Abstract
Although store-operated Ca2+ influx has been well-studied in nonneuronal cells, an understanding of its nature in neurons remains poor. In the bag cell neurons of Aplysia californica, prior work has suggested that a Ca2+ entry pathway can be activated by Ca2+ store depletion. Using fura-based imaging of intracellular Ca2+ in cultured bag cell neurons, we now characterize this pathway as store-operated Ca2+ influx. In the absence of extracellular Ca2+, the endoplasmic reticulum Ca2+-ATPase inhibitors, cyclopiazonic acid (CPA) or thapsigargin, depleted intracellular stores and elevated intracellular free Ca2+. With the subsequent addition of extracellular Ca2+, a prominent Ca2+ influx was observed. The ryanodine receptor agonist, chloroethylphenol (CEP), also increased intracellular Ca2+ but did not initiate store-operated Ca2+ influx, despite overlap between CEP- and CPA-sensitive stores. Bafilomycin A, a vesicular H+-ATPase inhibitor, liberated intracellular Ca2+ from acidic stores and attenuated subsequent Ca2+ influx, presumably by replenishing CPA-depleted stores. Store-operated Ca2+ influx was partially blocked by low concentrations of La3+ or BTP2, and strongly inhibited by either 1-[b-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole (SKF-96365) or a high concentration of Ni2+. Regarding IP3 receptor blockers, 2-aminoethyldiphenyl borate, but not xestospongin C, prevented store-operated Ca2+ influx. However, jasplakinolide, an actin stabilizer reported to inhibit this pathway in smooth muscle cell lines, was ineffective. The bag cell neurons initiate reproductive behavior through a prolonged afterdischarge associated with intracellular Ca2+ release and neuropeptide secretion. Store-operated Ca2+ influx may serve to replenish stores depleted during the afterdischarge or participate in the release of peptide that triggers behavior.
INTRODUCTION
Elevation of intracellular Ca2+ is a profoundly important signaling event, capable of producing neurotransmitter release, activating signal transduction pathways, or initiating gene expression (Hille 2001; Levitan and Kaczmarek 2002). Ca2+ can be released from intracellular stores, which in neuronal somata are principally the endoplasmic reticulum (Berridge 1998; Meldolesi 2001; Verkhratsky 2005), or Ca2+ can enter from the extracellular space through voltage-gated Ca2+ channels (Hille 2001; Reid et al. 2003). Because most nonneuronal/nonexcitable cells cannot employ the voltage sensitivity of Ca2+ channels, they gate Ca2+ influx through an alternative pathway known as capacitative Ca2+ entry or store-operated Ca2+ influx (Casteels and Droogmans 1981; Nilius 2003; Parekh 2003; Putney 1986). Although the mechanism is not fully defined, this pathway is activated when the depletion of Ca2+ from intracellular stores opens specific, Ca2+-permeable channels in the plasma membrane (Nilius 2003; Parekh 2003).
In comparison to nonneuronal cells, the characterization of store-operated Ca2+ influx in neurons is less extensive (Putney 2003). Initially, neurons, neuroendocrine cells, and neuronal cell lines were thought to lack store-operated Ca2+ influx (Friel and Tsien 1992; Jackson et al. 1988; Stauderman and Pruss 1989). However, beginning with a report of Ca2+ entry following store depletion in PC12 cells (Clementi et al. 1992), affirmative evidence began to accumulate (Arakawa et al. 2000; Baba et al. 2003; Bouron 2000; Grudt et al. 1996; Liu and Gylfe 1997; Piper and Lucero 1999; Powis et al. 1996; Prothero et al. 2000; Tozzi et al. 2003; Usachev and Thayer 1999; Villalobos and Garcia-Sancho 1995; Zufall et al. 2000). Nevertheless, reports continue to assert that certain neurons either lack or show limited store-operated Ca2+ influx (Chinopoulos et al. 2004; Emptage et al. 2001; Grimaldi et al. 2001; Koizumi et al. 1999). The ongoing controversy prompted us to explore this pathway in the bag cell neurons of the marine mollusk, Aplysia californica—a preparation in which the regulation of intracellular Ca2+ has been extensively investigated (Fink et al. 1988; Jonas et al. 1997; Knox et al. 1996, 2004; Magoski et al. 2000).
Brief synaptic input to the bag cell neurons triggers an ~30-min afterdischarge, resulting in neuropeptide secretion and the initiation of egg-laying behavior (Conn and Kaczmarek 1989; Kupfermann 1967; Kupfermann and Kandel 1970; Pinsker and Dudek 1977; Rothman et al. 1983). Previously, Knox et al. (1996) used a Ca2+-sensitive vibrating extracellular probe to shown that the endoplasmic reticulum Ca2+-ATPase inhibitor, thapsigargin (Thastrup et al. 1990), may initiate Ca2+ entry in bag cell neurons. In the present study, we employ intracellular Ca2+ imaging to demonstrate that this indeed is a store-operated Ca2+ influx pathway. During an afterdischarge, Ca2+ enters through voltage-gated Ca2+ channels and is released from intracellular stores, apparently in an IP3-dependent manner (Fink et al. 1988; Fisher et al. 1994). The bag cell neurons may require store-operated Ca2+ influx to replete these intracellular stores or may use this source of Ca2+ entry to elicit neuropeptide secretion itself.
METHODS
Animals and cell culture
Adult A. californica weighing 100–250 g were obtained from Marine Specimens Unlimited (San Francisco, CA) or Marinus (Long Beach, CA). Animals were housed in an ~300l aquarium containing continuously circulating, aerated artificial sea water (Instant Ocean, Aquarium Systems, Mentor, OH, or Kent sea salt, Kent Marine, Acworth, GA) at 14–16°C on an ~12/12 h light/dark cycle and fed Romaine lettuce three to five times a week.
For primary cultures of isolated bag cell neurons, animals were anesthetized by an injection of isotonic MgCl2 (~50% of body weight), the abdominal ganglion removed and treated for 18 h at 20–22°C with neutral protease (13.33 mg/ml; 165859, Roche Diagnositics, Indianapolis, IN) dissolved in tissue culture artificial sea water (tcASW; composition in mM: 460 NaCl, 10.4 KCl, 11 CaCl2, 55 MgCl2, 15 HEPES, 1 mg/ml glucose, 100 U/ml penicillin, and 0.1 mg/ml streptomycin, pH 7.8 with NaOH). The ganglion was then transferred to fresh tcASW and the bag cell neuron clusters dissected from their surrounding connective tissue. Using a fire-polished Pasteur pipette and gentle trituration, neurons were dispersed in tcASW onto regular 35 × 10-mm polystyrene tissue culture dishes (25000, Corning, Corning, NY) or glass coverslips (No. 1; 48366045, VWR, West Chester, PA) that were coated with poly-D-lysine (1 μg/ml, 70,000–150,000 MW; P0899, Sigma-Aldrich, Oakville, ON, Canada, or St. Louis, MO) and glued to drilled out tissue culture dishes. Cultures were maintained in tcASW for 1–3 days in a 14°C incubator. Salts were obtained from Fisher (Ottawa, ON, Canada), ICN (Aurora, OH), or Sigma-Aldrich.
