Abstract
Mammals contribute to important ecosystem processes and services, but many mammalian species are threatened with extinction. We compare how global patterns in three measures of mammalian diversity—species richness, phylogenetic diversity (PD) and body mass variance (BMV)—would change if all currently threatened species were lost. Given that many facets of species' ecology and life history scale predictably with body mass, the BMV in a region roughly reflects the diversity of species' roles within ecosystems and so is a simple proxy for functional diversity (FD). PD is also often considered to be a proxy for FD, but our results suggest that BMV losses within ecoregions would be much more severe than losses of PD or species richness, and that its congruence with the latter two measures is low. Because of the disproportionate loss of large mammals, 65 per cent of ecoregions would lose significantly more BMV than under random extinction, while only 11 per cent would lose significantly more PD. Ecosystem consequences of these selective losses may be profound, especially throughout the tropics, but are not captured by PD. This low surrogacy stresses a need for conservation prioritization based on threatened trait diversity, and for conservation efforts to take an ecosystem perspective.
Keywords: body mass, extinction, functional diversity, mammals, phylogenetic diversity
1. Introduction
The recently published Global Mammal Assessment emphasized that human actions jeopardize many mammalian species: globally, 1141 species (21% of all mammals) are now threatened with extinction (IUCN 2008). These assessments count each species' loss equally, but species differ in many ways, such as biological traits, ecosystem roles and evolutionary age. Conservation prioritization on the basis of species numbers alone does not capture all aspects of evolutionary history, phylogenetic diversity (PD) or ecosystem roles (Vane-Wright et al. 1991; Millennium Ecosystem Assessment 2005). While it is clear that some species are more ecologically important because ecosystem processes depend on their biological traits, the magnitude of functional-trait loss may not be easily predicted from either species or PD loss (Jernvall & Wright 1998; Gross & Cardinale 2005; Hooper et al. 2005). Our study therefore aims to map the global loss of evolutionary history as well as changes of the variance in a key species trait, body size, if all currently threatened mammalian species were to go extinct.
Their comparatively large range and body sizes mean that mammals have impacts on ecosystems over large spatial scales. Mammalian seed dispersers, predators and herbivores have been shown to directly or indirectly influence invertebrate and plant community structure, primary productivity and nutrient cycling, suggesting that species loss would impact ecosystem properties and functioning (Asquith et al. 1997; Terborgh et al. 2001; Pringle et al. 2007; Johnson 2009). Mammals are of special conservation concern because they are charismatic, they provide recreational value and their populations are declining rapidly (Collen et al. 2009). They are also of direct economic importance in most areas of the world as sources of food and income from meat, fur and tourism (Milner-Gulland et al. 2003).
Large mammals are disproportionately at risk, especially in the tropics (Cardillo et al. 2005; Fritz et al. 2009). Size or body mass is an important predictor of many ecological traits in mammals, and an indicator of a species' ecological niche (Western 1979; Eisenberg 1981). Species of different sizes generally fulfil different ecosystem functions: for example, the largest mammals are wide-ranging herbivores or carnivores, whereas small mammals are often insectivores or seed dispersers. Preferential extirpation of large species can lead to disproportionate fast loss of functional diversity (FD; Petchey & Gaston 2002). We therefore investigate the impacts of selectivity in the current extinction risk in terms of the variance in log10-transformed body mass (BMV), using ecoregions as spatial units. Given the links between body size and ecology in mammals, the change in BMV for these large-scale ecosystem units can be interpreted as a simple and practicable, if rough, indicator for possible changes in FD (see Mason et al. 2003 for a similar index).
Measuring FD accurately within an area is extremely difficult, and comparing FD across different ecosystems is even harder, because functional group definitions or complex ecological distance measures are problematic at the global scale and require vast trait databases (Petchey & Gaston 2006). Evolutionary history or PD is sometimes used as a proxy for FD (Faith 1992; Forest et al. 2007), because it is an indirect measure of phenotypic diversity if traits evolve along the branches of phylogeny. However, PD and FD of a community can be uncorrelated, so PD loss cannot always reliably predict FD loss (Jernvall & Wright 1998; Hooper et al. 2005). Our aim was to compare possible losses of mammalian diversity using three diversity measures: species richness, PD and BMV, where both PD and BMV can be seen as tentative measures of mammalian FD.
