Abstract
Pheromones play major roles in intraspecific communication in many animals. Elaborated communication systems in eusocial insects provide excellent materials to study neural mechanisms for social pheromone processing. We previously reported that alarm pheromone information is processed in a specific cluster of glomeruli in the antennal lobe of the ant Camponotus obscuripes. However, representation of alarm pheromone information in a secondary olfactory centre is unknown in any animal. Olfactory information in the antennal lobe is transmitted to secondary olfactory centres, including the lateral horn, by projection neurons (PNs). In this study, we compared distributions of terminal boutons of alarm pheromone-sensitive and -insensitive PNs in the lateral horn of ants. Distributions of their dendrites largely overlapped, but there was a region where boutons of pheromone-sensitive PNs, but not those of pheromone-insensitive PNs, were significantly denser than in the rest of the lateral horn. Moreover, most of a major type of pheromone-sensitive efferent neurons from the lateral horn extended dendritic branches in this region, suggesting specialization of this region for alarm pheromone processing. This study is the first study to demonstrate the presence of specialized areas for the processing of a non-sexual, social pheromone in the secondary olfactory centre in any animal.
Keywords: olfaction, social insects, sensory map, lateral horn, pheromone communication
1. Introduction
Pheromones are potent signalling molecules that are fundamental for organizing a wide range of social behaviours in many vertebrates and invertebrates. In mammals, recent studies have demonstrated the importance of pheromones in influencing social behaviour, but neural mechanisms of pheromone communication are not well understood (Brennan & Kendrick 2006; Brennan & Zufall 2006). In insects, mechanisms by which sex pheromones are processed in the olfactory system have been extensively studied (Hildebrand & Shepherd 1997; Jefferis et al. 2007), but little is known about how non-sexual, social pheromones are processed. Eusocial insects such as ants have developed sophisticated communication systems by means of social pheromones (Vander Meer & Alonso 1998). Therefore, they provide excellent materials to study neural mechanisms underlying the processing of social pheromones.
In insects, pheromonal and non-pheromonal olfactory molecules are received by olfactory receptor neurons situated on the antennae. Axons of receptor neurons project to the primary olfactory centre, the antennal lobe, which is the functional analogue of the olfactory bulb in mammals (Hildebrand & Shepherd 1997). It has been shown that the axons of receptor neurons that express the same type of olfactory receptor gene converge on a single glomerulus in the antennal lobe, and the glomeruli thus serve as functional units (fruitfly Drosophila: Couto et al. 2005). Since each type of receptor neuron responding to general environmental odours covers a wide range of chemical compounds that activate it (de Bruyne et al. 2001), a general environmental odour evokes responses in a large subset of different types of receptor neurons, and one type of receptor neurons responds to a large number of odours. Therefore, information about a general environmental odour is coded as a spatio-temporal activity pattern of a number of glomeruli (Joerges et al. 1997). The information is then transmitted to secondary olfactory centres in the protocerebrum—that is, the lateral horn (l ho) and the mushroom body (mb)—by second-order olfactory neurons, called PNs. A general environmental odour evokes activities in a broad area of the calyces of the mb (Szyszka et al. 2005), and the same has been proposed for the l ho (Jefferis et al. 2007). The manner by which such an across-fibre pattern code is decoded is not well understood.
In contrast to general environmental odours, sex pheromone information is coded by specific neural channels in insects. Sex pheromones are sensed by highly specific receptor neurons, and their axons project to specialized glomeruli collectively called the macroglomerular complex (Boeckh & Ernst 1987). PNs originating from the macroglomerular complex terminate in a specific region of the l ho, which is segregated out from the region where PNs conveying information about general environmental odours terminate (cockroaches: Nishino et al. 2003; moths: Kanzaki et al. 2003; Seki et al. 2005; fruitflies: Jefferis et al. 2007).
Representation of non-sexual, social pheromone information in the primary olfactory centre has been studied in insects and in mammals (Brennan & Kendrick 2006). In insects, two different theories for representation of social pheromones in the antennal lobe have been proposed. Firstly, calcium-imaging studies of the antennal lobe of the ant Camponotus rufipes (Galizia et al. 1999a), the ant Camponotus floridanus (Zube et al. 2008) and the honeybee (Galizia et al. 1999b; Sachse et al. 1999; Sandoz et al. 2007) have shown that glomeruli at the ventral surface of the antennal lobe respond to alarm pheromones and also to non-pheromonal odours, and thus these authors proposed that non-sexual, social pheromones are coded as an across-glomeruli activity pattern, as are environmental odours. The worker of the carpenter ant Camponotus obscuripes possesses approximately 400 glomeruli, as does the worker of a closely related species (C. japonicus: approx. 430; Nakanishi et al. in press), and we recently found that all uniglomerular PNs (uni-PNs) originating from one of five specific glomeruli responded to formic acid and/or n-undecane, alarm pheromone components (Yamagata et al. 2006). These glomeruli, which we refer to as the AS (alarm pheromone-sensitive) glomeruli, were clustered in the dorsalmost part of the antennal lobe. Most, but not all, of the uni-PNs originating from the AS glomeruli did not respond to any of the food odours we tested. Moreover, all of the multiglomerular PNs (multi-PNs) that responded to alarm pheromone components as well as non-pheromonal odours had dendrites in AS glomeruli, in addition to many other glomeruli. Thus, we have suggested that the antennal lobe of the ant C. obscuripes is equipped with specialized glomeruli to process alarm pheromone information. In the leaf-cutting ant Atta sexdens, the antennal lobe of large workers contains an enlarged glomerulus that exhibited responses to a major component of trail pheromone (Kleineidam et al. 2005; Kuebler et al. 2010). In mammals, it is thought that relatively non-volatile and volatile social pheromones are processed in the accessory olfactory bulb and main olfactory bulb, respectively, and that non-volatile pheromonal odours are coded in specific glomeruli in the accessory olfactory bulb (labelled line code), while volatile pheromonal and non-pheromonal odours are coded as across-glomeruli patterns in the main olfactory bulb (Brennan & Kendrick 2006).
Signals processed in the primary olfactory centres are sent to secondary olfactory centres: the l ho and mb in the case of insects and the piriform cortex, olfactory tubercle, entorhinal cortex and amygdala in the case of mammals. In insects, it is thought that the neural pathway involving the l ho participates in innate response to odours, while the mb participates in learned behavioural responses to odours (de Belle & Heisenberg 1994; Heimbeck et al. 2001). In mammals, the medial amygdala is thought to be important for the control of social behaviour (Brennan & Zufall 2006). Elucidation of the representation of social pheromones in secondary olfactory centres is important for understanding neural mechanisms of social behaviour, but this remains largely unknown in any animal (Brennan & Kendrick 2006; Brennan & Zufall 2006).
