10.1073/pnas.021450998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeine F., Vavre F., Fleury F., Loppin B., Hochberg M. E., Bouletreau M.2001Removing symbiotic Wolbachia specifically inhibits oogenesis in a parasitic wasp. Proc. Natl Acad. Sci. USA 98, 6247–6252 (doi:10.1073/pnas.101304298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., McFall-Ngai M. J., Relman D. A.2007An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449, 811–818 (doi:10.1038/nature06245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson S. L.2003Wolbachia pipientis: impotent by association. In Insect symbiosis (eds Bourtzis K., Miller T. A.), pp. 199–209 Boca Raton, FL: CRC Press [Google Scholar]
- Edwards M. A., Kaufman M. L., Storvick C. A.1957Microbiologic assay for the thiamine content of blood of various species of animals and man. Am. J. Clin. Nutr. 5, 51–55 [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J.1973Preparation, molecular weight, base composition, and secondary structure of giant ribonucleic acid. Biochemistry 12, 2330–2338 (doi:10.1021/bi00736a023) [DOI] [PubMed] [Google Scholar]
- Hosokawa T., Koga R., Kikuchi Y., Meng X., Fukatsu T.2010Wolbachia as a bacteriocyte associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107, 769–774 (doi:10.1073/pnas.0911476107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaki K., Ishikawa H.2000Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem. Mol. Biol. 30, 253–258 (doi:10.1016/S0965-1748(99)00125-3) [DOI] [PubMed] [Google Scholar]
- Kubi C., Van Den Abeele J., De Deken R., Marcotty T., Dorny P., Van Den Bossche P.2006The effect of starvation on the susceptibility of teneral and non-teneral tsetse flies to trypanosome infection. Med. Vet. Entomol. 20, 388–392 (doi:10.1111/j.1365-2915.2006.00644.x) [DOI] [PubMed] [Google Scholar]
- Leak S. G. A.1999Tsetse biology and ecology, their role in the epidemiology and control of trypanosomes New York, NY: CABI publishing [Google Scholar]
- Ma W. C., Denlinger D. L.1974Secretory discharge and microflora of milk gland in tsetse flies. Nature 247, 301–303 (doi:10.1038/247301a0) [Google Scholar]
- Margulis L., Fester R.1991Symbiosis as a source of evolutionary innovation Cambridge, MA: MIT Press; [PubMed] [Google Scholar]
- Miranda-Ríos J., Morera C., Taboada H., Dávalos A., Encarnación S., Mora J., Soberón M.1997Expression of thiamine biosynthetic genes (thiCOGE) and production of symbiotic terminal oxidase cbb3 in Rhizobium etli. J. Bacteriol. 179, 6887–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloo S. K.1971An artificial feeding technique for Glossina. Parasitology 63, 507–512 (doi:10.1017/S0031182000080021) [DOI] [PubMed] [Google Scholar]
- Moran N. A., Tran P., Gerardo N. M.2005Symbiosis and insect diversification: an ancient symbiont of sap feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71, 8802–8810 (doi:10.1128/AEM.71.12.8802-8810.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A., McCutcheon J., Nakabachi A.2008Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190 (doi:10.1146/annurev.genet.41.110306.130119) [DOI] [PubMed] [Google Scholar]
- Nogge G.1976Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experentia 32, 995–996 (doi:10.1007/BF01933932) [DOI] [PubMed] [Google Scholar]
- Nogge G.1981Significance of symbionts for the maintenance of the optimal nutritional state for successful reproduction in hematophagous arthropods. Parasitology 82, 101–104 [Google Scholar]
- O'Neill S. L., Gooding R. H., Aksoy S.1993Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med. Vet. Entomol. 7, 377–383 (doi:10.1111/j.1365-2915.1993.tb00709.x) [DOI] [PubMed] [Google Scholar]