Our analyses combined a large dataset of 4230 mammalian species body mass values (Jones et al. 2009) with geographical range maps (IUCN 2008) and a species-level phylogeny (Bininda-Emonds et al. 2007; Fritz et al. 2009), and used phylogenetically explicit methods to account for bias caused by terminal polytomies and by the lack of body-mass estimates for many small species. We compared current levels of diversity with those projected if either all threatened species on the IUCN Red List were to go extinct, or all of these plus species classified as Near Threatened (IUCN 2008). We also simulated random species loss within ecoregions to identify the areas where disproportionately high losses of mammalian PD and BMV would be incurred because of the selectivity of anthropogenic threats.
2. Material and methods
The spatial units of our analyses, the World Wildlife Fund (WWF) terrestrial ecoregions, are biogeographically coherent areas defined on the basis of natural assemblages (Olson et al. 2001). They are widely used in conservation planning (but see Jepson & Whittaker (2002) for a critique). Their large spatial scale ameliorates errors in species distribution maps (Jetz et al. 2008), and is more appropriate for investigations of ecosystem functioning than, for example, a fine global grid. We overlaid the ecoregion shapefiles (www.worldwildlife.org/science/ecoregions/item1267.html, accessed August 2006) with mammal range maps from the Global Mammal Assessment (GMA; IUCN 2008), and extracted ecoregion occurrences for each species.
We excluded domesticated species and range parts labelled as ‘historical’, ‘presence uncertain’, ‘introduced origin’ or ‘extinct’. GMA maps and risk data were matched to the taxonomy of the body mass data and phylogeny (Wilson & Reeder 2005); hence, 130 species that were absent from this taxonomy were excluded. A total of 56 species ranges were added to the set by splitting existing GMA maps, and 40 species ranges missing from the GMA set were added from Jones et al. (2009). Excluding species classified as Data Deficient, Extinct or Extinct in the Wild, our final range dataset contained 4230 species: 2939 of these were ranked as Least Concern, 306 as Near Threatened and 985 species were in one of the three threatened categories (Vulnerable, Endangered and Critically Endangered; IUCN 2008).
The software R (v. 2.7.2) was used for all analyses (R Development Core Team 2008). We used log10-transformed body mass throughout, because its distribution better approximates the normal distribution; also, the logarithmic transformation reflects the fact that the biological impact of a given difference in body mass (e.g. 10 g) will be much greater for small species than for large ones. Body mass values for 3382 of the 4230 species (80%) came from the PanTHERIA dataset (Jones et al. 2009). As large species are better known, the dataset is biased towards them; we therefore interpolated body mass values for the remaining species as the value of their closest relative (or the mean of several equally close relatives), on the basis of a supertree of 5020 extant mammalian species (Bininda-Emonds et al. 2007; Fritz et al. 2009). The mean of the resulting frequency distribution of log10-transformed body mass values was indeed significantly lower than the original mean, confirming the need to interpolate missing data to avoid bias (electronic supplementary material, figure S1; mean log10-transformed body mass with interpolated values = 2.15; mean without interpolated values = 2.22; t-test: t = −2.64, d.f. = 7155, p < 0.01). We excluded three species-poor ecoregions from all analyses because 50 per cent or more of their body mass values were interpolated. There was strong spatial pattern in the number and proportion of species for which body mass data were interpolated (see the electronic supplementary material, figure S2).
Ecoregion PD was calculated using the total sum of branch lengths in an ecoregion phylogeny (Faith 1992), initially directly derived from the species-level supertree (Bininda-Emonds et al. 2007; Fritz et al. 2009). PD measured on this tree is inflated because of its polytomies, which mostly reflect the lack of resolution rather than the real speciation pattern; we corrected for this following Davies et al. (2008) (see electronic supplementary material for details). We mapped each of the three diversity measures within ecoregions (species richness, PD and BMV) for all species, Least Concern and Near Threatened species (corresponding to the loss of currently threatened species), and just Least Concern species (for the loss of all species whose status is at least Near Threatened).
