Abstract
As CO2 levels increase in the atmosphere, so too do they in the sea. Although direct effects of moderately elevated CO2 in sea water may be of little consequence, indirect effects may be profound. For example, lowered pH and calcium carbonate saturation states may influence both deposition and dissolution rates of mineralized skeletons in many marine organisms. The relative impact of elevated CO2 on deposition and dissolution rates are not known for many large-bodied organisms. We therefore tested the effects of increased CO2 levels—those forecast to occur in roughly 100 and 200 years—on both shell deposition rate and shell dissolution rate in a rocky intertidal snail, Nucella lamellosa. Shell weight gain per day in live snails decreased linearly with increasing CO2 levels. However, this trend was paralleled by shell weight loss per day in empty shells, suggesting that these declines in shell weight gain observed in live snails were due to increased dissolution of existing shell material, rather than reduced production of new shell material. Ocean acidification may therefore have a greater effect on shell dissolution than on shell deposition, at least in temperate marine molluscs.
Keywords: ocean acidification, carbon dioxide, calcification, shell dissolution, shell deposition, Nucella lamellosa
1. Introduction
Many conspicuous and ecologically significant marine animals produce massive skeletons of calcium carbonate, including corals, molluscs, tube-dwelling polychaetes, crustaceans, bryozoans and echinoderms (Milliman 1974). These skeletons provide structural support and are often essential components of morphological defence in the ever-escalating evolutionary ‘arms race’ between prey and durophagous predators (Vermeij 1987). Because calcium carbonate structures also provide important habitat for many organisms, calcifying species have a large impact on community structure (e.g. Yap 2009).
Although calcium carbonate may not be expensive to produce energetically (Palmer 1992), the rate of shell deposition may limit the maximum rate of body growth in some groups (Palmer 1981), which could affect both growth rate and survival. A decrease in calcium carbonate saturation state (ΩCaCO3), along with decreased ocean pH levels owing to increased dissolved CO2, could therefore have a profound impact on rates of growth or on extent of skeletal development in many marine animal groups.
The degree to which future increases in ocean CO2 levels will reduce calcification rates in marine animals remains unclear. For example, predicted changes in ΩCaCO3 depend strongly upon temperature (and therefore ocean depth and latitude) and on the relative solubility of the particular crystal form of the biomineral used by a given species (Milliman et al. 1999; Feely et al. 2004). At current rates of CO2 increase, the predicted saturation state for pure calcite, aragonite and high-Mg calcite should remain favourable for biogenic calcification at most latitudes for at least the next century (Andersson et al. 2008). However, the degree of calcite supersaturation does appear to have a significant effect on the rate at which calcification can occur. In many marine taxa, calcification rates decrease with decreasing CaCO3 saturation state even when ΩCaCO3 > 1 (Feely et al. 2004). As a result, in prior ocean acidification studies where marine calcifiers were exposed to elevated CO2, many taxa exhibited lower growth rates in response to climatically relevant increases in [CO2] (reviewed in Doney et al. 2009).
Although mineralized skeletons are widespread and functionally significant, the process of biomineralization is still not fully understood (Wilt et al. 2003; Allemand et al. 2004). From a purely physico-chemical standpoint, CaCO3 should precipitate spontaneously when Ω > 1, and start to dissolve when Ω < 1. However, biogenic calcium carbonate deposition is not so straightforward. It is an active, highly controlled and far more complex process. Organisms must (i) obtain Ca2+ and HCO3− from ambient sea water, which requires active transport across cell membranes, and (ii) eliminate excess protons released during mineralization (Allemand et al. 2004). To precipitate CaCO3, organisms must therefore actively transport H+ against a concentration gradient so that CaCO3 remains supersaturated at the calcification site (McConnaughey 1995).
