Abstract
Although migration is a widespread and taxonomically diverse behaviour, the ecological factors shaping migratory behaviour are poorly understood. Like other montane taxa, many birds migrate along elevational gradients in the tropics. Forty years ago, Alexander Skutch postulated that severe storms could drive birds to migrate downhill. Here, we articulate a novel mechanism that could link storms to mortality risks via reductions in foraging time and provide, to our knowledge, the first tests of this hypothesis in the White-ruffed Manakin (Corapipo altera), a small partially migratory frugivore breeding on the Atlantic slope of Costa Rica. As predicted, variation in rainfall was associated with plasma corticosterone levels, fat stores, plasma metabolites and haematocrit. By collecting data at high and low elevation sites simultaneously, we also found that high-elevation residents were more adversely affected by storms than low elevation migrants. These results, together with striking temporal capture patterns of altitudinal migrants relative to storms, provide, to our knowledge, the first evidence that weather-related risks incurred by species requiring high food intake rates can explain altitudinal migrations of tropical animals. These findings resolve conflicting evidence for and against food limitation being important in the evolution of this behaviour, and highlight how endogenous and exogenous processes influence life-history trade-offs made by individuals in the wild. Because seasonal storms are a defining characteristic of most tropical ecosystems and rainfall patterns will probably change in ensuing decades, these results have important implications for understanding the ecology, evolution and conservation of tropical animals.
Keywords: allostasis, climate change, foraging, metabolism, partial migration, tropical forest
1. Introduction
Animal migrations often involve tremendous energetic costs, physical risks and precise spatial and temporal control. Identifying the selective factors responsible for shaping migratory behaviour remains extremely challenging owing to the difficulty of following individuals between their breeding and non-breeding grounds. Many tropical taxa (birds, bats and insects) migrate annually up and down elevational gradients (Stiles 1988; Timm & LaVal 2000; Haber & Stevenson 2004) but the causes of these migrations remain unknown. An often-invoked explanation for altitudinal migration is that food limitation leads frugivores and nectarivores to exploit seasonal and spatial pulses of food. However, increasing evidence suggests that patterns of food availability are not sufficient to explain this behaviour (e.g. Solórzano et al. 2000; Chaves-Campos et al. 2003; Boyle 2010). In 1969, Alexander Skutch speculated that tropical storms could affect the altitudinal migrations of birds (Skutch 1969). Boyle (2008b) articulated a possible mechanism for this observation by proposing the limited foraging opportunities (LFO) hypothesis, which postulates that altitudinal migration of tropical animals is driven by weather events that reduce foraging opportunities. Arising from the ‘body size hypothesis’ (Ketterson & Nolan 1976), the LFO postulates that in tropical wet forests subject to severe multi-day storms, heavy rain may restrict the number of hours available for foraging. Whereas the body size hypothesis was formulated explicitly in the context of temperate species where larger individuals would experience thermal advantages during winter and fasting advantages when snow and ice covered food supply (Belthoff & Gauthreaux 1991), the LFO hypothesis relies only on the fasting advantages of increased body size when access to food is restricted by rains that impede foraging. For animals with high metabolic rates and high food intake rates (e.g. small frugivorous birds), such foraging restrictions could reduce energy intake to the point of starvation. Cast in terms of allostasis models (‘achieving stability through change’; McEwen & Wingfield 2003), the LFO hypothesis postulates that storms (which are predictable seasonally, but unpredictable on daily or weekly time scales) represent both seasonal increases in allostatic loads (allostasis level B; Landys et al. 2006) as well as unpredictable stressors that result in energy demand surpassing supply leading to allostatic overload (level C; Landys et al. 2006). Downhill migration to drier sites is hypothesized to decrease allostatic load and increase survival for individuals whose morphology, diet and life history render them most vulnerable to storm-related risks.
Here we provide, to our knowledge, the first tests of the LFO hypothesis in White-ruffed Manakins (Corapipo altera Hellmayr, Pipridae), a small lekking frugivore that breeds in montane Central American wet forests. Some individuals migrate downhill after breeding and others remain year-round at breeding sites. Neither food abundance (Boyle 2010) nor predation risk (Boyle 2008a) can explain partial altitudinal migration in this species. Migratory tendency is also not related to age, as would be expected if competition for food drives some individuals downhill (i.e. ‘dominance hypothesis’; Lundberg 1985), nor is the male-biased migration consistent with competition for display sites (i.e. ‘arrival time hypothesis’; Ketterson & Nolan 1976), explaining intraspecific variation in migratory tendency. Furthermore, several condition-related variables are related to or interact with sex in their relation to migratory tendency (Boyle 2008b). Higher-elevation breeding sites receive double the rainfall of lowland non-breeding sites and White-ruffed Manakins primarily consume low-fat, low-protein, watery fruits (approx. 7% sugar; Lumpkin & Boyle 2009) leading to high food intake rates (Boyle 2009). Sex-related differences in migratory tendency are consistent with the LFO hypothesis because males are 15 per cent smaller than females. Energy stores scale with body size roughly to the power of 1, but metabolic rate in passerines scales to the power of approximately 0.72 (Calder 1974); thus, the ratio of energy stored to energy used is greater in larger individuals so smaller males should experience greater mortality risks of fasting.