Congruence of estimated proportional losses was assessed with Pearson correlation coefficients. Because of spatial autocorrelation in our ecoregion dataset, we did not test these for significance: spatial non-independence inflates degrees of freedom in statistical testing, but the coefficients themselves are thought to be unbiased measures of the correlation strength (Legendre 1993). To simulate PD and BMV losses expected if threatened species were a strictly random subset of an ecoregion's species, we used 1000 shuffles of threat status data within each ecoregion; these randomizations therefore preserved the spatial non-randomness in extinction risk prevalence, while simulated risk was random with respect to body mass and phylogeny within each ecoregion. Significance tests were one-tailed (proportion of random values that were smaller than the observed value).
3. Results
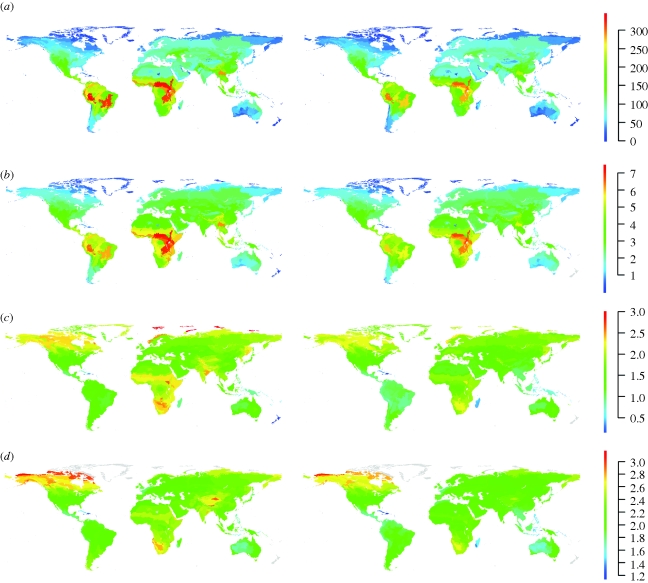
Our results suggest that extinction of all but currently Least Concern species would reduce BMV more dramatically than either species richness or PD. Some current ‘hotspots’ of BMV would completely vanish, while areas of high current species richness or PD would retain comparatively high values (figure 1). Maps of mean body mass within ecoregions suggested that most of the large reduction in variance was owing to selective losses of large species (figure 1d). Geographical patterns of losses for all diversity measures were similar whether species classified as Near Threatened were also lost or not (see the electronic supplementary material, figure S3), so we concentrated on the more severe scenario. Absolute losses for all three diversity measures would be highest in the Indo-Malayan realm, with the Afrotropics and Neotropics also strongly affected (figure 1; table 1). If all currently threatened and Near Threatened species were to go extinct, each ecoregion would lose a global average of 14 species (on an average 15% of its current species), 283 million years of evolutionary history (9%), and 14% of its current body-mass variety (table 1).
Figure 1.
Mammalian diversity within ecoregions for all species (left column) and just species currently rated as Least Concern (right column): (a) species richness, (b) phylogenetic diversity (PD), (c) variance in log10-transformed body mass (BMV) and (d) mean log10-transformed body mass. PD (in billions of years) was the total sum of branch lengths for the ecoregion phylogeny modified to account for terminal polytomies (see §2). Mean log10-transformed body mass within ecoregions is shown only for ecoregions with greater than or equal to 20 species.
Table 1.
Global and within-realm means and standard errors for absolute and proportional losses of mammalian species richness, phylogenetic diversity (PD) and variance in log10-transformed body mass (BMV) within ecoregions. (For PD and BMV, percentages of ecoregions with significantly non-random losses are also given. All diversity changes are assuming that species currently listed as threatened or Near Threatened will go extinct; negative numbers indicate diversity losses. Significance of loss within each ecoregion was assessed using a one-tailed test based on 1000 permutations for random species loss of the same magnitude as the actual loss.)
