Abstract
Many social animals use long-distance signals to attract mates and defend territories. They face the twin challenges of discriminating between species to identify conspecific mates, and between individuals to recognize collaborators and competitors. It is therefore often assumed that long-distance signals are under strong selection for species-specificity and individual distinctiveness, and that this will drive character displacement when closely related species meet, particularly in noisy environments. However, the occurrence of signal stereotypy and convergence in rainforest species seems to contradict these ideas, and raises the question of whether receivers in these systems can recognize species or individuals by long-distance signals alone. Here, we test for acoustically mediated recognition in two sympatric antbird species that are known to have convergent songs. We show that male songs are stereotyped yet individually distinctive, and we use playback experiments to demonstrate that females can discriminate not only between conspecific and heterospecific males, but between mates and strangers. These findings provide clear evidence that stereotypy and convergence in male signals can be accommodated by fine tuning of perceptual abilities in female receivers, suggesting that the evolutionary forces driving divergent character displacement in animal signals are weaker than is typically assumed.
Keywords: antbirds, animal communication, individual recognition, mate recognition, species coexistence, suboscine birds
1. Introduction
Long-distance signals mediate competition for mates and territories in a wide range of animals. Failure to discriminate between conspecifics and heterospecifics on the basis of these signals leads to a range of fitness costs, including wasted time, misdirected aggression and the production of unfit hybrids (Fisher 1930; West-Eberhard 1983). These costs are expected to exert strong selection on signallers and receivers for accurate transmission and perception of species-specific signals. However, while there is little doubt that ‘conspecific mate recognition’ (CMR) is an important attribute minimizing interaction and maintaining reproductive isolation between lineages (Mayr 1963; Irwin & Price 1999; Magurran & Ramnarine 2005), the implications for signal evolution remain unclear.
CMR provides a theoretical framework for two opposing hypotheses. On the one hand, it is widely assumed that selection will favour species-specificity in signal design, thereby leading to signal divergence when closely related species meet (Dobzhansky 1940; Marler 1957; West-Eberhard 1983; Nelson & Marler 1990). In support of this idea, some empirical studies find evidence of divergent character displacement in sympatry (Seddon 2005; Kirschel et al. 2009b). On the other hand, unambiguous demonstrations of signal displacement or partitioning are rare (Miller 1982; Kirschel et al. 2009b), suggesting that other more subtle routes to CMR may be widespread. One potential explanation is that selection mainly targets receiver preferences, and that divergent character displacement and community-wide partitioning are therefore more apparent in female preferences than in the design of male signals (Gerhardt & Huber 2002; Chek et al. 2003). Empirical evidence for this mechanism has been found in frogs (Gerhardt 1994) and insects (Nosil et al. 2003; Jang & Gerhardt 2006), but the extent to which CMR is mediated by signal perception rather than design remains poorly understood.
It seems plausible that the perceptual ability of receivers also influences the outcome of selection for individual recognition within species. Intraspecific recognition systems appear to be adaptive in many animals, as demonstrated by widespread feats of long-term individual recognition (Falls 1982; Godard 1991; Insley 2000; Clark et al. 2006). Indeed, reproductive success and resource defence often depend on efficient recognition of mates, group members and competitors. For this reason, ‘individual mate recognition’ (IMR) is thought to be a key component of social behaviour in long-term collaborations over parental care and resource defence (Tibbetts & Dale 2007). The prediction that intraspecific signals should therefore be individually distinctive is borne out by studies in a wide range of taxa (Falls 1982; Wanker et al. 1998; Johnston & Bullock 2001; Tibbetts 2002). However, few studies have considered the role of receiver perception in intraspecific recognition systems, and even fewer have experimentally tested for IMR (Tibbetts & Dale 2007).
Rainforests are an ideal environment to examine the interplay between signallers and receivers because they pose distinct challenges to long-distance signalling systems. In particular, they are so densely foliated that visual signals are only detectable at close range, whereas acoustic signals travel further but nonetheless degrade rapidly during transmission (Richards & Wiley 1980; Bradbury & Vehrencamp 1998). Moreover, they are exceptionally rich in co-existing species, such that receivers are forced to detect or discriminate signals against a backdrop of insect noise and multi-species choruses (Slabbekoorn & Smith 2002; Wollerman & Wiley 2002; Kirschel et al. 2009a). These constraints, coupled with the imperatives of CMR and IMR, suggest that rainforest species (and individuals) should develop acoustic signals, which are particularly distinctive (i.e. disjunct in acoustic space) to increase the reliability of transmission (Luther & Wiley 2009).
Some studies support the idea that constraints to perception influence signal design in rainforest birds, in particular by revealing broad rather than precise perception of songs by male receivers (Luther & Wiley 2009), greater divergent character displacement in sympatry versus allopatry (Seddon 2005) and community-wide partitioning of male songs in acoustic space (Luther 2009). However, the generality of this view is challenged by two observations. First, the songs of rainforest passerines appear to be more stereotyped and less individually variable than most open-country or temperate zone species (Bard et al. 2002; Seddon & Tobias 2007; Kirschel et al. 2009a). Second, rainforest species can coexist with near-identical songs (Tobias & Seddon 2009a). These findings suggest that the challenge of signal discrimination in difficult signalling environments may be overcome by an alternative mechanism, perhaps relying on increased sensory acuity of receivers (Jang & Gerhardt 2006; Tibbetts & Dale 2007; Vignal et al. 2008).
In this study, we tested for acoustically mediated CMR and IMR in two closely related antbirds—Hypocnemis peruviana and H. subflava—which occur sympatrically in the understorey of Amazonian forest (Isler et al. 2007). The communication systems of these two species are unusual because, rather than being disjunct in acoustic space, their songs broadly overlap in structure to the extent that territorial males do not discriminate between conspecific and heterospecific signals (Tobias & Seddon 2009a). Moreover, these taxa are not sister species, and therefore the overlap in signal design and perception appears to represent a clear case of convergent character displacement (Tobias & Seddon 2009a). This pattern of variation is intriguing because male song in most passerine birds, including Hypocnemis antbirds, functions not only in interspecific male–male competition, but in mate attraction (Collins 2004; J. A. Tobias & N. Seddon 2010, unpublished data). This raises the question of whether female antbirds are able to discriminate between species and between individuals on the basis of such minor signal differentiation, or whether other vocal and visual traits mediate individual recognition and, in particular, reproductive isolation.