In this study, we investigated the distribution of terminal boutons of PNs, the morphology and olfactory responses of which have been described (Yamagata et al. 2006), in the l ho of the ant C. obscuripes. Specifically, the distribution of terminal boutons of pheromone-sensitive PNs was compared with that of pheromone-insensitive PNs. We also investigated the distribution of dendritic branches of a major class of pheromone-sensitive output (efferent) neurons from the l ho, whose morphology and olfactory responses have also been reported (Yamagata et al. 2005, 2007).
2. Material and methods
(a). Neurons used for anatomical analysis
Neurons that were anatomically and physiologically characterized in our previous studies in the ant C. obscuripes (Yamagata et al. 2005, 2006, 2007) were used for the present anatomical analysis. Methods of intracellular recording, olfactory stimulation, analysis of odour responses and filling with Lucifer yellow have been previously described (Yamagata et al. 2006, 2007). In short, each ant was fixed on a recording stage, and the frontal side of the head capsule was cut open to expose the brain. The oesophagus and muscles in the head capsule were removed, and a glass rod was inserted into the oesophagus foramen to stabilize the brain. The brain was immersed in saline solution used in a related species, C. floridanus (Gronenberg et al. 1996).
For intracellular recording and staining, borosilicate glass capillaries were pulled on a laser puller (P-2000, Sutter Instruments) and were filled with 8 per cent Lucifer yellow (Sigma) dissolved in 1 M LiCl2 at the tips, with DC resistances of 60–90 MΩ. The electrical signal was amplified with an amplifier (MEZ-8100, Nihon Kohden) and displayed on an oscilloscope and a digital recorder (Omniace, NEC). Data were stored on a DAT recorder (RD120-T, TEAC). Recording durations varied from a few minutes to around 20 minutes. The recorded neuron was filled with Lucifer yellow by applying a hyperpolarizing current (−0.3 to −2 nA). Staining was performed during recordings of odour responses or after completing the recordings. The brains were fixed in 4 per cent formaldehyde solution, dehydrated in an ascending ethanol series and cleared in methyl salicylate for confocal microscopic observations.
A constant airflow system was used so that air that was passed through a cartridge containing a filter paper soaked with 40 μl odourant solution could be delivered to an antenna of the ant without changing the flow rate. The residual air around the preparation was continuously sucked out by a vacuum system. Animals received stimulations with all or a part of a set of odours that includes 100 per cent and 10 per cent formic acid (diluted in distilled water), 100 per cent and 10 per cent n-undecane (diluted in paraffin oil), vanilla, peppermint, 1-hexanol (diluted to 1% by paraffin oil), banana, apple, peach and maple odour. The spike data were analysed to characterize the response of each neuron to a given odour as excitatory, inhibitory or no response (for details, see Yamagata et al. 2007).
(b). Confocal microscopy and three-dimensional reconstruction
The preparations were observed by confocal scanning microscopy (LSM510, Carl Zeiss, Jena, Germany). An argon laser (458 nm) was used to observe neurons filled with Lucifer yellow with 20× objectives (NA = 0.75 or 0.50) and a longpass filter (more than 475 nm). Optimal confocal pinhole sizes were assigned by the microscope's scanning software. Pixel sizes were 0.45–0.64 μm. For three-dimensional reconstruction of the distribution of terminal boutons or dendritic branches of neurons in the l ho, optical sections were made at 4 μm intervals. In a few preparations, sections were made at approximately 2.9 μm intervals throughout the entire depth of the specimens for reconstruction of major neuropil structures and were visualized by auto-fluorescence.
The images were outlined and reconstructed three dimensionally with Amira software (Mercury, San Diego, CA). Volumes and distances of centroids of reconstructed structures were measured by Amira software. To minimize subjective biases, reconstructions of terminal boutons were performed in an experimenter-blind way.
We studied the distribution of terminal boutons of PNs in the l ho by using confocal stacks acquired with 4 μm intervals. The number of terminal boutons counted in this study may be a slight underestimation of the actual number, because the intervals were thicker than the diameter of a single bouton (approx. 1 μm). Indeed, we performed a reconstruction of terminal bouton distributions by the use of a confocal stack with 1 μm intervals in some neurons and found a larger number of reconstructed boutons (for 86.5%) compared with that in the case of 4 μm optical sections. However, the pattern of distribution of boutons in 1 μm optical sections did not differ from that in 4 μm optical sections, thus indicating that analysis with 4 μm intervals was sufficient for the purpose of the present study.
(c). Bodian staining
For Bodian staining, the heads of ants were isolated and mounted in a dish, and the brains were exposed. The heads were immersed in saline containing 3 per cent paraformaldehyde for 1 h, and then the brains were dissected out. The brains were fixed with a solution containing 4 per cent paraformaldehyde, 5 per cent glacial acetic acid and 85 per cent ethanol for 2 days, dehydrated, and embedded in paraffin. A variation (Otsuka 1962) of the Bodian-reduced silver impregnation method was used for 12 μm sections.
(d). Definition of the lateral horn
The termination area of uni-PNs in the lateral protocerebrum is referred to as the lateral horn (l ho). A wider definition of the l ho is the termination area of uni-PNs or multi-PNs in the lateral protocerebrum, but here we adopted the narrower definition to focus on the termination areas of the predominant type of PNs (i.e. uni-PNs). Since the number of uni-PNs examined in this study was limited (n = 6), the area described as l ho may not represent the entire l ho.
3. Results
In ants, PNs are morphologically classified into three classes, namely: uniglomelular-PNs (uni-PNs), the axons of which pass through the lateral antenno-cerebral tract (l-ACT); uni-PNs, the axons of which pass through the medial antenno-cerebral tract (m-ACT); and multiglomerular-PNs (multi-PNs), the axons of which pass through the medio-lateral antenno-cerebral tract (ml-ACT; Zube et al. 2008). Among these three types, we encountered only the latter two types in our studies in the ant C. obscuripes (Yamagata et al. 2007). We thus studied the distributions of terminal boutons of uni-PNs from the m-ACT (n = 6; an example is shown in the electronic supplementary material, figure S1a) and multi-PNs from the ml-ACT (n = 19; an example is shown in the electronic supplementary material, figure S1b) in the l ho. Morphologies and olfactory responses of these neurons have been reported (Yamagata et al. 2006). In short, uni-PNs had dendrites in a single glomerulus in the antennal lobe (a lob; see the electronic supplementary material, figure S1a3, arrow) and their axons terminated in the l ho and the calyces of the mb. Multi-PNs innervated multiple glomeruli (see the electronic supplementary material, figure S1b2, arrow) and their axons terminate in the l ho, in addition to the region called the lateral network (see figure 1h) and some other protocerebral regions (Yamagata et al. 2006).