Intracellular Ca2+ measurements
Somatic intracellular Ca2+ was measured by ratiometric imaging of the dye, fura PE3 (K+ salt; 0110, Teflabs, Austin, TX) (Vorndran et al. 1995). Fura-PE3 was pressure injected via sharp electrodes using either a Picospritzer II (General Valve) or a PMI-100 pressure micro-injector (Dagan, Minneapolis, MN) while simultaneously monitoring membrane potential with an Axoclamp 2B amplifier (Axon Instruments, Union City, CA). Microelectrodes were pulled from 1.2-mm internal diameter, borosilicate glass capillaries (1B120F-4, World Precision Instruments, Sarasota, FL) and had a resistance of 30–50 MOmega; when the tip was filled with 10 mM fura-PE3 then backfilled with 3 M KCl. Injections usually required 10–15 300- to 900-ms pulses at 30–60 kPa to fill the neurons with an optimal amount of dye—estimated to be 50–100 μM. All neurons used for subsequent imaging showed resting potentials of −50 to −60 mV and displayed action potentials that overshot 0 mV after depolarizing current injection (0.5–1 nA, directly from the amplifier). After dye injection, neurons were allowed to equilibrate for ≥30 min. Ca2+ imaging was performed using a Nikon Diaphot inverted microscope (Nikon, Mississauga, ON, Canada) equipped with a Nikon Fluor 10X objective (numerical aperture (NA) = 0.5) or Nikon TS100-F inverted microscope equipped with a Nikon Plan Fluor 10X objective (NA = 0.3). The light source was a 75 W Xenon arc lamp and a multi-wavelength DeltaRAM V monochromatic illuminator (Photon Technology International, London, ON, Canada) coupled to the microscope with a UV-grade liquid-light guide. Between acquisition episodes, the excitation illumination was blocked by a shutter, which along with the excitation wavelength, was controlled by a IBM-compatible personal computer, a Photon Technology International computer interface, and ImageMaster Pro software (version 1.49, Photon Technology International). The emitted light passed through a 510/40 nm barrier filter prior to being detected by either a Hamamatsu C2400 (Hamamatsu, Bridgewater, NJ) or Photon Technology International IC200 intensified charge coupled device camera. The camera intensifier voltage was set based on the initial fluorescence intensity of the cells at the beginning of each experiment and maintained constant thereafter. The camera black level was set prior to an experiment using the camera controller such that at a gain of 1 there was a 50:50 distribution of blue and black pixels on the image display with no light going to the camera. The ratioed image of the fluorescence intensities (converted to pixel values) from 340 and 380 nm excitation wavelengths was derived and, if necessary, averaged four to eight frames per acquisition, resulting in a single full-frame (520 × 480 pixels) acquisition time of 0.5–4 s. A sample of the fluorescence intensities ratio was taken typically at 1 min intervals using regions of interests (ROIs) defined over the neuronal somata prior to the start of the experiment. The ratio was then either recorded simply as 340/380 to reflect free intracellular Ca2+ or used to calculate the actual free intracellular Ca2+ from the relationship, [Ca2+] = Kd · Q(R − Rmin)/Rmax − R (Grynkiewicz et al. 1985). Rmin, Rmax, and Q were determined in intact bag cell neurons by applying 1–10 μM digitonin (D-8449, Molecular Probes, Eugene, OR) under Ca2+-free conditions followed by perfusion with saline containing a saturating amount of Ca2+ (11 mM). The constant Q was determined from the ratio of 380 nm evoked fura PE3 fluorescence in Ca2+-free ASW and 11 mM Ca2+-containing normal ASW (nASW). Values for Rmin, Rmax, and Q ranged from 0.11 to 0.33, 5.1–7.5, and 42.6–50, respectively, whereas the Kd was 204 nM (from Vorndran et al. 1995). The black level determination, image acquisition, frame averaging, emitted light ROI sampling, and ratio calculations were carried out using the Image-Master Pro software. Ratio calculations were saved for subsequent analysis (see following text). Imaging was carried out at room temperature (20–22°C) and performed in both nASW (composition as per tcASW but with the glucose, penicillin, and streptomycin omitted) and Ca2+-free ASW (composition as per nASW but with CaCl2 omitted and 0.5 mM EGTA added).
Membrane potential recordings
An Axoclamp 2B amplifier (Axon Instruments) in bridge mode was used to measure membrane potential. Current-clamp recordings were made using sharp microelectrodes (glass as per dye injection) with a resistance of 5–10 MΩ when filled with 2 M K-acetate (supplemented with 100 mM KCl and 10 mM HEPES; pH = 7.3 with KOH). Voltage was low-pass filtered at 3 kHz using the Axoclamp 2B Bessel filter and acquired at a sampling rate of 100 Hz with an IBM-compatible personal computer, a Digidata 1300 A/D converter (Axon Instruments), and the Clampex acquisition program of pCLAMP (version 8.0; Axon Instruments).
Drug application and reagents
Drug application or solution exchanges were accomplished by manual perfusion using a plastic transfer pipette to exchange the bath (tissue culture dish) solution. Complete exchange of the bath could be achieved in <30 s. In some cases, drugs were introduced directly into the bath by pipetting a small volume (<10 μl) of concentrated stock solution or a larger volume of saline (~500 μl) that was initially removed from the bath, mixed with the stock solution, and then reintroduced. Care was taken to perform all pipetting near the side of the dish and as far away as possible from the neurons. 2-aminoethyldiphenyl borate (2-APB; 100065, Calbiochem, San Diego, CA), bafilomycin A (B1793, Sigma-Aldrich), BTP2 (203890, Calbiochem), 4-chloro-3-ethylphenol (CEP; 279552, Sigma-Aldrich), cyclopiazonic acid (CPA; C1530, Sigma-Aldrich or 239805, Calbiochem), 1-[b-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole (SKF 96365; 567310, Calbiochem or S7809, Sigma-Aldrich), thapsigargin (T3250, Sigma-Aldrich), and xestospongin C (CA409, Cedarlane, Hornby, ON, Canada) all required DMSO (BP231, Fisher) as a vehicle, whereas jasplakinolide (420107, Calbiochem) required methanol (A412, Fisher). The maximal final concentration of DMSO and methanol was 0.01 μM and 0.01%, respectively, which in control experiments had no effect on intracellular Ca2+. LaCl3 (L4131, Sigma-Aldrich) and NiCl2 (N6136, Sigma-Aldrich) were dissolved as a stock in water or to the final desired concentration in saline.
Analysis
Origin (version 7, OriginLab Corporation, Northampton, MA) was used to import and plot ImageMaster Pro files as line graphs. For display, intracellular Ca2+ concentrations or the 340/380 ratios were averaged and presented as the means ± SE versus time. Analysis used steady-state changes acquired by taking the average value, by eye or with adjacent-averaging, from regions that had reached steady state for 5–10 min in plots of individual experiments. Statistics were performed on percent change values using Instat (version 3.0, GraphPad Software, San Diego, CA). The Kolmogorov-Smirnov method was used to test data sets for normality. A standard ANOVA with the Dunnett’s multiple comparisons test was used to test for differences between multiple means. In a few cases, Student’s paired or unpaired t-test was used to test for differences between two means. Data were considered significantly different if the two-tailed P value was <0.05.
RESULTS
Intracellular Ca2+ store depletion activates a Ca2+ influx pathway in cultured bag cell neurons
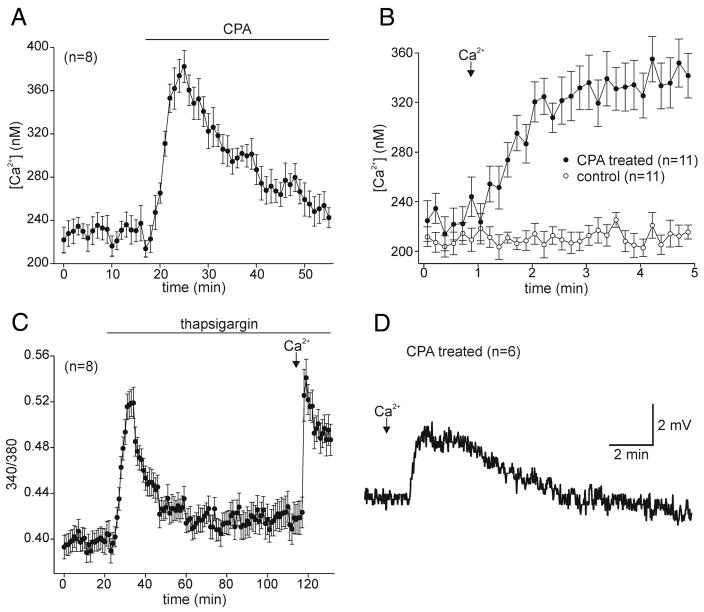
To determine if Ca2+ store depletion can initiate a Ca2+ influx pathway, cultured bag cell neurons were bathed in Ca2+-free ASW and exposed to agents that liberate intracellular Ca2+. The smooth endoplasmic reticulum Ca2+ pump inhibitor, CPA (10–50 μM) (Seidler et al. 1989), depleted intracellular stores and caused Ca2+ to rise quickly (Fig. 1A; n = 12). Despite the continued presence of CPA, Ca2+ levels recovered to near-control levels, most likely attributable to active and passive removal of Ca2+ from the intracellular to the extracellular compartment (Clapham 1995; Knox et al. 1996; Meldolesi 2001; Verkhratsky 2005). In separate experiments, the subsequent addition of extracellular Ca2+ by exchanging the Ca2+-free ASW for nASW initiated a marked and rapid rise in intracellular Ca2+ but only in those neurons depleted with CPA and not those merely exposed to Ca2+-free ASW alone (Fig. 1B; n = 44 versus 11). This suggested that depletion of intracellular Ca2+ stores activates a plasma membrane Ca2+ entry pathway. Although this pathway is presumably open during depletion in Ca2+-free conditions, it cannot be detected until extracellular Ca2+ is added and Ca2+ begins to flow back into the neurons. Similar results were achieved with 2–3 μM of the irreversible, smooth endoplasmic reticulum Ca2+ pump inhibitor, thapsigargin (Thastrup et al. 1990) (Fig. 1C; n = 15). On average, addition of extracellular Ca2+ after depletion with CPA resulted in an ~47% increase in intracellular Ca2+ that was statistically different from the ~25% increase observed following thapsigargin-induced depletion (Fig. 6; 2nd vs. 1st bar).
FIG. 1.