Two competing processes affect the mass of marine invertebrate exoskeletons: shell deposition at the site of mineralization, which is actively mediated by the living organism, and shell dissolution at the skeleton's outer surface (Carter 1990). Barring erosion due to scouring and attack by shell-boring organisms, dissolution is a physico-chemical process driven largely by (i) the solubility of the biomineral, (ii) chemical characteristics of the surrounding sea water and (iii) potential effects of metabolic by-products released by the adhering biofilm (e.g. Milliman et al. 1999). By comparing shell deposition rates of live animals and shell dissolution rates of empty shells, we could gauge the relative contribution of these two processes to overall shell mass change.
We set out to determine the effect of climatically relevant increases in [CO2] on rates of both CaCO3 deposition and dissolution in a common rocky intertidal whelk, Nucella lamellosa. By employing a technique that reliably estimates shell mass of living snails non-destructively (Palmer 1982), we were able to quantify short-term in situ rates of shell deposition in live animals, as well as dissolution rates of empty shells, under three CO2 levels.
We predicted that shell deposition rates of live snails would decrease at rates similar to other experimental studies of molluscs (e.g. Berge et al. 2006; Gazeau et al. 2007) and that shell dissolution rates of empty shells would increase with increasing CO2 concentrations. However, we expected that the declines in shell deposition rate under higher CO2 levels would be less than the increases in dissolution rates of empty shells, given that most invertebrates can regulate internal concentrations of physiologically important ions despite variation in the external medium (Robertson 1949).
2. Material and methods
(a). Collection, feeding and initial measurements
Thick-shelled N. lamellosa were collected intertidally from the shore of Grappler Inlet (48°50′00″ N, 125°06′49″ W), a quiet-water inlet near the Bamfield Marine Sciences Center (BMSC), on the west coast of Vancouver Island, British Columbia, Canada, on 10 July 2008. Only large-sized, actively growing snails showing more than one-quarter whorl of recent shell growth were collected. Snails were transported to BMSC and placed in large plastic tubs (40 × 25 × 15 cm) with lids where they were held in running sea water (pH = 8.00 ± 0.02, temperature = 9 ± 1°C, salinity = 35.0 ± 0.5 psu). For 40 days prior to the experiment (except days 19–27 when starved during a pilot study), snails were held continuously immersed and were fed their preferred prey, the barnacle Balanus glandula, on small stones. Fresh barnacles were provided every 10–12 days and greatly exceeded the numbers eaten.
Five days after collection, 100 snails were tagged, measured and weighed. Snails were removed from sea water, scrubbed to remove encrusting organisms, rinsed briefly in tap water and allowed to dry for 3 h, during which time they were (i) individually tagged using numbered Brady Wire Markers coated with cyanoacrylate glue, (ii) measured for shell length (apex to tip of siphonal canal) to the nearest 0.01 mm with digital calipers (Fowler Ultracal) and (iii) weighed in air (§2b). Snails were returned to holding tubs and underwater weights (§2b) recorded the next day, to avoid potentially confounding effects of air in the mantle cavity.
(b). Weighing protocol
Body wet weights and shell dry weights were estimated non-destructively on live snails following Palmer (1982). In brief, two weights were recorded for each snail: (i) total weight in air after extravisceral water was removed and (ii) underwater weight (weight suspended in sea water underneath the balance). Weight in air includes both body wet weight and shell dry weight, so body wet weight was estimated by subtracting shell dry weight from total weight in air. The empirically derived correction for the buoyant effect of sea water yields a highly accurate estimate of shell dry weight (r2 = 0.9998; Palmer 1982). All weights were recorded to ±1 mg with a digital balance (Mettler, BB240).