| species richness |
phylogenetic diversity (Myr) |
variance in log10-transformed body mass |
||||||
|---|---|---|---|---|---|---|---|---|
| absolute | proportion | absolute | proportion | significant (%) | absolute | proportion | significant (%) | |
| global | −14.4 ± 0.47 | −0.15 ± 0.01 | −282.7 ± 9.1 | −0.09 ± 0 | 11 | −0.23 ± 0.01 | −0.14 ± 0.01 | 65 |
| within realms | ||||||||
| Australasia | −7.6 ± 0.76 | −0.27 ± 0.03 | −154.2 ± 16.4 | −0.09 ± 0.01 | 4 | −0.01 ± 0.03 | 0 ± 0.05 | 26 |
| Afrotropic | −18.0 ± 1.08 | −0.13 ± 0.01 | −373.4 ± 16.9 | −0.10 ± 0.01 | 24 | −0.28 ± 0.02 | −0.15 ± 0.01 | 94 |
| Indo-Malay | −30.8 ± 1.78 | −0.23 ± 0.01 | −659.8 ± 32.4 | −0.18 ± 0.01 | 20 | −0.60 ± 0.03 | −0.33 ± 0.02 | 96 |
| Nearctic | −2.9 ± 0.32 | −0.04 ± 0 | −37.9 ± 3.7 | −0.02 ± 0 | 1 | −0.05 ± 0.01 | −0.02 ± 0 | 24 |
| Neotropic | −17.9 ± 0.75 | −0.13 ± 0.01 | −314.5 ± 10.4 | −0.09 ± 0.01 | 20 | −0.22 ± 0.01 | −0.20 ± 0.01 | 80 |
| Oceania | −1.3 ± 0.40 | −0.45 ± 0.12 | ||||||
| Palaearctic | −11.4 ± 0.58 | −0.14 ± 0.01 | −196.3 ± 8.9 | −0.07 ± 0 | 1 | −0.21 ± 0.02 | −0.11 ± 0.01 | 56 |
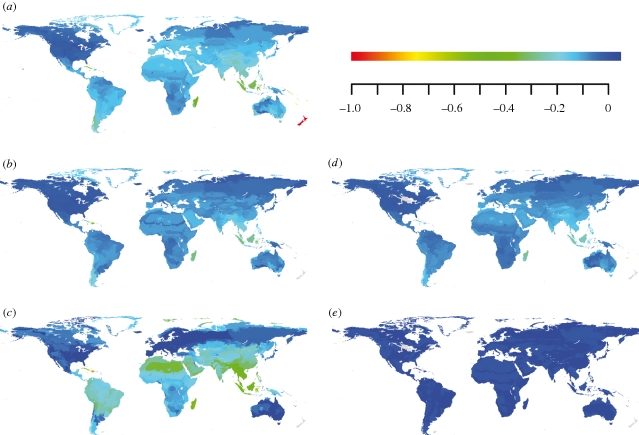
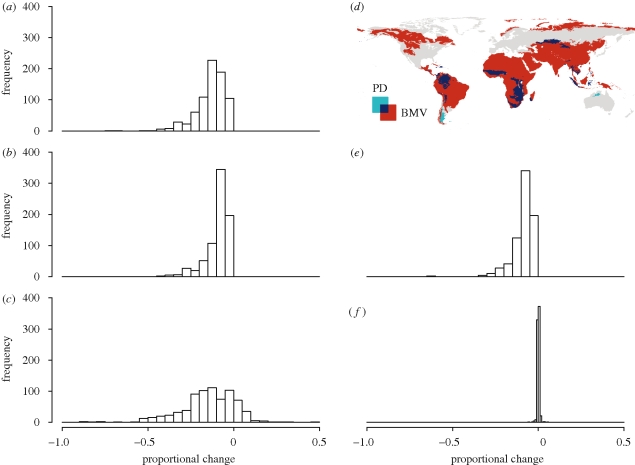
The magnitude of proportional losses would differ among the three diversity measures and regionally: they would be high for all three measures in the tropical realms apart from Australasia, but BMV losses would be greater there than losses in richness or PD (figure 2a–c; table 1). Histograms of proportional losses showed that the frequency of larger losses was higher for BMV than for richness and PD (figure 3a–c). In particular, random species-loss simulations for BMV within ecoregions differed hugely from proportional losses as predicted from Red List risk status, especially in the tropical realms (figure 2c,e).
Figure 2.
