Abstract
The characteristic bone destruction in giant cell tumour of bone (GCT) is largely attributed to the osteoclast-like giant cells. However, experimental analyses of bone resorption by cells from GCT often fail to exclude the neoplastic spindle-like stromal cells, and several studies have demonstrated that bone resorption by GCT cells is increased in the presence of stromal cells. The spindle-like stromal cells from GCT may therefore actively contribute to the bone resorption observed in the tumour. Type I collagen, a major organic constituent of bone, is effectively degraded by three matrix metalloproteinases (MMPs) known as the collagenases: MMP-1, MMP-8 and MMP-13. We established primary cell cultures from nine patients with GCT and the stromal cell populations were isolated in culture. The production of collagenases by primary cultures of GCT stromal cells was determined through real-time PCR, western blot analysis and a multiplex assay system. Results show that the cells produce MMP-1 and MMP-13 but not MMP-8. Immunohistochemistry confirmed the presence of MMP-1 and MMP-13 in paraffin-embedded GCT tissue samples. Medium conditioned by the stromal cell cultures was capable of proteolytic activity as determined by MMP-1 and MMP-13-specific standardized enzyme activity assays. The spindle-like stromal cells from GCT may therefore actively participate in the bone destruction that is characteristic of the tumour.
Keywords: Giant cell tumour, Stromal cells, Collagen, Osteolysis, Matrix metalloproteinases
Introduction
The pathogenesis of giant cell tumour of bone (GCT), a rare primary osteolytic bone tumour, remains a matter of some controversy. The GCT microenvironment is characterized by three cell types; namely, the osteoclast-like multinucleated giant cells [1,2], the mononuclear CD68-positive round cells of monocytic-macrophage origin [2,3], and the mononucleated spindle-like stromal cells of mesenchymal origin [4–7]. Although the localized tissue destruction observed in patients with GCT is often attributed to the multi-nucleated giant cells [8–13], few reports of bone resorption by this tumour have excluded the stromal cells from their analyses, which proliferate rapidly and ultimately dominate the cell population when grown in vitro. Indeed, the stromal cells are considered the neoplastic component of the tumour [7], and several studies have reported that the overall bone resorption by GCT cells is enhanced by the presence of stromal cells [12,14,15]. The stromal cells, therefore, may also actively participate in the bone destruction that is characteristic of GCT.
Previous research has demonstrated that the stromal cell population from GCT produces various matrix metalloproteinases (MMPs) that are known to degrade an assortment of components comprising the extracellular matrix [16–19]. MMPs are a family of zinc-dependent proteases that have been characterized in a multitude of organisms and are prominently involved in such regulatory processes as embryonic development, wound repair, and bone remodeling [20]. Although MMPs share several distinguishing features, they can be classed according to their domain structure and substrate specificity into subgroups that include the collagenases (MMP-1, MMP-8, MMP-13), the gelatinases (MMP-2, MMP-9), and the stromelysins (MMP-3, MMP-10), among others. Despite a wealth of information concerning MMPs in GCT, there is minimal data on the collagenases.
Collagen is considered the major organic component of normal bone [21–23]. Although several species of collagen have been described, the dominant form found in bone is that of type I collagen, which comprises approximately 90% of the entire collagen content [24]. An investigation into the bone degradation that is characteristic of GCT must therefore include an examination of the tumour’s ability to degrade type I collagen. Type I collagen consists of three polypeptide chains in the form of [α1(I)]2α2(I) and, in bone, is largely arranged in an insoluble fibrillar structure where it contributes to the structural integrity of the skeleton. However, only certain enzymes are capable of cleaving native fibrillar type I collagen. In this report, we investigated the ability of patient-derived GCT stromal cells to degrade type I collagen by evaluating the production and activity of the three collagenases known to degrade such collagen: MMP-1, MMP-8 and MMP-13.
Materials and methods
Cells and cell culture
Primary cell cultures were established from biopsy and resection specimens from patients pre-operatively diagnosed with GCT, following patient consent and approval from our institution’s Research Ethics Board (Table 1). Diagnoses of GCT were confirmed post-operatively by a qualified pathologist and tumours were graded radiographically according to the Campanacci method of classification [25]. Tissue samples were macerated with scalpel blades in a sterile glass Petri dish containing Dulbecco’s modified Eagle medium (D-MEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen Canada/Gibco; Burlington, Ontario, Canada). Aliquots of media containing cells and macerated tissue were passed through a 24-gauge needle and transferred to 25 cm2 vented tissue culture flasks and subsequently maintained at 37 °C in humidified air with 5% CO2. Following a 24-hour incubation period, the cell culture medium was replaced with fresh, supplemented D-MEM following several washes with phosphate buffered saline (PBS). Thereafter, cell culture medium was renewed every 2 to 3 days. Upon reaching ~80% confluence, cell cultures were digested with a 0.1% trypsin-EDTA solution; cells that easily detached from the flask surface were collected and maintained in supplemented D-MEM. Following several successive passages, the multinucleated giant cells and the CD68-positive monocytes were eliminated from the culture, as we have previously reported [16]. Cells used in experiments were analyzed between passages 5 and 17.
Table 1.
Demographic data for GCT primary cell cultures
| Case | Gender | Age | Location | Campanacci classification |
|---|---|---|---|---|
| GCT-1 | Female | 25 | Distal femur | Grade II |
| GCT-2 | Male | 59 | Distal femur | Grade II |
| GCT-3 | Male | 41 | Distal femur | Grade III |
| GCT-4 | Male | 55 | Proximal humerus | Grade III |
| GCT-5 | Female | 56 | Proximal tibia | Grade II |
| GCT-6 | Female | 31 | Distal ulna | Grade III |
| GCT-7 | Female | 47 | Distal femur | Grade II |
| GCT-8 | Female | 39 | Rib | Grade III |
| GCT-9 | Male | 20 | Distal femur | Grade II |
Simian virus 40 large T antigen-transfected human fetal osteoblast (hFOB) 1.19 cells were obtained from the American Type Culture Collection (ATCC # CRL-11372; Rockville, Maryland, USA) and maintained in supplemented D-MEM as described for the GCT cells at 34 °C in humidified air containing 5% CO2. Similarly, human osteosarcoma (HOS) cells were obtained from ATCC (# CRL-1543) and maintained in Eagle’s minimum essential medium (ATCC) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen Canada/Gibco) at 37 °C in humidified air containing 5% CO2.
