Abstract
High background noise is an important obstacle in successful signal detection and perception of an intended acoustic signal. To overcome this problem, many animals modify their acoustic signal by increasing the repetition rate, duration, amplitude or frequency range of the signal. An alternative method to ensure successful signal reception, yet to be tested in animals, involves the use of two different types of signal, where one signal type may enhance the other in periods of high background noise. Humpback whale communication signals comprise two different types: vocal signals, and surface-generated signals such as ‘breaching’ or ‘pectoral slapping’. We found that humpback whales gradually switched from primarily vocal to primarily surface-generated communication in increasing wind speeds and background noise levels, though kept both signal types in their repertoire. Vocal signals have the advantage of having higher information content but may have the disadvantage of loosing this information in a noisy environment. Surface-generated sounds have energy distributed over a greater frequency range and may be less likely to become confused in periods of high wind-generated noise but have less information content when compared with vocal sounds. Therefore, surface-generated sounds may improve detection or enhance the perception of vocal signals in a noisy environment.
Keywords: acoustic communication, humpback whales, background noise, acoustic behaviour, communication strategy
1. Introduction
One of the most fundamental concepts in animal communication is that the relevant receiver successfully receives, and then perceives, the intended communication signal. Background noise is an important constraint in successful signal detection, as noise can mask relevant signals to potential receivers. Animals have evolved various strategies to overcome this obstacle: some species actively avoid areas with particularly high background noise levels (Schaub et al. 2008). Certain species of lizard have been shown to speed up dynamic visual displays in noisy habitats (Ord et al. 2007), illustrating the potential to change the presentation rate of a signal. Rather than increase the rate, some animals increase the redundancy of the signal. Various bird species such as the Japanese quail (Coturnix coturnix japonica, Potash 1972) and king penguin (Aptenodytes patagonicus, Jouventin et al. 1999), new world monkeys (Brumm et al. 2004) and marine mammals such as the right whale (Eubalaena australis, Parks et al. 2007) avoid acoustic signal masking by increasing the number of syllables per call series. Amphibians (Feng et al. 2006) and marine mammals such as the right whale (Eubalaena australis, Parks et al. 2007) avoid acoustic signal masking by shifting the frequency range of the acoustic signals. In fact, this type of compensation in response to increases in environmental noise may account for the inter-population variation in vocalization frequencies found in Indo-Pacific bottlenose dolphins (Morisaka et al. 2004) and killer whales (Orcinus orca, Foote & Nystuen 2008). A final strategy to compensate for an increase in background noise involves the increase in source level or amplitude of the acoustic signal found in birds (Cynx et al. 1998), cetaceans (Holt et al. 2009) and primates (Brumm et al. 2004). This phenomenon is known as the Lombard effect (Lombard 1911) and probably serves to maintain an appropriate and detectable signal-to-noise ratio for the receiver.
Therefore, in many species, behavioural flexibility and vocal plasticity has allowed individuals to cope with natural variations in environmental noise. However, all previously mentioned studies have focused on the signaller either actively avoiding an area of high noise, or changing a specific signal parameter; the presentation frequency, source level, repetition rate or duration. Another way to mitigate the effects of increasing background noise is to enhance those primary signals or signalling behaviour with a secondary signal or signalling behaviour. For example, humans enhance acoustic signals with concordant visual speech gesture information to improve the detection, or enhance the perception, of speech in a noisy environment (Grant & Seitz 2000). As far as the authors are aware, there have been no studies demonstrating the enhancement of an acoustic signal with another type of signalling behaviour when background noise increases in any animal species. To do this, the animal requires two different acoustic signals or signalling behaviours that perform the same function, but can be interchanged depending on the level of background noise. The secondary signal (or signalling behaviour) should have properties that allow better signal reception and perception in a noisy environment compared with the primary acoustic signal.
Marine mammals rely on sound for communication and the influence of background noise, anthropogenic or natural, has been a major focus of marine mammal acoustic research (Richardson et al. 1995). Humpback whales are a particularly vocal marine mammal species in that males produce long, repetitive vocalizations known as ‘songs’ (Payne & McVay 1971). Humpback whales produce a second set of acoustic signals called ‘social sounds’ (Payne 1978a,b; Tyack 1981, 1986), which are made up of social vocalizations (or non-song vocal signals) and surface-generated sounds. Early publications on these communicative sounds coined the phrase ‘social sounds’ to describe the sounds produced by surface-active groups and to suggest that these sounds were produced in a social context (Tyack 1983; Tyack & Whitehead 1983; Silber 1986). These studies focused on ‘competitive groups’, in which a number of males compete for the primary escort position in relation to a mature female and ‘social sounds’ included sounds produced from surface behaviours such as ‘head lunges’, ‘charges’ and ‘aggressive contact’ as well as other sounds such as ‘underwater blows’ and vocalizations (Tyack & Whitehead 1983; Baker & Herman 1984). ‘Social sounds’ were thought to signal aggression and agitation among competing males (Silber 1986).