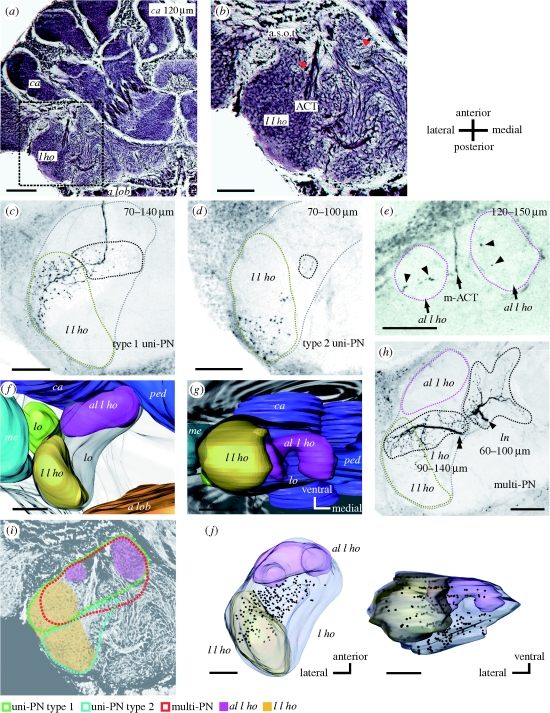
Figure 1.
Structure of the l ho of the ant brain. (a) A frontal section of a Bodian-stained ant brain at a depth of approximately 120 μm from the frontal (ventral) surface. a lob, antennal lobe; l ho, lateral horn; ca, calyces of the mushroom body. (b) A magnified image of the lateral protocerebrum (inset in (a)), including the l ho. Two round structures at the anterior part of the l ho (red arrowheads), which we call antero-lateral l ho (al l ho), are delineated by the antenno-cerebral tract (ACT) and the anterior superior optic tract (a.s.o.t). In addition, an ellipsoidal structure at the lateral edge of the l ho, lateral l ho (l l ho), is also visible. (c) Terminal arborizations of a class of uni-PN (type 1 uni-PN), the axon of which passes through the medial antenno-cerebral tract (m-ACT), seen as a confocal image at a depth of 70–140 µm from the frontal surface. Their terminal branches are located in the anterior part of the lateral l ho (region denoted by yellow broken line) and nearby l ho region (region denoted by black broken line). Grey broken line in (c), as well as in (d) and (h), depicts outline of the l ho at this depth. (d) Terminal arborizations of another class of uni-PN (type 2 uni-PN). This type terminates in the posterior part of the lateral l ho (yellow broken line) and nearby l ho region (black broken line). (e) A type 1 uni-PN with terminal arborizations (arrowheads) in the antero-lateral l ho (magenta broken lines). (f,g) A three-dimensional reconstruction of the lateral l ho, and the antero-lateral l ho, and nearby protocerebral neuropils, viewed (f) ventrally and (g) posteriorly. The antero-lateral l ho is positioned postero-laterally to the mb, and the lateral l ho is positioned postero-medially to the medulla (me) and lobula (lo). ped, pedunculus. (h) The axon of a multiglomerular PN (multi-PN) bifurcates (arrowhead and double arrowhead), each forming dendritic branches in the l ho and in the area called the lateral network (ln) (Kirschner et al. 2006; Zube et al. 2008). (i) A schematic diagram of termination areas of type 1 uni-PNs (green), type 2 uni-PNs (blue) and multi-PNs (red) in the l ho. (j) Three-dimensional reconstruction of the distribution of terminal boutons of a multi-PN in the l ho, viewed ventrally and posteriorly. Scale bars: (a) 100 μm; (b–h, j) 50 μm.
We also studied the distribution of dendritic branches of a major type of efferent (output) neuron of the l ho (n = 12; an example is shown in the electronic supplementary material, figure S1c), called a wide-field protocerebral neuron, the morphology and olfactory responses of which have been described (Yamagata et al. 2005, 2007). In short, this type of neuron possesses spiny dendritic branches in the l ho, the vertical lobe of the mb, and the medial protocerebrum and varicose terminal branches in some premotor areas, including the dorsal protocerebrum, lateral accessory lobe and dorsal lobe. The neurons are likely to integrate pheromonal and non-pheromonal information received in the l ho and other protocerebral areas, and send the information to various premotor areas (Yamagata et al. 2007).
We have reported that these input or output neurons of the l ho were physiologically classified into: (i) neurons that responded to alarm pheromone but not to any of the non-pheromonal odours tested (pheromone-specific neurons, n = 6); (ii) neurons that responded to alarm pheromone components (formic acid and/or n-undecane; Fujiwara-Tsujii et al. 2006) and also to environmental odours (n = 17); and (iii) neurons that did not respond to any of the pheromone components (pheromone-insensitive neurons, n = 14; Yamagata et al. 2006). Analyses of the distribution of terminal boutons of PNs in the l ho revealed complete overlapping of terminal boutons of the first and second types (see below), thus indicating that there is no region to exclusively receive alarm pheromone information (that is, to receive alarm pheromone information but not non-pheromonal information). Thus, these two types are grouped into ‘pheromone-sensitive’ PNs, and the distributions of their terminal boutons were compared with those of the third type, pheromone-insensitive PNs. We observed no obvious difference in the distribution of terminal boutons between formic acid-sensitive neurons (n = 7) and n-undecane-sensitive neurons (n = 3).
(a). Lateral horn of the ant brain
We found two subregions of the l ho in Bodian-stained (figure 1a,b) and unstained brains of the ant C. obscuripes, and used these as landmarks to evaluate distributions of terminal boutons of PNs. At first, two spherical structures delineated by antenno-cerebral tracts (ACT) and an optic tract from the medulla (figure 1b, two red arrowheads), which we called the antero-lateral l ho (al l ho), are seen at the anterior end of the l ho (approx. 120 μm in depth from the frontal surface of the brain). The latter tract matches the anterior superior optic tract (a.s.o.t) described in honey bees (Mobbs 1982). Another structure, which we call the lateral l ho (l l ho), is seen at the lateral edge of the lateral protocerebrum almost at the same depth (figure 1b). These two structures received different patterns of termination by two types of uni-PNs from the m-ACT. One type of uni-PNs, which we call type 1 uni-PN, innervates the anterior part of the lateral l ho (figure 1c) and the antero-lateral l ho (figure 1e, arrowheads), while the other type of uni-PN, which we call type 2 uni-PN, innervates the posterior part of the lateral l ho (figure 1d).