We used several physiological metrics to test predictions of the LFO hypothesis. High baseline levels of the glucocorticosteroid corticosterone (CORT) indicate compromised individual condition, mediate allostasis and reflect a response to stressors such as foraging unpredictability or bad weather (Wingfield & Ramenofsky 1997; Romero et al. 2000). Therefore, we predicted that during storms CORT levels should rise, and that resident birds at higher elevations should experience greater storm-induced allostatic loads than migrants in the lowlands. Because birds respond to foraging uncertainty by increasing fat stores (Rogers 1993; Reneerkens et al. 2002), we predicted that storms should be associated with increased stored fat and a greater reliance on fats for fuel (estimated via levels of the plasma metabolite Β-hydroxybutyrate, hereafter ‘BUTY’). Plasma metabolites have been used to examine fasting and fuelling dynamics during migratory stopover (e.g. Lyons et al. 2008), but we, to our knowledge, use them here for the first time to examine metabolic responses to weather. BUTY is synthesized from fatty acids and largely replaces glucose in fuelling metabolism during fasting (Ramenofsky 1990). If migrants escape the most severe effects of storms, we also predicted that fat and BUTY should be elevated in residents relative to migrants. Circulating levels of triglycerides (hereafter ‘TRIG’) provide insight into the dynamics of fuel storage over short time-scales (Jenni-Eiermann & Jenni 1994) by reflecting fat deposition and foraging rates (Guglielmo et al. 2005). Thus, we expected that TRIG would increase immediately prior to- or during breaks in storms, and to be higher in residents than migrants after storms owing to greater demand for fat. If residents exhaust both carbohydrates derived from recently consumed foods as well as fat reserves and enter an ‘emergency life-history stage’ (Wingfield et al. 1998), we predicted that lean mass would decrease during storms (with greater decreases in residents) as birds catabolize protein to stay alive. Finally, haematocrit can reflect differences in condition owing to nutritional stress (Piersma et al. 2000). Thus, haematocrit should decrease during storms and be lower in residents than migrants. Because of previous interactions between sex and condition-related variables, we examined how each response varied among birds of different sexes.
2. Material and methods
We worked at two sites adjoining Braulio Carrillo National park in northeast Costa Rica (see the electronic supplementary material, figure S1). The park and adjacent reserves protect approximately 50 000 ha of forest from 30 m above sea level to approximately 2900 m. Our higher-elevation site was at the Rara Avis and Selva Tica reserves (hereafter ‘RA’) at approximately 650–850 m in the breeding range of White-ruffed Manakins. Our lower-elevation site was at La Selva Biological Station (‘LS’) in lowland forest at the base of the mountains. Mean (±s.e.) annual rainfall at RA and LS is 8267 mm (±227 mm) and 4306 mm (±101 mm), respectively. Over the whole gradient, the heaviest rains fall in the middle (approx. June–July) and end (December) of the year with February–April being the driest months (RA and LS, unpublished weather data; see the electronic supplementary material, figure S1). White-ruffed Manakins initiate breeding in March with peak fledging in June and downhill migration occurring throughout the second half of the year (Rosselli 1994). Migrants are present at low elevations for a variable number of months each year, arriving between July and November and returning uphill in approximately February. All birds appear to return to higher elevations during the breeding season; we know of no lowland captures of White-ruffed Manakins during April–July. Thus, all ‘migrants’ in this study must truly be migrants, but it is possible that ‘residents’ may include some individuals who migrated subsequent to their capture at RA.
We worked concurrently at both sites from 11 October–27 December 2008, opening 2–10 (mean 5) mist nets (12 × 3.5 m, 35 mm mesh) per site for an average of 5.5 h d−1, primarily between 06.00–13.00. We located understorey nets in both old growth and tall second growth forest, moving nets every two days to maximize capture rates. We opened nets in all weather, positioning them in sight of an observer. We drew up to 30 µl blood from the brachial vein within 3 min of a bird's capture for analysis of baseline CORT (Wingfield et al. 1982), and an additional of up to 70 µl within 7 min for plasma metabolite analysis (Guglielmo et al. 2002). We collected blood in heparinized capillary tubes, stored samples on ice, and centrifuged them the same day, at which time we measured haematocrit. To estimate body composition, we injected birds with 30 µl ‘heavy’ water (99.999 atomic per cent of Deuterium (D), Sigma-Aldrich) into the pectoralis muscle using a 0.3 cc insulin syringe and kept birds 40 min in a covered cage while injected water mixed with body water (Speakman et al. 2001). We then collected up to 50 µl of urine by inserting a closed-ended, perforated cannula, made of polyethylene tubing (PE160, Intramedic, MD, USA) into the bird's cloaca. We transferred urine to capillary tubes and flame sealed both ends to minimize exchange with atmospheric hydrogen (Speakman 1997). We estimated fat reserves using a visual index of subcutaneous body fat (Kaiser 1993), weighed birds using a digital balance (0.01 g), measured wing chord, and placed a numbered aluminium leg band on birds prior to release. Our dataset does not include all variables for all individuals owing to occasional uncertainty of capture time or difficulties obtaining sufficient sample volume.