Real-time PCR
Total RNA was isolated from stromal cell cultures lysed with lysis buffer (buffer RLT) containing β-mercaptoethanol using the RNeasy Mini Kit (Qiagen; Mississauga, Ontario, Canada) according to the manufacturer’s instructions. Isolated mRNAs were reverse-transcribed into cDNAs using the SuperScript II First-Strand Synthesis System (Invitrogen Canada; Burlington, Ontario, Canada) as per the manufacturer’s instructions, and employing oligo-dT probes. The real-time polymerase chain reaction (PCR) was performed on a Mini-Opticon Real-Time PCR Detection System (Bio-Rad Laboratories; Mississauga, Ontario, Canada) using the iQ SYBR Green Supermix (Bio-Rad), according to the manufacturer’s instructions. The reaction was achieved in a total volume of 20 μL, consisting of 1:10 diluted cDNA template (2 μL) obtained from RNA (1 μg). Amplification was performed using a real-time thermal cycler (Bio-Rad) using 40 cycles of 94 °C for 15 s, 58–60 °C (based on individual optimization) for 30 s, and 72 °C for 30 s, with a gradient change in temperature to determine the melting curve of the final PCR products. The gene encoding human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an internal control. Cycle threshold (Ct) values were established and the relative change in expression from GAPDH was determined according to the 2−ΔΔCt method of analysis and compared to expression by hFOB 1.19 cells.
Primers were designed using the National Center for Biotechnology Information (NCBI)’s GenBank and prepared by Sigma-Genosys Canada (Sigma-Aldrich; Oakville, Ontario, Canada). Sense and anti-sense primers are listed in Table 2.
Table 2.
Primer information for real-time PCR
| Gene | Primer sequence | GenBank accession no. |
Base nos. | Size of amplicon (bp) |
|
|---|---|---|---|---|---|
| MMP-1 | Sense Anti-sense |
5′GGACCATGCCATTGAGAAAG3′ 5′AAGGAGAGTTGTCCCGATGA3′ |
NM_002421 | 461–591 | 131 |
| MMP-8 | Sense Anti-sense |
5′ATCTCACAGGGAGAGGCAGA3′ 5′ATTCCATTGGGTCCATCAAA3′ |
NM_002424 | 522–607 | 86 |
| MMP-13 | Sense Anti-sense |
5′CTTCCCAACCGTATTGATGC3′ 5′TTTGGAAGACCCAGTTCAGA3′ |
NM_002427 | 1016–1158 | 143 |
| GAPDH | Sense Anti-sense |
5′CATGAGAAGTATGACAACAGCCT3′ 5′AGTCCTTCCACGATACCAAAGT3′ |
NM_002046 | 511–623 | 113 |
Western blotting
GCT stromal cells were plated in 55 cm2 Petri dishes and grown to confluence. Cells were scraped and collected in Nonidet P-40 (NP40) lysis buffer (15% NP40, 5 M NaCl, 1 M Tris pH 7.4, 0.5 M EDTA pH 8.0) containing protease inhibitor cocktail tablets (Hoffmann-La Roche; Mississauga, Ontario, Canada) and maintained at 4 °C for 60 min. The lysate was centrifuged at 10,000 ×g for 20 min. Protein concentration was determined by the Bradford microassay procedure and 50 μg samples were electrophoresed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) at 90 V for 90 min, and then transferred to a nitrocellulose membrane using a semi-dry transfer cell (Bio-Rad) at 20 V for 45 min, as per the manufacturer’s instructions. Blots were blocked overnight with 5% skim milk in 1× TBS-T (Tris-buffered saline with Tween 20) and then incubated with monoclonal anti-human MMP-1 (Calbiochem; Mississauga, Ontario, Canada), MMP-8 or MMP-13 antibodies (R&D Systems; Minneapolis, Minnesota, USA) for 3 h at room temperature. Recombinant protein standards for MMP-8 and MMP-13 (R&D Systems) served as positive controls for those blots, while HOS functioned as a positive control for MMP-1 expression and water served as a negative control for all blots. Blots were subsequently incubated with appropriate secondary antibody and MMP protein was visualized using enhanced chemoluminescence (ECL) detection (Amersham Biosciences/GE Healthcare Bio-Sciences Inc.; Baie d’Urfé, Quebec, Canada) according to the manufacturer’s instructions. Antibodies were removed using stripping buffer (62.5 mM Tris–HCl pH 6.8, 2% SDS, 100 mM β-mercaptoethanol) at 65 °C for 30 min and blots were re-probed with monoclonal anti-actin (MP Biomedicals; Montreal, Quebec, Canada), which served as a loading control.
Multiplex assay
The Fluorokine MAP Multiplex Assay System with Luminex 100 detection equipment (R&D Systems) employs colour-coded microparticles to accurately detect and quantify specific analytes within a medium. The microparticles are equipped with analyte-specific antibodies and are added to a sample of interest where the antibodies bind to their respective substrates. Biotinylated antibodies are subsequently added to the sample and bind the microparticle-affiliated analytes. Lastly, a streptavidin–phycoerythrin conjugate is added to the sample, which binds the biotinylated antibodies. Quantification of specific analytes is achieved using a dual laser approach: one laser is used to determine the specific colours of the microparticle, thereby identifying the substrate, while a second laser determines the amount of bound analyte by assessing the magnitude of the phycoerythrin signal.
GCT stromal cells were grown to confluence in 55 cm2 Petri dishes. Cell lysates and serum-free D-MEM conditioned by stromal cell cultures for 24 h were collected separately. Total protein content in the lysates was quantified using the Bradford microassay procedure. Additionally, the total number of cells present at the time of the conditioned medium collection was determined by hemocytometer. Simultaneous quantification of MMP-1, MMP-8 and MMP-13 in the conditioned medium and lysates was achieved on the Multiplex Assay System using Flurokine MAP Human MMP kits (R&D Systems), as per the manufacturer’s instructions.