Locations of these structures relative to other protocerebral structures are shown in figure 1f,g as three-dimensional reconstruction from two different view angles. The antero-lateral l ho (magenta) is located posteriorly to the mb calyces (ca, blue) and laterally to the mb pedunculus (ped, deep blue), the location in the ventro-dorsal axis being approximately 100–180 μm from the frontal surface of the brain. The lateral l ho (khaki) is located postero-medially to the medulla (me, cyan) and postero-ventrally to the lobula (lo, green), the location in the ventro-dorsal axis being approximately 70–150 μm from the frontal surface of the brain. We used these structures as landmarks for subsequent positional analyses of terminal boutons of PNs.
Figure 1h shows terminal branches of a multi-PN. They are distributed in a part of the l ho and in areas of the lateral protocerbrum called the lateral network (Kirschner et al. 2006; Zube et al. 2008). Figure 1j shows three-dimensional reconstruction of terminal boutons of a multi-PN in the l ho in two different view angles. Distinct termination areas of type 1 uni-PNs, type 2 uni-PNs and mulit-PNs in the l ho are illustrated in figure 1i.
(b). Distributions of terminal boutons of pheromone-sensitive and pheromone-insensitive PNs in the lateral horn
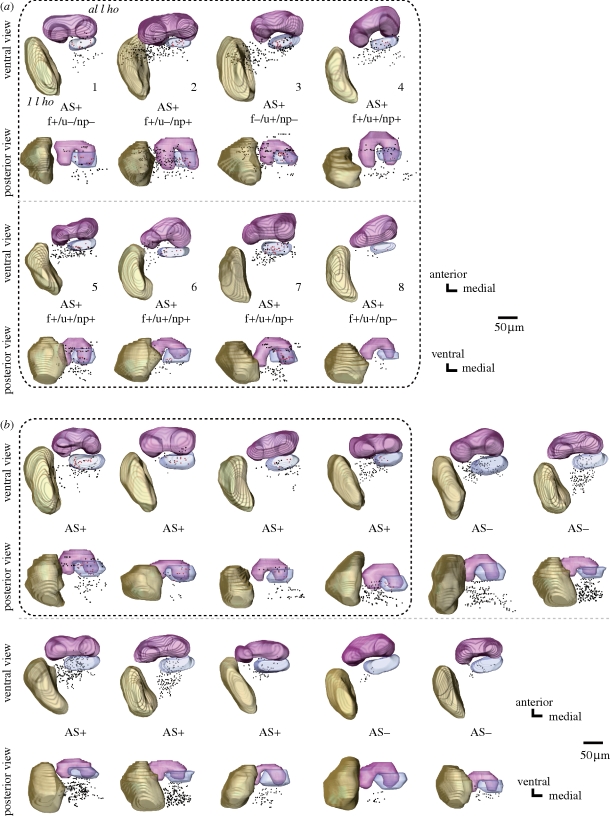
Terminal branches of PNs are equipped with a number of boutons (i.e. bleb-like structures characteristic of presynaptic sites; Strausfeld & Campos-Ortega 1977; Okada et al. 2003). We reconstructed the three-dimensional distribution of terminal boutons of PNs filled with Lucifer yellow from a stack of their confocal images. Figure 2a,b shows three-dimensional distributions of terminal boutons of three pheromone-sensitive and three pheromone-insensitive uni-PNs in two different view angles. Olfactory responses of pheromone-sensitive uni-PNs shown in figure 1a are summarized in the electronic supplementary material, table S1. The antero-lateral l ho and the lateral l ho are shown as landmarks. All of these neurons are m-ACT PNs. The last neuron shown in figure 2b is type 2 and all others are type 1 uni-PNs. Two of the three neurons in figure 2a specifically responded to alarm pheromone and originated from the AS1 or AS2 glomerulus (left and middle). The distribution of their terminal boutons overlapped with that of a PN that responded to alarm pheromone and non-pheromonal odours and originated from the AS4 glomerulus (right). All of the three pheromone-sensitive uni-PNs exhibited terminal boutons in a region posterior to the antero-lateral l ho (red boutons). In contrast, only one of the three pheromone-insensitive uni-PNs exhibited terminal boutons in this region. This region expands approximately 30–40 μm in the antero-posterior axis and approximately 70–80 μm from the medial edge of the medial compartment to the medial edge of the lateral compartment of the antero-lateral l ho in the medio-lateral axis (shown as blue regions), making up approximately 4.76 ± 0.34 per cent (n = 11) of the total volume of the l ho (blue). In subsequent sections, we examined whether this area can be regarded as a specialized area for the processing of alarm pheromone information: we defined ‘alarm pheromone focus’ as the area to receive terminal boutons of pheromone-sensitive PNs significantly more densely than the rest of the l ho, and examined whether the area described above, which we refer to as putative alarm pheromone focus, fulfils this definition.
Figure 2.
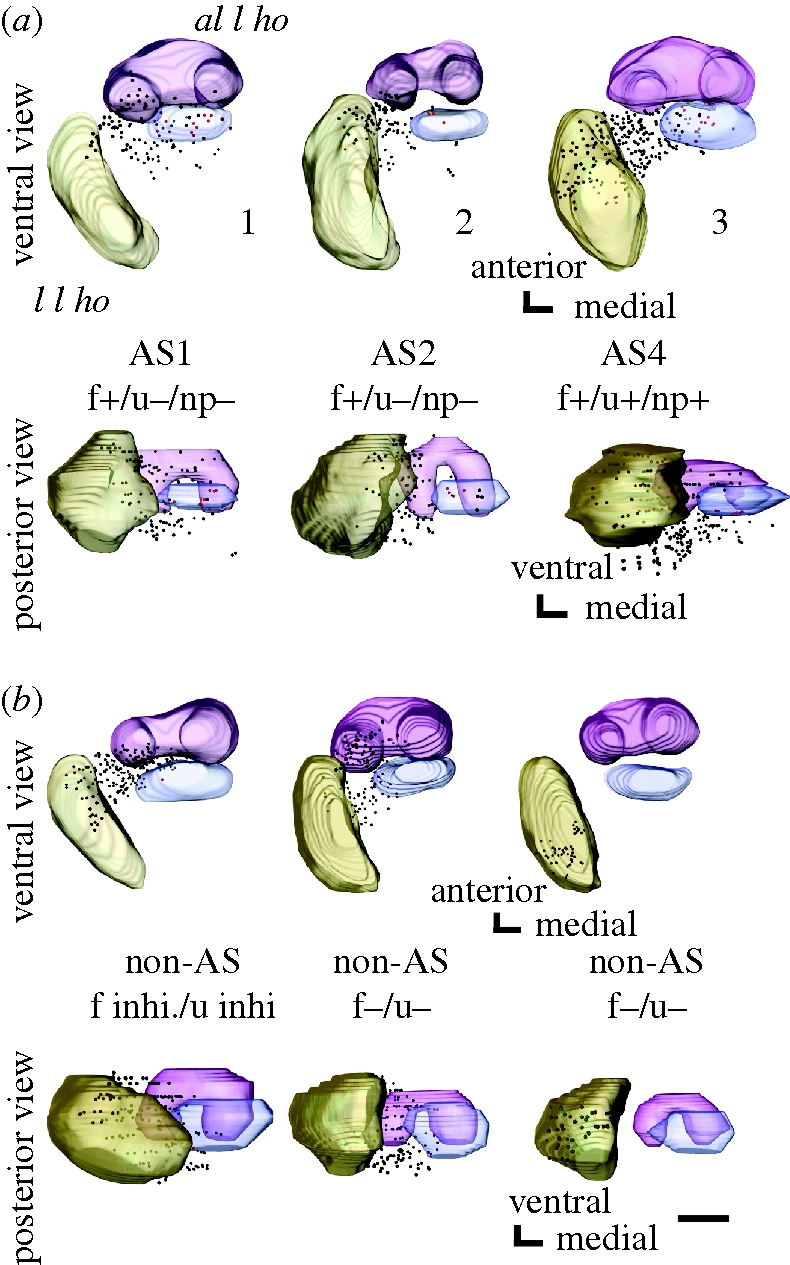
Three-dimensional reconstructions of distributions of terminal boutons of (a) three pheromone-sensitive and (b) three pheromone-insensitive uni-PNs in the l ho, viewed ventrally and posteriorly. The antero-lateral l ho (magenta) and the lateral l ho (khaki) are reconstructed as landmarks. Distributions of terminal boutons of pheromone-sensitive and pheromone-insensitive PNs are largely overlapped, but a region posterior to the antero-lateral l ho (blue) receives boutons from all of the three pheromone-sensitive PNs but from only one of the three pheromone-insensitive PNs. Boutons in the pheromone focus are coloured red and those in the rest of the l ho are coloured black. The neuron was sensitive or insensitive to formic acid (f±), n-undecane (u±) and non-pheromonal environmental odours (np±) tested. inhi.: the response was inhibitory. AS: alarm pheromone-sensitive glomerulus (Yamagata et al. 2006). The number for each pheromone-sensitive uni-PN in (a) corresponds to that in the electronic supplementary material, table S1 (scale bar, 50 μm).