Projected and simulated proportional losses of mammalian diversity within ecoregions: (a–c) projected proportional change if all mammalian species currently ranked as threatened or Near Threatened were lost, for (a) species richness, (b) phylogenetic diversity (PD) and (c) variance in log10-transformed body mass (BMV). Simulated proportional change for (d) PD and (e) BMV. Negative proportions indicate losses. In (c) and (e), all positive proportions (gains in BMV) were set to dark blue and do not reflect the magnitude of proportional gains. Simulated losses were the mean values of 1000 randomizations for the projected species loss within each ecoregion.
Figure 3.
Histograms of (a–c) projected and (e,f) simulated proportional changes of mammalian diversity within ecoregions, and (d) map showing areas projected to lose significantly more-than-random phylogenetic diversity (PD; light blue), variance in log10-transformed body mass (BMV; red), or both (dark blue). Projected proportional changes were for (a) species richness, (b) PD and (c) BMV; simulated proportional changes were for (e) PD and (f) BMV. Negative proportions indicate losses; all histograms were scaled to the same axes to facilitate comparison. Simulated losses were the mean values of 1000 randomizations for the projected species loss within each ecoregion; significance of losses within each ecoregion in (d) was assessed using a one-tailed test based on the permutations for random species loss.
The often severe reductions in within-ecoregion BMV contrast particularly strongly with the expectation under random species loss (figure 3c,f). Conversely, random species loss led to very similar histograms and maps of PD loss to those projected by losing at-risk species (figures 2b,d and 3b,e). Globally, 65 per cent of ecoregions would lose significantly more BMV than in a random species loss scenario, whereas only 11 per cent of ecoregions would lose significantly more PD (table 1). Much of the northern Holarctic and most tropical areas apart from Australia are predicted to lose significantly more BMV than under random species loss; only some of these areas, most of them tropical, also experienced significantly higher PD losses in our simulations (figure 3d). Among realms, Indo-Malaysia stood out particularly, as each ecoregion would lose an average of 23 per cent of its species and 18 per cent of evolutionary history, but 33 per cent of its current BMV (table 1). By contrast, Australasian ecoregions stand to lose a higher proportion of species (27%) but much less PD and BMV (9% and 0%, respectively; table 1). Congruence across a sample size of 757 ecoregions, as assessed by Pearson correlation coefficients, was high between proportional losses of species richness and PD (r = 0.893), but low between PD and BMV (r = 0.388) and very low between richness and BMV (r = 0.192).
4. Discussion
(a). Possible impacts of BMV losses
The extinction of the 1292 currently threatened and Near Threatened mammalian species would result in a disproportionate reduction of the body-mass variety within ecoregions: more than half of the world's ecoregions would lose significantly more of their current BMV than that expected under random species loss. This decrease seems to be largely owing to disproportionate losses of large species: indeed, we have previously shown on the same dataset that large body mass in mammals correlates with current species extinction risk across the tropics, but not in temperate areas (Fritz et al. 2009). To the extent that BMV reflects FD in mammals, consequences for these ecosystems could be severe and may impinge on ecosystem services and goods (Millennium Ecosystem Assessment 2005). Many previous studies have shown that plant community structure can be directly or indirectly affected by the removal of large herbivores (McNaughton et al. 1988; Pringle et al. 2007; Johnson 2009) (even if they are replaced by livestock populations; Knapp et al. 1999), by selective losses of large frugivores via changes in seed-dispersal patterns (Asquith et al. 1997; Muller-Landau 2007) and by losses of large predators via mammalian community interactions and extinction cascades (Crooks & Soulé 1999; Terborgh et al. 2001).
Together, these studies suggest that the high BMV losses projected in our worst-case scenario, which seemed to be mostly owing to losses of many large species, may have serious consequences on ecosystem processes. Given the reliance of the growing human population on ecosystem services, especially those provided by tropical systems, the economic consequences of ecosystem changes caused by BMV loss could be severe. Millions of tons of meat per year from subsistence hunting help to feed the poorest people in the tropical realms predicted to be most affected (Fa et al. 2002; Milner-Gulland et al. 2003). Tourism is also an important source of income in many tropical countries, and largely relies on the aesthetic appeal of large mammals, most of them threatened.