Depletion of cultured bag cell neuron intracellular Ca2+ stores initiates a store-operated Ca2+ influx pathway. A: addition of 10 μM of the smooth endoplasmic reticulum Ca2+ pump inhibitor, cyclopiazonic acid (CPA), causes Ca2+ store depletion and elicits a large, rapid Ca2+ rise that subsequently recovers (n = 8; representative of 12 in total). B: exchanging the Ca2+-free ASW for Ca2+-containing nASW (↓), results in a rapid elevation of intracellular Ca2+ in neurons depleted with CPA (n = 11; representative of 44 in total) but not in neurons simply maintained in Ca2+-free ASW (n = 11). The CPA-treated neurons were exposed to the drug for ~60 min prior to the addition of nASW. C: similarly, 3 μM of thapsigargin, a different smooth endoplasmic reticulum Ca2+ pump inhibitor, also depletes the Ca2+ stores of bag cell neurons bathed in Ca2+-free ASW. The introduction of extracellular Ca2+ again initiates an accelerated rise in intracellular Ca2+ (n = 8; representative of 15 in total). D: sharp-electrode current clamp recording of membrane potential from a cultured bag cell neuron bathed in Ca2+-free ASW plus 10 μM CPA for 30 min. Delivery of Ca2+-containing nASW elicits a small depolarization that recovers to baseline in ~5 min (n = 6).
FIG. 6.
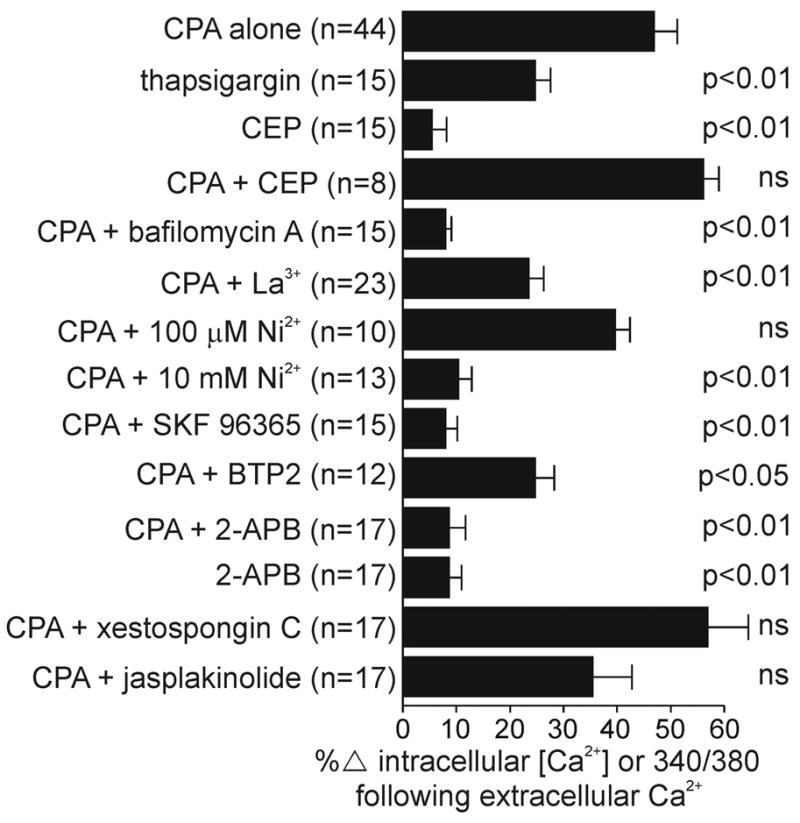
Summary of store-operated Ca2+ influx in bag cell neurons. The ordinate lists various treatment conditions, with the n values of the total number of neurons corresponding to both those given in the text and those given in the figure legends as “representative of n in total.” The abscissa is an index of store-operated Ca2+ influx as the percent change in either the intracellular Ca2+ concentration or the 340/380 ratio following the addition of extracellular Ca2+. All data sets passed the test for normality using the Kolmogorov-Smirnov method. The P values on the right represent the outcome of a Dunnett’s multiple comparisons test following a standard ANOVA. Comparisons were made between CPA alone and each subsequent condition.
It is possible that the store-operated pathway depolarizes the neurons to such an extent that voltage-gated Ca2+ channels are activated. This would contaminate the assay with an additional Ca2+ influx source. To resolve this, the membrane potential of bag cell neurons was recorded during the introduction of extracellular Ca2+ after depletion. After depletion with CPA in Ca2+-free ASW, exchange to Ca2+-containing nASW resulted in only a small depolarization of 8.7 ± 4.3 mV (Fig. 1D; n = 6). In Ca2+-free ASW plus CPA, the actual membrane potential was −52.8 ± 6.3 mV, whereas in nASW plus CPA, it depolarized to −45.6 ± 3.9 mV (not significant; Student’s paired t-test). Such a change would fail to bring the membrane potential near the threshold for activation of the voltage-gated Ca2+ current in these neurons (−30 to −25 mV) (Kaczmarek and Strumwasser 1984; Knox et al. 1996; A. Y. Hung and N. S. Magoski, unpublished data).
Activating ryanodine receptors does not initiate the store-operated Ca2+ influx pathway
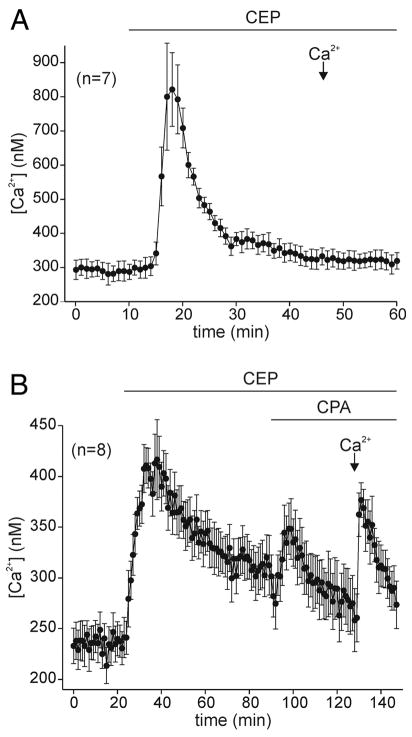
Although CPA and thapsigargin liberate Ca2+ by blocking Ca2+ pumps on the endoplasmic reticulum, an alternate means is to open the Ca2+-permeable channels within the store. In Ca2+-free ASW, 100 μM of the ryanodine receptor agonist, CEP (Zorzato et al. 1993), rapidly elevated intracellular Ca2+, which then returned to near baseline levels even with maintained exposure to the agonist (Fig. 2A; n = 15). Subsequent delivery of extracellular Ca2+ caused, on average, intracellular Ca2+ to rise by only ~5%, which was significantly different from the influx that occurred with CPA (Fig. 6; 3rd vs. 1st bar). When CPA was applied after CEP in Ca2+-free ASW, there was a small but detectable Ca2+ elevation as well as obvious store-operated Ca2+ influx on return to Ca2+-containing nASW (Fig. 2B; n = 8). Overall, addition of extracellular Ca2+ to neurons treated with CEP followed by CPA resulted in an ~55% increase in intracellular Ca2+ that was not significantly different from that seen after CPA alone (Fig. 6; 4th vs. 1st bar). CEP was chosen as a ryanodine receptor agonist over caffeine (Rousseau et al. 1988; Weber 1968) or ryanodine (Meissner 1985) because we have found that the former has a number of nonspecific effects on bag cell neuron ion channels, whereas the latter is not an efficacious agonist of the bag cell neuron Ca2+ release channel—perhaps because of the high salt conditions (N. S. Magoski, R. J. Knox, and L. K. Kaczmarek, unpublished observation).
FIG. 2.
A ryanodine receptor agonist elevates intracellular Ca2+ but does not cause store-operated Ca2+ influx. A: in Ca2+-free ASW, 100 μM of chloroethylphenol (CEP), a ryanodine receptor agonist, causes a rapid increase in intracellular Ca2+ followed by almost complete recovery. However, when extracellular Ca2+ is introduced on exchange to nASW, intracellular Ca2+ remains unchanged (n = 7; representative of 15 in total). B: prior opening of ryanodine receptors by 100 μM CEP reduces the Ca2+ elevation produced by the application of 10 μM CPA in Ca2+-free ASW. Nevertheless, after application of CEP and CPA, store-operated Ca2+ influx elicited by exchange to Ca2+-containing nASW remains intact (n = 8).