Figure 3 shows the distributions of terminal boutons of pheromone-sensitive (figure 3a; n = 8) and pheromone-insensitive (figure 3b; n = 11) multi-PNs in the l ho. Olfactory responses of pheromone-sensitive multi-PNs are summarized in the electronic supplementary material, table S1. In general, terminal boutons of multi-PNs were situated dorso-medially to those of uni-PNs. There was a high degree of overlap between the termination areas of multi-PNs that specifically responded to alarm pheromone components (n = 3) and those of multi-PNs that responded both to pheromonal and non-pheromonal odours (n = 5). All pheromone-sensitive multi-PNs had terminal boutons at the putative alarm pheromone focus defined by uni-PNs (figure 3a, red boutons in the blue region). On the other hand, terminal boutons of only four of the 11 pheromone-insensitive multi-PNs (broken square in figure 3b) were distributed in this region.
Figure 3.
Three-dimensional reconstructions of distributions of terminal boutons of (a) pheromone-sensitive and (b) pheromone-insensitive multi-PNs in the l ho, viewed ventrally and posteriorly. All eight pheromone-sensitive multi-PNs have terminal boutons in a region in which terminal boutons of pheromone-sensitive uni-PNs are densely distributed (blue). This is in contrast to the fact that only four (shown in broken square) of 11 pheromone-insensitive multi-PNs have terminal boutons in this region. AS+ and AS− indicate that the neuron had dendrites in at least one of the AS glomeruli and that the neuron had no dendrites in any of the AS glomeruli, respectively. All eight pheromone-sensitive multi-PNs innervated the AS glomeruli. Seven out of 11 pheromone-insensitive multi-PNs had densities in the AS glomeruli. Pheromone insensitivity of these multi-PNs is probably because they receive only a very weak synaptic input from the AS glomeruli. Notice that multi-PNs have dendrites in more than 50 glomeruli, among which only a few are the AS glomeruli. The number for each pheromone-sensitive multi-PN in (a) corresponds to that in the electronic supplementary material, table S1. For abbreviations, see legend of figure 2.
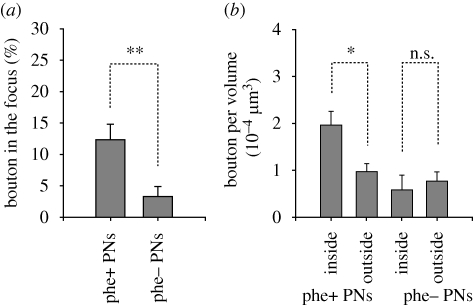
We counted the numbers of terminal boutons of pheromone-sensitive and pheromone-insensitive PNs in the entire l ho and in the putative alarm pheromone focus. On average, we counted 126.1 ± 22.7 (mean ± s.e.m.; n = 11) boutons for each of the pheromone-sensitive neurons and 97.4 ± 16.4 (n = 14) boutons for each of the pheromone-insensitive neurons in the l ho, the numbers not being significantly different (p = 0.32, t-test). The ratios of boutons distributed in the putative pheromone focus were 12.3 ± 2.4 per cent (n = 11) in the case of pheromone-sensitive PNs and 3.3 ± 1.6 per cent (n = 14) in the case of pheromone-insensitive PNs, and the ratios were significantly different (figure 4a, p = 0.005, t-test). In accordance with this, the density (number per volume) of terminal boutons in the putative pheromone focus (19.6 ± 2.8 × 10−5 boutons μm−3, n = 11) was significantly greater than that in the rest of the l ho (9.7 ± 1.7 × 10−5 boutons μm−3, n = 11) in the case of pheromone-sensitive PNs (figure 4b, p = 0.01, paired t-test), but it did not significantly differ in the case of pheromone-insensitive PNs (p = 0.68, paired t-test). Therefore, terminal boutons of AS PNs are more densely distributed in the putative alarm pheromone focus than in the rest of the l ho. In contrast, those of pheromone-insensitive PNs are not.
Figure 4.
Distributions of terminal boutons of PNs in the putative pheromone focus and the rest of the l ho. (a) The percentage of the number of boutons in the putative pheromone focus was significantly greater for pheromone-sensitive PNs (n = 11) than that for pheromone-insensitive PNs (n = 14; p = 0.005, t-test). (b) The bouton density in the putative pheromone focus (inside) was significantly higher than that in the rest of the l ho (outside) in the case of pheromone-sensitive PNs (left, p = 0.01, n = 11, paired t-test), but not in the case of pheromone-insensitive PNs (right, p = 0.68, n = 14, paired t-test). phe+, pheromone-sensitive PNs; phe−, pheromone-insensitive PNs. s.e.m. as error bars. n.s.: non-significant, *p < 0.05, **p < 0.01.