(c). Shell deposition and dissolution rates
Baseline rate of shell deposition was estimated prior to the CO2 treatments by measuring underwater weight (§2b) at the start and end of a 48 h period of starvation, immediately before the CO2 treatments. Rate of shell deposition under the three CO2 treatments was estimated by measuring underwater weight (§2b) of live N. lamellosa at the start and end of a 6-day period of starvation. Shell dissolution rates were obtained by weighing thoroughly dried, empty N. lamellosa shells in air before and after the experiment. Empty shells were obtained by removing the flesh from live snails similar in size to, and collected concurrently with, the experimental snails. Empty shells were scrubbed and then cleaned in heated tap water for 1 h in a 1 l ultrasonic cleaner to remove residual organic material and salt, and dried for 24 h prior to weighing.
(d). Experimental design
From 100 tagged snails, only 72 individuals showing the greatest rates of shell weight gain during the preceding 13 days while feeding were selected. To estimate baseline rates of shell deposition, these snails were removed from food, weighed underwater (§2b), returned to their cleaned and otherwise empty holding tubs in running sea water (§2a) and weighed underwater again after 48 h.
Snails were selected so that shell lengths, body weights, shell weights and baseline shell deposition rates were similar in each CO2 treatment (table 1). The 24 snails in each treatment were divided equally among six (25×15×15 cm) plastic containers (for details regarding containers, see electronic supplementary material) to yield four snails per container. One pre-weighed, empty Nucella shell was also placed in each container. Empty shells were assigned to containers so that average shell dry weights were approximately the same in the three CO2 treatments (table 2). Underwater weights (§2b) of live snails were measured at the beginning and end of 6 days exposure to the three CO2 treatments.
Table 1.
Initial sizes and baseline shell deposition rates of N. lamellosa used in the CO2 treatments. Differences among treatments were not significant for any measurement (p = 0.87, p = 0.70, p = 0.50, p = 0.60, respectively).
| shell length (mm) |
body wet wt (g) |
shell dry wt (g) |
baseline shell deposition ratea (mg d−1) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| treatment | n | mean | s.e. | mean | s.e. | mean | s.e. | mean | s.e. |
| control | 24 | 34.9 | 0.48 | 1.17 | 0.044 | 5.51 | 0.212 | 4.36 | 0.963 |
| 2× CO2 | 24 | 34.9 | 0.42 | 1.13 | 0.030 | 5.83 | 0.228 | 5.44 | 0.931 |
| 4× CO2 | 24 | 34.6 | 0.43 | 1.13 | 0.037 | 5.54 | 0.196 | 5.50 | 0.814 |
aBaseline shell deposition rate of starved snails over 2 days immediately prior to the CO2 treatments.
Table 2.
Initial weights of empty N. lamellosa shells. Differences among treatments were not significant (p = 0.64).
| shell dry wt (g) |
|||
|---|---|---|---|
| treatment | n | mean | s.e. |
| control | 6 | 5.23 | 0.401 |
| 2× CO2 | 6 | 5.83 | 0.375 |
| 4× CO2 | 6 | 5.53 | 0.525 |
(e). Manipulation of pH and CO2
The three CO2 treatments comprised: (i) a control, in which ambient air was bubbled into the tanks (1× ambient [CO2] = 385 ppm (NOAA 2008); (ii) moderately increased CO2, where 400 ppm of CO2 was added to the ambient air being bubbled into the tanks (2× ambient [CO2] = 785 ppm); and (iii) high CO2, where 1200 ppm of CO2 was added to the ambient air (4× ambient [CO2] = 1585 ppm). The latter two treatments were chosen to approximate future CO2 concentrations predicted for the year 2100 under the IS92a scenario for carbon emissions (Feely et al. 2004) and within the range projected to occur between 2100 and 2200 (Caldeira & Wickett 2003), respectively. To achieve the desired CO2 concentrations in the 2× and 4× treatments, paired mass flow controllers (Sierra Instruments, Model 810C Mass-Trak, 0–100 sccm, and 0–5 slpm) were programmed to combine carbon dioxide from a pre-mixed compressed gas cylinder (2.0% CO2: 20.4% O2: balance N2, PRAXAIR) and ambient air from the in-house compressor in the appropriate proportions. A fifth mass flow controller (0–5 slpm) regulated air flow between the compressor and the 1× CO2 tanks.