Ca2+ liberated from acidic stores replenishes stores depleted with CPA
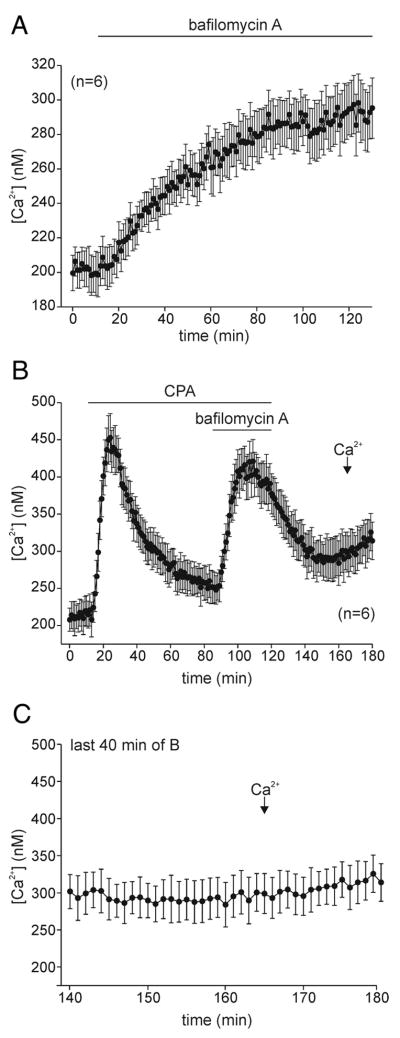
The acidic compartment is a bag cell neuron Ca2+store that has yet to be investigated. Bafilomycin A, a vacuolar H+-ATPase inhibitor (Bowman et al. 1988), caused bag cell neuron intracellular Ca2+ to slowly rise in Ca2+-free ASW when applied at 50 nM (Fig. 3A; n = 6). The source was designated an acidic store because Ca2+ was liberated after the bafilomycin-A-induced collapse of its H+ gradient, which is presumably required for maintaining Ca2+ uptake (Christensen et al. 2002; Dunn et al. 1994; Goncalves et al. 1999; Ohsumi and Anraku 1983). The time to onset of the bafilomycin-A-induced elevation in intracellular Ca2+ was shorter if the neurons were first exposed to CPA (Fig. 3B; n = 15); however, the mean change for bafilomycin A alone (39.7 ± 4.65% increase) versus CPA then bafilomycin A (56.6 ± 4.8% increase) only approached significance (P < 0.06; Student’s unpaired t-test). Wash of the bafilomycin A + CPA resulted in intracellular Ca2+ recovering to near CPA-depleted levels, whereas subsequent exchange to Ca2+-containing nASW initiated some store-operated Ca2+influx, although it was very muted (Fig. 3, B and C). Compared with responses after depletion in CPA alone, the amplitude of the response was small and the time to onset was markedly slower (compare Figs. 1B with 3C). At only an ~8% increase, the average Ca2+ influx after introduction of extracellular Ca2+ to bafilomycin A + CPA treated neurons was significantly different from that after CPA alone (Fig. 6; 5th vs. 1st bar).
FIG. 3.
H+-ATPase inhibitor elevates intracellular Ca2+ and replenishes depleted Ca2+ stores. A: under Ca2+-free conditions, a gradual elevation of intracellular Ca2+ is induced by 50 nM of the vacuolar H+-ATPase inhibitor, bafilomycin A (n = 6). B: Ca2+ release from acidic stores partially replenishes previously depleted stores. Addition of 50 nM bafilomycin A, after depletion by 20 μM CPA, causes intracellular Ca2+ to rise at a rapid rate in Ca2+-free ASW (compare with A). On wash of both bafilomycin A and CPA, Ca2+ levels show partial recovery; moreover, exchange to Ca2+-containing nASW produces only a slow, attenuated elevation of intracellular Ca2+ (n = 6; representative of 15 in total). C: The last 40 min of B on an expanded time scale. The record begins just after Ca2+ levels have recovered after washout of the drugs. If at all, extracellular Ca2+ produces a small, slow elevation of intracellular Ca2+.
Store-operated Ca2+ influx pathway is sensitive to La3+, Ni2+, SKF 96365, and BTP2
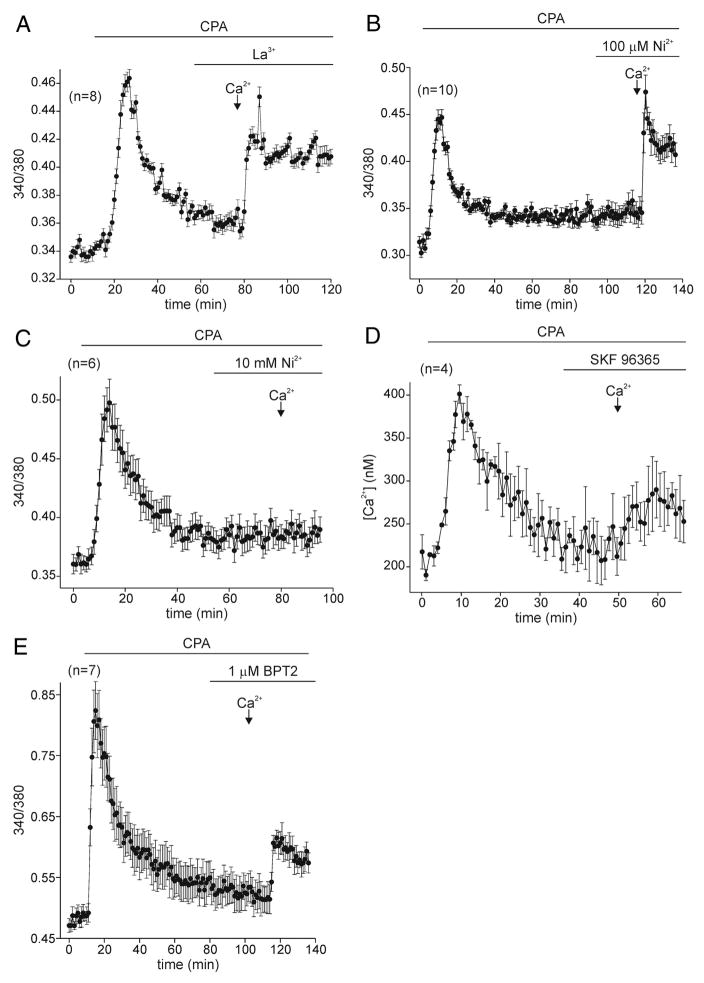
Depleting intracellular stores clearly initiates Ca2+ influx in bag cell neurons. However, to better compare this route of Ca2+ entry with similar pathways in other neuronal and non-neuronal cells, it was important to learn more about its pharmacological properties. Store-operated Ca2+ influx is often blocked by tri- or divalent metals such as La3+ and Ni2+ (Gore et al. 2004; Hoth and Penner 1993; Ross and Cahalan 1995). With 100 μM La3+ in the bath, addition of extracellular Ca2+ to CPA-depleted bag cell neurons brought about a rise in intracellular Ca2+ that was more modest in comparison to control (Fig. 4A; n = 23). Although this block was by no means complete, the difference between the mean change in intracellular Ca2+ in La3+ (~23% increase) was significantly different from that in control (~47% increase; Fig. 6; 6th vs. 1st bar). When the same experiment was performed using 100 μM Ni2+, no block of the Ca2+ influx pathway was seen (Fig. 4B; n = 10), although increasing the concentration of Ni2+ to 10 mM quite effectively inhibited the increase in intracellular Ca2+ normally observed with the addition of Ca2+-containing nASW (Fig. 4C; n = 13). The average change in intracellular Ca2+ after the introduction of extracellular Ca2+ to neurons treated with CPA +100 μM Ni2+ was an ~40% increase, which was not significantly different from CPA alone (Fig. 6; 7th vs. 1st bar). Conversely, there was only a mean ~10% increase in intracellular Ca2+ for CPA 310 mM Ni2+, and this value readily reached the level of significance when compared with neurons treated with just CPA (Fig. 6; 8th vs. 1st bar).
FIG. 4.
Block of store-operated Ca2+ influx. A: after depletion in Ca2+-free ASW with 10 μM CPA, introduction of extracellular Ca2+ in the presence of 100 μM La3+ results in attenuated store-operated Ca2+ influx (n = 8; representative of 2+ in total). B: same concentration of Ni2+ does not prevent store-operated Ca2+ influx induced by prior depletion (n = 10). C: however, when the concentration of Ni2+ is increased 100-fold, it results in complete block of store-operated Ca2+ influx initiated by depleting stores with 10 μM CPA in Ca2+-free ASW (n = 6; representative of 13 in total). D: Ca2+ stores are first depleted with 50 μM CPA in Ca2+-free ASW. With the subsequent addition of 20 μM 1-[b-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole (SKF 96365), a common antagonist of store-operated Ca2+ influx, the exchange to nASW elicits only a small Ca2+ elevation (n = 4; representative of 15 in total). E: similarly, after depletion by 20 μM CPA in Ca2+-free ASW, the presence of a different store-operated Ca2+ influx blocker, BTP2, results in only a modest Ca2+ increase when Ca2+-containing nASW is introduced (n = 7; representative of 12 in total).