Social vocalizations in humpback whales are not limited to competing males. Vocalizations have been recorded in many other social and behavioural contexts and probably convey information on signaller identity (species and sex), signaller location, size, sexual selection criteria and social integration, social context and behavioural context (Tyack 1983; Sibler 1986; Dunlop et al. 2007, 2008). Although surface-generated sounds could be viewed as acoustic signals (as we assume they are audible to the receiver), it could also be argued that they represent a visual signal when carried out within the visual range of the receiver. They include sounds from behaviours such as breaching (leaping out and body slamming into the water), flippering (repeatedly slapping one or both pectoral fins on the water surface) and lob tailing (thrashing the flukes onto the water surface) (Whitehead 1985). Although the function of surface behaviour signalling in humpback whales is not well understood, it has been suggested that breaching especially may have an important signalling role owing to the loud splash made (Herman & Tavolga 1980; Norris & Møhl 1983; Clark 1990), and be used to maintain contact within a dispersed group (Payne 1978a,b). Whitehead's (1985) subjective evaluation is that ‘a breach acts like a physical exclamation mark, to accentuate other visual or acoustic communication signals’. Studies on ‘slapping’ behaviour suggest that it serves a communicatory function, possibly as a female signal used to ‘call-in’ males, solicit competition in competitive groups or an aggressive signal between competing males (Silber 1986; Thompson et al. 1986; Clapham 2000; Nachtigal et al. 2000; Deakos 2002; Noad 2002; Wahlberg et al. 2002), or a signal used between close-by groups or members of the same group (Dunlop et al. 2008). Percussive signals in gorillas (chest beating) are used during threat displays to indicate aggression (Schaller 1963). Percussive signals in humpbacks may have similar functions. Therefore, humpback whales are known to use two different types of communication signal: vocal acoustic signals that convey detailed acoustic information, and surface-generated sounds elicited by surface behaviours that convey acoustic and possibly some limited visual information.
Humpback whales, like all marine mammals, are exposed to many sources of noise—background shipping, surf, wind and waves, and biological. Whitehead (1985) presented some limited evidence that humpback whales tend to increase the rate of breaching in increased wind speeds, and it was suggested in his review that breaching aids communication when wave noise obscures vocalizations. This study did not measure the background noise levels and did not account for vocal behaviour or other surface behaviours. The present study focuses on the effects of wind-dependent ambient noise on communication strategies and signalling behaviour used by migrating humpback whales. Wind-dependent noise is generated by surface wave breaking. Although early studies found that it is correlated with sea state (Knudsen et al. 1948), later studies showed that it correlates better with wind speed (Wenz 1962; Perrone 1969; Wille & Geyer 1984; Cato 1997). However, because there are other factors contributing to ambient background noise levels in the ocean, it is difficult to test the effects of wind-generated noise alone on humpback whale communication. Therefore, we will initially use wind speed and sea state as a proxy for background noise levels and then test the effects of measured background noise levels for comparison.
2. Material and methods
(a). Visual and acoustic data collection
Data were collected as part of the Humpback whale Acoustic Research Collaboration (HARC) during the September/October southward migrations of 2002–2004 (for detailed methods see Noad et al. 2004). The experiment was repeated in 2008. The study site was Peregian Beach, 150 km north of Brisbane on the east coast of Australia (26° S, 153° E). Land-based observations of group composition and behaviours within groups were collected daily (07.00–17.00, weather permitting) from an elevated survey point, Emu Mountain (elevation 73 m). A theodolite (Leica TM 1100) was used, in conjunction with a notebook computer running Cyclopes software (E. Kniest, University of Newcastle, Australia), to track the groups in real-time (for further details see Dunlop et al. 2007, 2008). Weather was noted by the land-based observation team every hour and included an estimation of sea state, wind speed and direction, cloud cover, visibility and rainfall. Sea state was estimated visually using the table in Richardson et al. 1995 (in the electronic supplementary material S1). Wind speeds were measured every hour from the beach using a hand-held anemometer and from an anemometer on the roof of the base station (speed and direction) every 5 min. The beach readings (considered to be the more accurate) were used to calibrate the roof readings. The wind speed and sea state were averaged over each analysis period.
Acoustic recordings were made from four or five hydrophone buoys (High Tech HTI-96-MIN hydrophone with built-in +40 dB pre-amplifier, external custom-built pre-amplifier (+20 dB) and VHF radio transmitter) moored in 18–28 m of water and arranged in a T-shape. Radio signals were received onshore at the base station and recorded in digital form with an upper frequency limit of 11 kHz (for further details on the acoustic set-up and validation, see Noad et al. 2004; Dunlop et al. 2007, 2008). Ishmael (Mellinger 2001) was used to determine the location of sound sources detected (using the differences in time of arrival of the same sound at the buoys). The system was calibrated to calculate the sound levels received in water (in dB re 1 µPa). Mt Emu and base station computers were linked in real-time using a wireless network. Humpback whale groups were tracked from the visual station using a theodolite and simultaneously tracked acoustically from the base station in real-time. The error around the group was such that acoustic localization could only be at the group level, not the individual level. However, accurate (and calibrated) acoustic tracking paired with accurate (and calibrated) theodolite tracking meant that there was no ambiguity as to which group was being recorded behaviourally and acoustically. Humpback whale groups were tracked continually visually and acoustically as they passed through the observation area (10 km radius from the base station) and since they were migrating, it is reasonable to assume that there was no duplication of groups studied.
In 2008 and 2009, four DTAGs (non-invasive, digital acoustic recording tags with depth and orientation sensors, acoustic sampling rate of 64 kHz and sensor sampling rate of 5 Hz; (Johnson & Tyack 2003)) were deployed onto four humpback whales (two mothers from two mother-calf groups (tag 1 and 3), a singleton of unknown sex (tag 2) and a mother from a mother-calf-escort group (tag 4)). Whales were tagged using a specially-equipped boat with a long pole attached at the bow. As a target whale surfaced in front of the boat, the pole was lowered to place the tag onto the back of the whale. The tags were attached by suction cups and pre-programmed to detach at a set time (usually after a period of a few hours—ideally after the whale had transited the study area). The tags contained a hydrophone, fast-lock global positioning system and three-axis inclinometers to measure pitch, yaw and roll. Tags also provided a high-quality recording of the sounds to which the whale is exposed and the vocalizations of the tagged and nearby whales. Tags 1, 2 and 4 remained attached for 3 h and tag 3 remained attached for 2 h. The (tag 1) mother–calf group joined another mother–calf group after 45 min; therefore, acoustic sampling was limited to the first 45 min as it was not possible to be sure which mother and calf group were producing the sounds. All tags contained acoustic recordings of social sounds (vocalizations and surface-active events) produced by the tagged animal.