At LS we stored plasma at −80°C and at RA we stored plasma at approximately −10°C for 1–6 days before transferring to −80°C at LS. We analysed TRIG using a colorimetric endpoint assay (Sigma reagents T2449, F6428 and G7793) run on a Spectronic Helios β spectrophotometer at LS. We ran assays using 10 µl undiluted plasma and 800 µl and 200 µl of reagents A and B (warmed to 37°C) with absorbance measured at 540 nm. We calculated new standard curves each day that we ran assays, and analysed residual TRIG after accounting for fat score because fat stores affect TRIG levels independent of fat deposition rates (Hays 2008). All other assays were conducted at the University of Western Ontario. To comply with import regulations, we diluted plasma destined for BUTY and CORT assays 1 : 1 with 70 per cent ethanol the day before transport (Wikelski et al. 2004) and refroze samples within 24 h. We analysed BUTY by kinetic endpoint assay within one week of dilution using a microplate spectrophotometer (R-Biopharm Kit 0907979) following methods described in Guglielmo et al. (2005). Prior to this study, we determined that diluting plasma with ethanol did not affect BUTY when assays were conducted within a week of dilution. We extracted CORT from plasma using dichloromethane within three weeks of dilution and measured CORT using a double-antibody 125I radioimmunoassay (ImmuChem 07-107 103; MP Biomedicals, Orangeburg, NY) follow methods in Newman et al. (2008). Ethanol dilution results in slight reductions in measured CORT (Goymann et al. 2007) implying that comparison with studies using undiluted plasma should be conducted with caution. Nevertheless, because the ethanol storage period was of short and equal duration for all samples, our inferences are unaffected by sample preservation.
Because blood plasma samples have been used in the few previous studies using the isotope dilution method for estimating body composition, we validated our methods by sampling both urine and blood from six White-ruffed Manakins during February 2009 following the same procedures outlined above. For all urine and plasma samples used in body composition analyses, we vacuum distilled the water from samples in evacuated and flame sealed pasteur pipettes by placing sideways on a slide warmer with the narrow end cooled by room air. After 48 h the distilled water collected in the narrow end which we removed by flame sealing. We measured the concentration of D in distilled water using a Fourier transform infrared spectrophotometer (Nicolet 380 FT-IR Thermo Electron Corp. Madison WI, with the SmartMIRacle single reflection Micro ATR accessory) following Rae et al. (2009). We ran background scans prior to each sample and calculated atomic per cent of D based on peak height of the (5 µl) sample spectrum at 2493 nm relative to atomic per cent of D in the standard curve (R = 0.998) run the same day as the samples. We estimated total body water (TBW) using equations in Speakman et al. (2001). Estimates of heavy water dilution in blood and urine samples differed by only −0.005 atomic per cent ± s.e. −0.011. Correlation of paired samples resulted in an R2 of 0.69 (D2Oblood = −0.033 + 1.114 D2Ourine). Although the intercept was not statistically different from zero (p = 0.155), we used this equation to correct for slight differences between blood and urine D2O values to facilitate comparison with results of other studies. We used TBW as an index of lean mass because we were unable to calibrate TBW by sacrificing White-ruffed Manakins for direct measurement of body composition. Thus, we analysed residual TBW corrected for wing chord and sex as our response variable.
We analysed each response variable separately using a two-tiered approach. First, we constructed a series of linear regression models (ordinal logistic regression for fat score) using five rainfall predictor variables: rain at the site of capture (i) on the day of capture, (ii) the day before capture, (iii) over 48 h (day of and day before), (iv) 72 h and (v) 96 h periods. Although only rain falling during daylight should affect foraging, more detailed weather data were not available. Thus, for each response variable, we chose the rainfall parameter having the greatest explanatory power (highest R2) for use in subsequent models. We then constructed multivariate general linear models using Akaike information criterion (AIC; Burnham & Anderson 2002) to select from the following parameters: rainfall, site, sex, age (hatch year/after hatch year), body mass (except in models of TBW and fat score), time of capture, ordinal day and two- and three-way interactions between sex, rain and site. We included time of capture as this has been shown to be related to plasma metabolite levels (Guglielmo et al. 2002). Parameter values for individual model effects are presented as least square means ± s.e.
3. Results
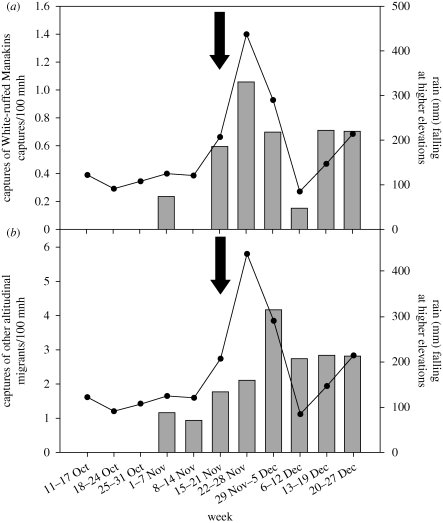
We captured 85 White-ruffed Manakins in 6370 mist net hours (mnh). Consistent with long-term records, rainfall at RA during the field season was nearly double that of LS (1947 mm versus 1087 mm). However, rainfall during October and the first half of November was lower than average and the first multi-day storm during these months began mid-November (figure 1). Coincident with the change in weather, we detected a marked increase in abundance of all altitudinal migrant species at LS; capture rates of manakins rose from 0.1/100 mnh before 16 November to 1.2/100 mnh after 16 November (Fisher's exact test, p = 0.012; figure 1a) which mirrored an overall dramatic increase in the proportion of lowland captures represented by all altitudinal migrant species (from 1.0/100 to 5.9/100 mnh, 61 altitudinal migrants of 521 captures, Fisher's exact test, p < 0.0001; figure 1b). At RA, manakin capture rates declined from 7.4/100 to 5.4/100 mnh, and the sex ratio changed from a pre-storm male bias (70% of 48 captures male) to a post-storm female bias (33% of 24 captures male). Additionally, 92 per cent (11 out of 12) of the manakins captured at LS were males, confirming previous evidence for male-biased migration in this species (Boyle 2008b). Adult males appeared to be more likely to migrate than younger males; the proportion of adults to sub-adults at RA declined from 47 per cent (n = 34) before 16 November to 12.5 per cent (n = 8) after 16 November. Because the nature of these migratory movements is unknown, we cannot infer which proximate cues triggered downhill movements; possibly birds anticipated storms via atmospheric pressure changes and reacted behaviourally and/or physiologically depending on their condition.