In addition to tri- or divalent metals, an imidazole known as SKF-96365 is commonly used to block store-operated Ca2+ influx (Cabello and Schilling 1993; Daly et al. 1995, Doi et al. 2000; Fasolato et al. 1990; Merritt et al. 1990). The Ca2+ entry route initiated by store depletion in bag cell neurons was antagonized by 20 μM SKF 96365 (Fig. 4D; n = 15). The mean change in intracellular Ca2+ after exchange to Ca2+-containing nASW for those neurons exposed to CPA + SKF 96365 was an ~8% increase, and this was significantly different from the Ca2+ elevation elicited in neurons subjected to only CPA (Fig. 6; 9th vs. 1st bar). Recently, a pyrazole derivative designated as BTP2 was found to block both store-operated Ca2+ influx and depletion-activated current in Jurkat T-lymphocytes (Ishikawa et al. 2003; Zitt et al. 2004). For bag cell neurons, 1 μM BTP2 inhibited store-operated Ca2+ influx (Fig. 4E; n = 12). In neurons exposed to CPA + BTP2 there was an ~25% elevation in intracellular Ca2+ after exchange to nASW, which was significantly different from the approximately 47% increase seen in neurons given only CPA (Fig. 6; 10th vs. 1st bar).
Neither IP3 receptors nor microfilaments likely play a role in store-operated Ca2+ influx
Ma et al. (2000) reported that inhibition of IP3 receptors by the membrane-permeable antagonist, 2-APB (Maruyama et al. 1997), prevents store-operated Ca2+ influx. However, subsequent work has suggested that 2-APB is in fact a direct inhibitor of store-operated channels (Baba et al. 2003; Braun et al. 2001; Cordova et al. 2003; Prakriya and Lewis 2001; Tozzi et al. 2003). Therefore, we felt it necessary to test the ability of this agent to block Ca2+ entry after store depletion in bag cell neurons. For bag cell neurons that had been depleted with CPA in Ca2+-free ASW, the application of 100 μM 2-APB itself produced an elevation in intracellular Ca2+; moreover, 2-APB quite effectively prevented store-operated Ca2+ influx when extracellular Ca2+ was introduced with nASW (Fig. 5A; n = 17). On average, there was only an ~9% increase in intracellular Ca2+ when Ca2+-containing nASW was delivered to neurons treated with CPA +2-APB, which was significantly different from the ~47% increase seen in the CPA alone treated neurons (Fig. 6; 11th vs. 1st bar). Perhaps on account of its antagonistic actions or because it simply is not a true depleting agent, store-operated Ca2+ influx was not initiated following release of intracellular Ca2+ by 2-APB alone (Fig. 6; 12th vs. 1st bar). The release of intracellular Ca2+ by 2-APB was not altogether unexpected. In their initial report that 2-APB could inhibit IP3 receptors with an IC50 of 42 μM, Maruyama et al. (1997) noted that >90 μM, it also caused Ca2+ release. Because 2-APB is known to inhibit both IP3 receptors and store-operated channels, we tested xestospongin C, an alkaloid IP3 receptor antagonist that is thought to attenuate store-operated Ca2+ influx by interfering with the coupling between depletion and activation of the pathway (Gafni et al. 1997; Kiselyov et al. 1998; Ma et al. 2000). Despite the effectiveness of 2-APB, 15 μM xestospongin C did not alter store-operated influx in CPA-depleted bag cell neurons (Fig. 5B; n = 17). Overall, intracellular Ca2+ went up by ~57% when Ca2+-containing nASW was introduced to neurons bathed in CPA + xestospongin C. This elevation was not significantly different from that observed in neurons bathed in CPA alone (Fig. 6; 13th vs. 1st bar).
FIG. 5.
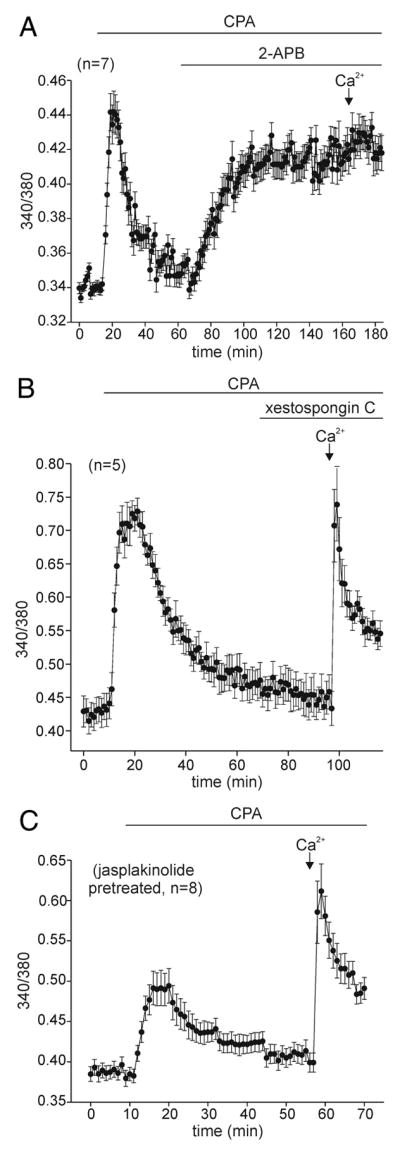
Store-operated Ca2+ influx likely does not invovle IP3 receptors or microfilaments. A: after Ca2+ store depletion with 10 μM CPA, the introduction of 100 μM 2-aminoethyldiphenyl borate (2-APB), a purported IP3 receptor antagonist, elicits a 2nd rise in intracellular Ca2+. When the Ca2+-free ASW is exchanged to nASW, the store-operated Ca2+ influx is absent (n = 7; representative of 17 in total). B: application of 15 μM xestospongin C, a different IP3 receptor blocker, to neurons depleted with 20 μM CPA, neither alters the intracellular Ca2+ nor affects store-operated Ca2+ influx (n = 5; representative of 17 in total). C: When neurons are first pretreated with 1 μM of the microfilament stabilizer, jasplakinolide, the store-operated Ca2+ influx produced by depletion with 10 μM CPA is unaltered (n = 8; representative of 17 in total).
Both Yao et al. (1999) and Patterson et al. (1999) provided evidence that store-operated Ca2+ influx in Xenpous oocytes or mammalian cell lines was initiated by secretion or trafficking of a signal from the endoplasmic reticulum to the plasma membrane. In the case of cell lines, influx was blocked following microfilament stabilization with the membrane-permeable actin polymerizer, jasplakinolide (Bubb et al. 1994; Patterson et al. 1999). However, when 1 μM jasplakinolide was applied to bag cell neurons, store-operated Ca2+ influx was not prevented (Fig. 5C; n = 17). There was no significant difference between the ~35% mean change in intracellular Ca2+ after introduction of extracellular Ca2+ to neurons exposed to CPA + jasplakinolide, and the ~47% increase for neurons treated with only CPA (Fig. 6; 14th vs. 1st bar).
DISCUSSION
Treatment with CPA or thapsigargin in Ca2+-free saline, followed by the introduction of extracellular Ca2+, elevates bag cell neuron intracellular Ca2+ in a manner consistent with store-operated Ca2+ influx. Store-operated Ca2+ influx will not occur unless depletion opens the pathway and extracellular Ca2+ is present to enter the neurons. CPA and thapsigargin deplete Ca2+ by inhibiting the endoplasmic reticulum Ca2+-ATPase (Seidler et al. 1989; Thastrup et al. 1990). Ca2+ then leaks out of the organelle and is removed to the extracellular space by plasma membrane exchangers or pumps (Clapham 1995; Meldolesi 2001; Verkhratsky 2005). It is unlikely that Ca2+-free saline itself triggers influx given that without prior depletion, no change in intracellular Ca2+ is observed on delivery of extracellular Ca2+. These results extend the findings of Knox et al. (1996), who detected Ca2+ entry into bag cell neurons with a Ca2+-sensitive vibrating extracellular probe after application of thapsigargin in the presence of extracellular Ca2+. However, they did not determine if store depletion itself initiates Ca2+ entry nor did they demonstrate sensitivity to store-operated channel antagonists.
Both neuronal and nonneuronal store-operated channels are sensitive to certain di- and trivalent metals as well as SKF 96365, BTP2, and 2-APB. La3+ is the most potent metal, producing near-complete block at 0.1–1 mM (Baba et al. 2003; Bouron 2000; Gore et al. 2004; Grudt et al. 1996; Hoth and Penner 1993; Lepple-Wienhues and Cahalan 1996; Liu and Gylfe 1997; Prothero et al. 2000; Ross and Cahalan 1995). Ni2+, the other metal used here, is less effective and requires 5–10 mM for substantial inhibition (Arakawa et al. 2000; Hoth and Penner 1993; Prothero et al. 2000; Trepakova et al. 2001; Usachev and Thayer 1999; Villalobos and Garcia-Sancho 1995). Regarding SKF 96365, Merritt et al. (1990) first proposed it as a blocker of store-operated Ca2+ influx, and although not absolutely specific, inhibition by 10–100 μM remains a key indicator of this pathway (Arakawa et al. 2000; Cabello and Schilling 1993; Daly et al. 1995; Grudt et al. 1996; Prakriya and Lewis 2003). The later developed BTP2 will completely antagonize store-operated influx after short-term incubation at 10 μM, whereas 1 μM produces a less potent reduction (Ishikawa et al. 2003). Finally, 10–100 μM 2-APB eliminates store-operated influx (Baba et al. 2003; Braun et al. 2001; Cordova et al. 2003; Prakriya and Lewis 2001; Tozzi et al. 2003). Accordingly, the profile of the bag cell neuron Ca2+ elevation, i.e., partial block by 100 μM La3+ and 1 μM BTP2, as well as near-complete block by 10 mM Ni2+, 20 μM SKF 96365, or 100 μM 2-APB, is consistent with store-operated Ca2+ influx channels.