Of course, our study does not provide exact predictions for any of these impacts. Our measure of BMV loss is necessarily rough: we consider only native wild species, and not all species currently Red Listed will vanish completely. Importantly, irreplaceability will differ among species, and resilience will differ among ecosystems (Hooper et al. 2005; Larsen et al. 2005). However, even though not all currently threatened species may actually die out globally, local extinctions or declines will still impact local ecosystems. Our results provide the first global sketch of how the mammalian trait variety currently present in ecosystems may be reduced in the future, and the emerging picture is not encouraging.
(b). Selective losses of large mammals
Reductions in BMV as measured in this study seemed to be mostly owing to the selective loss of large mammals. Large mammals face disproportionate threats, because they are often exploited by humans for meat and fur (Bodmer et al. 1997; Fa et al. 2002); because their larger home ranges lead to increased exposure to habitat loss and other threats (Woodroffe & Ginsberg 1998); and because life-history traits that increase species' susceptibility to anthropogenic threats scale with body size (Cardillo et al. 2005). Our results also agree with previous findings showing that selective loss of large mammalian, bee and beetle species from natural assemblages leads to faster declines in FD than random species losses do (Petchey & Gaston 2002; Larsen et al. 2005).
If all but Least Concern species were to disappear, nearly all ecoregions in the large tropical realms (Afrotropic, Neotropic and Indo-Malay) would experience disproportionately large reductions in BMV. These are places where larger species are particularly likely to be declining or at risk of extinction (Collen et al. 2009; Fritz et al. 2009), apparently because of high recent and ongoing rates of agricultural land conversion (Millennium Ecosystem Assessment 2005). Historic agricultural impacts have probably already caused local declines and extinctions in temperate regions and Australia in the past, such that non-tropical large mammals tend to be either locally extinct or not perceived as currently under threat (Fritz et al. 2009). A recent meta-analysis of mammalian and bird communities also found that previous land conversion was linked to FD being currently lower than expected from species richness (Flynn et al. 2009). Our results here imply that boreal forests in North America and most of the Siberian tundra stand to experience strong reductions of mammalian BMV, highlighting the high intrinsic susceptibility of these species-poor but currently relatively pristine areas (Cardillo et al. 2006). It seems that human actions have affected large mammals disproportionately at least since the industrial revolution: our figure 1 vividly illustrates how the world might look if these drivers continue unchecked.
(c). Implications for conservation planning
Only 11 per cent of ecoregions worldwide stand to lose a significantly higher amount of their current mammalian PD than expected under random species loss. The high redundancy of phylogenetic trees (Nee & May 1997) means that while a global average of 15 per cent of the species in an ecoregion are at risk, representing 14 per cent of current regional BMV on average, only 9 per cent of PD would be lost on average within ecoregions. Congruence with proportional losses in BMV was higher for PD than for species richness, but still relatively low (0.39). Clearly, BMV captures a very different aspect of diversity when compared with PD, and it is unclear which is the better indicator for FD.
PD is still relevant for conservation planning because it acts as a surrogate of diversity for features not correlated with extinction risk (Vane-Wright et al. 1991; Faith 1992) and because lost evolutionary heritage is irretrievable. Congruence between predicted losses of species richness and PD was high in this study, which is expected to be a common outcome (Rodrigues et al. 2005). This result suggests that ‘classic’ global conservation schemes trying to capture high numbers of threatened species could perform well for the preservation of mammalian evolutionary history, at least at a large spatial scale. However, the low surrogacy between prospective losses of PD and BMV indicates an urgent need for more work on global FD indicators. For conservation planning to consider ecosystem processes and services, we need to understand which species traits underpin them.
Acknowledgements
This study was funded by a Marie-Curie fellowship awarded by the European Commission (FP6 Early-Stage Training Network ‘Understanding and Conserving Earth's Biodiversity Hotspots’, contract number MEST-CT-2005-020561), with additional funding to the Centre for Macroecology, Evolution and Climate by the Danish National Research Foundation. We thank K. Jones and R. Grenyer for help with the PanTHERIA data, B. Collen for help with the GMA, R. Bernard for help with GIS, and J. Davies and R. Grenyer for providing the R code to correct for terminal polytomies. N. Cooper, R. Freckleton, R. Grenyer, G. Mace, L. McInnes, D. Orme and S. Whitmee provided helpful comments and discussion.