To address this question, we used acoustic analyses to assess whether male songs encode individual-specific information, and we used playback experiments to test whether females are able to discriminate between conspecific mates, conspecific strangers and heterospecific strangers on the basis of vocal cues. Female responses to male songs are difficult to assess using field experiments, first because recognition may be based on contextual cues such as location, and second because playback treatments typically elicit aggressive responses from resident males (Grant & Grant 2002; Seddon & Tobias 2007; Uy et al. 2009). Therefore, we conducted playbacks on captive females in a natural setting. Again, Hypocnemis antbirds are ideal for this approach because males and females interact with simple male-led duets (Seddon & Tobias 2006; Tobias & Seddon 2009b), allowing us to assess female perception by quantifying their willingness to duet with male song.
2. Material and methods
(a). Study system
Hypocnemis antbirds are medium-sized (11–12 cm, 10–14 g), sexually dimorphic, socially monogamous passerines found in Amazonia, the Guianan region and the Andean foothills (Zimmer & Isler 2003; Isler et al. 2007). Most members of this genus were lumped as a widespread polytypic taxon—H. cantator—which was recently split into six species on the basis of acoustic and genetic data (Isler et al. 2007; Tobias et al. 2008). In October to December 2007, we studied sympatric populations of H. peruviana and H. subflava at the Centro de Investigación y Conservación de Río Los Amigos (CICRA; 12°34′07″ S, 70°05′57″ W), Madre de Díos, Peru. Males and females typically form long-term partnerships, with both sexes contributing equally to parental care, and cooperating to defend stable, year-round territories (reviewed in Zimmer & Isler 2003; J. A. Tobias & N. Seddon 2010, unpublished data). They produce sex-specific, multipurpose songs that function in territory defence and mate attraction (Seddon & Tobias 2006; Tobias & Seddon 2009b; J. A. Tobias & N. Seddon 2010, unpublished data). Given that antbirds are tracheophone suboscine passerines, their songs are thought to develop without learning (Kroodsma 1984; Kroodsma & Konishi 1991; Seddon & Tobias 2007).
(b). Acoustic analyses
This study focused on 18 colour-ringed pairs, nine of H. peruviana and nine of H. subflava. Using a Sennheiser ME67-K3U microphone and a Sound Devices 722 portable digital recorder, we recorded male songs from a distance of less than 10 m. We then selected recordings of high quality (low background noise) to produce a library of digital PCM wav files (24-bit, mono, 44.1 kHz) containing 165 songs in total (mean±s.d. = 9.2 ± 1.2, range = 6–10 songs per male). These songs were converted into spectrograms using Avisoft SASLabPro v. 4.15 (R. Specht, Berlin, Germany) and then described quantitatively using 20 standard temporal and spectral parameters (electronic supplementary material, figure S1; see Seddon & Tobias (2006) for techniques and settings). Following Vignal et al. (2004), we analysed the individual distinctiveness of male songs using (i) non-parametric analysis of variance (Kruskal–Wallis ANOVA); and (ii) a discriminant function analysis (DFA). The DFA allowed us to quantify the extent to which songs could be assigned to the correct individual on the basis of acoustic characteristics. Numerous acoustic variables were strongly correlated with one another (Pearson's correlation > 0.8), and multicollinearity violates a key assumption of DFA (Tabachnick & Fidell 2006).Therefore, following numerous studies (e.g. Vignal et al. 2004; Hollén & Manser 2007), we first conducted a principal components analysis (PCA) and used PC scores (unrotated) rather than raw variables as predictors in the DFA. In both species, PCA produced five PCs that accounted for 81.6 per cent of the variation in the original acoustic dataset (see electronic supplementary material, table S1 for factor loadings). We used F-tests (Wilks' Lambda) to examine whether the overall discriminant models were significant, and cross-validation to estimate error rates (for details see Tobias & Seddon 2009a).
To quantify intra- and inter-individual variation of each acoustic parameter in both species, we calculated the coefficient of variation within individuals (CVi) and between individuals (CVb) according to the formula: CV = (100(s.d./X) (1 + 1/4n)), where s.d. is the standard deviation, X is the mean of the sample and n is the sample size. The potential for individual coding (PIC; Robisson et al. 1993) for each acoustic variable was calculated as the ratio of CVb/CVi-mean. For a given variable, a PIC value >1 suggests that it has greater inter- than intra-individual variability and could therefore be used in individual recognition. Using the same formula, we also quantified the species-specificity of each acoustic variable, where CVi was the coefficient of variation within species, CVb the coefficient of variation between species and PIC > 1 indicated that a variable has greater inter- than intra-specific variability and could therefore be used in species recognition. Following Vignal et al. (2004, 2008), we treat any variable with PIC > 2 as highly individual- or species-specific, and therefore particularly likely to be used as recognition cues by females.
(c). Playback experiments
We conducted playback experiments on 18 females (the mates of all males included in the above analysis). Each female was lured into a 4 × 12 m mistnet using playback of female song between 05.00 and 06.00 h. After capture, individuals were placed in an experimental cage (60 × 40 × 40 cm) located greater than 500 m from the nearest Hypocnemis territory. Food (mainly hand-caught orthopterans) and water were provided ad libitum. Between 06.00 and 08.00 h on the day after capture, each female received three playback treatments comprising male songs of (i) her mate; (ii) a conspecific stranger; and (iii) a heterospecific stranger (i.e. a male H. peruviana to a female H. subflava; or a male H. subflava to a female H. peruviana). Each treatment consisted of one high-quality song repeated 10 times, with 5 s intervals between songs. All 18 males (nine per species) sampled for the acoustic analysis were used for each treatment. Songs were filtered (bandpass: less than 1 and greater than 7 kHz) prior to playback, and broadcast using a Mineroff SME-AFS-amplified loudspeaker connected to an Archos Gmini MP3 player. The loudspeaker was placed 15 m from the cage, 0.5 m above the ground and hidden from view. The experiments were conducted in natural habitat so that signals were transmitted through dense vegetation and against a backdrop of insect noise and birdsong. Peak sound pressure level was adjusted to approximate that of natural songs, i.e. 65 dB SPL at 10 m. Successive treatments were separated by 300 s and the order of treatments was randomized. Each subject received songs from different strangers to avoid pseudoreplication. We ensured that songs of conspecific and heterospecific strangers came from territories at least 500 m away from the subject's own to eliminate effects of neighbour recognition.
Female behaviour was recorded on a Sony video recorder mounted on a tripod. Using 11 min video files, the number of songs and calls given by the female were counted from 5 min before until 5 min after playback. For definition of songs and calls in this system, see Tobias & Seddon (2009a). The 5 min periods of data collection before and after playback were included to quantify background rates of vocal behaviour and to ensure that we captured the full response to playback. The effect of playback on female vocal behaviour was compared between treatments using Friedman tests, followed by Wilcoxon-signed rank tests with p-values corrected for multiple comparisons. All female subjects were released back into their territories immediately after experimental treatments were completed (usually by 08.30 h the day after capture). All released individuals duetted with their mate within 30 min, and no adverse effects of temporary capture were detected. Note that reciprocal experiments on males cannot be conducted as solo males do not respond vocally to female song.