(b). Social sound data analysis
This paper deals only with a sample of groups of humpback whales generating social sounds, and of those, only the ones generating both social vocalizations and surface-active behaviours. Post-field recordings in which social vocalizations were heard and tracked acoustically to specific groups (n = 25 in 2004, n = 6 in 2008) were divided into 5 min segments, beginning when the first social sound was audible and tracked successfully to a group, and ending either when social sounds ceased and were not heard again in that group (determined from the acoustic tracking), or when signal-to-noise ratio was poor and the sounds were difficult to detect. The minimum period of analysis per group was 10 min, the maximum was 50 min and the mode analysis period was 20 min. The mean social sound sample size per analysed group is given in table 1.
Table 1.
Average number of social sounds (with standard deviation in brackets), social vocalizations, surface-active sounds and breaches per analysed group showing both groups analysed from the array data-tagged groups.
| platform | no. groups | no. social sounds | no. breaches | no. surface-active sounds | no. vocalizations |
|---|---|---|---|---|---|
| array | 31 | 71 (75) | 8 (6) | 18 (37) | 47 (63) |
| tag | 4 | 47 (18) | 16 (6) | 16 (6) | 29 (15) |
Sounds generated by energetic surface behaviours were divided into ‘breaching’ or ‘slapping’ (repeated slapping of the pectoral flippers or the tail on the surface). Sounds of ‘breaching’ were distinguished from sounds of ‘slaps’ either by correlation with the visual observations, or, in rare cases of single ‘breaches’ not observed from Mt Emu, by the singularity of the event. ‘Repetitive slaps’ were a series of surface-active behaviours occurring in a bout. Sometimes it was hard to determine visually whether ‘slapping’ involved pectoral slapping, peduncle slapping or tail slapping (especially when groups were at a distance from the visual station). In this study, all continuous ‘repetitive slapping’ sounds were grouped together despite the fact they may have different behavioural functions.
The total number of acoustically tracked surface-active events, breaching events and social vocalizations were counted for each array and tag recording. The numbers of humpback whales (including calves) present in the observed groups and tagged groups were also counted. The rate of social sound production (vocalizations and surface-generated sounds) per minute for each group was calculated and converted to social sounds per minute per whale (rSS) by dividing by the number of whales in the group. Social sounds were separated into surface-generated sounds and social vocalizations. Breaching sounds (being the most obvious surface behaviour) were further separated from surface-generated sounds. The rate (per minute per whale) of surface-active generated sounds (rSA), rate of social vocalization production (rSV) and rate of breaching (rBR) was also calculated for each group. We also calculated the proportion of each of these to the total rate of social sounds, defined as the proportion of the total social sound production rate (per minute per whale) dedicated to vocalizations (pSV), surface-active generated sounds (pSA) and breaching (pBR).
 |
Note that while the rates rSA, rSV and rBR depend on the number of whales in a group, the proportions pSV, pSA and pBR do not, since division by the number of whales to obtain the rates cancels out in calculating the proportions.
(c). Background noise data analysis
Wind-dependent noise was measured when there were no audible and/or visually tracked vessels in the area. Recordings of humpback whale groups were eliminated from the analysis if vessels were audible in the background noise. However, in many noise samples, singing humpback whales were audible and this source of noise was difficult to eliminate, especially if background singers were present. When singers were present, song units were deleted from the recording until a 20 s noise sample was obtained that did not appear to contain any energy from song units. A 20 s noise sample was taken from each hydrophone in the array at the beginning of the analysis period and every 10 min during the analysis period. The noise in each 20 s sample was measured in one-third octave band levels (dB re 1 µPa). One-third octave bands represent the logarithmic shape of auditory filters in the mammalian ear (Fletcher 1940), and in humpback vocalizations most sound energy of the fundamental frequency is contained within a one-third octave band, making this an appropriate filter. The total background noise level over a broader frequency band was calculated by summing the sound intensity for each third octave band and converting this total sound intensity level to total broadband noise level (dB re 1 µPa). Mean broadband noise levels for each analysis period were calculated from all noise samples. By applying a bandwidth correction, third octave band levels for each sample were converted to noise spectral levels (dB re 1 µPa2 Hz−1) and mean noise spectral levels were calculated.
(d). Statistical analysis
For further details on the statistical analysis, see the electronic supplementary material S2. Response variables (total number of social sounds per minute per whale, rate of production of vocalizations, surface behaviour sounds and breaching sounds, proportion of vocalizations, surface behaviour sound events and breaching sounds) were square-root transformed for normalization (see Zar 1998). The social sound response with sea state was tested using an analysis of variance model (with the fixed effect of sea state), and a linear regression analysis (see Zar 1998) was used to test the effect of mean wind speed, mean broadband levels and mean third octave broadband levels. A validation analysis (using the 2004 dataset to create the linear model and the 2008 dataset as the test sample) was used to support the results of the regression analysis (see Lewicki & Hill 2006). The predicted values (with 95% confidence limits) from the 2004 model were compared with the observed dependent values for the 2008 dataset to test if the 2004 linear model holds true for subsequent datasets. A ‘best subset’ stepwise regression model (using all one-third octave band noise levels as explanatory variables) was used to determine which combination of frequency bands of noise accounted best for the observed variation in the social sound repertoire of humpback whales. The critical p-value (for entry or removal of explanatory variable from the model) was 0.05 (see Lewicki & Hill 2006). The adjusted r2-value (adjusted to the number of explanatory variables in each model) and mean square residual was used to select the best model.