There are two categories of store-operated Ca2+ influx channels. One, Ca2+ release-activated Ca2+ (CRAC) channels, which are highly Ca2+ selective, very-small-conductance (estimated ~20 fS) channels (Hoth and Penner 1993; Prakriya and Lewis 2003; Zweifach and Lewis 1993). Two, Ca2+-permeable, larger-conductance (tens of pS), nonselective cation channels (Albert and Large 2002; Curtis and Scholfield 2001; Su et al. 2001; Trepakova et al. 2001). Some of the latter belong to the transient receptor potential channel family originally cloned from Drosophila (Beech et al. 2003; Kiselyov et al. 1998; Ma et al. 2000; Nilius 2003; Petersen et al. 1995; Philipp et al. 1996; Tozzi et al. 2003; Zitt et al. 1996). Recently, a membrane-spanning protein, termed Orai1 or CRACM1, has been identified as an fundamental constituent or regulator of store-operated Ca2+ influx channels (Feske et al. 2006; Vig et al. 2006). The channel-type mediating bag cell neuron store-operated Ca2+ influx is currently unknown, although the modest depolarization seen with extracellular Ca2+ following depletion may be due to its opening. Alternatively, store-operated Ca2+ influx could cause depolarization by triggering one of the bag cell neuron Ca2+-activated cation channels (Knox et al. 1996; Lupinsky and Magoski 2006). Nevertheless, the source of Ca2+ during influx appears to be solely from the store-operated pathway, given that the depolarization is too small to activate voltage-gated Ca2+ current (−30 to −25 mV threshold) (Kaczmarek and Strumwasser 1984; Knox et al. 1996; A. Y. Hung and N. S. Magoski, unpublished data).
Models for depletion-activation of store-operated channels include diffusional coupling of an unknown small-molecule released from stores to stimulate channels (Randriamampita and Tsien 1993), trafficking or secretion coupling of channels or channel regulators, such a STIM1, to the plasma membrane (Patterson et al. 1999; Spassova et al. 2006, Yao et al. 1999; Zhang et al. 2005), and conformational coupling of IP3 receptors through a physical link with channels (Kiselyov et al. 1998; Ma et al. 2000). In smooth muscle cells and platelets, secretion coupling of store-operated Ca2+ influx is prevented by jasplakinolide-induced microfilament stabilization (Patterson et al. 1999; Rosado et al. 2004). However, this is not the case for epithelia or thyroid cells (Abeele et al. 2004; Gratschev et al. 2004) or, as shown in the current study, the bag cell neurons. The ineffectiveness of jasplakinolide cannot be explained by species differences in pharmacology because jasplakinolide and its membrane-impermeant analogue, phalloidin, are effective cytoskeletal stabilizers in Aplysia and Drosophila (Forscher et al. 1992; Tilney et al. 2004). Thus, trafficking or secretion coupling are unlikely to contribute to store-operated Ca2+ influx in bag cell neurons. For conformational coupling, a hallmark is the block of store-operated Ca2+ influx by IP3 receptor antagonists, such as 2-APB and xestospongin C (Kiselyov et al. 1998; Ma et al. 2000; Maruyama et al. 1997). For the present study, 2-APB inhibited store-operated Ca2+ influx, yet xestospongin C was ineffective. Given that 2-APB also blocks store-operated channels directly (Prakriya and Lewis 2001), the parsimonious conclusion would be that diffusional coupling, rather than conformational coupling via IP3 receptors, is the mechanism in bag cell neurons.
The overlap between stores with CPA-sensitive Ca2+ pumps and stores with CEP-sensitive ryanodine receptors influences bag cell neuron store-operated Ca2+ influx. Although, store-operated Ca2+ influx can be initiated by ryanodine receptors in DRG neurons and smooth muscle (Curtis and Scholfield 2001; Usachev and Thayer 1999), CEP fails to activate this pathway in bag cell neurons despite causing Ca2+ release. The failure to trigger influx may be due to CEP not preventing Ca2+ uptake, which leaves the Ca2+ in the stores at a lowered but not depleted level. Interaction also occurs between acidic stores and the endoplasmic reticulum. Acidic stores sequester Ca2+ via a Ca2+/H+ exchanger driven by the H+ gradient (Christensen et al. 2002; Dunn et al. 1994; Goncalves et al. 1999; Ohsumi and Anraku 1983). Inhibition of bag cell neuron vacuolar H+-ATPases with bafilomycin A (Bowman et al. 1988) initiates a slow rise in intracellular Ca2+. Because this rise is accelerated by CPA, the endoplasmic reticulum presumably takes up some of the Ca2+ released by bafilomycin A. Moreover, the Ca2+ liberated from acidic stores appears to replenish the CPA-sensitive stores, given that store-operated influx is attenuated on return to Ca2+-containing saline. Although mitochondrial Ca2+ uptake is known to promote CRAC channel activation (Gilabert and Parekh 2000; Glitsch et al. 2002), this is the first report, to our knowledge, of acidic stores regulating store-operated Ca2+ influx. Vacuolar H+-ATPases are not localized to mitochondria but are found on a number of organelles, including vesicles (Calakos and Scheller 1996; Nelson 1992). Vesicles have low pH with high Ca2+, and if the H+-pump is blocked, Ca2+ will leak into the cytosol (Floor et al. 1990; Goncalves et al. 1999; Grohovaz et al. 1996; Scheenen et al. 1998; Thirion et al. 1995). Jonas et al. (1997) demonstrated that insulin triggers bag cell neuron peptide secretion by releasing Ca2+ from a nonendoplasmic reticulum/nonmitochondrial store that likely represents vesicles or granules.
Store-operated Ca2+ influx is universal in nonneuronal cells (Prakriya and Lewis 2003; Putney 2003), but for neurons it clearly varies between species and cell type. Although not detected in bullfrog sympathetic neurons or rat hippocampal and cortical neuron cultures (Chinopoulos et al. 2004; Friel and Tsien 1992; Koizumi et al. 1999), store-operated Ca2+ influx is observed in neurons from squid olfactory receptors, rat DRG, substantia nigra, and a number of cortical area-derived cultures (Arakawa et al. 2000; Baba et al. 2003; Bouron 2000; Piper and Lucero 1999; Prothero et al. 2000; Tozzi et al. 2003; Usachev and Thayer 1999). For neuroendocrine cells, there is evidence for and against store-operated Ca2+ influx in bovine chromaffin cells (Powis et al. 1996; Stauderman and Pruss 1989) as well as proof positive in murine pancreatic β cells (Liu and Gylfe 1997).
Given the presence of voltage-gated Ca2+ channels, the role of neuronal store-operated Ca2+ influx appears somewhat enigmatic. That said, store-operated channels do initiate secretion in chromaffin cells at membrane potentials too negative for voltage-gated Ca2+ channel activation (Fomina and Nowycky 1999). Depleting stores in the hippocampus can also enhance spontaneous transmitter release or induce long-term potentiation (Emptage et al. 2001; Ris et al. 2003). The bag cell neuron afterdischarge is an ~30-min barrage of action potentials that triggers neuropeptide secretion and egg-laying behavior (Conn and Kaczmarek 1989; Kupfermann 1967; Kupfermann and Kandel 1970; Pinsker and Dudek 1977; Rothman et al. 1983). Along with voltage-gated Ca2+ channels, store-operated Ca2+ influx could promote neuropeptide secretion, particularly late in the afterdischarge when release is maintained despite lowered firing frequency (Loechner et al. 1990; Michel and Wayne 2002). The afterdischarge is also associated with IP3-dependent release of Ca2+ from intracellular stores (Fink et al. 1988; Fisher et al. 1994). After the afterdischarge, the bag cells neurons enter a prolonged term of inactivity, known as the refractory period (Kaczmarek and Kauer 1983; Kaczmarek et al. 1982). The lack of voltage-gated Ca2+ channel opening during the quiescence of the refractory period would hinder Ca2+ store repletion; however, this could be circumvented by store-operated Ca2+ influx, which would readily replenish Ca2+ stores in the absence of activity.
Acknowledgments
The authors are very grateful to S. L. Smith for excellent technical assistance, and N. M. Magoski for critical evaluation of earlier drafts of the manuscript.