All statistical tests were carried out using SPSS v. 16 (SPSS 2007).
3. Results
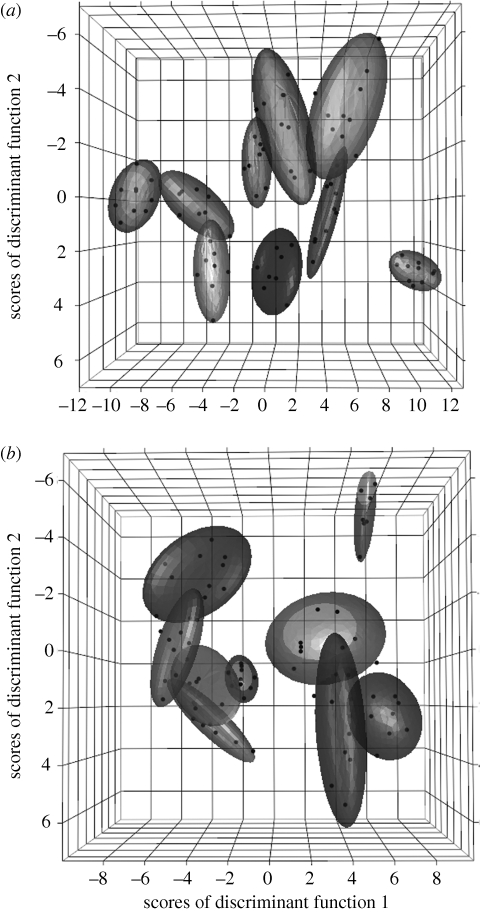
Acoustic analyses revealed that songs of both species are stereotyped signals. Plotting descriptive data extracted from songs shows that their acoustic structure is tightly clustered, with little variation either within-species or within-individuals (figure 1). We also found that songs are individually distinct signatures: cross-validated DFA allowed us to discriminate 97.2 per cent of males in H. peruviana (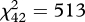 , p < 0.0001) and 86.7 per cent of males in H. subflava (
, p < 0.0001) and 86.7 per cent of males in H. subflava (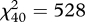 , p < 0.0001; figure 1). This indicated that male songs contain potential cues to individual identity and hence recognition by females. CV analysis further revealed that all but two acoustic characters in H. peruviana and all but four in H. subflava had PIC values greater than 1 (table 1). Of these, the characters most likely to serve as cues to individual identity within species are those with PIC > 2: four in H. peruviana (three temporal and one spectral character) and six in H. subflava (two temporal and four spectral characters). In contrast, only one character (duration of the final note) had PIC > 2, when comparing between species rather than individuals. In other words, we found that songs of male Hypocnemis antbirds are strongly individual-specific but only weakly species-specific.
, p < 0.0001; figure 1). This indicated that male songs contain potential cues to individual identity and hence recognition by females. CV analysis further revealed that all but two acoustic characters in H. peruviana and all but four in H. subflava had PIC values greater than 1 (table 1). Of these, the characters most likely to serve as cues to individual identity within species are those with PIC > 2: four in H. peruviana (three temporal and one spectral character) and six in H. subflava (two temporal and four spectral characters). In contrast, only one character (duration of the final note) had PIC > 2, when comparing between species rather than individuals. In other words, we found that songs of male Hypocnemis antbirds are strongly individual-specific but only weakly species-specific.
Figure 1.
Scatter plots showing individually distinctive structure of male songs in (a) H. peruviana (n = 9), and (b) H. subflava (n = 9). Songs are plotted according to three discriminant functions (DF) derived from 20 acoustic parameters (DF3 is not labelled but is represented by depth). Each male is represented by one normal contour ellipsoid (coverage = 80%) and each point corresponds to one analysed song. In (a) DF1 is positively related to PC1 (which largely reflects note number, note duration, maximum frequency, peak frequency and bandwidth); DF2 is positively related to PC1 and negatively related to PC2 (song duration, overall pace and change in pace) and DF3 is negatively related to PC1 and PC4 (minimum frequency). In (b), DF1 is negatively related to PC1; DF2 is positively related to PC1 and PC2; DF3 is negatively related to PC4 (bandwidth, change in frequency) and positively related to PC5 (pace of segment 2). See electronic supplementary material for factor loadings (table S1) and description of acoustic characters (figure S1).
Table 1.
Acoustic structure of songs of male Hypocnemis antbirds, as revealed by analysis of coefficients of variation (CV) for 20 characters measured for 18 individuals (6–10 songs each). Data shown are mean, s.d. and the potential for individual coding (PIC; see §2). Bold denotes where song characters are highly individualized (i.e. PIC > 2.0).