Immunohistochemistry
Paraffin-embedded tissue samples of GCT were cut and mounted onto slides. Tissue sample slides were de-paraffinized in several washes of xylene. Slides were blocked for endogenous peroxidase activity by incubation with 3% hydrogen peroxide for 10 min and subsequently washed in 1× TBS-T before treatment with 5% normal horse serum for 30 min. Next, sample slides were incubated at room temperature for 1 h in a moist chamber with various dilutions of primary antibodies that included MMP-1 (Calbiochem), MMP-8, and MMP-13 (R&D Systems). Slides were then rinsed three times in TBS-T and incubated for a further 30 min at room temperature with a 1:500 dilution of secondary anti-mouse/rabbit/goat immunoglobulin (IgG) (Sigma), as dictated by the primary antibody. Following a further wash with TBS-T and ABC conjugation (Vector Laboratories; Burlington, Ontario, Canada), substrate colour was developed for 2 to 10 min at room temperature using a liquid 3,30-diaminobenzidine (DAB) substrate-chromogen solution kit (DAKO Canada; Mississauga, Ontario, Canada). Slides were counterstained in hematoxylin, dehydrated in graded ethyl alcohol (70%, 90% and 100%), and mounted in Permount. Preparations without the primary antibody served as negative controls.
Standardized collagenase activity assays
Serum-free D-MEM conditioned by confluent stromal cell cultures for 24 h was concentrated approximately 40 fold using Amicon Ultra-4 Centrifugal Filter Devices (Millipore; Etobicoke, Ontario, Canada) and analyzed using SensoLyte Plus 520 MMP-1 and SensoLyte Plus 520 MMP-13 Assay Kits (AnaSpec; San Jose, California, USA), as per the manufacturer’s instructions. The kits employ 96-well cell culture plates coated with monoclonal anti-human MMP-1 or MMP-13 antibodies that recognize both the latent and active forms of the enzymes. The specificity of the monoclonal antibodies prevents cross-reactivity with other MMPs. Isolated pro-MMP-1 or pro-MMP-13 is subsequently activated by incubation with 4-aminophenylmercuric acetate (APMA) at 37 °C. Proteolytic activity of the enzymes is measured using a fluorescence resonance energy transfer (FRET) peptide containing a quenched fluorophore. Upon cleavage by MMP-1 or MMP-13, fluorescence of the fluorophore was recovered and was measured on a CytoFluor Multi-Well Plate Reader series 4000 (PerSeptive Bio-systems/Applied Biosystems; Streetsville, Ontario, Canada) following an eight-hour incubation period, with an excitation and emission wavelength of 485±20 nm and 530±25 nm, respectively. Due to equipment design limitations, the contents of each well from the kit’s cell culture plate were transferred to a new 96-well cell culture plate immediately prior to quantification.
Statistical Analyses
Statistical analyses for the real-time PCR data were performed using the two-sample independent student’s t-test. The average value of MMP gene expression within each experiment was expressed relative to the expression of the GAPDH internal control. Results from repeated experiments were subsequently compared to the results of hFOB 1.19 cells and accepted as significant if P≤0.05.
Results
Collagenase mRNA expression
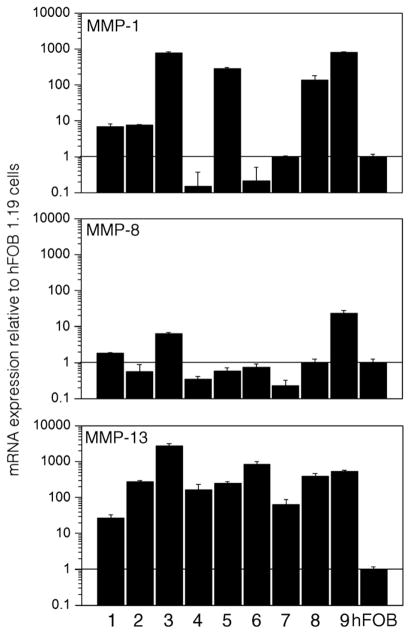
Amplification of total mRNA isolated from GCT stromal cell lysates and reverse-transcribed into cDNA was evaluated for collagenase expression and quantified by real-time PCR. Results are summarized in Fig. 1. Significantly greater MMP-13 expression was detected in all nine GCT stromal cell cultures when compared to hFOB 1.19 cells and using the two-sample independent student’s t-test (P ≤0.05). Furthermore, six cell cultures (GCT-1, -2, -3, -5, -8, and -9) similarly expressed MMP-1 mRNA levels that were significantly greater than that of hFOB 1.19 cells, and of those, three cultures (GCT-1, -3, and -9) also expressed significantly greater MMP-8 levels. The hFOB 1.19 cells were employed for comparative reasons due to the mesenchymal origin of the GCT stromal cells [7].
Fig. 1.
Real-time PCR of cDNA from GCT stromal cell lysates showing expression of MMP-1, MMP-8 and MMP-13 relative to hFOB 1.19 cells (“hFOB”). Total cDNA from GCT-1 through 9 are indicated as 1 through 9, respectively. Results are the average of two replicate experiments.
Collagenase protein expression
Western blotting was performed on GCT stromal cell lysates. Both MMP-1 and MMP-13 proteins were detected in all stromal cell lysates as shown in the representative results of Fig. 2. However, MMP-8 was not observed by western blotting, despite positive detection of the control peptide. Band sizes for MMP-1, MMP-8 and MMP-13 corresponded with the anticipated 54, 75 and 55 kDa sizes of the latent enzymes, respectively. Collagenase protein expression was quantified in stromal cell lysates, as well as in media conditioned by the cells for 24 h, using a multiplex assay system, as shown in Table 3. Due to the clinical nature of the cell lines and the variability in available sample sizes, not all cell lines were available at the time of analysis and only the cell lines presented in Table 3 were analyzed. Results are given as total pg/mL for each sample. Data were subsequently normalized to pg of MMP per 100 μg total proteins or per 1000 cells for the lysate and conditioned medium samples, respectively. Although overall expression of MMPs in the lysates and subsequent secretion into the conditioned medium varied for each cell line, MMP-8 expression was consistently negligible, whereas MMP-1 and MMP-13 expression was detected in greater quantities.