Figure 1.
Arrival of altitudinal migrants to low elevation sites is temporally associated with the onset of storms; bars represent capture rates (per 100 mist net hours (mnh)) of (a) White-ruffed Manakins and (b) all other altitudinal migrant species weekly during October–December 2008 in lowland forest (LS). Dots and lines represent the amount of rain falling during the week at the higher-elevation site (RA). Arrows depict the week during which the first major storm of the season began.
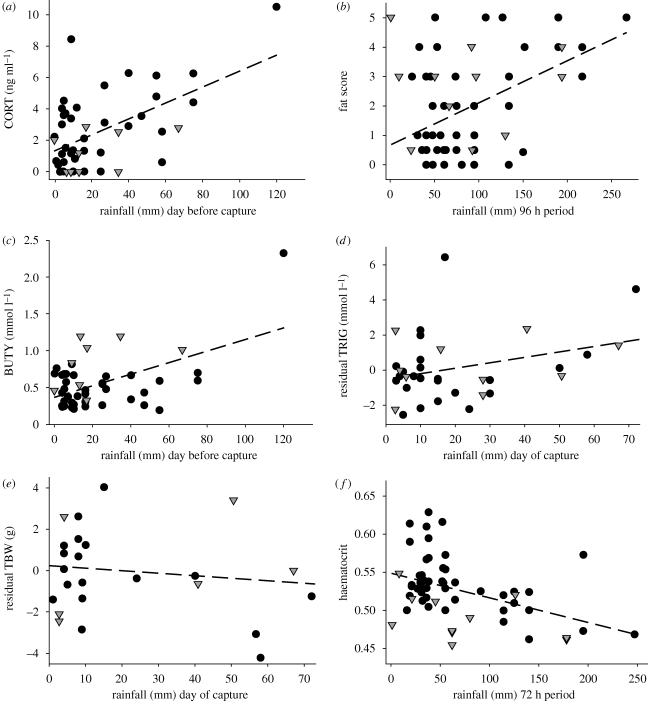
CORT was positively associated with the amount of rain that fell the day before capture (F1,49 = 20.1, p < 0.0001, R2 = 0.29; figure 2a). CORT was positively associated with fat score (likelihood ratio χ2 = 3.9, p = 0.049) but was not related to other response variables. The best model included only rain and site (table 1 summarizes all model results and rain effect sizes). Other models within two AIC scores included both rain and site with the addition of rain * site, sex, age, day or body mass. After accounting for rain, manakins remaining at RA had double the baseline CORT levels of manakins that migrated to lower elevations (RA 2.69 ± 0.30 ng ml−1; LS 1.29 ± 0.65 ng ml−1).
Figure 2.
Relationship between rain and the six physiological response variables we measured in individual White-ruffed Manakins: (a) CORT, (b) fat scores, (c) BUTY, (d) residual TRIG, (e) TBW and (f) haematocrit. Black circles and grey triangles represent values for birds captured at higher elevations (RA) and lower elevations (LS), respectively. In analyses, we treated fat score as an ordinal variable. Residual TRIG represents values accounting for fat score, and residual TBW represents values accounting for body size (wing chord and sex). Slopes of the relationships between each variable and the relevant rainfall parameter appear in table 1.
Table 1.
Summary of model results including parameters chosen using AIC for each response variable. (We analysed fat score using logistic models and therefore report a χ2 value instead of an F-value and omit slope values for this variable.)
| model parameters | temporal scale of rain response | model statistics |
slope (s.e.) of rain relationship | ||
|---|---|---|---|---|---|
| F (χ2) | p | R2 | |||
| CORT | |||||
| rain, site | day before capture | 20.1 | <0.0001 | 0.29 | 0.051 (0.011) |
| fat score | |||||
| rain, site, sex, age, ordinal day | 96 h period | 53.8 | <0.0001 | 0.22 | — |
| BUTY | |||||
| rain, site, sex, age, rain * sex | day before capture | 12.8 | <0.0001 | 0.61 | 0.008 (0.002) |
| residual TRIG | |||||
| rain | day of capture | 3.5 | 0.072 | 0.10 | 0.031 (0.017) |
| residual TBW | |||||
| rain | day of capture | 1.7 | 0.207 | 0.08 | −0.025 (0.019) |
| haematocrit | |||||
| rain, site, age, body mass, capture time | 72 h period | 9.5 | <0.0001 | 0.51 | −0.0003 (<0.001) |
Fat scores were positively related to the amount of rain falling over 96 h up to and including the day of capture (likelihood ratio χ2 = 15.0, n = 69, p = 0.0001; figure 2b). The best model included rain, site, age, sex and day (table 1). No other combination of factors yielded a ΔAIC within two scores of the best model. After accounting for rain, migrant manakins had on average 0.74 greater odds of having 1 higher fat score relative to residents. Additionally, adults carried less fat than young birds (2.32 odds of 1 lower fat score), females were fatter than males (0.23 odds of 1 higher fat score), and birds got fatter as the season progressed (0.95 odds per day).