|
H. peruviana |
H. subflava |
||||||
|---|---|---|---|---|---|---|---|
| song charactera | mean ± s.d. | PIC | Fb | mean ± s.d. | PIC | Fb | PICc |
| note number | 8.19 ± 1.73 | 1.80 | 23.94 | 8.56 ± 1.29 | 2.24 | 33.81 | 1.67 |
| song duration | 2.89 ± 0.67 | 1.53 | 13.96 | 2.92 ± 0.60 | 1.63 | 15.77 | 1.64 |
| duration note 1 | 0.12 ± 0.02 | 1.60 | 15.46 | 0.12 ± 0.01 | 0.89 | 6.46 | 1.69 |
| duration 1st interval | 0.26 ± 0.03 | 1.90 | 421.77 | 0.25 ± 0.02 | 2.04 | 41.80 | 1.71 |
| duration mid note | 0.17 ± 0.03 | 2.12 | 23.09 | 0.15 ± 0.02 | 1.79 | 22.93 | 1.84 |
| duration final note | 0.21 ± 0.03 | 1.19 | 19.00 | 0.18 ± 0.02 | 0.93 | 6.75 | 2.12 |
| overall pace | 2.86 ± 0.26 | 2.20 | 19.20 | 2.98 ± 0.29 | 1.33 | 14.63 | 1.69 |
| pace segment 1 | 3.52 ± 0.41 | 6.57 | 37.65 | 3.69 ± 0.21 | 3.83 | 118.36 | 1.78 |
| pace segment 2 | 2.71 ± 0.34 | 1.59 | 13.10 | 2.74 ± 0.44 | 1.28 | 15.08 | 1.66 |
| change in pace | 1.33 ± 0.21 | 1.94 | 38.73 | 1.40 ± 0.24 | 1.42 | 13.80 | 1.67 |
| max frequency note 1 | 3.18 ± 0.16 | 2.54 | 426.73 | 3.21 ± 0.20 | 3.29 | 86.92 | 1.66 |
| min frequency note 1 | 2.81 ± 0.16 | 1.93 | 22.85 | 2.79 ± 0.11 | 1.21 | 9.12 | 1.67 |
| max frequency mid note | 3.30 ± 0.11 | 1.42 | 27.84 | 3.21 ± 0.23 | 3.34 | 92.28 | 1.79 |
| min frequency mid note | 2.42 ± 0.04 | 0.46 | 56.37 | 2.42 ± 0.10 | 1.12 | 10.13 | 1.77 |
| max frequency end note | 3.28 ± 0.14 | 1.62 | 27.10 | 3.25 ± 0.21 | 2.85 | 68.81 | 1.68 |
| min frequency end note | 2.21 ± 0.16 | 1.55 | 17.93 | 2.28 ± 0.05 | 0.44 | 1.69n.s. | 1.92 |
| peak frequency | 2.86 ± 0.12 | 1.12 | 1.84n.s. | 2.86 ± 0.15 | 1.37 | 16.15 | 1.65 |
| bandwidth note 1 | 0.37 ± 0.07 | 1.10 | 24.59 | 0.42 ± 0.13 | 1.46 | 15.80 | 1.80 |
| bandwidth mid note | 0.89 ± 0.10 | 0.87 | 24.51 | 0.79 ± 0.25 | 2.04 | 42.68 | 1.81 |
| change in frequency | 1.05 ± 0.04 | 1.00 | 60.07 | 1.06 ± 0.03 | 0.57 | 2.82n.s. | 1.68 |
aSee electronic supplementary material (figure S1) for definitions of acoustic characters.
bF-ratio values are for ANOVAs comparing among- and within-male variation, and among- and between-species variation, for each acoustic character; all F ratios are significant at α = 0.05, when Bonferroni-corrected to p ≤ 0.0025 across 20 comparisons, except for those denoted with ‘n.s.’.
cBetween-species comparison.
To investigate the extent to which females use these cues to discriminate between species and individuals, we played the songs of conspecific mates, conspecific strangers and heterospecific strangers to each of 18 females. In both species, no vocalizations were given by any female during the 5 min before or after the playback treatment. However, there was a significant effect of playback treatment on female vocal behaviour (Friedman test; H. peruviana: 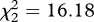 , n = 9, p < 0.0001; H. subflava:
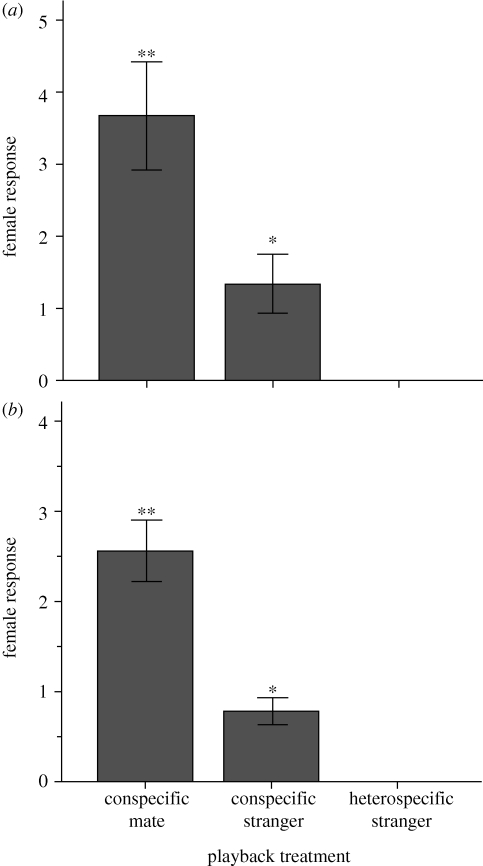
, n = 9, p < 0.0001; H. subflava: 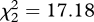 , n = 9, p < 0.0001; see figure 2). Specifically, females of both species gave significantly more vocalizations during playback of their mate's song than during playback of the song of a conspecific stranger (Wilcoxon signed-rank test: H. peruviana—W = 36, n = 9, p = 0.008; H. subflava—W = 45, n = 9, p = 0.004; figure 2). We also found evidence that song mediates species-recognition: females gave more vocalizations in response to conspecific stranger than to heterospecific stranger (H. peruviana—W = 21, n = 9, p = 0.031; H. subflava—W = 28, n = 9, p = 0.016; figure 2). Indeed, no captive female responded to playback of heterospecific song.
, n = 9, p < 0.0001; see figure 2). Specifically, females of both species gave significantly more vocalizations during playback of their mate's song than during playback of the song of a conspecific stranger (Wilcoxon signed-rank test: H. peruviana—W = 36, n = 9, p = 0.008; H. subflava—W = 45, n = 9, p = 0.004; figure 2). We also found evidence that song mediates species-recognition: females gave more vocalizations in response to conspecific stranger than to heterospecific stranger (H. peruviana—W = 21, n = 9, p = 0.031; H. subflava—W = 28, n = 9, p = 0.016; figure 2). Indeed, no captive female responded to playback of heterospecific song.
Figure 2.
Number of songs and calls given by captive female antbirds to playback of songs of their mate, a conspecific stranger and a heterospecific stranger. Data reveal a strong significant effect of playback treatment on female response in both (a) H. peruviana and (b) H. subflava. Females responded most strongly to playback of their mate's song than to playback of conspecific stranger song, and more strongly in response to conspecific stranger song than to heterospecific stranger song. Asterisks denote p-values from Wilcoxon-signed rank tests comparing female response to conspecific mate versus stranger, and to stranger versus heterospecific (*p < 0.05, **p < 0.001). Bars show mean ± s.e.; n = 9 females for all experiments.
4. Discussion
(a). Species recognition
Two of the longest standing paradigms in signal evolution are that species-specific signals are an essential component of reproductive isolation, and that selection for species-specificity is likely to drive signal divergence in closely related sympatric taxa (Marler 1957; Mayr 1963; West-Eberhard 1983; Nelson & Marler 1990; Kirschel et al. 2009b). Recent work has added a new slant to these ideas by suggesting that perception of signal space is imprecise in noisy environments, placing greater limits on the similarity of acoustic signals in coexisting rainforest species (Luther & Wiley 2009). Our results bring the logic of these arguments into question by revealing that females are capable of discriminating between species and individuals on the basis of exceptionally subtle cues, even in dense, diverse and noisy environments. We propose that finely tuned perception by females is an important yet overlooked component of CMR that can allow closely related and acoustically similar species to coexist without experiencing divergent selection.