GRANTS
The work was supported by a Ruth Taylor Research Fund studentship to B. A. Kachoei, National Institutes of Health operating grants to S. Levy and L. K. Kaczmarek, Human Frontiers Science Program and Medical Research Council of Canada postdoctoral fellowships as well as a Canadian Institutes of Health Research operating grant to N. S. Magoski.
References
- Abeele FV, Lemonnier L, Thébault S, Lepage G, Parys JB, Shuba Y, Skryma R, Prevarskaya N. Two types of store-operated Ca2+ channels with different activation modes and molecular origin in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2004;279:30326–30337. doi: 10.1074/jbc.M400106200. [DOI] [PubMed] [Google Scholar]
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa N, Sakaue M, Yokoyama I, Hashimoto H, Koyama Y, Baba A, Matsuda T. KB-R7943 inhibits store-operated Ca2+ entry in cultured neurons and astrocytes. Biochem Biophys Res Comm. 2000;279:354–357. doi: 10.1006/bbrc.2000.3968. [DOI] [PubMed] [Google Scholar]
- Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci. 2003;23:7737–7741. doi: 10.1523/JNEUROSCI.23-21-07737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33:433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bouron A. Activation of a capacitative Ca2 entry pathway by store depletion in cultured hippocampal neurones. FEBS Lett. 2000;470:269–272. doi: 10.1016/s0014-5793(00)01340-5. [DOI] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane atpases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun FJ, Broad LM, Armstrong DL, Putney JW., Jr Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J Biol Chem. 2001;276:1063–1070. doi: 10.1074/jbc.M008348200. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Cabello OA, Schilling WP. Vectorial Ca2+ flux from the extracellular space to the endoplasmic reticulum via a restricted cytoplasmic compartment regulates inositol 1,4,5-triphosphate-stimulated Ca2+ release from internal stores in vascular enothelial cells. Biochem J. 1993;295:357–366. doi: 10.1042/bj2950357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calakos N, Scheller RH. Synaptic vesicle biogenesis, docking, and fusion: a molecular description. Physiol Rev. 1996;76:1–29. doi: 10.1152/physrev.1996.76.1.1. [DOI] [PubMed] [Google Scholar]
- Casteels R, Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinopoulos C, Gerencser AA, Doczi J, Fiskum G, Adam-Vizi V. Inhibition of glutamate-induced delayed calcium deregulation by 2-APB and La3+ in cultured cortical neurons. J Neurochem. 2004;91:471–483. doi: 10.1111/j.1471-4159.2004.02732.x. [DOI] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signalling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Clementi E, Scheer H, Zacchetti D, Fasolato C, Pozzan T, Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992;267:2164–2172. [PubMed] [Google Scholar]
- Conn PJ, Kaczmarek LK. The bag cell neurons of Aplysia. Mol Neurobiol. 1989;3:237–273. doi: 10.1007/BF02740607. [DOI] [PubMed] [Google Scholar]
- Cordova D, Delpech VR, Sattelle DB, Rauh JJ. Spatiotemporal calcium signalling in a Drosophila melanogaster cell line stably expressing a Drosophila muscarinic acetylcholine receptor. Invert Neurosci. 2003;5:19–28. doi: 10.1007/s10158-003-0024-2. [DOI] [PubMed] [Google Scholar]
- Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JW, Leuders J, Padgett WL, Shin Y, Gusovsky F. Maitotoxin-elicited calcium influx in cultured cells. Biochem Pharmacol. 1995;50:1187–1197. doi: 10.1016/0006-2952(95)00257-z. [DOI] [PubMed] [Google Scholar]
- DeRiemer SA, Strong JA, Albert KA, Greengard P, Kaczmarek LK. Enhancement of calcium current in Aplysia neurons by phorbol ester and protein kinase C. Nature. 1985;313:313–316. doi: 10.1038/313313a0. [DOI] [PubMed] [Google Scholar]
- Doi S, Damron SS, Horibe M, Murray PA. Capacitive Ca2+ entry and tryosine kinase activation in canine pulmonary arterial smooth muscle cells. Am J Physiol. 2000;278:L118–L130. doi: 10.1152/ajplung.2000.278.1.L118. [DOI] [PubMed] [Google Scholar]
- Dunn T, Gable K, Beeler T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Pizzo P, Pozzan T. Receptor-mediated calcium influx in PC12 cells. ATP and bradykinin activate two independent pathways. J Biol Chem. 1990;265:20351–20355. [PubMed] [Google Scholar]
- Fink LA, Connor JA, Kaczmarek LK. Inositol triphosphate releases intracellularly stored calcium and modulates ion channels in molluscan neurons. J Neurosci. 1988;8:2544–2555. doi: 10.1523/JNEUROSCI.08-07-02544.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T, Levy S, Kaczmarek LK. Transient changes in intracellular calcium associated with a prolonged increase in excitability in neurons of Aplysia californica. J Neurophysiol. 1994;71:1254–1257. doi: 10.1152/jn.1994.71.3.1254. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S-H, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Floor E, Leventhal PS, Schaeffer SF. Partial purification and characterization of the vacuolar H+-ATPase of mammalian synaptic vesicles. J Neurochem. 1990;55:1663–1670. doi: 10.1111/j.1471-4159.1990.tb04954.x. [DOI] [PubMed] [Google Scholar]
- Fomina AF, Nowycky MC. A current activated on depletion of intracellular Ca2+ stores can regulate exocytosis in adrenal chromaffin cells. J Neurosci. 1999;19:3711–3722. doi: 10.1523/JNEUROSCI.19-10-03711.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P, Lin CH, Thompson C. Novel form of growth cone motility involving site-directed actin filament assembly. Nature. 1992;357:515–518. doi: 10.1038/357515a0. [DOI] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurons modulates effects of Ca2+ entry on [Ca2+]I. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J. 2000;19:6401–6407. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves PP, Meireles SM, Neves P, Vale MG. Synaptic vesicle Ca2+/H+ antiport: dependence on the proton electrochemical gradient. Mol Brain Res. 1999;71:178–84. doi: 10.1016/s0169-328x(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Gore A, Moran A, Hershfinkel M, Sekler I. Inhibitory mechanism of store-operated Ca2+ channels by zinc. J Biol Chem. 2004;279:11106–11111. doi: 10.1074/jbc.M400005200. [DOI] [PubMed] [Google Scholar]
- Gratschev D, Blom T, Björklund S, Törnquist K. Phosphatase inhibition reveals a calcium entry pathway dependent on protein kinase A in thyroid FRTL-5 cells: comparison with store-operated calcium entry. J Biol Chem. 2004;279:49816–49824. doi: 10.1074/jbc.M406364200. [DOI] [PubMed] [Google Scholar]
- Grimaldi M, Atzori M, Ray P, Alkon DL. Mobilization of calcium from intracellular stores, potentiation of neurotransmitter-induced calcium transients, and capacitative calcium entry by 4-aminopyridine. J Neurosci. 2001;21:3135–3143. doi: 10.1523/JNEUROSCI.21-09-03135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohovaz F, Bossi M, Pezzati R, Meldolesi J, Tarelli FT. High resolution ultrastructural mapping of total calcium: electron spectroscopic imaging/electron energy loss spectroscopy analysis of a physically/chemically processed nerve-muscle preparation. Proc Natl Acad Sci USA. 1996;93:4799–4803. doi: 10.1073/pnas.93.10.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Usowicz MM, Henderson G. Ca2+ entry following store depletion in SH-SY5Y neuroblastoma cells. Mol Brain Res. 1996;36:93–100. doi: 10.1016/0169-328x(95)00248-q. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescent properties. J Biol Chem. 1985;260:3440–3448. [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, Yamada T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J Immunol. 2003;170:4441–4449. doi: 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Patterson SI, Thastrup O, Hanley MR. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115–401L neuronal cells. Biochem J. 1988;253:81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Knox RJ, Smith TCM, Wayne NL, Connor JA, Kaczmarek LK. Regulation by insulin of a unique neuronal Ca2+ pool and neuropeptide secretion. Nature. 1997;385:343–346. doi: 10.1038/385343a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Jennings K, Strumwasser F. An early sodium and a late calcium phase in the afterdischarge of peptide-secreting neurons of Aplysia. Brain Res. 1982;238:105–115. doi: 10.1016/0006-8993(82)90774-0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Kauer JA. Calcium entry causes a prolonged refractory period in peptidergic neurons of Aplysia. J Neurosci. 1983;3:2230–2239. doi: 10.1523/JNEUROSCI.03-11-02230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Strumwasser F. A voltage-clamp analysis of currents in isolated peptidergic neurons of Aplysia. J Neurophysiol. 1984;52:340–349. doi: 10.1152/jn.1984.52.2.340. [DOI] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Lutz Birnbaumer L, Muallem S. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Jonas EA, Kao L-S, Smith PJS, Connor JA, Kaczmarek LK. Ca2+ influx and activation of a cation current are coupled to intracellular Ca2+ release in peptidergic neurons of Aplysia californica. J Physiol. 1996;494:27–693. doi: 10.1113/jphysiol.1996.sp021520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RJ, Magoski NS, Wing D, Barbee SJ, Kaczmarek LK. Activation of a calcium entry pathway by sodium pyrithione in the bag cell neurons of Aplysia. J Neurobiol. 2004;60:411–423. doi: 10.1002/neu.20029. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Bootman MD, Bobanovic LK, Schell MJ, Berridge MJ, Lipp P. Characterization of elementary Ca2+ release signals in NGF differentiated PC12 cells and hippocampal neurons. Neuron. 1999;22:125–137. doi: 10.1016/s0896-6273(00)80684-4. [DOI] [PubMed] [Google Scholar]