References
- Asquith N. M., Wright S. J., Clauss M. J.1997Does mammal community composition control recruitment in Neotropical forests? Evidence from Panama. Ecology 78, 941–946 (doi:10.1890/0012-9658(1997)078[0941:DMCCCR]2.0.CO;2) [Google Scholar]
- Bininda-Emonds O. R. P., et al. 2007The delayed rise of present-day mammals. Nature 446, 507–512 [Corrigendum (2008) Nature, 456, 274]. (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- Bodmer R. E., Eisenberg J. F., Redford K. H.1997Hunting and the likelihood of extinction of Amazonian mammals. Conserv. Biol. 11, 460–466 (doi:10.1046/j.1523-1739.1997.96022.x) [Google Scholar]
- Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A.2005Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- Cardillo M., Mace G. M., Gittleman J. L., Purvis A.2006Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen B., Loh J., Whitmee S., McRae L., Amin R., Baillie J. E. M.2009Monitoring change in vertebrate abundance: the Living Planet Index. Conserv. Biol. 23, 317–327 (doi:10.1111/j.1523-1739.2008.01117.x) [DOI] [PubMed] [Google Scholar]
- Crooks K. R., Soulé M. E.1999Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563–566 (doi:10.1038/23028) [Google Scholar]
- Davies T. J., et al. 2008Phylogenetic trees and the future of mammalian biodiversity. Proc. Natl Acad. Sci. USA 105, 11 556–11 563 (doi:10.1073/pnas.0801917105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg J. F.1981The mammalian radiations. An analysis of trends in evolution, adaptation, and behaviour London, UK: Athlone Press [Google Scholar]
- Fa J. E., Peres C. A., Meeuwig J.2002Bushmeat exploitation in tropical forests: an intercontinental comparison. Conserv. Biol. 16, 232–237 (doi:10.1046/j.1523-1739.2002.00275.x) [DOI] [PubMed] [Google Scholar]
- Faith D. P.1992Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (doi:10.1016/0006-3207(92)91201-3) [Google Scholar]
- Flynn D. F. B., Gogol-Prokurat M., Nogeire T., Molinari N., Trautman Richers B., Lin B. B., Simpson N., Mayfield M. M., DeClerck F.2009Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 12, 22–33 (doi:10.1111/j.1461-0248.2008.01255.x) [DOI] [PubMed] [Google Scholar]
- Forest F., et al. 2007Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445, 757–760 (doi:10.1038/nature05587) [DOI] [PubMed] [Google Scholar]
- Fritz S. A., Bininda-Emonds O. R. P., Purvis A.2009Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecol. Lett. 12, 538–549 (doi:10.1111/j.1461-0248.2009.01307.x) [DOI] [PubMed] [Google Scholar]
- Gross K., Cardinale B. J.2005The functional consequences of random vs ordered species extinctions. Ecol. Lett. 8, 409–418 (doi:10.1111/j.1461-0248.2005.00733.x) [Google Scholar]
- Hooper D. U., et al. 2005Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35 (doi:10.1890/04-0922) [Google Scholar]
- IUCN 20082008 IUCN Red List of Threatened Species Cambridge, UK: International Union for Conservation of Nature (IUCN) See http://www.iucnredlist.org/mammals [Google Scholar]
- Jepson P., Whittaker R. J.2002Ecoregions in context: a critique with special reference to Indonesia. Conserv. Biol. 16, 42–57 (doi:10.1046/j.1523-1739.2002.01143.x) [DOI] [PubMed] [Google Scholar]
- Jernvall J., Wright P. C.1998Diversity components of impending primate extinctions. Proc. Natl Acad. Sci. USA 95, 11 279–11 283 (doi:10.1073/pnas.95.19.11279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz W., Sekercioglu C. H., Watson J. E. M.2008Ecological correlates and conservation implications of overestimating species geographic ranges. Conserv. Biol. 22, 110–119 (doi:10.1111/j.1523-1739.2007.00847.x) [DOI] [PubMed] [Google Scholar]