Fig. 2.
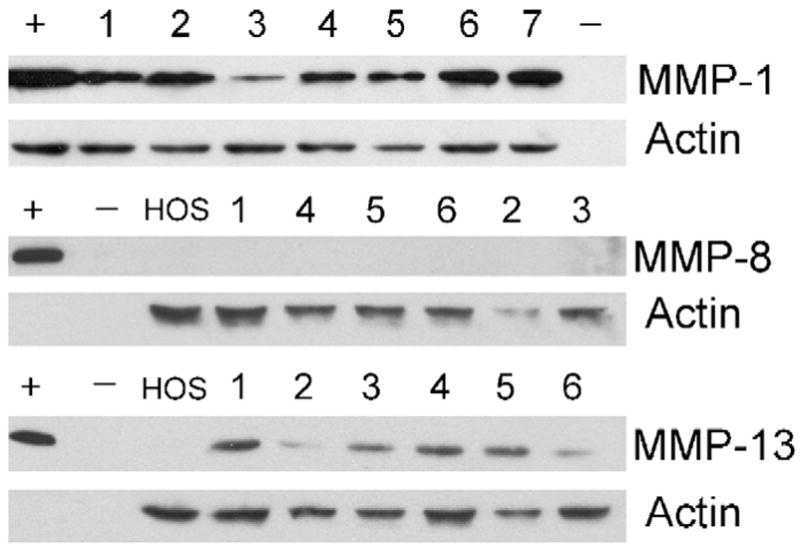
Representative western blot analyses of GCT stromal cell lysates for MMP-1, MMP-8 and MMP-13. The blots show protein expression by HOS and GCT stromal cell lines. Corresponding numerical values represent GCT-1 through 9. Controls include serum-free D-MEM (−) and recombinant protein standards for MMP-8 and MMP-13 (+). HOS serves as a positive control for MMP-1. Anti-actin antibodies serve as an internal control. Results are each representative of three independent experiments.
Table 3.
Quantification of MMP-1, MMP-8 and MMP-13 protein levels from GCT stromal cell lysates and conditioned medium by a multiplex assay system
| Conditioned media | MMP-1 |
MMP-8 |
MMP-13 |
|||
|---|---|---|---|---|---|---|
| pg/mL | Na | pg/mL | Na | pg/mL | Na | |
| GCT-1 | 96.00±3.3 | 1.164 | 7.33±3.3 | 0.089 | 15.83±2.2 | 0.192 |
| GCT-2 | 107.67±1.7 | 1.485 | 0.17±1.6 | 0.002 | 599.33±12.2 | 8.267 |
| GCT-4 | 3.33±0.2 | 0.065 | 2.00±3.3 | 0.039 | 35.33±6.6 | 0.689 |
| GCT-5 | 3495.33±105.7 | 50.841 | 0.00±2.8 | 0.000 | 301.83±41.3 | 4.390 |
| GCT-7 | 13.00±0.9 | 0.067 | 0.00±0.8 | 0.000 | 32.67±2.6 | 0.168 |
| GCT-9 | 567.5±18.4 | 3.266 | 1.33±1.7 | 0.008 | 142.17±6.6 | 0.818 |
| Lysates | pg/mL | Nb | pg/mL | Nb | pg/mL | Nb |
| GCT-1 | 1374.17±165.9 | 60.0 | 53.0±6.0 | 2.3 | 901.83±124.5 | 39.4 |
| GCT-2 | 15830.67±1598.0 | 1224.7 | 60.5±1.0 | 4.7 | 4185.67±231.6 | 323.8 |
| GCT-3 | 13179.33±253.1 | 1529.7 | 64.33±3.9 | 7.5 | 27984.33±114.6 | 3248.2 |
| GCT-4 | 140.5±50.0 | 11.1 | 49.0±4.1 | 3.9 | 2167.83±55.5 | 171.7 |
| GCT-5 | 684.5±33.4 | 89.6 | 51.67±2.6 | 6.8 | 13887.83±449.7 | 1818.1 |
| GCT-6 | 54.5±4.8 | 3.5 | 48.33±2.3 | 3.1 | 1685.83±21.1 | 107.2 |
| GCT-7 | 254.83±13.4 | 9.4 | 45.5±1.3 | 1.7 | 1358.33±72.9 | 50.1 |
Mean data normalized (N) to pg per 1000 cells.
Mean data normalized to pg per 100 μg total protein.
Immunohistochemistry analyses of GCT
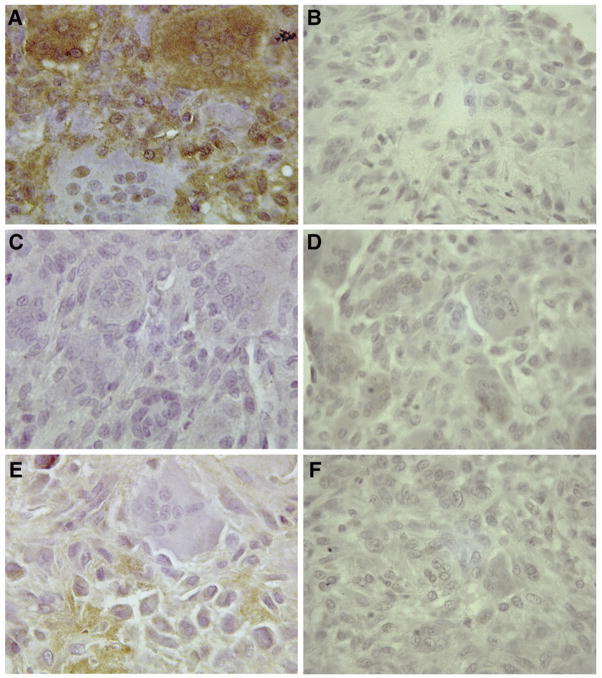
To evaluate whether collagenase expression in GCT was an artifact of cell culturing, immunohistochemical staining of GCT tissue samples with monoclonal anti-human collagenase antibodies was performed. As shown in Fig. 3, results from paraffin-embedded GCT-8 tissue revealed localization of both MMP-1 and MMP-13 in the stromal cell population of GCT. MMP-1 antibody staining was additionally present in many giant cells. However, MMP-8 was not detectable in any of the GCT cell types, despite positive staining in human breast cancer tissue (data not shown), which has previously demonstrated expression of MMP-8 [26]. The collagenase staining pattern observed with GCT-8 was confirmed through analysis of 23 archival GCT specimens obtained through our institution (data not shown).