Concentrations of circulating BUTY were positively related to the amount of rain that fell the day before capture (F1,47 = 17.5, p = 0.0001, R2 = 0.27; figure 2c). The best model included rain, site, sex, age and rain * sex (table 1). Other models within two AIC scores included rain, site and sex with combinations of body mass, time of capture, day, rain * site or sex * site. To explore the relationship between rain and sex, we regressed BUTY against rain separately for males and females. Whereas an increase of 10 mm rain was associated with an average increase of 0.13 mmol l−1 BUTY concentrations among males (p < 0.001), BUTY concentrations in females did not change with increased rain (p = 0.601). After accounting for rain and sex, residents had lower BUTY concentrations (0.53 mmol l−1 ± 0.05) than migrants (0.74 mmol l−1 ± 0.09). Additionally, adults had lower BUTY concentrations than birds in their first year of life (0.55 mmol l−1 ± 0.06 versus 0.73 mmol l−1 ± 0.08).
A strong positive relationship between raw TRIG values and rain largely disappeared after controlling for fat score. Residual TRIG was best explained by the amount of rain falling on the day of capture, but this model had low explanatory power (F1,32 = 3.5, p = 0.072, R2 = 0.10; figure 2d). The best model included only rain, with competing models within two AIC scores including site, day or both.
Residual TBW (after accounting for sex and wing chord) was best explained by the amount of rain falling on the day of capture, but this negative trend was not statistically significant (F1,21 = 1.7, p = 0.207, R2 = 0.07; figure 2e). The best model included only rain, with competing models within two AIC scores including site, time of capture, day, age or rain * site.
Haematocrit was best explained by rainfall over 72 h up to and including the day of capture (F1,55 = 12.9, p = 0.001, R2 = 0.19; figure 2f). The best model included rain, site, age, body mass and time of capture (table 1). Both rain and site were included in all top models which differed by either omitting age, body mass or time of capture, or by including day, sex or rain * sex. After accounting for the effects of rain, resident manakins at RA had higher haematocrit (0.53 ± 0.01) than birds that migrated to LS (0.49 ± 0.01). Additionally, young birds had slightly lower haematocrit than older birds (0.51 ± 0.01 versus 0.52 ± 0.01), lighter birds had lower haematocrit than heavier birds (0.01 g−1 body mass) and birds captured early in the day had lower haematocrit than birds captured later (0.004 h−1).
4. Discussion
We provide, to our knowledge, the first evidence that tropical altitudinal migration is driven by severe storm events, thereby linking variation in migration tendency to physiological responses related to life-history trade-offs. By simultaneously monitoring migrants and residents in a single breeding population and assessing a broad spectrum of physiological variables, our study provides one of the most comprehensive assessments of individual condition in a wild population of birds. CORT, fat scores, BUTY and haematocrit all responded to severe rain storms in the direction predicted (figure 2). Trends in residual TRIG and TBW data were also consistent with the predicted effect of rain although these relationships were not statistically significant. CORT showed an additive effect of site in the direction predicted with resident birds having CORT levels indicative of higher allostatic loads or higher baseline energetic requirements than birds at lower elevations, even after accounting for the two-fold difference in rainfall between sites. The dramatic increase in captures of altitudinal migrants at low elevations following a major storm (figure 1) provides compelling evidence for a direct link between weather and migration in this system.
Some results were not consistent with those predicted by the LFO hypothesis. After accounting for differences in rain at the two sites, residents and migrants differed in their fat scores, BUTY concentrations and haematocrit, but in the direction opposite to that predicted. Under similar weather conditions, migrants tended to be fatter than residents, and they were burning more of that fat for fuel. Although less rain falls, scarcer fruit at low elevation sites throughout the latter part of the year (Boyle 2010) may diminish a birds' ability to refuel quickly during good weather. Alternatively, higher fat scores in migrants relative to residents could reflect pre-migratory fattening, either to fuel migration itself, or as a bet-hedging strategy in the face of resource uncertainty in arrival locations. Parallel elevational patterns of fat scores and BUTY concentrations highlights the fact that BUTY levels can only be elevated when stored fat is available to mobilize. Likewise, the marginal statistical significance of the relationship between rain and residual TRIG may imply that the opposing effects of weather and food abundance may balance each other in affecting fat deposition rates. In other words, birds at higher elevations may be unable to fully realize the fattening potential of the site owing to the weather. The temporal resolution of the rain data (24 h periods) may also obscure the finer-scale timing of foraging and fat deposition that probably occurs in response to rain. It is probable that intense foraging bouts and fat deposition occurs either immediately prior to storms (if birds can anticipate changes in weather), or occurs during periods of lighter rain during the storm interspersed with fasting during heavy rain. Either pattern would lead to high variability in the relationship between rain and residual TRIG. With finer-scale rainfall and behavioural data it would be useful to determine at what rainfall intensity foraging ceases and calculate the reductions in foraging time associated with storms of different intensities.
After accounting for the effects of rain, higher haematocrit in resident birds could reflect acclimatization to the small differences in atmospheric oxygen partial pressure or metabolic costs of slightly lower temperature (approx. 3°C) attributable to the 700 m difference in altitude between sites (Carey & Morton 1976). Regardless of the mechanism underlying differences, decreased haematocrit in heavy rain results in less haemoglobin available for aerobic activity and potentially compromised flight performance. We found no effect of rain or site on TBW, although trends in the rain relationship matched those predicted by the LFO. It is probable that none of the birds we sampled were close enough to death that they resorted to lean mass catabolism. This result may confirm that migration represents a behavioural adaptation to avoid allostatic overload and entering an emergency life-history stage.