(c). Evaluation of the spatial extent of the pheromone focus
The analysis described above shows that the putative pheromone focus, which occupies approximately 4.8 per cent of the l ho, fulfils our definition of the alarm pheromone focus. We next attempted to delimit the spatial extent of the pheromone focus. At first, we defined a larger region around the putative pheromone focus (see the electronic supplementary material, large region in figure S2a), which occupies approximately 16.8 per cent of the l ho (as is described below), and a smaller region within the putative pheromone focus (small region), which occupies approximately 1.2 per cent of the l ho. Then, we examined whether these regions fulfil the definition of the alarm pheromone focus, as does the putative pheromone focus (medium-sized region).
At first, we defined these regions as quantitatively as possible. The areas of the small, medium-sized and large regions in the l ho were 1.33 ± 0.06 × 104 μm3 (1.15 ± 0.07% of the l ho, small, n = 11), 5.52 ± 0.29 × 104 μm3 (4.76 ± 0.34%, mid, n = 11) and 1.94 ± 0.10 × 105 μm3 (16.82 ± 1.17%, large, n = 11), respectively (see the electronic supplementary material, figure S2b). Distances between centroids of these regions and those of the antero-lateral l ho did not significantly differ (see the electronic supplementary material, figure S2c; p = 0.34, ANOVA, n = 11 for each region), indicating that their relative location did not differ in the antero-posterior axis. On the other hand, distances between centroids of the regions and those of the lateral l ho differed significantly (see the electronic supplementary material, figure S2c; p = 0.008, ANOVA, n = 11). This was because the centroid of the large region was shifted laterally in the medio-lateral axis. The variance of location of the regions among individuals was small (see the electronic supplementary material, figure S2d; approx. 3–10 μm in each axis). However, it should be noted that such variance indicates some degree of ambiguity in defining the regions, especially in defining the small region.
We calculated the number of boutons of pheromone-sensitive PNs per volume (see the electronic supplementary material, figure S2e) for the small or large region (inside, white bars) and for the rest of the l ho (outside, grey bars). We observed that the bouton density was less in the large region than in the small region or the medium-sized region, although the bouton density did not significantly differ among these three regions (p = 0.41, ANOVA, n = 11 for each category), which again implies the existence of a confined region where boutons of pheromone-sensitive PNs are densely distributed. Although the bouton density was significantly greater in the putative alarm pheromone focus (the medium-sized region) than in the rest of the l ho, as we have shown in figure 4b, the bouton density in the small region did not significantly differ from that in the rest of the l ho (p = 0.15, paired t-test, n = 11), due mainly to a large variance of bouton density in the small region among pheromone-sensitive PNs. The bouton density in the large region did not significantly differ from that in the rest of the l ho (p = 0.14, paired t-test, n = 11). We thus conclude that the medium-sized region provides a good estimate of the spatial extent of the pheromone focus and refer to this region as the pheromone focus.
(d). Distribution of dendrites of pheromone-sensitive output neurons in the lateral horn
Finally, we evaluated distributions of dendritic branches of a class of pheromone-sensitive output neurons of the l ho, called wide-field protocerebrum neurons (see the electronic supplementary material, figure S3; n = 12). Dendritic branches of these neurons were preferentially distributed at the posterior region of the antero-lateral l ho, which corresponded to the pheromone focus. The distribution of dendrites of pheromone-specific neurons (n = 2) did not differ from that of neurons that responded to both pheromonal and non-pheromonal odours (n = 10). Notably, 11 of the 12 pheromone-sensitive neurons had dendrites in the pheromone focus defined by the distribution of terminal boutons of uni-PNs (see the electronic supplementary material, figure S3, black broken ellipses). The finding that most of the pheromone-sensitive output neurons of the l ho had dendrites in the pheromone focus confirms that this area plays a major role in the processing of alarm pheromone information. Dendrites of some neurons (n = 4) were almost confined to the pheromone focus (e.g. see the electronic supplementary material, the first neuron in figure S3). Pheromone-insensitive neurons of this class were encountered only twice and thus were not included in the analysis. One neuron innervated the pheromone focus and the other did not.
4. Discussion
(a). Major findings
In our previous study, we found that all of the PNs that exhibited responses to alarm pheromone had dendrites in a group of glomeruli in the antennal lobe of the ant C. obscuripes, and we referred to these glomeruli as AS glomeruli (Yamagata et al. 2006). We also anatomically and physiologically characterized one type of pheromone-sensitive neurons with dendrites in the l ho, one of the termination areas of PNs (Yamagata et al. 2005, 2007). Distributions of terminal boutons of pheromone-sensitive and pheromone-insensitive PNs in the l ho, as well as those of dendritic branches of these pheromone-sensitive output (efferent) neurons of the l ho, were examined in this study.
This study is the first study on spatial representation of olfactory information in a secondary olfactory centre in any animal, based on analysis of physiologically and anatomically identified afferent (input) and efferent (output) neurons. We found no region to specifically represent alarm pheromone information: terminal boutons of pheromone-sensitive PNs were widely distributed in the l ho and all regions that received alarm pheromone information also received non-pheromonal information. This is in contrast to the findings in some species of insects that the area to represent sex pheromone information is in large part segregated from the area to represent information on other odours in the l ho (cockroaches: Nishino et al. 2003; moths: Kanzaki et al. 2003; Seki et al. 2005; fruit-flies: Jefferis et al. 2007). However, we found an area in the antero-medial region of the l ho in which terminal boutons of pheromone-sensitive PNs are significantly more densely distributed than in the rest of the l ho but those of pheromone-insensitive PNs are not. We refer to this area as the alarm pheromone focus. It should be noted that our samples include only five pheromone-specific PNs and more studies based on a larger number of samples are needed to better characterize the spatial extent and functional roles of the alarm pheromone focus.
We also found that most of a major type of pheromone-sensitive output neurons of the l ho, wide-field protocerebral neurons (Yamagata et al. 2007), had dendritic branches in the alarm pheromone focus, and we suggest that this area plays major roles in alarm pheromone processing. These neurons typically responded to both alarm pheromones and non-pheromonal odours, and had dendritic arborizations in the l ho and in some other areas of the protocerebrum (see the electronic supplementary material, figure S1c; Yamagata et al. 2007). Therefore, we assume that these neurons integrate alarm pheromone information received in the l ho and non-pheromonal information received in other areas of the protocerebrum. Strictly speaking, these neurons are not ideal for quantitatively evaluating the processing of alarm pheromone information in the l ho, because it is unclear to what extent the observed spiking activities of these neurons reflect synaptic input received in the l ho. Pheromone-sensitive output neurons of the l ho whose dendrites are confined within the l ho are more suited for such analysis, but such neurons have rarely been encountered in our studies.