Fig. 3.
Representative immunohistochemistry staining of paraffin-embedded GCT-8 tissue samples with monoclonal anti-human (A) MMP-1 (1:100), (C) MMP-8 (1:50) and (E) MMP-13 (1:50) antibodies. The negative controls for each antibody are shown in (B) for MMP-1, (D) for MMP-8 and (F) for MMP-13. Original magnification × 400.
Collagenolytic activity
To confirm the functional abilities of the secreted proteases, enzyme-specific activity assays that utilize a fluorescently-quenched peptide, which fluoresces upon proteolytic cleavage, were employed. Collagenase activity was measured following activation of latent enzymes with APMA. Concentrated media conditioned by GCT stromal cells showed increased fluorescence relative to media alone using both MMP-1 and MMP-13-specific assays (Fig. 4). Media conditioned by HOS cells, which are known to have MMP-1 activity, are shown for comparison. The conditioned media all presented a range of fluorescent activity that was elevated when compared to serum-free D-MEM. The only exceptions were the GCT-4 samples, which did not show MMP-1 activity (0.99±0.03) despite positive MMP-13 activity (1.39±0.01).
Fig. 4.
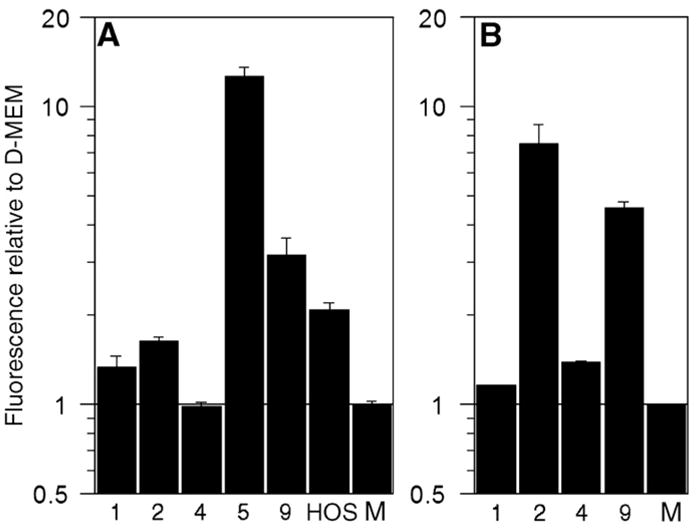
Proteolytic activity of (A) MMP-1 and (B) MMP-13 in concentrated media conditioned by HOS or GCT stromal cells for 24 h, as determined by protease-specific standardized activity assays and relative to serum-free D-MEM (“M”). Quantification was achieved through measurement of fluorescence following incubation with a quenched fluorophore that fluoresced upon proteolytic cleavage by MMP-1 or MMP-13. GCT cell lines tested are indicated numerically and refer to GCT-1, -2, -4, -5, and -9, respectively.
Discussion
A select number of mammalian proteases are capable of cleaving the native fibrillar structure of type I collagen. These enzymes are principally cathepsin K [27], membrane-type 1 MMP (MMP-14) [28], and the interstitial collagenases (MMP-1, MMP-8 and MMP-13); however, gelatinase-A (MMP-2) has also shown a weak affinity for fibrillar collagen [29]. Although we [16], and others [18,19], have previously reported the production of MMP-2 by the GCT stromal cells, this study focused on the secreted collagenases, which function under physiologic conditions of neutral pH. In contrast, cathepsin K is active within the acidic pH range that is characteristic of an osteoclast environment, and indeed, its production has been associated with the multinucleated giant cells [30–32].
Osteoclasts resorb the bone matrix through the combined actions of vacuolar H+-ATPase, which demineralizes the crystals of hydroxyapatite [33], and cathepsin K, which largely degrades the organic components of bone [31,32]. Physiologically, therefore, cathepsin K can be considered the principal protease involved in bone resorption. However, the activity of both enzymes is dependent upon the formation of a ruffled membrane in the osteoclast: a characteristic morphological change comprised of actin rings. Degradation of type I collagen by interstitial collagenases was shown to stimulate the formation of ruffled membranes, thereby inciting osteoclast activity [34,35]. Osteoclasts themselves do not produce soluble collagenases [34,36], although MMP-13 is detectable at the resorption zone [37,38], implying an alternate source for the enzymes such as osteoblasts and other fibroblast-like cells [34].
Here we report the expression of MMP-1 and MMP-13 by the stromal cell population of GCT from nine affected patients. The enzymes were sufficient for proteolytic activity, as determined by standardized activity assays, although stromal cells have previously shown an inability to independently resorb dentine slices [14,30]. However, the resorptive activity of the osteoclast-like giant cells is enhanced by the presence of stromal cells [12,14,15], suggesting that the stromal cells may encourage giant cell activity through the degradation of type I collagen. The stromal cells are the neoplastic element of GCT [7], and have previously shown an ability to stimulate the formation of multinucleated cells from mononuclear cells [6,39–41]. Moreover, the stromal cells may be at least partly responsible for the initial recruitment of mononuclear osteoclast precursors to the tumour site [42,43]. Taken together, these results suggest that the spindle-like stromal cells of GCT largely influence the initiation, propagation and activity of the osteolytic tumour through the formation and activation of the giant cells.