Bubbling rate was maintained at approximately 150 ml min−1 in all experimental tanks. One half of the volume of each container was replaced with fresh sea water daily; equilibrium pH values re-established within approximately 1 h. pH was measured using an Accumet AP63 pH meter, calibrated daily with pHNBS 4.0, 7.0 and 10.0 buffers (Fisher Scientific). pH, temperature, salinity and bubbling rates were recorded at least once every 24 h.
(f). Statistical analysis
Rates of dissolution and deposition were computed as milligram dry shell weight change per snail per day. Differences in shell deposition rates of live snails among CO2 treatments were tested with a one-factor ANCOVA (dependent variable: mg d−1 shell weight change per snail over the 6-day experiment; covariate: baseline mg d−1 shell weight change per snail; grouping factor: CO2 treatment; type III sum of squares; SuperANOVA 1.11, Abacus Concepts, Berkeley, CA, USA). All remaining statistical tests for differences in snail characteristics among treatments were conducted with a one-factor ANOVA (SuperANOVA 1.11). Tests for differences in sea water properties among treatments were conducted with repeated-measures ANOVA (JMP 7.0.1, SAS Instruments).
3. Results
(a). Water chemistry
As predicted, pH decreased significantly (repeated-measures ANOVA: F = 503.6, p<0.0001) from ambient levels where [CO2] was increased experimentally (figure S1a, electronic supplementary material). When averaged across all measurements, the mean (±s.e.) pH of the control containers bubbled with ambient air was 7.98 ± 0.012, while those bubbled with 2× and 4× current [CO2] had average (±s.e.) pH values of 7.80 ± 0.015 and 7.54 ± 0.009, respectively. Although pH fluctuated somewhat through time (both time and the treatment × time interaction were significant; p < 0.0001), these fluctuations were minor (generally less than 0.05 pH units) when compared with the main [CO2] effect, and were only detectable because variance among tanks within a treatment was so low. Bubbling rate did not vary with time, treatment or their interaction (repeated-measures ANOVA: p > 0.2; figure S1b, electronic supplementary material). Temperature and salinity also varied slightly (less than 1°C and less than 0.25 psu) owing to variation at the station's sea water intake, but this variation was independent of treatment (figure S1c,d, electronic supplementary material).
(b). Shell deposition and dissolution
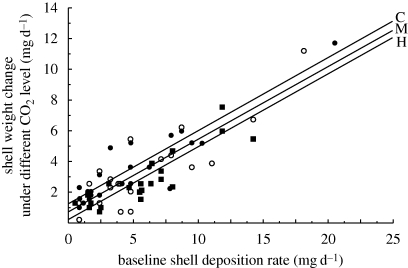
Baseline shell deposition rate of individual N. lamellosa explained 73 to 83 per cent of the variation in shell weight gain among snails in the CO2 treatments (figure 1). Because this baseline deposition rate varied so much that it would have swamped the treatment effects, and because the relationship was linear and deviations about the regression appeared to be uniform (figure 1), we statistically removed among-individual variation in baseline shell deposition rate via ANCOVA. Slopes did not differ among the three treatments (p > 0.99; figure 1 and table 3), therefore tests for deviations of shell weight change from a single common slope were justified.
Figure 1.
Shell weight change of live N. lamellosa held under three CO2 levels (black circles, C—2008 ambient, y = 0.476x + 1.25, r2=0.83; white circles, M—2× 2008 ambient, y = 0.473x + 0.73, r2=0.73; black squares, H—4× 2008 ambient, y = 0.476x + 0.20, r2=0.79) for 6 days as a function of baseline shell deposition rate (shell deposited in the absence of food for 2 days in ambient sea water prior to the experiment). Each point represents an individual snail. Least-squares linear regression equations describe the relationship for each treatment (all three were highly significant, p<0.001). Five snails that exhibited a baseline shell deposition of 0 mg d−1 or less were excluded from the analysis.