It should be noted that the presence of the alarm pheromone focus does not rule out the possibility that the other l ho regions play roles in at least some aspects of alarm pheromone processing. This possibility may be strengthened if we find pheromone-sensitive l ho efferent neurons for which the dendrites are not densely distributed in the pheromone focus, but we have not yet encountered such types of efferent neurons.
(b). Representation of social pheromone information in the lateral horn
The finding that the alarm pheromone focus in the l ho receives not only pheromonal information but also non-pheromonal information suggests that pheromonal information is integrated with non-pheromonal information in this area. Notice, however, that this area may also allow for specifically extracting pheromonal information by summation of synaptic inputs from a large number of pheromone-sensitive PNs and a smaller number of pheromone-insensitive PNs, which should improve signal-to-noise ratio if it is combined with a high threshold for spike firing. Thus, the alarm pheromone focus may be able to perform three different tasks, i.e. to extract alarm pheromone information, to integrate pheromonal and non-pheromonal information, and to pass them to different sets of output (efferent) neurons.
Are similar ‘foci’ likely to be present for general environmental odours? A simulation study of terminal activities of PNs in the l ho in fruitflies, based on response spectra of receptor neurons (Jefferis et al. 2007), suggested the presence of five separate domains that process information received by different subsets of receptor neurons, one of which processes sex pheromones and the other four of which process odours other than sex pheromones. Thus, a degree of functional subdivision is present in the l ho, which may provide the basis for a degree of categorization of general environmental odours. However, it is unlikely that each of the thousands of environmental odours has its own specific focus in the l ho. Studies of PNs in some species of insects (honey bees: Müller et al. 2002; Yamagata et al. 2009; moths: Hildebrand & Shepherd 1997; locusts: Laurent 2002) have shown that each PN responds to a large number of odours and each odour evokes responses in a large number of PNs, indicating an across-fibre activity pattern coding for general environmental odours by a population of PNs. Therefore, whatever the projection patterns of PNs in the l ho are, it is unlikely that terminal boutons of PNs that respond to a particular odour, such as peppermint or vanilla, can converge to a specific area in the l ho. We thus suggest that the presence of the alarm pheromone focus in the l ho of C. obscuripes reflects the presence of specific glomeruli (and their PNs) for processing alarm pheromone information in the antennal lobe.
It should be noted that our finding of the pheromone focus is based on observation of a relatively limited number of PNs. In honey bees and in C. rufipes and C. floridanus ants, calcium imaging studies of glomeruli at the surface of the antennal lobe have revealed that many glomeruli in this area responded both to alarm pheromones and non-pheromonal odours (Galizia et al. 1999a,b; Sachse et al. 1999; Zube et al. 2008), and we have not yet encountered any pheromone-sensitive uni-PNs originating from this area. Whether uni-PNs from this area terminate in the alarm pheromone focus of the l ho needs to be studied.
In conclusion, we propose that the manner by which alarm pheromones are coded in the l ho is intermediate between labelled-line coding for sex pheromone and across-regional activity pattern coding for each general environmental odour. To evaluate the validity of our proposal, further studies of spatial representations of alarm pheromones and general environmental odours in the l ho of the worker ant, as well as spatial representations of sex pheromones in the l ho of the male ant, will be necessary.
Evolution of social behaviour and of brain mechanisms underlying social behaviour are intriguing subjects in neurobiology, and our suggestion that representation of alarm pheromone information in the l ho is an intermediate between separate representation for sex pheromone and overlapping representation for general environmental odours has an implication for evolution of the neural system to process non-sexual, social pheromones in insects. In insects, the system to process sex pheromone information should have emerged in a very early stage and its separation from the system to process general environmental odours is found in many species of insects. In contrast, the system to process alarm pheromone information described here is likely to have been developed later during the course of evolution of social systems in hymenopteran insects. Thus, the imperfect separation of the system to deal with social pheromones may indicate that such a system has emerged relatively recently from the system to process general environmental odours. Alternatively, the imperfect separation may indicate that alarm pheromone information needs to be integrated with non-pheromonal odours for appropriate defence behaviour against nest intruders (Vander Meer & Alonso 1998). Further studies on social pheromone processing in the ant brain are promising for a better understanding of the evolution of neural systems underlying social behaviour in insects.
Acknowledgements
This study was supported by grants from the Ministry of Education, Science, Culture, Sports and Technology of Japan.
References
- Boeckh J., Ernst K. D.1987Contribution of single unit analysis in insects to an understanding of olfactory function. J. Comp. Physiol. A. 161, 549–565 (doi:10.1007/BF00603661) [Google Scholar]
- Brennan P. A., Kendrick K. M.2006Mammalian social odours: attraction and individual recognition. Phil. Trans. R. Soc. B. 361, 2061–2078 (doi:10.1098/rstb.2006.1931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. A., Zufall F.2006Pheromonal communication in vertebrates. Nature 444, 308–315 (doi:10.1038/nature05404) [DOI] [PubMed] [Google Scholar]
- Couto A., Alenius M., Dickson B. J.2005Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 15, 1535–1547 (doi:10.1016/j.cub.2005.07.034) [DOI] [PubMed] [Google Scholar]
- de Belle J. S., Heisenberg M.1994Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692–695 (doi:10.1126/science.8303280) [DOI] [PubMed] [Google Scholar]
- de Bruyne M., Foster K., Carlson J. R.2001Odor coding in the Drosophila antenna. Neuron 30, 537–552 (doi:10.1016/S0896-6273(01)00289-6) [DOI] [PubMed] [Google Scholar]