The mRNA expression of each collagenase, as determined by real-time PCR (Fig. 1), largely corresponds with the relative protein levels obtained using the multiplex assay system (Table 3). Although elevated MMP-8 mRNA expression was detected in GCT-1, -3 and -9, the results are relative to hFOB 1.19 cells, which are not known to produce MMP-8. Accordingly, all other experimental approaches suggest that MMP-8 expression was consistently negligible in all GCT stromal cell lines. With respect to MMP-1 and MMP-13, the majority of each enzyme was detected in the cell lysates, although it is possible that increased amounts of protein in the surrounding medium are achievable if the incubation period was sustained beyond 24 h. Indeed, immunohistochemical analyses of GCT tissue revealed considerable positive staining of both MMP-1 and MMP-13 in the stromal cell population (Fig. 3). Regardless, the amount of collagenase secreted into the media was sufficient for proteolytic activity, as determined by the enzyme-specific standardized activity assays (Fig. 4).
Due to the limited availability of some clinical specimens, only a select number of GCT cell lines were available for analysis by the multiplex assay system and the standardized activity assays. Despite this fact, we have demonstrated that MMP-13 is consistently and highly expressed by the spindle-like stromal cell population from GCT. These results expand on previous isolated reports of interstitial collagenase expression in GCT. Microarray analyses of whole GCT samples have demonstrated that MMP-13 is highly expressed by the tumour [44,45], and real-time PCR analysis of total GCT mRNA also indicated elevated MMP-13 expression [30]. Few previous reports have examined MMP-1 expression in GCT. Lindeman et al. [30] demonstrated no detectable MMP-1 expression by real-time PCR analysis of total GCT mRNA from seven individual tumours. Conversely, we have demonstrated MMP-1 expression by the stromal cells from GCT using a variety of experimental protocols. The variability of MMP-1 expression and activity by these cells suggest that MMP-1 may not be present in all GCTs. However, the consistency of MMP-13 expression by the neoplastic stromal cells warrants further investigation into what role, if any, these collagenases have in the pathogenesis of the tumour.
The variability in collagenase expression amongst the different cell lines is likely a result of differences between patients. Indeed, many reports examining GCT samples from different patients detail differences in the expression of several genes [46]. These differences may be influenced by other factors produced by the stromal cells, including transforming growth factor-β1 (TGF-β1) [47], which has demonstrated the ability to stimulate expression of MMP-13 in renal carcinoma cells [48]. Although few papers have described the production of cytokines by GCT stromal cells, both interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α) are known to stimulate the expression of MMP-1 and MMP-13 in osteoarthritis and rheumatoid arthritis [49,50], while interleukin-17 was shown to induce MMP-13 expression in osteoarthritic chondrocytes [51]. Similar cytokine-mediated mechanisms of stimulating collagenase expression may occur in GCT. Furthermore, it cannot be conclusively ruled out that there may be contaminating cells in the primary cultures, which may influence collagenase expression by the stromal cells, as the cell lines were established from individual patients. There was no discernable correlation between collagenase expression and aggressiveness of the tumours, as determined by the Campanacci classification. This lack of correlation is not surprising, as many reports have described a similar inability to relate aggressiveness to their findings [46].
In the context of GCT, the role of stromal cell-derived MMP-1 and MMP-13 may be giant cell stimulation, as described above. However, the collagenases themselves may be contributing to bone resorption following demineralization by the giant cells. This intriguing consideration may help account for the extensive bone resorption that is characteristic of GCT in cases where fewer giant cells are present [52,53]. In point of fact, it is the spindle-like stromal cells, and not the giant cells, that are present at the margins of the tumour where bone resorption occurs [53]. Further, these enzymes may have regulatory functions, as MMP-13 was previously shown to activate other latent MMPs [54] and can be activated by other proteases found in GCT [55].
In summary, we have demonstrated that the stromal cells derived from patients with GCT produce MMP-1 and MMP-13, but not MMP-8, and that the enzymes were sufficient for proteolytic activity in vitro. The role of the stromal cell-derived collagenases in GCT remains undetermined, although they may aid in bone resorption either directly, or through the stimulation of giant cell activity. Future study is required to elucidate the mechanism of collagenase expression in GCT and to determine the role of these enzymes in vivo.
Acknowledgments
We thank J.E.M. Young for his assistance in collecting some of the GCT samples for analysis.