Our findings demonstrate a clear sexual disparity in response behaviour: females routinely discriminated between conspecific and heterospecific male songs, whereas a previous study showed that males fail to discriminate between conspecific and heterospecific songs during simulated territorial contests (see Tobias & Seddon 2009a). This mismatch in responses makes sense from an evolutionary perspective because females stand to pay higher costs for hybridization, and are therefore expected to be more choosy than males (Parker & Partridge 1998; Kozak et al. 2008). It is tempting to conclude that males and females differ in their ability to discriminate subtly different signals. However, an alternative explanation is that responses vary not with ability but with motivation. In other words, males may be relatively indiscriminate, not because of perceptual constraints, but rather because aggressive responses to similar species are adaptive. This seems especially likely in the case of long-distance songs, which are often used by rainforest birds to defend territories against congeners or other ecologically similar species (Robinson & Terborgh 1995; Tobias & Seddon 2009a).
The idea that interspecific territoriality can drive signal convergence in species that compete over space and resources (Cody 1969), has received recent support from empirical and theoretical studies (Grether et al. 2009; Tobias & Seddon 2009a). Nonetheless, the notion of convergent competitors remains contentious, not least because signal similarity is thought to raise the likelihood of costly reproductive interference (Murray 1976; Gerhardt & Huber 2002; Gröning & Hochkirch 2008). Our study suggests that these costs can be circumvented by the precision of signal perception in females, at least in birds, thus removing an apparent obstacle to signal convergence. This extends the findings of Gerhardt (1994) and Jang & Gerhardt (2006) who showed that reproductive character displacement can target female perception, rather than male signal design. It remains possible that signal discrimination by females in our system reflects this type of displacement in sympatry, but comparable data of female preferences in allopatry are needed to test this idea.
The ability of females to discriminate convergent male signals seems to contradict the message emerging from studies of hybrid zones. For example, work on Ficedula flycatchers shows that signal similarity predicts hybridization (Qvarnström et al. 2006), which in turn may drive reproductive character displacement via reinforcement (Sætre et al. 1997). Why does female perception accommodate convergence in some instances but not others? We believe the answer to this question lies in the fact that mitochondrial DNA sequence divergence between Ficedula hypoleuca and F. albicollis is 3.2 per cent (Sætre et al. 2001), whereas the equivalent divergence in H. peruviana and H. subflava is 6.8 per cent (Tobias et al. 2008). This means that the Hypocnemis lineages are much older (i.e. they split 3.4 Ma rather than 1.6 Ma assuming a 2% molecular clock; Weir & Schluter 2008). Thus, they have had more time to develop signalling incompatibilities, including different contact calls and plumage (Tobias & Seddon 2009a). Such differences may act as a ‘safety net’ against hybridization in older lineages, limiting the scope for divergent character displacement by reinforcement, or any other process. Moreover, prolonged reinforcement may lead to highly resolved signal perception (Gerhardt 1994; Jang & Gerhardt 2006), in which case Ficedula and Hypocnemis may occupy different points along a continuum from early reinforcement to post-reinforcement.
Our demonstration of the precision of female acoustic perception has further implications on studies of species limits and female preferences. First, our results indicate that the similarity of mating signal phenotypes in two populations is not always a reliable predictor of reproductive compatibility, contrary to the prevailing view (Dobzhansky 1940; Mayr 1963; Becker 1982; Baptista & Kroodsma 2001). This raises the possibility that many narrowly divergent cryptic species are erroneously lumped, as was the case with H. peruviana and H. subflava for over a century (Isler et al. 2007). Second, it calls into question the widespread practice of quantifying male responses to playback to make inferences about female choice (e.g. Irwin et al. 2001; Grant & Grant 2002; Seddon & Tobias 2007; Uy et al. 2009). This method assumes that the strength of responses by males is positively correlated with the likelihood of hybridization. However, we have shown that males and females may differ in the extent to which they discriminate between conspecific and heterospecific signals, and that male responses are therefore likely to be an imperfect indicator of female preferences and perceptual ability.
(b). Individual recognition
In oscine songbirds, learning and auditory feedback play a major role in vocal development (reviewed in Beecher & Brenowitz 2005), and the stochasticity inherent in this process promotes individual distinctiveness (Falls 1982; Gil & Gahr 2002). Conversely, the songs of suboscines tend to be much more stereotyped in structure, presumably because they are shaped mainly, if not exclusively, by underlying genetic differences and developmental constraints rather than learning (Gil & Gahr 2002; Seddon & Tobias 2007). However, it also seems plausible that stereotypy, like all ritualization, improves the detection and interpretation of signals against background noise (Cullen 1966; Nelson 1989). From this perspective, reduced differences between individuals in suboscines may promote CMR on the one hand, but inhibit IMR on the other, as proposed by Wiley (2005).
Our results confirm that male songs in Hypocnemis antbirds are highly stereotyped, yet nonetheless individually distinctive. This finding is not unexpected, as some degree of individual distinctiveness has been reported in the songs of three other tropical forest suboscines: screaming piha Lipaugus vociferans (Fitzsimmons et al. 2008), long-tailed manakin Chiroxiphia linearis (Trainer & McDonald 1995) and spotted antbird Hylophylax naevioides (Bard et al. 2002). However, contrary to the hypothesis that forest-dwelling species should have more individually distinctive songs than non-forest species, we found that Hypocnemis songs (mean PIC value ± s.d. across all 20 acoustic characters: H. peruviana—1.85 ± 1.28; H. subflava—1.79 ± 0.94) were much less individually distinctive than vocalizations of two open-country species, alder flycatcher Empidonax alnorum (3.99 ± 2.56, n = 13 males, 18 characters; Lovell & Lein 2004) and zebra finch Taeniopygia guttata (2.33 ± 1.93, calls of n = 6 males, 17 characters; Vignal et al. 2008). The key finding is that, despite relatively low levels of individuality in the songs of males, female suboscines are able to use these vocal signatures to discriminate between conspecific mates and conspecific strangers, providing one of the few examples of learned IMR in animals (Tibbetts & Dale 2007).