Table 3.
Results from statistical tests for differences among CO2 treatments.
| source | d.f. | MS | F | p |
|---|---|---|---|---|
|
ANCOVA of shell weight change of live snails (mg d−1)a | ||||
| CO2 level | 2 | 5.97 | 5.03 | 0.0094 |
| covariate | 1 | 268.20 | 226.06 | <0.0001 |
| residual | 63 | 1.18 | ||
| covariate × treatment | 2 | 0.0009 | 0.0008 | >0.99 |
|
ANOVA of weight change of empty shells (mg d−1)b | ||||
| between groups | 2 | 1.093 | 12.53 | 0.0006 |
| within groups | 15 | 0.087 | ||
Least-squares mean shell weight change—change during the experiment standardized to a common baseline deposition rate by ANCOVA—differed significantly among CO2 treatments (p = 0.009; table 3): weight change decreased roughly linearly with increasing CO2 levels (figure 2). Shell weight change of empty shells also varied significantly among CO2 treatments (p < 0.001, table 3): in all cases it was negative, indicating dissolution, and dissolution rate increased roughly linearly with increasing CO2 levels (figure 2). When the entire average amount of shell lost to dissolution per gram of shell was added to the shell weight change of live N. lamellosa over the experiment (white squares, figure 2), differences in estimated rates of shell deposition among CO2 levels were no longer significant (p = 0.79). When only half of the shell lost to dissolution was added (black squares, figure 2), to better estimate dissolution from only the outside surface of the shell (both shell layers in Nucella are calcite; Vermeij 1995), estimated rates of shell deposition did decrease with increasing CO2, but these differences were still not significant (p = 0.18).
Figure 2.
Circles and solid lines: least-squares mean weight change (at a common baseline shell deposition rate, from ANCOVA) of live (white circles) and empty (black circles) N. lamellosa shells held under three CO2 levels for 6 days (mean ± s.e.; n = 22, 23 and 22, respectively, for live shells; n = 6 for dead shells). Squares and dashed lines: estimated shell deposition rate of live N. lamellosa after correcting for dissolution—that is, after adding either 0.5 (filled squares) or 1.0 (open squares) times the shell weight change per gram of empty shells. Error bars for the black squares were omitted for clarity.
4. Discussion
Our results suggest that elevated ocean CO2 levels have a greater effect on shell dissolution rate than on shell deposition rate, at least for temperate marine molluscs at lower temperatures (approx. 10°C or less). Significantly, our results reveal how estimates of both deposition and dissolution rate are essential in studies of how manipulated CO2 or pH levels impact skeletal growth. Had we not measured shell dissolution rate, we might have been tempted to interpret the decline in shell weight gain per snail per day with increasing CO2 levels (figure 2) as reduced ability to deposit new shell. In fact, after accounting for the average rates of shell dissolution at increased CO2 levels, net rates of shell deposition did not appear to differ among treatments. So elevated CO2 within a climatically relevant range clearly affected shell dissolution rates, but had little or no effect on shell deposition rates in Nucella.
We cannot say with certainty that elevated CO2 levels had no effect on deposition rate because dissolution rate per gram of shell for empty shells may differ from that in occupied shells (see Methodological Issues in the electronic supplementary material). But regardless of whether all, or only half (to better estimate shell dissolution solely from the outer shell surface), of the shell weight lost to dissolution was added back to the shell weight change of live snails, differences in estimated rates of shell deposition did not differ significantly among CO2 levels (figure 2). We also cannot be sure whether deposition or dissolution rates might have been different (higher or lower) over a longer time period, or whether lower pH might affect maximal rates of shell deposition when feeding (Palmer 1981). Our use of starved snails, to avoid potentially confounding effects of variable feeding rates on growth (see Methodological Issues in the electronic supplementary material), restricted these experiments to less than 10 days. However, others report that the effects of carbonate chemistry on calcification rates are immediate (Marubini & Atkinson 1999) and not significantly different between the short term (days) and longer term (months; Langdon et al. 2000).