3. Results
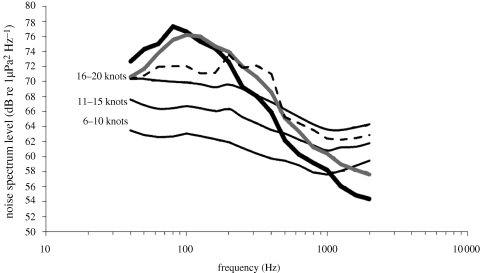
Figure 1 shows the mean spectrum levels of noise received in the absence of singer noise and vessel noise, mean background noise levels including singer noise (in the absence of vessel noise) and mean spectrum levels of social vocalizations and surface active sounds.
Figure 1.
Mean noise spectrum levels (dB re 1 µPa2 Hz−1) for wind speeds of 6–10 knots, 11–15 knots and 16–20 knots, mean spectrum levels for background noise including singer noise (dashed line) and mean spectral received levels of social vocalizations (thick solid dark grey line) and surface-active sounds (thick solid light grey line).
The mean spectra of the vocalizations and surface active sounds were measured only from groups that produced both; therefore, the graph indicates that both types of sounds would be detectable to similar distances. The measured noise in the absence of singer and vessel noise is similar to that generally observed for wind-dependent noise in Australian waters up to 800 Hz, showing the general decrease in noise level with increasing frequency (Cato 1996). Above 800 Hz, the spectra increase with frequency and are consistent with the noise of snapping shrimps that were audible in the recordings. Consequently, only the spectra between 40 and 800 Hz are considered to be wind-dependent noise. Estimates of ‘broadband’ wind-dependent noise were therefore limited to the frequency range of 40 and 800 Hz to limit the contribution of shrimp noise. Most of the energy of humpback whale vocalizations lies within this frequency band. Wind speed significantly correlated with noise levels in all third octave frequency bands (p < 0.05; correlation Z-test). Broadband wind-dependent noise levels (40–800 kHz) varied from a minimum of 88 dB re 1 µPa to a maximum of 100.2 dB re 1 µPa. At a wind speed of 6–10 knots, the mean level averaged 90.8 dB re 1 µPa (standard deviation (s.d.) 1.5). At wind speeds of 11–15 knots, it was 94.1 dB re 1 µPa (s.d. = 3.2) and at wind speeds of 16–20 knots, 96.3 dB re 1 µPa (s.d. = 2.9).
The mean broadband received levels (rms) over the frequency band 40–2000 Hz of received surface-generated sounds in this study was 103.2 dB re 1 µPa (s.d. = 9.9) and vocalizations was 104.5 dB re 1 µPa (s.d. = 9.7); mean peak levels were found to be 124.9 dB re 1 µPa (s.d. = 4.9) and 125.9 dB re 1 µPa (s.d. = 4.2) for surface sounds and vocalizations, respectively. Social vocalizations and surface-behaviour sounds were heard out to similar distances from the array (about 7 km in low winds and about 4–5 km in high winds).
(a). Social sound behaviour in increasing wind-generated noise
The rate of social sound production (per minute per whale), rate of social vocalizations, rate of surface-generated sounds or rate of breaching showed wide variations with wind speed, sea state or broadband ambient noise levels. No significant trend was detected with rates of breaching or rates of vocal sounds, but a trend was detected with rates of surface-generated sounds (p = 0.017; r2 = 0.17), though the low r2-value indicates a large amount of variance around the trend line.
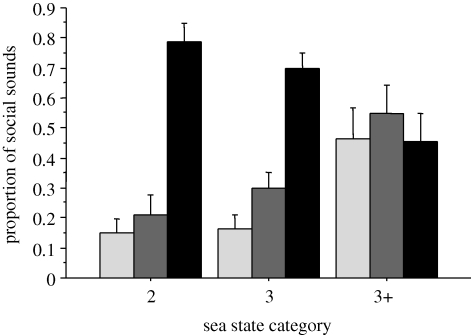
However, the proportions of rates of the different social sound types to the total social sound rate did vary significantly with these environmental variables. All groups recorded using the array (excluding the tagged data; n = 31) were tested together. The proportion of the social sounds dedicated to surface-active events significantly increased with increasing sea state and the proportion dedicated to vocalizations significantly decreased (F3,30 = 5.85, p < 0.01; figure 2). The proportion of ‘breaching’ social sounds also significantly increased with increasing sea state (F3,30 = 5.91, p<0.01; figure 2). The mean proportion of surface-active and breaching events for all tagged groups lies within the mean (+s.d.) found in groups recorded using the array (as shown in the electronic supplementary material S3).
Figure 2.
Mean (±s.e.m.) (untransformed) proportion of breaches (light grey bars), social vocalizations (black bars) and surface-active behaviours (dark grey bars) in the social sound activity budget for humpback whale groups present in a range of sea states.
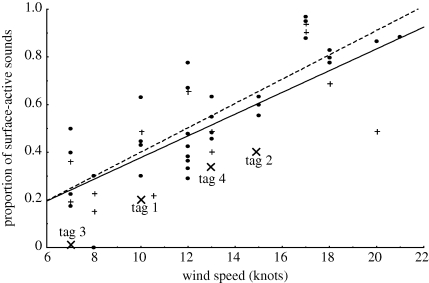
The linear regression analysis revealed a strong positive linear relationship between the proportion of surface-active social sounds and wind speed (p < 0.0001, r2 = 0.666; figure 3) and strong negative linear relationship between the proportion of vocalizations and wind speed (p < 0.0001, r2 = 0.619). A weaker relationship was found between the proportion of the social sounds from breaching (p < 0.01, r2 = 0.279). A previous study found that breaching behaviour was more predominant in single animals (Dunlop et al. 2008). We consequently limited the analysis of breaching sounds to singleton animals only and found a strong positive linear relationship between the proportion of breaching sounds and wind speed (p < 0.0001; r2 = 0.787) in these individuals (figure 3).