In addition to the main effects of rain and site, three of our response variables were also associated with additional correlates. The BUTY response to rain differed between sexes in a manner consistent with males experiencing greater allostatic loads than females in response to the same storm events. Additionally, younger birds had higher BUTY concentrations than older birds which might be expected if younger birds are less experienced foragers and therefore, less able to replenish energy sources during breaks in rain. Similarly, fat scores differed between birds of different ages in a manner consistent with younger birds requiring a greater buffer against foraging uncertainty. After controlling for other factors, females carried more fat than males, which may be associated with sexual differences in plumage and its relationship with predation risk. Fat stores compromise a birds' flight ability (Witter et al. 1994; Lind et al. 1999) and have been proposed to be regulated in response to perceived predation risk (Nebel & Ydenberg 2005). Thus, the bright plumage of adult males may increase the cost of fuel storage relative to the behavioural migratory response in the face of foraging uncertainty. Finally, haematocrit was lower in both younger and lighter birds, again probably indicating higher metabolic costs owing to foraging inexperience or reduced fuel reserves.
For insectivorous species living in drier tropical forests, increased rain has beneficial effects on condition, resulting in earlier preparation for long distance migration and lower CORT levels (Marra & Holberton 1998; Studds & Marra 2007). By contrast, our results suggest that in extremely wet environments, increased rain has adverse effects on small birds. Apparently, both very low and very high rainfall can affect the trade-offs involved in migratory decisions but act via different mechanisms. In dry environments, the link between weather and migration is mediated by effects on food abundance, whereas in our system, it seems that this link is mediated by reductions in foraging time.
One could interpret the influx of migrants to low elevations coincident with bad weather as evidence that rain is merely a cue that times migration. However, if the primary benefits of migrating downhill are explained by temporal and spatial variation of other factors (e.g. predation or parasitism) that are not linked to weather-related stressors, then it is probable that birds would use more temporally predictable cues such as changes in photoperiod rather than temporally unpredictable storms to synchronize migratory movements with changes in those other factors. Severe storms can occur throughout the year, yet only post-breeding do birds migrate downhill. In this respect, altitudinal migrants resemble long-distance migrants in their seasonally specific response to cues triggering migration. Furthermore, if alternate selective pressures explain the fitness consequences of altitudinal migration, it is unlikely that so many physiological variables would respond to rainfall in the predicted directions. One possibility that remains to be explored is that some storm-related variable related to rainfall intensity, but not related to LFO leads to the same behavioural and physiological responses acting via a different mechanism. However, we can think of no plausible alternatives that would lead to the set of relationships demonstrated here. Regardless of mechanism, the consistency of results from multiple physiological predictions and other lines of evidence (Boyle 2008b) imply that the migratory decisions of White-ruffed Manakins involve mortality risks influenced by weather.
Evidence that high-elevation rains limit foraging to the extent that birds risk death or migrate downhill resolves conflicting evidence for and against food limitation being important in shaping tropical bird movements. On one hand, comparative correlations between degree of frugivory and migratory propensity suggest a role for food limitation in promoting altitudinal migration (Boyle 2006). Yet several tests of the hypothesis of reciprocal variation in food abundance between elevations have failed to explain both up and downhill movements of altitudinal migrant species (e.g. Loiselle & Blake 1991; Solórzano et al. 2000; Chaves-Campos 2004; Boyle 2010). The LFO hypothesis resolves this paradox because, owing to the high fruit intake rates frugivores must maintain (Witmer & Van Soest 1998), only the most highly frugivorous species are hypothesized to be in danger of starvation when foraging time is reduced by a few hours per day. Thus, the link between diet and migration is not a direct consequence of food availability, but availability as mediated by suitable foraging conditions.
By integrating the results of multiple aspects of condition, this study more completely characterizes the effects of environmental variables on wild birds than has generally been achieved and, for the first time, to our knowledge, elucidates the physiological basis of an intra-tropical migration system. We provide, to our knowledge, the first evidence for the new hypothesis that severe storms may affect avian survival and influence a major behavioural trait. This association has profound implications for population persistence, community structure and migratory behaviour if ongoing climate change alters storm severity or the timing of rainfall events in tropical regions as is predicted (Karmalkar et al. 2008). Our results highlight how explanations for life-history traits must consider how interactions between endogenous and exogenous processes affect behavioural trade-offs made by individual animals in the wild.
Acknowledgements
J. Guevara, A. Bien, R. Carlson, and the UWO animal use subcommittee granted permits.
We thank M. Jones, C. Craig and J. Anderson for hard work in the field. B. Rajakumar, A. Newman, E. Price and A. Gerson assisted with laboratory work. The UWO (Helen Battle Fellowship, W.A.B.), the National Geographic Society (W.A.B., C.G.G., D.R.N.), Discovery Grants from the Natural Sciences and Engineering Research Council of Canada (C.G.G., D.R.N.), and a Leaders Opportunity Fund Award (C.G.G., D.R.N.) from the Canada Foundation for Innovation and the Ontario Research Fund provided financial support.