Many invertebrates can regulate extracellular pH and maintain high rates of calcification during periods of hypercapnia (Gutowska et al. 2010). For example, even at 10× and 15× current CO2 levels—levels much higher than we used—the cuttlefish Sepia officinalis grew and deposited new skeleton at the same rate as controls (Gutowska et al. 2008). Wood et al. (2008) similarly reported increased calcification rates in a brittle star exposed to reduced sea water pH, even at pH 6.8. The significant acid–base regulatory capacities displayed by many marine invertebrates could explain the seemingly impossible ability of some (i) to deposit calcium carbonate shells despite living in naturally acidic habitats (including some estuarine areas and near hydrothermal vents; Marshall et al. 2008; Tunnicliffe et al. 2009), and (ii) to have survived periods of elevated CO2 in the past (e.g. Fraiser & Bottjer 2007) in sea water highly under-saturated with respect to calcium carbonate.
Clearly, calcifying marine invertebrates can regulate extracellular concentrations of physiologically important ions, and exercise tight control over cellular processes including calcification. By actively regulating intracellular Ca2+ concentrations and acid–base parameters, for example, many invertebrates can actively generate an extracellular micro-environment with elevated calcium carbonate saturation states that would favour calcification (Pomar & Hallock 2008). Furthermore, the site of mineralization is typically isolated from the external sea water (Findlay et al. 2009), and would therefore not directly experience the ambient sea water saturation state. So the ability of species to precipitate new shell material under unfavourable conditions, at least over the short term, is perhaps unsurprising. However, shell dissolution at the external surface is not regulated by the organism, and may be much more sensitive to changes in the carbonate saturation state of the sea water with which it is in direct contact.
Like many temperate marine molluscs (Lowenstam 1954), the shell of N. lamellosa is mainly calcite (Vermeij 1995). Therefore, it should be less soluble in sea water than aragonitic or high-Mg calcite shells (Andersson et al. 2008). Nonetheless, despite exposing snails to experimental conditions where the predicted saturation state for calcite (ΩCaCO3) is greater than 1 (11°C, CO2 less than 1600 ppm; Andersson et al. 2008), shell dissolution rate increased roughly linearly with increasing CO2 levels (figure 2). This trend is consistent with the increase in dissolution rates of ‘dead’ or detached brittle star arms and dead coralline algal thalli exposed to increasingly acidic sea water over a similar pH range (Wood et al. 2008; Martin & Gattuso 2009). Shell dissolution therefore appears highly sensitive to decreases in saturation state even when the sea water to which the shell is exposed is supersaturated with respect to calcite.
Recent findings that CO2-mediated declines in ocean pH may not affect calcification rates seem to contradict prior experimental studies of many invertebrates (reviewed in Doney et al. 2009). But this apparent contradiction may be illusory. Our results reveal that shell dissolution under elevated CO2 levels may be affected more than shell deposition. Thus, measurements of overall changes in shell mass, although relevant endpoints to report, do not necessarily reflect changes in calcification or shell deposition rates per se (see also Findlay et al. 2009). Clearly, both shell dissolution and deposition rates must be monitored to understand how changes in sea water chemistry affect the growth and maintenance of essential skeletal structures.
Finally, our results highlight some broader ecological implications of ocean acidification. Dissolution of non-growing shell regions can result in structural weakness (e.g. barnacle tests; McDonald et al. 2009), and may increase susceptibility to certain predators and bio-eroders. Furthermore, shells and skeletons of reef-forming corals and bivalves provide important habitat even after the animals have died, and gastropod shells may house hermit crabs and other organisms for many years after the original occupant died. Increased dissolution rates, as measured here, will reduce the lifespan of these biogenic structures and may have significant ecological consequences.