- Kupfermann I. Stimulation of egg laying: possible neuroendocrine function of bag cells of abdominal ganglion of Aplysia californica. Nature. 1967;216:814–815. doi: 10.1038/216814a0. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Kandel ER. Electrophysiological properties and functional interconnections of two symmetrical neurosecretory clusters (bag cells) in abdominal ganglion of Aplysia. J Neurophysiol. 1970;33:865–876. doi: 10.1152/jn.1970.33.6.865. [DOI] [PubMed] [Google Scholar]
- Lepple-Wienhues A, Cahalan MD. Conductance and permeation of monovalent cations through depletion-activated Ca2+ channels (ICRAC) in Jurkat T cells. Biophys J. 1996;71:787–794. doi: 10.1016/S0006-3495(96)79278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB, Kaczmarek LK. The Neuron: Cell and Molecular Biology. New York: Oxford Univ. Press; 2002. [Google Scholar]
- Loechner KJ, Azhderian EM, Dreyer R, Kaczmarek LK. Progressive potentiation of peptide release during a neuronal discharge. J Neurophysiol. 1990;63:738–744. doi: 10.1152/jn.1990.63.4.738. [DOI] [PubMed] [Google Scholar]
- Liu Y-J, Gylfe E. Store-operated Ca2+ entry in insulin releasing pancreatic β-cells. Cell Calcium. 1997;22:272–286. doi: 10.1016/s0143-4160(97)90066-x. [DOI] [PubMed] [Google Scholar]
- Lupinsky DA, Magoski NS. Ca2+-dependent regulation of a non-selective cation channel from Aplysia bag cell neurons. J Physiol. 2006;575:491–506. doi: 10.1113/jphysiol.2006.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H-T, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol triphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Magoski NS, Knox RJ, Kaczmarek LK. Activation of a Ca2+-permeable cation channel produces a prolonged attenuation of intracellular Ca2+ release in Aplysia bag cell neurons. J Physiol. 2000;522:271–283. doi: 10.1111/j.1469-7793.2000.t01-2-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Prog Neurobiol. 2001;65:309–338. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1985;261:6300–6306. [PubMed] [Google Scholar]
- Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KA, Rink TJ. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Wayne NL. Neurohormone secretion persists after post-afterdischarge membrane depolarization and cytosolic calcium elevation in peptidergic neurons in intact nervous tissue. J Neurosci. 2002;22:9063–9069. doi: 10.1523/JNEUROSCI.22-20-09063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. The vacuolar H+-ATPase - one of the most fundamental ion pumps in nature. J Exp Biol. 1992;172:19–27. doi: 10.1242/jeb.172.1.19. [DOI] [PubMed] [Google Scholar]
- Nilius B. From TRPs to SOCs, CCEs, and CRACs: consensus and controversies. Cell Calcium. 2003;33:293–298. doi: 10.1016/s0143-4160(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983;258:5614–5617. [PubMed] [Google Scholar]
- Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333–348. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Petersen CCH, Berridge MJ, Borgese MF, Bennett DL. Putative capacitive calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem J. 1995;311:41–44. doi: 10.1042/bj3110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp S, Cavalie A, Freichel M, Wissenbach U, Zimmer S, Trost C, Marquart A, Murakami M, Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- Pinsker HM, Dudek FE. Bag cell control of egg laying in freely behaving Aplysia. Science. 1977;197:490–493. doi: 10.1126/science.197.4302.490. [DOI] [PubMed] [Google Scholar]
- Piper DR, Lucero MT. Calcium signalling in squid olfactory receptor neurons. Biol Sig Rec. 1999;8:329–337. doi: 10.1159/000014606. [DOI] [PubMed] [Google Scholar]
- Powis DA, Clark CL, O’Brien KJ. Depeted internal store-activated Ca2+ entry can trigger neurotransmitter release in bovine chromaffin cells. Neurosci Lett. 1996;204:165–168. doi: 10.1016/0304-3940(96)12346-6. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–21. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Prothero LS, Mathie A, Richards CD. Purinergic and muscarinic receptor activation activates a common calcium entry pathway in rat neo-cortical neurons and glial cells. Neuropharmacol. 2000;39:1768–1778. doi: 10.1016/s0028-3908(00)00013-7. [DOI] [PubMed] [Google Scholar]
- Putney J. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:l–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney JW. Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/s0143-4160(03)00143-x. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–14. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD. Presynaptic Ca2+ channels: a functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ris L, Dewachter I, Reversé D, Godaux E, Van Leuven F. Capacitative calcium entry induces hippocampal long term potentiation in the absence of Presenilin-1. J Biol Chem. 2003;278:44393–44399. doi: 10.1074/jbc.M300971200. [DOI] [PubMed] [Google Scholar]
- Rosado JA, López JJ, Harper AGS, Harper MT, Redondo PC, Pariente JA, Sage SO, Salido GM. Two pathways for store-mediated calcium entry differentially dependent on the actin cytoskeleton in human platelets. J Biol Chem. 2004;279:29231–29235. doi: 10.1074/jbc.M403509200. [DOI] [PubMed] [Google Scholar]
- Ross PE, Cahalan MD. Ca2+ influx pathways mediated by swelling or stores depletion in mouse thymocytes. J Gen Physiol. 1995;106:415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman BS, Weir G, Dudek FE. Egg-laying hormone: direct action on the ovotestis of Aplysia. Gen Comp Endocrinol. 1983;52:134–141. doi: 10.1016/0016-6480(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Arch Biochem Biophysics. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Scheenen WJJM, Wollheim CB, Pozzan T, Fasolato C. Ca2+ depletion from granules inhibits exocytosis. A study with insulin-secreting cells. J Biol Chem. 1998;273:19002–19008. doi: 10.1074/jbc.273.30.19002. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specifiec inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Spassova MA, Soboloff J, He L-P, Xu W, Dziadek MA, Gill DL. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauderman KA, Pruss RM. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989;264:18349–18355. [PubMed] [Google Scholar]
- Su Z, Csutora P, Hunton D, Shoemaker RL, Marchase RB, Blalock JE. A store-operated nonselective cation channel in lymphocytes is activated directly by Ca2+ influx factor and diacylglycerol. Am J Physiol Cell Physiol. 2001;280:C1284–C1292. doi: 10.1152/ajpcell.2001.280.5.C1284. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirion S, Stuenkel EL, Nicaise G. Calcium loading of secretory granules in stimulated neurohypophysial nerve endings. Neuroscience. 1995;64:125–137. doi: 10.1016/0306-4522(94)00414-z. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Guild GM. Microvilli appear to represent the first step in actin bundle formation in Drosophila bristles. J Cell Sci. 2004;117:3531–3538. doi: 10.1242/jcs.01215. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–45. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J Biol Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- Usachev YM, Thayer SA. Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2+ stores. J Physiol. 1999;519:115–130. doi: 10.1111/j.1469-7793.1999.0115o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Garcia-Sancho J. Capacitative Ca2+ entry contributes to the Ca2+ influx induced by thyrotropin-releasing hormone (TRH) in GH3 pituitary cells. Eur J Physiol. 1995;430:923–935. doi: 10.1007/BF01837406. [DOI] [PubMed] [Google Scholar]
- Vorndran C, Minta A, Poenie M. New fluorescent calcium indicators designed for cytosolic retention or measuring calcium near membranes. Biophys J. 1995;69:2112–2124. doi: 10.1016/S0006-3495(95)80082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968;52:760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Ferrer-Montiel AV, Montal M, Tsien RY. Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell. 1999;98:475–485. doi: 10.1016/s0092-8674(00)81976-5. [DOI] [PubMed] [Google Scholar]
- Zitt C, Zobel A, Obukhov AG, Harteneck C, Kalkbrenner F, Luckhoff A, Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzato F, Scutari E, Tegazzin V, Clementi E, Treves S. Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ release. Mol Pharmacol. 1993;44:1192–1201. [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T, Greer CA. Amplification of odor-induced Ca2+ transients by store-operated Ca2+ release and its role in olfactory signal transduction. J Neurophysiol. 2000;83:501–512. doi: 10.1152/jn.2000.83.1.501. [DOI] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6259–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]