- Pais R., Lohs C., Wu Y., Wang J., Aksoy S.2008The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microbiol. 74, 5965–5974 (doi:10.1128/AEM.00741-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio R. V., Lefevre C., Heddi A., Aksoy S.2003Comparative genomics of insect-symbiotic bacteria: influence of host environment on microbial genome composition. Appl. Environ. Microbiol. 69, 6825–6832 (doi:10.1128/AEM.69.11.6825-6832.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio R. V., Wu Y. N., Filardo G., Aksoy S.2006Dynamics of multiple symbiont density regulation during host development: tsetse fly and its microbial flora. Proc. R. Soc. B 273, 805–814 (doi:10.1098/rspb.2005.3399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov D. A., Vitreschak A. G., Mironov A. A., Gelfand M. S.2002Comparative genomics of thiamin biosynthesis in prokaryotes. J. Biol. Chem. 277, 48 949–48 959 (doi:10.1074/jbc.M208965200) [DOI] [PubMed] [Google Scholar]
- Runyen-Janecky L. J., Payne S. M.2002Identification of chromosomal Shigella flexneri genes induced by the eukaryotic intracellular environment. Infect. Immun. 70, 4379–4388 (doi:10.1128/IAI.70.8.4379-4388.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W.2001Molecular cloning: a laboratory manual, 3rd edn Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Schowen R.1998Thiamin-dependent enzymes. In Comprehensive catalysis (ed. Sinnott L.), San Diego, CA: Academic Press [Google Scholar]
- Sweetman M. D., Palmer L. S.1928Insects as test animals in vitamin research. J. Biol. Chem 77, 33–52 [Google Scholar]
- Takiya D. M., Tran P. L., Dietrich C. H., Moran N. A.2006Co-cladogenesis spanning three phyla: leafhoppers (Insecta: Hemiptera: Cicadellidae) and their dual bacterial symbionts. Mol. Ecol. 15, 4175–4191 (doi:10.1111/j.1365-294X.2006.03071.x) [DOI] [PubMed] [Google Scholar]
- Toh H., Weiss B. L., Perkin S. A., Yamashita A., Oshima K., Hattori M., Aksoy S.2006Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 16, 149–156 (doi:10.1101/gr.4106106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M. J., Chang N. W.1995Increased male fertility in Trilobium confusum beetles after infection with the intracellular parasite Wolbachia. Nature 3473, 72–74 (doi:10.1038/373072a0) [DOI] [PubMed] [Google Scholar]
- Wang J., Wu Y., Yang G., Aksoy S.2009Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc. Natl. Acad. Sci. USA 106, 12 133–12 138 (doi:10.1073/pnas.0901226106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E., Class K., Downs D.1998thiBPQ encodes an ABC transporter required for the transport of thiamine and thiamine pyrophosphate in Salmonella typhimurium. J. Biol. Chem. 273, 8946–8950 (doi:10.1074/jbc.273.15.8946) [DOI] [PubMed] [Google Scholar]
- Weiss B. L., Mouchotte R. M., Rio R. V. M., Wu Y., Wu Z., Heddi A., Aksoy S.2006Interspecific transfer of bacterial endosymbionts between tsetse fly species: infection establishment and effect on host fitness. Appl. Environ. Microbiol. 72, 7013–7021 (doi:10.1128/AEM.01507-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. L., Weng Y., Schwank J. J., Tolwinski N. S., Aksoy S.2008An insect symbiosis is influenced by bacterium-specific polymorphisms in outer-membrane protein A. Proc. Natl Acad. Sci. USA 105, 15 088–15 093 (doi:10.1073/pnas.0805666105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welburn S. C., Maudlin I.1992The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Ann. Trop. Med. Parasitol. 86, 529–536 [DOI] [PubMed] [Google Scholar]
- Welburn S. C., Maudlin I., Ellis D. S.1987In vitro cultivation of Rickettsia-like-organisms from Glossina spp. Ann. Trop. Med. Parasitol. 81, 331–335 [DOI] [PubMed] [Google Scholar]
- Wernegreen J. J., Moran N. A.2000Decay of mutualistic potential in aphid endosymbionts through silencing of biosynthetic loci: Buchnera of Diuraphis. Proc. R. Soc. Lond. B 267, 1423–1431 (doi:10.1098/rspb.2000.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler W., Nahvi A., Breaker R. R.2002Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (doi:10.1038/nature01145) [DOI] [PubMed] [Google Scholar]
- Wu D., et al. 2006Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 4, 1079–1092 (doi:10.1371/journal.pbio.0040188) [DOI] [PMC free article] [PubMed] [Google Scholar]