References
- Belthoff J. R., Gauthreaux S. A.1991Partial migration and differential winter distribution of House Finches in the eastern United States. Condor 93, 374–382 (doi:10.2307/1368953) [Google Scholar]
- Boyle W. A.2006Why do birds migrate? The role of food, habitat, predation, and competition Tucson, AZ: Ecology and Evolutionary Biology, University of Arizona [Google Scholar]
- Boyle W. A.2008aCan variation in risk of nest predation explain altitudinal migration in tropical birds? Oecologia 155, 397–403 (doi:10.1007/s00442-007-0897-6) [DOI] [PubMed] [Google Scholar]
- Boyle W. A.2008bPartial migration in birds: tests of three hypotheses in a tropical lekking frugivore. J. Anim. Ecol. 77, 1122–1128 (doi:10.1111/j.1365-2656.2008.01451.x) [DOI] [PubMed] [Google Scholar]
- Boyle W. A.2009How to keep tropical montane frugivorous birds in captivity. Ornitol. Neotrop. 20, 265–273 [Google Scholar]
- Boyle W. A.2010Does food abundance explain altitudinal migration in a tropical frugivorous bird? Can. J. Zool. Rev. Can. Zool. 88, 204–213 (doi:10.1139/Z09-133) [Google Scholar]
- Burnham K. P., Anderson D. R.2002Model selection and multi-model inference: a practical information—theoretic approach. New York, NY: Springer-Verlag New York Inc [Google Scholar]
- Calder W. A.1974Consequences of body size for avian energetics. In Avian Energetics (ed. Paynter R. A.), pp. 86–144 Cambridge, MA: Nuttall Ornithological Club Publication 15 [Google Scholar]
- Carey C., Morton M. L.1976Aspects of circulatory physiology of montane and lowland birds. Comp. Biochem. Physiol. A 54, 61–74 (doi:10.1016/S0300-9629(76)80073-4) [DOI] [PubMed] [Google Scholar]
- Chaves-Campos J.2004Elevational movements of large frugivorous birds and temporal variation in abundance of fruits along an elevational gradient. Ornitol. Neotrop. 15, 433–445 [Google Scholar]
- Chaves-Campos J., Arévalo J. E., Araya M.2003Altitudinal movements and conservation of Bare-necked Umbrellabird Cephalopteris glabricollis of the Tilarán Mountains, Costa Rica. Bird Conserv. Int. 13, 45–58 (doi:10:1017/S0959270903003046) [Google Scholar]
- Goymann W., Schwabl I., Trappschuh M., Hau M.2007Use of ethanol for preserving steroid and indoleamine hormones in bird plasma. Gen. Comp. Endocrinol. 150, 191–195 (doi:10.1016/j.ygcen.2006.09.014) [DOI] [PubMed] [Google Scholar]
- Guglielmo C. G., O'Hara P. D., Williams T. D.2002Extrinsic and intrinsic sources of variation in plasma lipid metabolites of free-living western sandpipers (Calidris mauri). Auk 119, 437–445 (doi:10.1642/0004-8038(2002)119[0437:EAISOV]2.0.CO;2) [Google Scholar]
- Guglielmo C. G., Cerasale D. J., Eldermire C.2005A field validation of plasma metabolite profiling to assess refueling performance of migratory birds. Phys. Biochem. Zool. 78, 116–125 (doi:10.1086/425198) [DOI] [PubMed] [Google Scholar]
- Haber W. A., Stevenson R. D.2004Diversity, migration, and conservation of butterflies in northern Costa Rica. In Biodiversity conservation in Costa Rica: learning the lessons in a seasonal dry forest (eds Frankie G. W., Mata A., Vinson S. B.), pp. 99–114 Berkeley, CA: University of California Press [Google Scholar]
- Hays Q. R.2008Stopover behavior and refueling physiology in relation to migration distance in Wilson's Warblers (Wilsonia pusilla) London, Ontario: Biology, University of Western Ontario [Google Scholar]
- Jenni-Eiermann S., Jenni L.1994Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the Garden Warbler. Auk 111, 888–899 [Google Scholar]
- Kaiser A.1993A new multi-category classification of subcutaneous fat deposits of songbirds. J. Field Ornithol. 64, 246–255 [Google Scholar]
- Karmalkar A. V., Bradley R. S., Diaz H. F.2008Climate change scenario for Costa Rican montane forests. Geophys. Res. Lett. 35, L11702 (doi:10.1029/2008GL033940) [Google Scholar]
- Ketterson E. D., Nolan V., Jr1976Geographic variation and its climatic correlates in sex-ratio of eastern wintering Dark-eyed Juncos (Junco hyemalis hyemalis). Ecology 57, 679–693 (doi:10.2307/1936182) [Google Scholar]
- Landys M. M., Ramenofsky M., Wingfield J. C.2006Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149 (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- Lind J., Fransson T., Jakobsson S., Kullberg C.1999Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behav. Ecol. Sociobiol. 46, 65–70 (doi:10.1007/s002650050593) [Google Scholar]
- Loiselle B. A., Blake J. G.1991Temporal variation in birds and fruits along an elevational gradient in Costa Rica. Ecology 72, 180–193 (doi:10.2307/1938913) [Google Scholar]
- Lumpkin H. A., Boyle W. A.2009Effects of forest age on fruit composition and removal in tropical bird-dispersed understorey trees. J. Trop. Ecol. 25, 515–522 (doi:10.1017/S0266467409006208) [Google Scholar]
- Lundberg P.1985Dominance behavior, body weight and fat variations, and partial migration in European Blackbirds Turdus merula. Behav. Ecol. Sociobiol. 17, 185–189 [Google Scholar]