- Fujiwara-Tsujii N., Yamagata N., Takeda T., Mizunami M., Yamaoka R.2006Behavioral responses to the alarm pheromone of the ant Camponotus obscuripes (Hymenoptera: Formicidae). Zool. Sci. 23, 353–358 (doi:10.2108/zsj.23.353) [DOI] [PubMed] [Google Scholar]
- Galizia C. G., Menzel R., Hölldobler B.1999aOptical imaging of odor-evoked glomerular activity patterns in the antennal lobes of the ant Camponotus rufipes (Hymenoptera: Formicidae). Naturwissenschaften 86, 533–537 (doi:10.1007/s001140050669) [DOI] [PubMed] [Google Scholar]
- Galizia C. G., Sachse S., Rappert A., Menzel R.1999bThe glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 2, 473–478 (doi:10.1038/8144) [DOI] [PubMed] [Google Scholar]
- Gronenberg W., Heeren S., Hölldobler B.1996Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019 [DOI] [PubMed] [Google Scholar]
- Heimbeck G., Bugnon V., Gendre N., Keller A., Stocker R. F.2001A central neural circuit for experience-independent olfactory and courtship behavior in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 15 336–15 341 (doi:10.1073/pnas.011314898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand J. G., Shepherd G. M.1997Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu. Rev. Neurosci. 20, 595–631 (doi:10.1146/annurev.neuro.20.1.595) [DOI] [PubMed] [Google Scholar]
- Jefferis G. S., Potter C. J., Chan A. M., Marin E. C., Rohlfing T., Maurer C. R., Jr, Luo L.2007Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell 128, 1187–1203 (doi:10.1016/j.cell.2007.01.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerges J., Küttner A., Galizia C. G., Menzel R.1997Representations of odours and odour mixtures visualized in the honeybee brain. Nature 387, 285–288 (doi:10.1038/387285a0) [Google Scholar]
- Kanzaki R., Soo K., Seki Y., Wada S.2003Projections to higher olfactory centers from subdivisions of the antennal lobe macroglomerular complex of the male silkmoth. Chem. Senses 28, 113–130 (doi:10.1093/chemse/28.2.113) [DOI] [PubMed] [Google Scholar]
- Kirschner S., Kleineidam C. J., Zube C., Rybak J., Grünewald B., Rössler W.2006Dual olfactory pathway in the honeybee, Apis mellifera. J. Comp. Neurol. 499, 933–952 (doi:10.1002/cne.21158) [DOI] [PubMed] [Google Scholar]
- Kleineidam C. J., Obermayer M., Halbich W., Rössler W.2005A macroglomerulus in the antennal lobe of leaf-cutting ant workers and its possible functional significance. Chem. Senses 30, 383–392 (doi:10.1093/chemse/bji033) [DOI] [PubMed] [Google Scholar]
- Kuebler L. S., Kelber C., Kleineidam C. J.2010Distinct antennal lobe phenotypes in the leaf-cutting ant (Atta vollenweideri). J. Comp. Neurol. 51, 352–365 (doi:10.1002/cne.22217) [DOI] [PubMed] [Google Scholar]
- Laurent G.2002Olfactory network dynamics and the coding of multidimensional signals. Nature Rev. Neurosci. 3, 884–895 (doi:10.1038/nrn964) [DOI] [PubMed] [Google Scholar]
- Mobbs P. G.1982The brain of the honeybee Apis mellifera: the connections and spatial organization of the mushroom bodies. Phil. Trans. R. Soc. Lond. B. 298, 309–354 (doi:10.1098/rstb.1982.0086) [Google Scholar]
- Müller D., Abel R., Brandt R., Zöcker M., Menzel R.2002Differential parallel processing of olfactory information in the honeybee, Apis mellifera. J. Comp. Physiol. A. 188, 359–370 (doi:10.1007/s00359-002-0310-1) [DOI] [PubMed] [Google Scholar]
- Nakanishi A., Nishino H., Watanabe H., Yokohari F., Nishikawa M.In press Sex-specific antennal sensory system in the ant Camponotus japonicus: glomerular organizations of antennal lobes. J. Comp. Neurol. [DOI] [PubMed] [Google Scholar]
- Nishino H., Yamashita S., Yamazaki Y., Nishikawa M., Yokohari F., Mizunami M.2003Projection neurons originating from thermo- and hygrosensory glomeruli in the antennal lobe of the cockroach. J. Comp. Neurol. 455, 40–55 (doi:10.1002/cne.10450) [DOI] [PubMed] [Google Scholar]
- Okada R., Sakura M., Mizunami M.2003Distribution of dendrites of descending neurons and its implications for the basic organization of the cockroach brain. J. Comp. Neurol. 458, 158–174 (doi:10.1002/cne.10580) [DOI] [PubMed] [Google Scholar]
- Otsuka N.1962Histologisch-entowicklungsgeschichtliche Untersuchungen an Mauthnerschen Zellen von Fischen. Z. Zellforsh. Mikrosk. Anat. 58, 33–50 (doi:10.1007/BF00406940) [Google Scholar]
- Sachse S., Rappert A., Galizia C. G.1999The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur. J. Neurosci. 11, 3970–3982 (doi:10.1046/j.1460-9568.1999.00826.x) [DOI] [PubMed] [Google Scholar]
- Sandoz J., Deisig N., de Brito Sanchez M., Giurfa M.2007Understanding the logics of pheromone processing in the honeybee brain: from labeled-lines to across-fiber patterns. Front. Behav. Neurosci. 1, 5 (doi:10.3389/neuro.08.005.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Aonuma H., Kanzaki R.2005Pheromone processing center in the protocerebrum of Bombyx mori revealed by nitric oxide-induced anti-cGMP immunocytochemistry. J. Comp. Neurol. 481, 340–351 (doi:10.1002/cne.20392) [DOI] [PubMed] [Google Scholar]
- Strausfeld N. J., Campos-Ortega J. A.1977Vision in insects: pathways possibly underlying neural adaptation and lateral inhibition. Science 195, 894–897 (doi:10.1126/science.841315) [DOI] [PubMed] [Google Scholar]
- Szyszka P., Ditzen M., Galkin A., Galizia C. G., Menzel R.2005Sparsening and temporal sharpening of olfactory representations in the honeybee mushroom bodies. J. Neurophysiol. 94, 3303–3313 (doi:10.1152/jn.00397.2005) [DOI] [PubMed] [Google Scholar]
- Vander Meer R. K., Alonso L. E.1998Pheromone directed behavior in ants. In Pheromone communication in social insects (eds Vander Meer R. K., Breed M. D., Winston M. L., Espelie K. E.), pp. 159–192 Oxford, UK: Westview Press [Google Scholar]
- Yamagata N., Fujiwara N., Yamaoka R., Mizunami M.2005Pheromone communication and the mushroom body of the ant, Camponotus obscuripes (Hymenoptera: Formicidae). Naturwissenschaften 92, 532–536 (doi:10.1007/s00114-005-0039-0) [DOI] [PubMed] [Google Scholar]
- Yamagata N., Nishino H., Mizunami M.2006Pheromone-sensitive glomeruli in the primary olfactory centre of ants. Proc. R. Soc. B. 273, 2219–2225 (doi:10.1098/rspb.2006.3565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata N., Nishino H., Mizunami M.2007Neural pathways for the processing of alarm pheromone in the ant brain. J. Comp. Neurol. 505, 424–442 (doi:10.1002/cne.21500) [DOI] [PubMed] [Google Scholar]
- Yamagata N., Schmuker M., Szyszka P., Mizunami M., Menzel R.2009Differential odor processing in two olfactory pathways in the honeybee. Frontiers Systems Neurosci 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zube C., Kleineidam C. J., Kirschner S., Neef J., Rössler W.2008Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J. Comp. Neurol. 506, 425–441 (doi:10.1002/cne.21548) [DOI] [PubMed] [Google Scholar]