Previous tests of recognition in suboscines were conducted on territorial males. These provided some evidence of neighbour recognition in alder flycatchers (Lovell & Lein 2004) and acadian flycatchers Empidonax virescens (Wiley 2005), but not in spotted antbirds Hylophylax naeivioides (Bard et al. 2002). In all cases, however, tests were carried out using field experiments, which suffer the drawback that responses may depend on contextual cues rather than the properties of signals (see Bee & Gerhardt 2002). By applying a standardized technique, our study provides the first robust evidence of individual recognition based on acoustic cues in suboscine birds. Moreover, we have shown that this level of recognition is feasible even in Amazonian rainforest, where signal stereotypy and dense habitat are generally thought to make individual recognition more challenging (Wiley 2005). Our experiments were conducted at a typical distance for intrapair communication, and we have on several occasions observed females responding to playback of their mate's song from much greater distances (greater than 100 m) during male removals (N. Seddon & J. A. Tobias 2010, unpublished data). This suggests that learned IMR is highly developed in suboscines despite their unlearned songs.
Why is accurate individual recognition important in rainforest passerines? Previous demonstrations of IMR in birds involve colonial or flocking species in which mates are expected to be under strong selection to distinguish each other in a crowd (see Beer 1970). This explains the prevalence of IMR in Magellanic penguins Sphenurus magellanicus (Clark et al. 2006), spectacled parrotlets Forpus conspicillatus (Wanker et al. 1998), silvereyes Zosterops lateralis (Robertson 1996), zebra finches (Vignal et al. 2008) and pinyon jays Gymnorhinus cyanocephalus (Marzluff 1996). To the best of our knowledge, this study is the first to demonstrate acoustically mediated IMR in a pair-living species. We suggest that this form of IMR will be found to apply more generally, first because stable partnerships are common in the tropics, typically involving long-term collaborations over territory defence and offspring care (Greenberg & Gradwohl 1986; Morton 1996), and second because many such species live in dense habitats, where selection should favour acoustic recognition systems.
Our results highlight the importance of considering the receiver's perspective when making inferences about reproductive isolation, signal evolution and signaller–receiver interactions. They show that, even though female antbirds are faced with discriminating signals in one of the most demanding of signalling environments, their perception of stereotyped and convergent male signals is sufficiently acute to mediate both CMR and IMR. These findings challenge the long-standing idea that coexistence of closely related species automatically exerts divergent selection on signals, and the more recent proposal that this effect is accentuated in tropical forests. Instead, our data suggest that the costs of signal similarity and convergent character displacement may be much lower than is generally assumed, with potentially far-reaching implications for the study of reproductive and agonistic character displacement (Grether et al. 2009; Pfennig & Pfennig 2009; Hoskin & Higgie 2010). In particular, they may help to explain why there are so few unambiguous demonstrations of divergent character displacement in animal signals, and how the signals of older lineages may converge without breaking down barriers to gene flow.
Acknowledgements
This study complies with the protocols set out by Instituto Nacional de Recursos Naturales (INRENA) in Peru, and conforms to ASAB guidelines for ethical research.
We are grateful to INRENA and Asociación para la Conservación de la Cuenca Amazónica (ACCA) for granting permission to carry out research at CICRA. We also thank Nigel C. Pitman for logistical support, and Julissa Cabrera, Dominic Cram, Victor Gamarra-Toledo and Diego García-Olaechea for assistance with data collection and the arduous task of catching grasshoppers in bulk. This research was funded by the Royal Society.
References
- Baptista L. F., Kroodsma D. E.2001Avian bioacoustics. In Handbook of the birds of the world (eds del Hoyo J., Elliott A., Sargatal J.), pp. 11–52 Barcelona, Spain: Lynx Edicions [Google Scholar]
- Bard S. C., Hau M., Wikelski M., Wingfield J. C.2002Vocal distinctiveness and response to conspecific playback in the spotted antbird, a Neotropical suboscine. Condor 104, 387–394 (doi:10.1650/0010-5422(2002)104[0387:VDARTC]2.0.CO;2) [Google Scholar]
- Becker P. H.1982The coding of species-specific characteristics in bird sounds. In Acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 213–252 New York, NY: Academic Press [Google Scholar]
- Bee M. A., Gerhardt H. C.2002Individual voice recognition in a territorial frog (Rana catesbeiana). Proc. R. Soc. B 269, 1443–1448 (doi:10.1098/rspb.2002.2041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher M. D., Brenowitz E. A.2005Functional aspects of song learning in songbirds. Trends Ecol. Evol. 20, 143–150 (doi:10.1016/j.tree.2005.01.004) [DOI] [PubMed] [Google Scholar]
- Beer C. G.1970Individual recognition of voice in the social behaviour of birds. Adv. Study Behav. 3, 27–74 (doi:10.1016/S0065-3454(08)60154-0) [Google Scholar]
- Bradbury J. W., Vehrencamp S. L.1998Principles of animal communication Sunderland, MA: Sinauer Associates [Google Scholar]
- Chek A. A., Bogart J. P., Lougheed C.2003Mating signal partitioning in multi-species assemblages: a null model test using frogs. Ecol. Lett. 6, 235–247 (doi:10.1046/J.1461-0248.2003.00420.x) [Google Scholar]
- Clark J. A., Boersma P. D., Olmsted D. M.2006Name that tune: call discrimination and individual recognition in Magellanic penguins. Anim. Behav. 72, 1141–1148 (doi:10.1016/j.anbehav.2006.04.002) [Google Scholar]
- Cody M. L.1969Convergent characteristics in sympatric species: a possible relation to interspecific competition and aggression. Condor 71, 222–239 [Google Scholar]
- Collins S.2004Vocal fighting and flirting: the functions of birdsong. In Nature's music: the science of birdsong (eds Marler P., Slabbekoorn H.), pp. 39–79 London, UK: Elsevier [Google Scholar]