- Lyons J. E., Collazo J. A., Guglielmo C. G.2008Plasma metabolites and migration physiology of semipalmated sandpipers: refueling performance at five latitudes. Oecologia 155, 417–427 (doi:10.1007/s00442-007-0921-x) [DOI] [PubMed] [Google Scholar]
- Marra P. P., Holberton R. L.1998Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116, 284–292 (doi:10.1007/s004420050590) [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Wingfield J. C.2003The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15 (doi:10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- Nebel S., Ydenberg R.2005Differential predator escape performance contributes to a latitudinal sex ratio cline in a migratory shorebird. Behav. Ecol. Sociobiol. 59, 44–50 (doi:10.1007/s00265-005-0007-x) [Google Scholar]
- Newman A. E. M., Pradhan D. S., Soma K. K.2008Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149, 2537–2545 (doi:10.1210/en.2007-1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piersma T., Koolhaas A., Dekinga A., Gwinner E.2000Red blood cell and white blood cell counts in sandpipers (Philomachus pugnax, Calidris canutus): effects of captivity, season, nutritional status, and frequent bleedings. Can. J. Zool. Rev. Can. Zool. 78, 1349–1355 (doi:10.1139/cjz-78-8-1349) [Google Scholar]
- Rae L. F., Mitchell G. W., Mauck R. A., Guglielmo C. G., Norris D. R.2009Radio transmitters do not affect the body condition of Savannah Sparrows during the fall premigratory period. J. Field Ornithol. 80, 434–441 (doi:10.1111/j.1557-9263.2009.00249.x) [Google Scholar]
- Ramenofsky M.1990Fat storage and fat metabolism in relation to migration. In Bird migration (ed. Gwinner E.), pp. 214–231 Berlin, Germany: Springer [Google Scholar]
- Reneerkens J., Piersma T., Ramenofsky M.2002An experimental test of the relationship between temporal variability of feeding opportunities and baseline levels of corticosterone in a shorebird. J. Exp. Zool. 293, 81–88 (doi:10.1002/jez.10113) [DOI] [PubMed] [Google Scholar]
- Rogers C. M.1993Life-history theory in the non-breeding period: trade-offs in avian fat reserves? Ecology 74, 419–426 (doi:10.2307/1939303) [Google Scholar]
- Romero L. M., Reed J. M., Wingfield J. C.2000Effects of weather on corticosterone responses in wild free-living passerine birds. Gen. Comp. Endocrinol. 118, 113–122 (doi:10.1006/gcen.1999.7446) [DOI] [PubMed] [Google Scholar]
- Rosselli L.1994The annual cycle of the White-ruffed Manakin, Corapipo leucorrhoa, a tropical frugivorous altitudinal migrant, and its food plants. Bird Conserv. Int. 4, 143–160 [Google Scholar]
- Skutch A. F.1969Life histories of central American birds III: families Cotingidae, Pipridae, Formicariidae, Furnariidae, Dendrocolaptidae, and Picidae Berkeley, CA: Pacific Coast Avifauna Number 35, Cooper Ornithological Society [Google Scholar]
- Solórzano S., Castillo S., Valverde T., Ávila L.2000Quetzal abundance in relation to fruit availability in a cloud forest of southeastern Mexico. Biotropica 32, 523–532 [Google Scholar]
- Speakman J. R.1997Doubly labelled water: theory and practise London, UK: Chapman & Hall [Google Scholar]
- Speakman J. R., Visser G. H., Ward S., Król E.2001The isotope dilution method for the evaluation of body composition. In Body composition analysis of animals: a handbook of non-destructive methods (ed. Speakman J. R.), pp. 56–98 Cambridge, UK: Cambridge University Press [Google Scholar]
- Stiles G. F.1988Altitudinal movements of birds on the Caribbean slope of Costa Rica: implications for conservation. In Tropical rainforests: diversity and conservation (eds Alameda F., Pringle C. M.), pp. 243–258 San Francisco, CA: California Academy of Sciences [Google Scholar]
- Studds C. E., Marra P. P.2007Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird. Setophaga ruticilla. Climate Res. 35, 115–122 (doi:10.3354/cr00718) [Google Scholar]
- Timm R. M., LaVal R. K.2000Mammals. In Monteverde: Ecology and conservation of a tropical cloud forest (eds Nadkarni N. M., Wheelwright N. T.), pp. 223–244, 553–560 New York, NY: Oxford University Press [Google Scholar]
- Wikelski M., Steiger S. S., Gall B., Nelson K. N.2004Sex, drugs, and mating role: testosterone-induced phenotype-switching in Galapagos marine iguanas. Behav. Ecol. 16, 260–268 (doi:10.1093/beheco/arh160) [Google Scholar]
- Wingfield J. C., Ramenofsky M.1997Corticosterone and facultative dispersal in response to unpredictable events. Ardea 85, 155–166 [Google Scholar]
- Wingfield J. C., Smith J. P., Farner D. S.1982Endocrine responses of White-crowned Sparrows to environmental stress. Condor 84, 399–409 (doi:10.2307/1367443) [Google Scholar]
- Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D.1998Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- Witmer M. C., Van Soest P. J.1998Contrasting digestive strategies of fruit-eating birds. Funct. Ecol. 12, 728–741 (doi:10.1046/j.1365-2435.1998.00242.x) [Google Scholar]
- Witter M. S., Cuthill I. C., Bonser R. H. C.1994Experimental investigations of mass-dependent predation risk in the European Starling, Sturnus vulgaris. Anim. Behav. 48, 201–222 (doi:10.1006/anbe.1994.1227) [Google Scholar]