Figure 3.
Graph showing a significant positive relationship between the wind speed (knots) and the (untransformed) proportion of surface-active behaviours (dashed line and circles) in all analysed groups and breaching (solid line and plus signs) (singletons only) in the social sound budget. Tagged animals are demarcated by crosses.
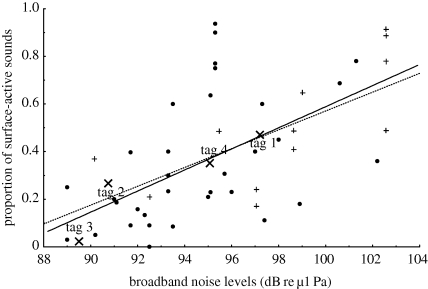
Background noise level (not including noise from vessels in the area and noise from singers in the area) was also found to be a predictor of the proportion of the types of social sounds in humpback whales. Using the entire dataset (i.e. including tag data), a significant positive relationship was found between the proportion of surface-active sounds and broadband background noise levels over the frequency band of 40–800 Hz (figure 4). However, the r2-value generated for this relationship (p < 0.01, r2 = 0.253) was much less when compared with that found for the relationship with wind speed. The proportion of ‘breaching’ sounds in all groups was not significantly related to background noise levels but the proportion of breaching ‘singletons’ significantly increased in increasing levels of background noise (p < 0.01; r2 = 0.655; figure 4).
Figure 4.
Graph showing a significant positive relationship between broadband (40–2000 Hz) noise levels (dB re µ1 Pa) and the (untransformed) proportion of surface-active behaviours (dotted line and circles) in the social sound budget for all analysed groups of animals and proportion of breaching (solid line and plus signs) (singletons only). Tagged animals are demarcated by crosses.
To test the predictive function of the wind and background noise linear models, we used the 2004 dataset as the ‘analysis’ dataset (n = 25) and the 2008 plus both tag datasets as the ‘test’ sample (n = 10). Using the 2004 dataset as a representative model is valid as there was no significant difference in the regression coefficients or intercepts for the 2004 dataset and the full dataset. The predicted values (with 95% CI) for the 2008 data points were generated using the 2004 model. Only one observed 2008 data point did not lie within the 95% CI for predicted observations.
A ‘best subset’ stepwise regression model was used to determine which combination of frequency bands best accounted for the variability in the proportion of each type of social sound. The model was developed for noise levels in all one-third octave band levels between 40 and 500 Hz. The proportion of surface behaviour sounds was used as the predictor variable. Initially, we included all groups in this analysis. The best model included third octave broadband noise levels between 50 and 500 Hz (table 2) after removing those at 100, 125, 200 and 315 Hz. ‘Moans’, which were the most common unit in the song had a fundamental frequency of 315 Hz; lower frequency sounds such as ‘groans’ and ‘grumbles’ had fundamental frequencies ranging from approximately 100–200 Hz, so sounds from distant singers may have contributed to the background noise so that it was not entirely wind-dependent noise. Limiting the (multiple) regression analysis to include only those one-third octave bands highlighted in the model produced a similar relationship between noise and social sound use to that found for wind speed and social sound use (table 2). The multiple regression analysis was repeated for the 2004 dataset (using only the one-third octave bands highlighted previously) and results were used to predict values for the 2008 and tag data. All observed values for the test dataset (n = 10) lay within the 95% CI for the predicted values generated using this model.
Table 2.
Results of the best subset stepwise regression model showing the best combination of frequency bands to account for the observed variation in the dependent data and results of the multiple regression analysis using the highlighted frequency bands as predictor variables.
| independent data | dependent data | adjusted r2 | F-value | p-value | included parameters |
|---|---|---|---|---|---|
| one-third octave broadband noise 40–2000 Hz | surface-active behaviour, all groups | 0.610 | 8.55 | <0.0001 | 40, 50, 63, 80, 160, 250, 400, 500 |
| one-third octave broadband noise 40–2000 Hz | breaching behaviour, singletons | 0.742 | 6.92 | 0.005 | 50, 63, 80 |
4. Discussion
This study found that in conditions of increased wind speed, sea state and wind-dependent background noise levels, humpback whales gradually switched from using predominantly vocal to predominantly surface-generated acoustic signals. There was no evidence that humpback whales compensated for increased background noise levels by increasing the rate of presentation of vocal signals, although there was evidence of an increase in the rate of surface sounds (with a large amount of variance). With the obvious exception of singletons, we could not locate sound to a specific member of the group; therefore, the rate analysis assumed that all animals within the group were contributing equally to the social sound production. The rates of production of social sounds were calculated per whale (rather than per group) as a way of standardizing between different group sizes. Perhaps, this sampling bias accounts for some of the spread of data points (and the low r2-values) in the regression models for rates. Furthermore, it is likely that rates of social sound production are influenced by social context and behavioural state of the source and receiver groups adding another source of variation in the rate of social sound production that is probably independent of wind speed or noise. However, the relative proportions of vocalizations and surface-active sounds correlate much better with noise and wind speed. One reason for this may be that the proportions of the types of sounds are independent of whether the rates are measured per whale or per group (since division by the number of whales in a group in determining the rates cancels out when the proportions are calculated). Another reason may be that variations in behavioural state may be similar in both the rates of vocalizations and surface-generated sounds, so that the variation in rates with changes in behaviour will be less evident in the regression of proportions on wind speed or noise. Therefore, the behavioural source of variance may be compensated for by using proportions.