Footnotes
Statement of ethics: This study has been approved by the Research Ethics Board of McMaster University and Hamilton Health Sciences. Experiments were undertaken with the understanding and written consent of each subject in compliance with the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit:
References
- 1.Yoshida H, Akeho M, Yumoto T. Giant cell tumor bone. Enzyme histochemical, biochemical and tissue culture studies. Virchows Arch A Pathol Anat Histol. 1982;395:319–30. doi: 10.1007/BF00429357. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR, Schiller AL, Mankin HJ, Dayer JM, Krane SM. Characterization of cells from human giant cell tumors of bone. Clin Orthop Relat Res. 1986:59–75. [PubMed] [Google Scholar]
- 3.Goldring SR, Roelke MS, Petrison KK, Bhan AK. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987;79:483–91. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Teng XY, Cheng YY, Lee KM, Kumta SM. Expression of preosteoblast markers and Cbfa-1 and Osterix gene transcripts in stromal tumour cells of giant cell tumour of bone. Bone. 2004;34:393–401. doi: 10.1016/j.bone.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Murata A, Fujita T, Kawahara N, Tsuchiya H, Tomita K. Osteoblast lineage properties in giant cell tumors of bone. J Orthop Sci. 2005;10:581–8. doi: 10.1007/s00776-005-0946-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura M, Yuasa K, Mori K, Miyamoto N, Ito M, Tsurudome M, et al. Cytological properties of stromal cells derived from giant cell tumor of bone (GCTSC) which can induce osteoclast formation of human blood monocytes without cell to cell contact. J Orthop Res. 2005;23:979–87. doi: 10.1016/j.orthres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Wülling M, Delling G, Kaiser E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum Pathol. 2003;34:983–93. doi: 10.1053/s0046-8177(03)00413-1. [DOI] [PubMed] [Google Scholar]
- 8.Chambers TJ, Fuller K, McSheehy PMJ, Pringle JAS. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol. 1985;145:297–305. doi: 10.1002/path.1711450403. [DOI] [PubMed] [Google Scholar]
- 9.Kanehisa J, Izumo T, Takeuchi M, Yamanaka T, Fujii T, Takeuchi H. In vitro bone resorption by isolated multinucleated giant cells from giant cell tumour of bone: light and electron microscopic study. Virchows Arch A Pathol Anat Histopathol. 1991;419:327–38. doi: 10.1007/BF01606524. [DOI] [PubMed] [Google Scholar]
- 10.Ohsaki Y, Takahashi S, Scarcez T, Demulder A, Nishihara T, Williams R, et al. Evidence for an autocrine/paracrine role for interleukin-6 in bone resorption by giant cells from giant cell tumors of bone. Endocrinology. 1992;131:2229–34. doi: 10.1210/endo.131.5.1425421. [DOI] [PubMed] [Google Scholar]
- 11.Soueidan A, Gan OI, Gouin F, Godard A, Heymann D, Jacques Y, et al. Culturing of cells from giant cell tumour of bone on natural and synthetic calcified substrata: the effect of leukaemia inhibitory factor and vitamin D3 on the resorbing activity of osteoclast-like cells. Virchows Arch. 1995;426:469–77. doi: 10.1007/BF00193170. [DOI] [PubMed] [Google Scholar]
- 12.Wen J, Xie D, Yao J, Zhang M, Bi J. Effect of cytokines on in vitro bone resorption by cells isolated from giant cell tumor of bone. Chin Med J (Engl) 1999;112:443–7. [PubMed] [Google Scholar]
- 13.Zheng MH, Fan Y, Wysocki S, Wood DJ, Papadimitriou JM. Detection of mRNA for carbonic anhydrase II in human osteoclast-like cells by in situ hybridization. J Bone Miner Res. 1993;8:113–8. doi: 10.1002/jbmr.5650080114. [DOI] [PubMed] [Google Scholar]
- 14.James IE, Dodds RA, Lee-Rykaczewski E, Eichman CF, Connor JR, Hart TK, et al. Purification and characterization of fully functional human osteoclast precursors. J Bone Miner Res. 1996;11:1608–18. doi: 10.1002/jbmr.5650111104. [DOI] [PubMed] [Google Scholar]
- 15.Oreffo RO, Marshall GJ, Kirchen M, Garcia C, Gallwitz WE, Chavez J, et al. Characterization of a cell line derived from a human giant cell tumor that stimulates osteoclastic bone resorption. Clin Orthop Relat Res. 1993:229–41. [PubMed] [Google Scholar]
- 16.Ghert M, Simunovic N, Cowan RW, Colterjohn N, Singh G. Properties of the stromal cell in giant cell tumor of bone. Clin Orthop Relat Res. 2007;459:8–13. doi: 10.1097/BLO.0b013e31804856a1. [DOI] [PubMed] [Google Scholar]
- 17.Kumta SM, Huang L, Cheng YY, Chow LTC, Lee KM, Zheng MH. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci. 2003;73:1427–36. doi: 10.1016/s0024-3205(03)00434-x. [DOI] [PubMed] [Google Scholar]
- 18.Rao VH, Bridge JA, Neff JR, Schaefer GB, Buehler BA, Vishwanatha JK, et al. Expression of 72 kDa and 92 kDa type IV collagenases from human giant-cell tumor of bone. Clin Exp Metastasis. 1995;13:420–6. doi: 10.1007/BF00118181. [DOI] [PubMed] [Google Scholar]
- 19.Sasaguri Y, Komiya S, Sugama K, Suzuki K, Inoue A, Morimatsu M, et al. Production of matrix metalloproteinases 2 and 3 (stromelysin) by stromal cells of giant cell tumor of bone. Am J Pathol. 1992;141:611–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36:128–37. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 21.Dequeker J, Merlevede W. Collagen content and collagen extractability pattern of adult human trabecular bone according to age, sex and amount of bone mass. Biochim Biophys Acta. 1971;244:410–20. doi: 10.1016/0304-4165(71)90243-1. [DOI] [PubMed] [Google Scholar]
- 22.Dickerson JWT. Changes in the composition of the human femur during growth. Biochem J. 1962;82:56–61. doi: 10.1042/bj0820056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers HJ, Weidmann SM, Parkinson A. Studies on the skeletal tissues. II. The collagen content of bones from rabbits, oxen and humans. Biochem J. 1952;50:537–42. doi: 10.1042/bj0500537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EJ, Martin GR, Piez KA, Powers MJ. Characterization of chick bone collagen and compositional changes associated with maturation. J Biol Chem. 1967;242:5481–9. [PubMed] [Google Scholar]
- 25.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–14. [PubMed] [Google Scholar]