Acknowledgements
This research was approved by the BMSC animal care committee and complied with Canadian Council on Animal Care guidelines and policies.
We thank the staff at Bamfield Marine Sciences Center for their assistance. This study was supported by grants from the Bamfield Marine Sciences Center, Natural Sciences and Engineering Research Council of Canada Discovery Grants (A7245 to A.R.P. and 22R43158 to C.D.G.H.) and a Canada Foundation for Innovation grant (to C.D.G.H.). S.N. and A.R.P. conceived and designed the study, conducted the experiments and analyses, and drafted the article. C.D.G.H. contributed to the conceptual design and to critical revision of the manuscript.
References
- Allemand D., Ferrier-Pages C., Furla P., Houlebreque F., Puverel S., Reynaud S., Tambutte E., Tambutte S., Zoccola D.2004Biomineralisation in reef-building corals: from molecular mechanisms to environmental control. C. R. Pelevol. 3, 453–467 (doi:10.1016/j.crpv.2004.07.011) [Google Scholar]
- Andersson A. J., Mackenzie F. T., Bates N. R.2008Life on the margin: implications of ocean acidification on Mg-calcite, high latitude and cold-water marine calcifiers. Mar. Ecol. Prog. Ser. 373, 265–273 (doi:10.3354/meps07639) [Google Scholar]
- Berge J. A., Bjerkeng B., Pettersen O., Schaanning M. T., Oxnevad S.2006Effects of increased sea water concentrations of CO2 on growth of the bivalve Mytilus edulis L. Chemosphere 62, 681–687 (doi:10.1016/j.chemosphere.2005.04.111) [DOI] [PubMed] [Google Scholar]
- Caldeira K., Wickett M. E.2003Anthropogenic carbon and ocean pH. Nature 425, 365 (doi:10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- Carter J. G.1990Skeletal biomineralization: patterns, processes and evolutionary trends. Scarborough, Ontario: Nelson Canada [Google Scholar]
- Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A.2009Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- Feely R. A., Sabine C. L., Lee K., Berelson W., Kleypas J., Fabry V. J., Millero F. J.2004Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (doi:10.1126/science.1097329) [DOI] [PubMed] [Google Scholar]
- Findlay H. S., Wood H. L., Kendall M. A., Spicer J. L., Twitchett R. J., Widdicombe S.2009Calcification, a physiological process to be considered in the context of the whole organism. Biogeosci. Discuss. 6, 2267–2284 [Google Scholar]
- Fraiser M. L., Bottjer D. J.2007When bivalves took over the world. Paleobiology 33, 397–413 (doi:10.1666/05072.1) [Google Scholar]
- Gazeau F., Quiblier C., Jansen J. M., Gattuso J.-P., Middelburg J. J., Heip H. R.2007Impact of elevated CO2 on shellfish calcification. Geophys. Res. Lett. 34, L07603 (doi:10.1029/2006GL028554) [Google Scholar]
- Gutowska M. A., Pörtner H. O., Melzner F.2008Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar. Ecol. Prog. Ser. 373, 303–309 (doi:10.3354/meps07782) [Google Scholar]
- Gutowska M. A., Melzner F., Langenbuch M., Bock C., Claireaux G., Pörtner H. O.2010Acid–base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J. Comp. Physiol. B. 180, 323–335 (doi:10.1007/s00360-009-0412-y) [DOI] [PubMed] [Google Scholar]
- Langdon C., Takahashi T., Sweeney C., Chipman D., Goddard J., Marubini F., Aceves H., Barnett H., Atkinson M. J.2000Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochem. Cycles 14, 639–654 (doi:10.1029/1999GB001195) [Google Scholar]
- Lowenstam H. A.1954Factors affecting the aragonite: calcite ratios in carbonate secreting organisms. J. Geol. 62, 284–322 (doi:10.1086/626163) [Google Scholar]