It has been hypothesized by a number of authors that surface behaviours in humpbacks perform some sort of communicative function (Herman & Tavolga 1980; Tyack 1981, 1983; Clark 1983; Norris & Møhl 1983; Whitehead 1985; Silber 1986; Thompson et al. 1986; Clapham 2000; Deakos 2002; Noad 2002; Dunlop et al. 2008), though definitive evidence is limited. Sibler (1986) observed an increase in both the rate of vocalizations in conjunction with an increase in the rate of visual surface displays when new whales joined the group and Deakos (2002) also found an increase in surface displays (mainly pectoral slapping) during social interactions. Thompson et al. (1977) reported vocalizations in tandem with blowhole-associated sounds and surface impacts on feeding grounds. Some surface-generated behaviours such as body thrashing, tail lashing and lob tailing have been designated aggressive signals during threat displays in agonistic encounters (Tyack 1981, 1983; Tyack & Whitehead 1983; Silber 1986; Deakos 2002). Particular vocal signals such as ‘underwater blows’ have also been heard primarily in competitive groups, suggesting these sounds may also convey aggression (Tyack 1981; Tyack & Whitehead 1983; Silber 1986; Dunlop et al. 2008). Therefore, previous studies of vocal and surface-generated sounds have provided evidence of similarity in their contextual use, although it is unlikely that surface signals possess the amount of information content that vocalization signals do.
This paper also shows that the proportion of surface-active sounds increases and the proportion of vocalizations decreases as noise level and wind speed rise. This inter-relation suggests some commonality of function for surface-active sounds and vocalizations, further strengthening the support for the hypothesis that surface-active sounds are a means of communication. Figure 1 shows that the frequency range covered by surface-active sounds is similar to that covered by social vocalizations. The spectrum levels are the mean values measured only from groups producing both surface-active sounds, so that both types of sounds have similar received levels. Thus, the sounds of surface-active behaviour provide information about the behaviour of the sender, which are likely to be audible at broadly similar distances as vocalizations, whether or not this is intended and whether or not it is used by a receiving humpback whale. It would seem likely that if the information sent and available for reception correlates with behaviour, then it would be exploited. Perhaps, these surface sounds are used as non-vocal ‘attention’ signals, in a way similar to non-vocal (drumming) signals in monkeys (Remedios et al. 2009) or ‘bark’ signals in wild canines (Mitchell et al. 2006).
With the exception of extremely ‘quiet’ conditions (in which sounds were sometimes exclusively vocal), both sets of signals were found to be present in the social sound repertoire of each analysed group of whales. The results suggest that humpback whales supplement vocal signals with surface-generated signals in periods of high background noise. This behaviour could be related to human enhancement of acoustic signals with concomitant visual gestures to improve signal perception, as gestures are more easily received and/or perceived given that they are not masked within the background noise (Grant & Seitz 2000). Similarly, in humpback whales, surface-generated signals may have an added visual component. The type of surface behaviour, such as pectoral slapping, lob tailing or tail slapping may be an added distinguishing feature, which enhances receiver perception of the vocal signal in periods of increased ambient noise. However, this may only be applicable to groups of animals, such as competitive groups, in which the group members are within the visual range of each other.
In this study, proportions of whale social sounds were more closely related to wind speed than to our estimates of wind-dependent background noise levels. The stepwise model generated for the proportion of surface-active sounds was most closely determined by noise levels contained in the lower frequency range (40–500 Hz). When the four one-third octave noise bands 100, 125, 200 and 315 Hz were excluded, the relationships were as good as the one with wind speed. These excluded the one-third octave bands that contained the dominant frequencies of the more prominent sounds in the humpback whale song. Although data with clear contribution from song were not included in the analysis, it was not possible to obtain data that were completely free of song (at any one time there was usually at least one background singer). The improvement of correlations by removing the one-third octave bands with the highest song components suggests that it is the wind-dependent component of background noise without the contribution from the song that the whales are responding to.
This study emphasizes the need to take other behaviours into account when determining the effects of increased background noise levels on acoustic communication. A common response of animals in periods of increasing background noise is to modify their acoustic signals; but this study shows that humpback whales also change the type of signal with increasing wind-generated background noise levels. This is important when considering the response of animals not only to increases in natural background noise, but begins to provide a biological context with which to compare the response to increases in anthropogenic noise and other biological noise sources, such as conspecific singer noise.
Acknowledgements
Ethics approval for the experiment came from the University of Queensland Animal Ethics Committee.
This work was supported by the US Office of Naval Research, the Australian Defence Science and Technology Organisation and the Australian Marine Mammal Center. The authors thank everyone involved in the Humpback Acoustic Research Collaboration (HARC), in particular Dale Stokes, Patrick Miller, Nicoletta Biassoni, John Noad, Melinda Rekdahl, Josh Smith and the numerous volunteers who donated their time and energy to this project. We also thank David Paton for his invaluable field expertise and Eric Kniest for his continued support in the development of Cyclopes. The authors would also like to thank Michael Bryden for taking the time to review earlier drafts.