- 26.Duffy MJ, Blaser J, Duggan C, McDermott E, O’Higgins N, Fennelly JJ, et al. Assay of matrix metalloproteases types 8 and 9 by ELISA in human breast cancer. Br J Cancer. 1995;71:1025–8. doi: 10.1038/bjc.1995.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnero P, Borel O, Byrjalsen I, Ferreras M, Drake FH, McQueney MS, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273:32347–52. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 28.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–51. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 29.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–6. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 30.Lindeman JH, Hanemaaijer R, Mulder A, Dijkstra PD, Szuhai K, Bromme D, et al. Cathepsin K is the principal protease in giant cell tumor of bone. Am J Pathol. 2004;165:593–600. doi: 10.1016/S0002-9440(10)63323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drake FH, Dodds RA, James IE, Connor JR, Debouck C, Richardson S, et al. Cathepsin K, but not cathepsins B, L, or S, is abundantly expressed in human osteoclasts. J Biol Chem. 1996;271:12511–6. doi: 10.1074/jbc.271.21.12511. [DOI] [PubMed] [Google Scholar]
- 32.Littlewood-Evans A, Kokubo T, Ishibashi O, Inaoka T, Wlodarski B, Gallagher JA, et al. Localization of cathepsin K in human osteoclasts by in situ hybridization and immunohistochemistry. Bone. 1997;20:81–6. doi: 10.1016/s8756-3282(96)00351-1. [DOI] [PubMed] [Google Scholar]
- 33.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science. 1989;245:855–7. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 34.Holliday LS, Welgus HG, Fliszar CJ, Veith GM, Jeffrey JJ, Gluck SL. Initiation of osteoclast bone resorption by interstitial collagenase. J Biol Chem. 1997;272:22053–8. doi: 10.1074/jbc.272.35.22053. [DOI] [PubMed] [Google Scholar]
- 35.Holliday LS, Welgus HG, Hanna J, Lee BS, Lu M, Jeffrey JJ, et al. Interstitial collagenase activity stimulates the formation of actin rings and ruffled membranes in mouse marrow osteoclasts. Calcif Tissue Int. 2003;72:206–14. doi: 10.1007/s00223-002-1008-7. [DOI] [PubMed] [Google Scholar]
- 36.Fuller K, Chambers TJ. Localisation of mRNA for collagenase in osteocytic, bone surface and chondrocytic cells but not osteoclasts. J Cell Sci. 1995;108(Pt 6):2221–30. doi: 10.1242/jcs.108.6.2221. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura H, Sato G, Hirata A, Yamamoto T. Immunolocalization of matrix metalloproteinase-13 on bone surface under osteoclasts in rat tibia. Bone. 2004;34:48–56. doi: 10.1016/j.bone.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Delaissé JM, Eeckhout Y, Neff L, François-Gillet C, Henriet P, Su Y, et al. (Pro) collagenase (matrix metalloproteinase-1) is present in rodent osteoclasts and in the underlying bone-resorbing compartment. J Cell Sci. 1993;106(Pt 4):1071–82. doi: 10.1242/jcs.106.4.1071. [DOI] [PubMed] [Google Scholar]
- 39.Lau YS, Sabokbar A, Gibbons CL, Giele H, Athanasou N. Phenotypic and molecular studies of giant-cell tumors of bone and soft tissue. Hum Pathol. 2005;36:945–54. doi: 10.1016/j.humpath.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NF-kappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol. 2000;156:761–7. doi: 10.1016/s0002-9440(10)64942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyamoto N, Higuchi Y, Tajima M, Ito M, Tsurudome M, Nishio M, et al. Spindle-shaped cells derived from giant-cell tumor of bone support differentiation of blood monocytes to osteoclast-like cells. J Orthop Res. 2000;18:647–54. doi: 10.1002/jor.1100180418. [DOI] [PubMed] [Google Scholar]
- 42.Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, et al. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005;23:203–9. doi: 10.1016/j.orthres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Zheng MH, Fan Y, Smith A, Wysocki S, Papadimitriou JM, Wood DJ. Gene expression of monocyte chemoattractant protein-1 in giant cell tumors of bone osteoclastoma: possible involvement in CD68+ macrophage-like cell migration. J Cell Biochem. 1998;70:121–9. [PubMed] [Google Scholar]
- 44.Morgan T, Atkins GJ, Trivett MK, Johnson SA, Kansara M, Schlicht SL, et al. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. Am J Pathol. 2005;167:117–28. doi: 10.1016/s0002-9440(10)62959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skubitz KM, Cheng EY, Clohisy DR, Thompson RC, Skubitz AP. Gene expression in giant-cell tumors. J Lab Clin Med. 2004;144:193–200. doi: 10.1016/j.lab.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Gamberi G, Benassi MS, Ragazzini P, Pazzaglia L, Ponticelli F, Ferrari C, et al. Proteases and interleukin-6 gene analysis in 92 giant cell tumors of bone. Ann Oncol. 2004;15:498–503. doi: 10.1093/annonc/mdh091. [DOI] [PubMed] [Google Scholar]
- 47.Zheng MH, Fan Y, Wysocki SJ, Lau AT, Robertson T, Beilharz M, et al. Gene expression of transforming growth factor-beta 1 and its type II receptor in giant cell tumors of bone. Possible involvement in osteoclast-like cell migration. Am J Pathol. 1994;145:1095–104. [PMC free article] [PubMed] [Google Scholar]
- 48.Kominsky SL, Doucet M, Thorpe M, Weber KL. MMP-13 is over-expressed in renal cell carcinoma bone metastasis and is induced by TGF-beta1. Clin Exp Metastasis. 2008;25:865–70. doi: 10.1007/s10585-008-9202-2. [DOI] [PubMed] [Google Scholar]
- 49.Pei Y, Harvey A, Yu XP, Chandrasekhar S, Thirunavukkarasu K. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: potential role of Runx2 in mediating p38 effects. Osteoarthritis Cartilage. 2006;14:749–58. doi: 10.1016/j.joca.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 50.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–64. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benderdour M, Tardif G, Pelletier JP, Di Battista JA, Reboul P, Ranger P, et al. Interleukin-17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1beta. J Rheumatol. 2002;29:1262–72. [PubMed] [Google Scholar]
- 52.Boutou-Bredaki S, Agapios P, Papachristou G. Prognosis of giant cell tumor of bone. Histopathological analysis of 15 cases and review of the literature. Adv Clin Path. 2001;5:71–8. [PubMed] [Google Scholar]
- 53.Steiner GC, Ghosh L, Dorfman HD. Ultrastructure of giant cell tumors of bone. Hum Pathol. 1972;3:569–86. doi: 10.1016/s0046-8177(72)80007-8. [DOI] [PubMed] [Google Scholar]
- 54.Knäuper V, Smith B, López-Otín C, Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur J Biochem. 1997;248:369–73. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 55.Knäuper V, Will H, López-Otín C, Smith B, Atkinson SJ, Stanton H, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–31. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]