- Cullen J. M.1966Reduction of ambiguity through ritualization. Phil. Trans. R. Soc. Lond. B 251, 363–374 (doi:10.1098/rstb.1966.0022) [Google Scholar]
- Dobzhansky T.1940Speciation as a stage in evolutionary divergence. Am. Nat. 74, 312–321 [Google Scholar]
- Falls J. B.1982Individual voice recognition by sounds in birds. In Acoustic communication in birds, vol. 2. Song learning and its consequences (eds Kroodsma D. E., Miller E. H., Quellet H.), pp. 237–278 New York, NY: Plenum Press [Google Scholar]
- Fisher R. A.1930The genetical theory of natural selection Oxford, UK: Oxford University Press [Google Scholar]
- Fitzsimmons L. P., Barker N. K., Mennill D. J.2008Individual variation and lek-based vocal distinctiveness in songs of the screaming piha (Lipaugus vociferans), a suboscine songbird. Auk 125, 908–914 (doi:10.1525/auk.2008.07128) [Google Scholar]
- Gerhardt H. C.1994Reproductive character displacement on female mate choice in the grey treefrog Hyla chrysocelis. Anim. Behav. 47, 959–969 (doi:10.1006/anbe.1994.1127) [Google Scholar]
- Gerhardt H. C., Huber F.2002Acoustic communication in insects and anurans Chicago, IL: Chicago University Press [Google Scholar]
- Gil D., Gahr M.2002The honesty of bird song: multiple constraints for multiple traits. Trends Ecol. Evol. 17, 133–141 (doi:10.1016/S0169-5347(02)02410-2) [Google Scholar]
- Godard R.1991Long-term memory of individual neighbours in a migratory songbird. Nature 350, 228–229 (doi:10.1038/350228a0) [Google Scholar]
- Grant B. R., Grant P. R.2002Simulating secondary contact in allopatric speciation: an empirical test of premating isolation. Biol. J. Linn. Soc. 76, 545–556 (doi:10.1046/j.1095-8312.2002.00076.x) [Google Scholar]
- Greenberg R., Gradwohl J.1986Stable territories and constant densities in tropical forest insectivorous birds. Oecologia 69, 618–625 (doi:10.1007/BF00410372) [DOI] [PubMed] [Google Scholar]
- Grether G. F., Losin N., Anderson C. N., Okamoto K.2009The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 84, 617–635 (doi:10.1111/j.1469-185X.2009.00089.x) [DOI] [PubMed] [Google Scholar]
- Gröning J., Hochkirch A.2008Reproductive interference between animal species. Q. Rev. Biol. 83, 257–282 (doi:10.1086/590510) [DOI] [PubMed] [Google Scholar]
- Hollén L., Manser M. B.2007Motivation before meaning: motivational information encoded in meerkat alarm calls develops earlier than referential information. Am. Nat. 169, 758–767 [DOI] [PubMed] [Google Scholar]
- Hoskin C. J., Higgie M.2010Speciation via species interactions: the divergence of mating traits within species. Ecol. Lett. 13, 409–420 (doi:10.1111/j.1461-0248.2010.01448.x) [DOI] [PubMed] [Google Scholar]
- Insley S. J.2000Long-term vocal recognition in the northern fur seal. Nature 406, 404–405 (doi:10.1038/35019064) [DOI] [PubMed] [Google Scholar]
- Irwin D. E., Price T.1999Sexual imprinting, learning and speciation. Heredity 82, 347–354 (doi:10.1038/sj.hdy.6885270) [DOI] [PubMed] [Google Scholar]
- Irwin D. E., Bensch S., Price T.2001Speciation in a ring. Nature 409, 333–337 (doi:10.1038/35053059) [DOI] [PubMed] [Google Scholar]
- Isler M. L., Isler P. R., Whitney B. M.2007Species limits in antbirds (Thamnophilidae): the Hypocnemis cantator complex. Auk 124, 11–28 (doi:10.1642/0004-8038(2007)124[11:SLIATT]2.0.CO;2) [Google Scholar]
- Jang Y., Gerhardt H. C.2006Divergence in female calling song discrimination between sympatric and allopatric populations of the southern wood cricket Gryllus fultoni (Orthoptera: Gryllidae). Behav. Ecol. Sociobiol. 60, 150–158 (doi:10.1007/s00265-005-0151-3) [DOI] [PubMed] [Google Scholar]
- Johnston R. E., Bullock T. A.2001Individual recognition by use of odours in golden hamsters: the nature of individual representations. Anim. Behav. 61, 545–557 (doi:10.1006/anbe.2000.1637) [Google Scholar]
- Kirschel A. N. G., Blumstein D. T., Cohen R. E., Buermann W., Smith T. B., Slabbekoorn H.2009aBirdsong tuned to the environment: green hylia song varies with elevation, tree cover and noise. Behav. Ecol. 20, 1089–1095 [Google Scholar]
- Kirschel A. N. G., Blumstein D. T., Smith T. B.2009bCharacter displacement of song and morphology in African tinkerbirds. Proc. Natl Acad. Sci. USA 106, 8256–8261 (doi:10.1073/pnas.0810124106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak G. M., Reisland M., Boughman J. W.2008Sex differences in mate recognition and conspecific preference in species with mutual mate choice. Evolution 63, 353–365 (doi:10.1111/j.1558-5646.2008.00564.x) [DOI] [PubMed] [Google Scholar]
- Kroodsma D. E.1984Songs of the alder flycatcher (Empidonax alnorum) and willow flycatcher (Empidonax traillii) are innate. Auk 101, 13–24 [Google Scholar]
- Kroodsma D. E., Konishi M.1991A suboscine bird (eastern phoebe, Sayornis phoebe) develops normal song without auditory feedback. Anim. Behav. 42, 477–487 (doi:10.1016/S0003-3472(05)80047-8) [Google Scholar]
- Lovell S. F., Lein M. R.2004Neighbor–stranger discrimination by song in a suboscine bird, the alder flycatcher, Empidonax alnorum. Behav. Ecol. 15, 799–804 (doi:10.1093/beheco/arh082) [Google Scholar]
- Luther D.2009The influence of the acoustic community on songs of birds in a neotropical rainforest. Behav. Ecol. 20, 864–871 (doi:10.1093/beheco/arp074) [Google Scholar]
- Luther D. A., Wiley R. H.2009Production and perception of communicatory signals in a noisy environment. Biol. Lett. 5, 183–187 (doi:10.1098/rsbl.2008.0733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A. E., Ramnarine I. W.2005Evolution of mate discrimination in a fish. Curr. Biol. 15, R867–R868 (doi:10.1016/j.cub.2005.10.034) [DOI] [PubMed] [Google Scholar]
- Marler P.1957Specific distinctiveness in the communication signals of birds. Behaviour 11, 13–39 (doi:10.1163/156853956X00066) [Google Scholar]
- Marzluff N.1996Vocal recognition of mates by breeding pinyon jays Gymnorhinus cyanocephalus. Anim. Behav. 36, 296–298 (doi:10.1016/S0003-3472(88)80275-6) [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, MA: Belknap Press [Google Scholar]
- Miller E. H.1982Character and variance shift in acoustic signals of birds. In Acoustic communication in birds (eds Kroodsma D. E., Miller E. H.), pp. 253–295 New York, NY: Academic Press [Google Scholar]
- Morton E. S.1996A comparison of vocal behavior among tropical and temperate passerine birds Ithaca, NY: Cornell University Press [Google Scholar]