References
- Baker C. S., Herman L. M.1984Aggressive behavior between humpback whales (Megaptera novaeangliae) wintering in Hawaiian waters. Can. J. Zool. 62, 1922–1937 [Google Scholar]
- Brumm H., Voss K., Kollmer I., Todt D.2004Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 207, 443–448 (doi:10.1242/jeb.00768) [DOI] [PubMed] [Google Scholar]
- Cato D. H.1996Ambient sea noise in waters near Australia. J. Acoust. Soc. Am. 60, 320–328 [Google Scholar]
- Cato D. H.1997Features of ambient noise in shallow water. In Shallow water acoustics (eds Zhang R., Zhou J.), pp. 385–390 Beijing, China: China Ocean Press [Google Scholar]
- Clapham P. J.2000The humpback whale. In Cetacean Societies. Field studies of dolphins and whales (eds Mann J., O'Connor R., Tyack P. L., Whitehead H.), pp. 173–198 London, UK: The University of Chicago Press [Google Scholar]
- Clark C. W.1983Acoustic communication and behavior of the southern right whale (Eubalaena australis). In Communication and behavior of whales (ed. Payne R.), pp. 163–198 Boulder, CO: Westview Press [Google Scholar]
- Clark C. W.1990Acoustic behaviour of mysticete whales. In Sensory abilities of cetaceans (eds Thomas J., Kastelein R. R.), pp. 571–583 New York, NY: Plenum Press [Google Scholar]
- Cynx J., Lewis R., Tavel B., Tse H.1998Amplitude regulation of vocalizations in noise by a songbird, Taeniopygia guttata. Anim. Behav. 56, 107–113 (doi:10.1006/anbe.1998.0746) [DOI] [PubMed] [Google Scholar]
- Deakos M. H.2002Humpback whale (Megaptera novaeangliae) communication: the context and potential functions of pec-slapping behavior on the Hawai'ian wintering grounds. Masters thesis, University of Hawaii, Manoa [Google Scholar]
- Dunlop R. A., Noad M. J., Cato D. H., Stokes D.2007The social vocalization repertoire of east Australian migrating humpback whales (Megaptera novaeangliae). J. Acoustic. Soc. Am. 122, 2893–2905 (doi:10.1121/1.2783115) [DOI] [PubMed] [Google Scholar]
- Dunlop R. A., Cato D. H., Noad M. J.2008Non-song acoustic communication in migrating humpback whales (Megaptera novaeangliae). Mar. Mamm. Sci. 24, 613–629 (doi:10.1111/j.1748-7692.2008.00208.x) [Google Scholar]
- Feng A. S., Narins P. M., Xu C. H., Lin W. Y., Yu Z. L., Qiu Q., Xu Z. M., Shen J. X.2006Ultrasonic communication in frogs. Nature 440, 333–336 (doi:10.1038/nature04416) [DOI] [PubMed] [Google Scholar]
- Fletcher H.1940Auditory patterns. Rev. Mod. Phys. 12, 47–65 (doi:10.1103/RevModPhys.12.47) [Google Scholar]
- Foote A. D., Nystuen J. A.2008Variation in call pitch among killer whale ecotypes. J. Acoustic. Soc. Am. 123, 1747–1752 (doi:10.1121/1.2836752) [DOI] [PubMed] [Google Scholar]
- Grant K. W., Seitz P. F.2000The use of visible speech cues for improving auditory detection of spoken sentences. J. Acoustic. Soc. Am. 108, 1197–1208 (doi:10.1121/1.1288668) [DOI] [PubMed] [Google Scholar]
- Herman L. M., Tavolga W. N.1980The communication systems of cetaceans. In Cetacean behavior: mechanisms and functions (ed. Herman L. M.), pp. 149–209 New York, NY: John Wiley & Sons Inc [Google Scholar]
- Holt M. M., Noren D. P., Veirs V., Emmons C. K., Veirs S.2009Speaking up: killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoustic. Soc. Am. 125, EL27–EL32 (doi:10.1121/1.3040028) [DOI] [PubMed] [Google Scholar]
- Johnson M. P., Tyack P. L.2003A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean. Eng. 28, 3–12 (doi:10.1109/JOE.2002.808212) [Google Scholar]
- Jouventin P., Aubin T., Lengagne T.1999Finding a parent in a king penguin colony: the acoustic system of individual recognition. Anim. Behav. 57, 1175–1183 (doi:10.1006/anbe.1999.1086) [DOI] [PubMed] [Google Scholar]
- Knudsen V. O., Alford R. S., Emiling J. W.1948Underwater ambient noise. J. Mar. Res. 7, 410–429 [Google Scholar]
- Lewicki P., Hill T.2006Statistics: methods and applications: a comprehensive reference for science, industry and data mining Tulsa, OK: StatSoft Inc [Google Scholar]
- Lombard E.1911Le signe de l'elevation de la voix. ANN. MAL. OREIL. Larynx 37, 101–199 [Google Scholar]
- Mellinger D. K.2001Ishmael 1.0 User's Guide. NOAA Technical Memorandum, pp. 120 OAR PMEL [Google Scholar]
- Mitchell B. R., Makagon M. M., Jaeger M. M., Barrett R. H.2006Information content of coyote barks and howls. Bioacoust: Int. J. Anim. Sound Record. 15, 289–314 [Google Scholar]
- Morisaka T., Shinohara M., Nakahara F., Akamatsu T.2004Ambient noise differences may cause the geographical variations on the acoustic signals of the Indo-Pacific bottlenose dolphin (Tursiops aduncus) populations. Proc. Int. Congress on Acoustics, 4–9 April 2004, Kyoto, Japan [Google Scholar]
- Nachtigal P. E., Au W. W. L., Pawloski J. L., Andrews K., Oliver C. W.2000Measurements of the low frequency components of active and passive sounds produced by dolphins. Aquat. Mammals 26, 167–174 [Google Scholar]