- Marshall D. J., Santos J. H., Leung K. M. Y., Chak W. H.2008Correlations between gastropod shell dissolution and water chemical properties in a tropical estuary. Mar. Environ. Res. 66, 422–429 (doi:10.1016/j.marenvres.2008.07.003) [DOI] [PubMed] [Google Scholar]
- Martin S., Gattuso J. P.2009Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Global Change Biol. 15, 2089–2100 (doi:10.1111/j.1365-2486.2009.01874.x) [Google Scholar]
- Marubini F., Atkinson M. J.1999Effects of lowered pH and elevated nitrate on coral calcification. Mar. Ecol. Prog. Ser. 188, 117–121 (doi:10.3354/meps188117) [Google Scholar]
- McConnaughey T. A.1995Ion transport and the generation of biomineral supersaturation. Bull. Inst. Oceanog. Monaco 14, 1–18 [Google Scholar]
- McDonald M. R., McClintock J. B., Amsler C. D., Rittschof D., Angus R. A., Orihuela B., Lutostanski K.2009Effects of ocean acidification over the life history of the barnacle Amphibalanus amphitrite. Mar. Ecol. Prog. Ser. 385, 179–187 (doi:10.3354/meps08099) [Google Scholar]
- Milliman J. D.1974Marine carbonates. New York, NY: Springer-Verlag [Google Scholar]
- Milliman J. D., Troy P. J., Balch W. M., Adams A. K., Li Y.-H., Mackenzie F. T.1999Biologically mediated dissolution of calcium carbonate above the chemical lysocline? Deep-Sea Res. I 46, 1653–1669 [Google Scholar]
- NOAA 2008Trends in atmospheric carbon dioxide: global. Global Monitoring Division, Earth System Research Laboratory, National Oceanic & Atmospheric Administration, Boulder, CO. See www.esrl.noaa.gov/gmd/ccgg/trends [Google Scholar]
- Palmer A. R.1981Do carbonate skeletons limit the rate of body growth? Nature 292, 150–152 (doi:10.1038/292150a0) [Google Scholar]
- Palmer A. R.1982Growth in marine gastropods: a non-destructive technique for independently measuring shell and body weight. Malacologia 23, 63–73 [Google Scholar]
- Palmer A. R.1992Calcification in marine molluscs: how costly is it? Proc. Natl Acad. Sci. USA 89, 1379–1382 (doi:10.1073/pnas.89.4.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomar L., Hallock P.2008Carbonate factories: a conundrum in sedimentary geology. Earth-Sci. Rev. 87, 134–169 (doi:10.1016/j.earscirev.2007.12.002) [Google Scholar]
- Robertson J. D.1949Ionic regulation in some marine invertebrates. J. Exp. Biol. 26, 182–200 [DOI] [PubMed] [Google Scholar]
- Tunnicliffe B., Davies K. T. A., Butterfield D. A., Embley R. W., Rose J. M., Chadwick W. W., Jr2009Survival of mussels in extremely acidic waters on a submarine volcano. Nat. Geosci. 2, 344–348 (doi:10.1038/NGEO500) [Google Scholar]
- Vermeij G. J.1987Evolution and escalation. An ecological history of life Princeton, NJ: Princeton University Press [Google Scholar]
- Vermeij G. J.1995A natural history of shells. Princeton, NJ: Princeton University Press [Google Scholar]
- Wilt F. H., Killian C. E., Livingston B. T.2003Development of calcareous skeletal elements in invertebrates. Differentiation 71, 237–250 (doi:10.1046/j.1432-0436.2003.7104501.x) [DOI] [PubMed] [Google Scholar]
- Wood H. L., Spicer J. I., Widdicombe S.2008Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 275, 1767–1773 (doi:10.1098/rspb.2008.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap H. T.2009Local changes in community diversity after coral transplantation. Mar. Ecol. Prog. Ser. 374, 33–41 (doi:10.3354/meps07650) [Google Scholar]