- Murray B. G.1976A critique of interspecific territoriality and character convergence. Condor 78, 518–525 (doi:10.2307/1367102) [Google Scholar]
- Nelson D. A.1989The importance of invariant and distinctive features in species recognition of bird song. Condor 91, 120–130 (doi:10.2307/1368155) [Google Scholar]
- Nelson D. A., Marler P.1990The perception of bird song and an ecological concept of signal space. In Comparative perception: complex signals, vol. 2 (eds Stebbins W. C., Berkley M. A.), pp. 443–478 New York, NY: Wiley [Google Scholar]
- Nosil P., Crespi B. J., Sandoval C. P.2003Reproductive isolation driven by the combined effects of ecological adaptation and reinforcement. Proc. R. Soc. Lond. B 270, 1911–1918 (doi:10.1098/rspb.2003.2457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G. A., Partridge L.1998Sexual conflict and speciation. Phil. Trans. R. Soc. Lond. B 353, 261–274 (doi:10.1098/rstb.1998.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig K. S., Pfennig D. W.2009Character displacement: ecological and reproductive responses to a common evolutionary problem. Q. Rev. Biol. 84, 253–276 (doi:10.1086/605079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnström A., Haavie J., Saether S. A., Eriksson D., Pärt T.2006Song similarity predicts hybridization in flycatchers. J. Evol. Biol. 19, 1202–1209 (doi:10.1111/j.1420-9101.2006.01140.x) [DOI] [PubMed] [Google Scholar]
- Richards D. G., Wiley R. H.1980Reverberations and amplitude fluctuations in the propagation of sound in a forest—implications for animal communication. Am. Nat. 115, 381–399 [Google Scholar]
- Robertson B. C.1996Vocal mate-recognition in a monogamous, flock-forming bird, the silvereye Zosterops lateralis. Anim. Behav. 51, 303–311 (doi:10.1006/anbe.1996.0030) [Google Scholar]
- Robinson S. K., Terborgh J.1995Interspecific aggression and habitat selection by Amazonian birds. J. Anim. Ecol. 64, 1–11 [Google Scholar]
- Robisson P., Aubin T., Brémond J. C.1993Individuality in the voice of emperor penguin Aptenodytes forsteri: adaptation to a noisy environment. Ethology 94, 279–290 [Google Scholar]
- Sætre G. P., Moum T., Bures S., Kral M., Adamjan M., Moreno J.1997A sexually selected character displacement in flycatchers reinforces premating isolation. Nature 387, 589–591 (doi:10.1038/42451) [Google Scholar]
- Sætre G. P., Borge T., Lindell J., Moum T., Primmer C. R., Sheldon B. C., Haavie J., Johnsen A., Ellegren H.2001Speciation, introgressive hybridization and nonlinear rate of molecular evolution. Mol. Ecol. 10, 737–749 [DOI] [PubMed] [Google Scholar]
- Seddon N.2005Ecological adaptation and species recognition drive vocal evolution in Neotropical suboscine birds. Evolution 59, 200–215 [PubMed] [Google Scholar]
- Seddon N., Tobias J. A.2006Duets defend mates in a suboscine passerine, the warbling antbird (Hypocnemis cantator). Behav. Ecol. 17, 73–83 (doi:10.1093/beheco/ari096) [Google Scholar]
- Seddon N., Tobias J. A.2007Song divergence at the edge of Amazonia: an empirical test of the peripatric speciation model. Biol. J. Linn. Soc. 90, 173–188 (doi:10.1111/j.1095-8312.2007.00753.x) [Google Scholar]
- Slabbekoorn H., Smith T. B.2002Habitat-dependent song divergence in the little greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution 56, 1849–1858 [DOI] [PubMed] [Google Scholar]
- Tabachnick B., Fidell L.2006Using multivariate statistics Boston, MA: Allyn & Bacon [Google Scholar]
- Tibbetts E. A.2002Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. B 269, 1423–1428 (doi:10.1098/rspb.2002.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E. A., Dale J.2007Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537 (doi:10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- Tobias J. A., Seddon N.2009aSignal design and perception in Hypocnemis antbirds: evidence of convergent evolution via social selection. Evolution 63, 3168–3189 [DOI] [PubMed] [Google Scholar]
- Tobias J. A., Seddon N.2009bSignal jamming mediates sexual conflict in a duetting bird. Curr. Biol. 19, 577–582 (doi:10.1016/j.cub.2009.02.036) [DOI] [PubMed] [Google Scholar]
- Tobias J. A., Bates J. M., Hackett S. J., Seddon N.2008Comment on the latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 319, 901c (doi:10.1126/science.1150568) [DOI] [PubMed] [Google Scholar]
- Trainer J. M., McDonald D. B.1995Singing performance, frequency matching and courtship success of long-tailed manakins (Chiroxiphia linearis). Behav. Ecol. Sociobiol. 37, 249–254 (doi:10.1007/BF00177404) [Google Scholar]
- Uy J. A. C., Moyle R. G., Filardi C. E.2009Plumage and song differences mediate species recognition between incipient flycatcher species of the Solomon Islands. Evolution 63, 153–164 (doi:10.1111/j.1558-5646.2008.00530.x) [DOI] [PubMed] [Google Scholar]
- Vignal C., Mathevon N., Mottin S.2004Audience drives male songbird response to partner's voice. Nature 430, 448–451 (doi:10.1038/nature02645) [DOI] [PubMed] [Google Scholar]
- Vignal C., Mathevon N., Mottin S.2008Mate-recognition by female zebra finch: analysis of individuality in male call and first investigations on female decoding process. Behav. Processes 77, 191–198 (doi:10.1016/j.beproc.2007.09.003) [DOI] [PubMed] [Google Scholar]
- Wanker R., Apcin J., Jennerjahn B., Waibel B.1998Discrimination of different social companions in spectacled parrotlets (Forpus conspicillatus): evidence for individual vocal recognition. Behav. Ecol. Sociobiol. 43, 197–202 (doi:10.1007/s002650050481) [Google Scholar]
- Weir J. T., Schluter D.2008Calibrating the avian molecular clock. Mol. Ecol. 17, 2321–2328 (doi:10.1111/j.1365-294X.2008.03742.x) [DOI] [PubMed] [Google Scholar]
- West-Eberhard M. J.1983Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 [Google Scholar]
- Wiley R. H.2005Individuality in songs of Acadian flycatchers and recognition of neighbours. Anim. Behav. 70, 237–247 (doi:10.1016/j.anbehav.2004.09.027) [Google Scholar]
- Wollerman L., Wiley R. H.2002Possibilities for error during communication by Neotropical frogs in a complex acoustic environment. Behav. Ecol. Sociobiol. 52, 465–473 (doi:10.1007/s00265-002-0534-7) [Google Scholar]
- Zimmer K. J., Isler M. L.2003Family Thamnophilidae (typical antbirds). In Handbook of birds of the world, vol. 8 (eds del Hoyo J., Elliott A., Christie D.), pp. 448–681 Barcelona, Spain: Lynx Edicions [Google Scholar]