- Noad M. J.2002The use of song in humpback whales (Megaptera novaeangliae) during migration off the east coast of Australia. PhD thesis, Faculty of Veterinary Science, University of Sydney, Sydney, Australia [Google Scholar]
- Noad M. J., Cato D. H., Stokes M. D.2004Acoustic tracking of humpback whales: measuring interactions with the acoustic environment. In Proc. of Acoustics 2004 Meeting of the Australian Acoustical Society, 3–5 November 2004, pp. 353–358 [Google Scholar]
- Norris K. S., Møhl B.1983Can odontocetes debilitate prey with sound? Am. Nat. 122, 85–104 (doi:10.1086/284120) [Google Scholar]
- Ord T. J., Peters R. A., Clucas B., Stamps J. A.2007Lizards speed up visual displays in noisy motion habitats. Proc. R. Soc. B 274, 1057–1062 (doi:10.1098/rspb.2006.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S. E., Clark C. W., Tyack P. L.2007Short- and long-term changes in right whale calling behavior: the potential effects of noise on acoustic communication. J. Acoustic. Soc. Am. 122, 3725–3731 (doi:10.1121/1.2799904) [DOI] [PubMed] [Google Scholar]
- Payne R. S.1978aBehavior and vocalizations of humpback whales (Megaptera sp.). In Report on a workshop on problems related to humpback whales (Megaptera novaeangliae) in Hawai'i (eds Norris K. S., Reeves R. R.), pp. 56–78 NTIS, PB- 280 794, Report No. MMC-77/03 [Google Scholar]
- Payne R.1978bBehavior and vocalizations of humpback whales (Megaptera sp.). In Report on a workshop on problems related to humpback whales (Megaptera novaeangliae) in Hawaii (eds Norris K. S., Reeves R. R.), pp. 56–78 NTIS, PB-280 794, Report No. MMC-77/03, appendix 6 [Google Scholar]
- Payne R. S., McVay S.1971Songs of humpback whales. Science 173, 585–597 (doi:10.1126/science.173.3997.585) [DOI] [PubMed] [Google Scholar]
- Perrone A. J.1969Deep-ocean ambient-noise spectra in the Northwest Atlantic. J. Acoustic. Soc. Am. 46, 762–770 (doi:10.1121/1.1911759) [Google Scholar]
- Potash L. M.1972A signal detection problem and possible solution in Japanese quail (Coturnix coturnix japonica). Anim. Behav. 20, 192–195 (doi:10.1016/S0003-3472(72)80191-X) [Google Scholar]
- Remedios R., Logothetis N. K., Kayser C.2009Monkey drumming reveals common networks for perceiving vocal and nonvocal communication sounds. Proc. Natl Acad. Sci. USA 106, 18 010–18 015 (doi:10.1073/pnas.0909756106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. J., Greene C. R., Jr, Malme C. I., Thomson D. H.1995Marine mammals and noise San Diego, CA: Academic Press [Google Scholar]
- Schaller G.1963The mountain gorilla Chicago, IL: University of Chicago Press [Google Scholar]
- Schaub A., Ostwald J., Siemers B. M.2008Foraging bats avoid noise. J. Exp. Biol. 211, 3174–3180 (doi:10.1242/jeb.022863) [DOI] [PubMed] [Google Scholar]
- Silber G. K.1986The relationship of social vocalizations to surface behaviour and aggression in the Hawaiian humpback whale (Megaptera novaeangliae). Can. J. Zool. 64, 2075–2080 [Google Scholar]
- Thompson P. O. W., Cummings W. C., Kennison S. J.1977Sound production of humpback whales. J. Acoustic. Soc. Am. 62, S89 (doi:10.1121/1.2016437) [Google Scholar]
- Thompson P. O. W., Cummings W. C., Ha S. J.1986Sounds, source levels, and associated behaviour of humpback whales, southeast Alaska. J. Acoustic. Soc. Am. 80, 735–740 (doi:10.1121/1.393947) [DOI] [PubMed] [Google Scholar]
- Tyack P.1981Interactions between singing Hawaiian humpback whales and conspecifics nearby. Behav. Ecol. Sociobiol. 8, 105–116 (doi:10.1007/BF00300822) [Google Scholar]
- Tyack P. L.1983Differential response of humpback whales, Megaptera novaeangliae, to playback of song or social sounds. Behav. Ecol. Sociobiol. 13, 49–55 (doi:10.1007/BF00295075) [Google Scholar]
- Tyack P. L.1986Population biology, social behaviour and communication in whales and dolphins. Trends Evol. Ecol. 1, 144–150 (doi:10.1016/0169-5347(86)90042-X) [DOI] [PubMed] [Google Scholar]
- Tyack P., Whitehead H.1983Male competition in large groups of wintering humpback whales. Behaviour 83, 132–154 (doi:10.1163/156853982X00067) [Google Scholar]
- Wahlberg M., Lunneryd S. G., Westerberg H.2002The source level of harbour seal flipper slaps. Aquat. Mamm. 28, 90–92 [Google Scholar]
- Wenz G. M.1962Acoustic ambient noise in the ocean: spectra and sources. J. Acoustic. Soc. Am. 34, 1936–1956 (doi:10.1121/1.1909155) [Google Scholar]
- Whitehead H. P.1985Humpback whale breaching. Invest. Cetacea 17, 117–155 [Google Scholar]
- Wille P. C., Geyer D.1984Measurements on the origin of the wind-dependent ambient noise variability in shallow water. J. Acoustic. Soc. Am. 75, 173–185 (doi:10.1121/1.390411) [Google Scholar]
- Zar J. H.1998Biostatistical analysis Upper Saddle River, NJ: Prentice Hall International, Inc [Google Scholar]