- Johnson C. N.2009Ecological consequences of Late Quaternary extinctions of megafauna. Proc. R. Soc. B 276, 2509–2519 (doi:10.1098/rspb.2008.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. E., et al. 2009PanTHERIA: a species-level database of life-history, ecology and geography of extant and recently extinct mammals. Ecology 90, 2648 (doi:10.1890/08-1494.1) [Google Scholar]
- Knapp A. K., Blair J. M., Briggs J. M., Collins S. L., Hartnett D. C., Johnson L. C., Towne E. G.1999The keystone role of bison in North American tallgrass prairie. BioScience 49, 39–50 (doi:10.2307/1313492) [Google Scholar]
- Larsen T. H., Williams N. M., Kremen C.2005Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547 (doi:10.1111/j.1461-0248.2005.00749.x) [DOI] [PubMed] [Google Scholar]
- Legendre P.1993Spatial autocorrelation: trouble or new paradigm? Ecology 74, 1659–1673 (doi:10.2307/1939924) [Google Scholar]
- Mason N. W. H., MacGillivray K., Steel J. B., Wilson J. B.2003An index of functional diversity. J. Veg. Sci. 14, 571–578 (doi:10.1111/j.1654-1103.2003.tb02184.x) [Google Scholar]
- McNaughton S. J., Ruess R. W., Seagle S. W.1988Large mammals and process dynamics in African ecosystems. BioScience 38, 794–800 (doi:10.2307/1310789) [Google Scholar]
- Millennium Ecosystem Assessment 2005Ecosystems and human well-being: biodiversity synthesis Washington, DC: World Resources Institute [Google Scholar]
- Milner-Gulland E. J., Bennett E. L. & SCB 2002 Annual Meeting Wild Meat Group 2003. Wild meat: the bigger picture. Trends Ecol. Evol. 18, 351–357 (doi:10.1016/S0169-5347(03)00123-X). [Google Scholar]
- Muller-Landau H. C.2007Predicting the long-term effects of hunting on plant species composition and diversity in tropical forests. Biotropica 39, 372–384 (doi:10.1111/j.1744-7429.2007.00290.x) [Google Scholar]
- Nee S., May R. M.1997Extinction and the loss of evolutionary history. Science 278, 692–694 (doi:10.1126/science.278.5338.692) [DOI] [PubMed] [Google Scholar]
- Olson D. M., et al. 2001Terrestrial ecoregions of the world: a new map of life on earth. BioScience 51, 933–938 (doi:10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [Google Scholar]
- Petchey O. L., Gaston K. J.2002Extinction and the loss of functional diversity. Proc. R. Soc. Lond. B 269, 1721–1727 (doi:10.1098/rspb.2002.2073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchey O. L., Gaston K. J.2006Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758 (doi:10.1111/j.1461-0248.2006.00924.x) [DOI] [PubMed] [Google Scholar]
- Pringle R. M., Young T. P., Rubenstein D. I., McCauley D. J.2007Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl Acad. Sci. USA 104, 193–197 (doi:10.1073/pnas.0609840104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team 2008R: a language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- Rodrigues A. S. L., Brooks T. M., Gaston K. J.2005Integrating phylogenetic diversity in the selection of priority areas for conservation: does it make a difference? In Phylogeny and conservation (eds Purvis A., Gittleman J. L., Brooks T. M.), pp. 101–119 Cambridge, UK: Cambridge University Press [Google Scholar]
- Terborgh J., et al. 2001Ecological meltdown in predator-free forest fragments. Science 294, 1923–1926 (doi:10.1126/science.1064397) [DOI] [PubMed] [Google Scholar]
- Vane-Wright R. I., Humphries C. J., Williams P. H.1991What to protect? Systematics and the agony of choice. Biol. Conserv. 55, 235–254 [Google Scholar]
- Western D.1979Size, life-history and ecology in mammals. Afr. J. Ecol. 17, 185–204 (doi:10.1111/j.1365-2028.1979.tb00256.x) [Google Scholar]
- Wilson D. E., Reeder D. M.2005Mammal species of the world: a taxonomic and geographic reference, 3rd edn.Baltimore, MD: John Hopkins University Press [Google Scholar]
- Woodroffe R., Ginsberg J. R.1998Edge effects and the extinction of populations inside protected areas. Science 280, 2126–2128 (doi:10.1126/science.280.5372.2126) [DOI] [PubMed] [Google Scholar]