Abstract
Rhythmic activity is responsible for numerous essential motor functions including locomotion, breathing and chewing. In the case of locomotion, it has been realized for some time that the spinal cord contains sufficient circuitry to produce a sophisticated stepping pattern. However, the central pattern generator for locomotion in mammals has remained a ‘black box’ where inputs to the network were manipulated and the outputs interpreted. Over the last decade, new genetic approaches and techniques have been developed that provide ways to identify and manipulate the activity of classes of interneurons. The use of these techniques will be critically discussed and related to current models of network function.
Keywords: spinal cord, locomotion, central pattern generator, mouse, networks
1. Introduction
Nearly 100 years ago, Graham-Brown published a seminal article describing his observations on locomotion in spinal cats (Graham-Brown 1911). Using a set of elegant experiments, he demonstrated that circuitry within the spinal cord is sufficient to produce coordinated rhythmic movements of the hindlimbs. He postulated that a set of flexor and extensor centres (half-centres) connected by mutually inhibitory connections produced the rhythmic stepping pattern.
Over the years, this model has been used as a framework for understanding how locomotor activity is produced in a wide variety of vertebrate species. Naturally, it has been revised and expanded over the years, but the essence of Graham-Brown's original half-centre hypothesis continues to be relevant today. As pointed out in an interesting historical review, Graham-Brown's summary of his 1911 paper nicely summarizes the current conceptual framework regarding the modulation and generation of stepping (Stuart & Hultborn 2008). As a field, we have provided support for Graham-Brown's ideas using a diverse range of vertebrate species such as the lamprey, tadpole, turtle, cat and rodent (Jankowska et al. 1967b; Lundberg 1979; Roberts et al. 1998; Stein et al. 1998; Roberts 2000; Grillner 2003; Kiehn 2006; Stuart & Hultborn 2008). The problem then and now is how to identify and selectively silence classes of interneurons that we suspect form part of the locomotor network. Genetic tools are providing a sophisticated range of methods to trace neural circuits, and identify and manipulate the activity of classes of interneurons (Gordon & Whelan 2006; Kiehn 2006; Luo et al. 2008). The impact of these tools on neuroscience and spinal cord networks in particular cannot be overstated. On the other hand, the initial flurry of studies while technically outstanding have not yet provided a conceptual advance that have leapfrogged us beyond that articulated by Graham-Brown. Most probably, this is because networks, no matter where they are located, are difficult to understand (Parker 2010; Selverston 2010). To fully appreciate their operation, a multi-pronged approach combining behavioural, genetic, modelling, intracellular and imaging techniques needs to be applied.
2. Outline of the problem
Peter Getting, among others, outlined a set of steps required to understand a rhythmic network (Getting 1989; Selverston 2005; Yuste et al. 2005). This started with an analysis of the output of the network and ended with the ability to successfully model the network. In between, it was argued that we need to understand how the building blocks of the network interact to produce the behaviour. This means that we need to understand the connectivity between the building blocks of the network. However, this by itself is not enough, since networks are not hard-wired and can flexibly reconfigure by adjusting synaptic and intrinsic properties of the neurons (Marder & Bucher 2001). To build a realistic model of networks, the classes of interneurons need to be identified along with their connections, as well as their intrinsic and synaptic properties. Even in comparatively simple invertebrate networks, this is easier said than done. In a provocative study that modelled a three neuron simplified stomatogastric ganglia network, it was found that over 3000 different weightings of intrinsic and synaptic properties could account for the output of the network (Prinz et al. 2004). This suggests that even if the output of the network appears to be constant, the network connectivity does not have to be the same to produce a given behaviour (Prinz 2010). Clearly this issue has broad implications when examining spinal mammalian networks, which consist of thousands of neurons.
The additional complexity of terrestrial mammalian networks is required to control over 80 muscles to produce fluid stepping behaviour. Acceleration and deceleration of limbs must be precisely timed to produce a seamless gait pattern (Grillner 1981). The spinal network is constructed in such a way that the output is both robust yet modifiable so that it accommodates volitional and reflexive changes to the basic pattern. Furthermore, during development, as limbs and muscles grow and the animal gets heavier, spinal networks are capable of adjusting to the changing biomechanics of the system. For example, human males increase body weight by over 700 per cent during development, yet the basic gait continues to be effortlessly produced.
Determining how the mammalian central pattern generator (CPG) is constructed to accommodate these demands is a fascinating and demanding area of research. Genetic approaches are providing new approaches at a rapid pace, especially in the field of optogenetics (Deisseroth et al. 2006). It is now possible to design experiments that selectively and rapidly turn on and off populations of interneurons using light (Airan et al. 2007). These tools should prove to be useful to test whether a class of interneurons is necessary or sufficient for spinal network function. The terms necessity and sufficiency are commonly used in the description of networks and are not interchangeable. A class of interneurons is necessary for CPG function if their removal from the circuit eliminates some definable aspect of the rhythmic output. For example, if the ability to produce left–right alternating activity is abolished when a class of interneurons is removed, then that class would be deemed necessary for that behaviour. Similarly, if blocking a class of interneurons eliminated rhythmic activity, then it would suggest that activation of the class was necessary for operation of the CPG. That said, one has to be cautious in interpreting the blockade of activity as follower interneurons rather than interneurons comprising the rhythm generator may be affected. A careful analysis of the pattern can resolve these issues (McCrea & Rybak 2008).
On the other hand, selectively activating a class of interneurons may be sufficient to produce the rhythm. However, it does not imply that the class is necessary. This is because there may be redundant classes of interneurons, each of which may be sufficient to evoke the behaviour. It is essential, therefore when designing tests of necessity and sufficiency to define the behaviour.
3. Brief history of mammalian networks
Like many areas of science, progress in the understanding of spinal networks has been a function of conceptual advances, availability of technical tools and historical circumstances. Conceptually, major advances were made by Graham-Brown (Graham-Brown 1911; Stuart & Hultborn 2008). Following his discovery, advances were slow, probably because of a lack of recognition of the importance of this early work (Stuart & Hultborn 2008). Part of this was because of an increased focus on recording intracellular potentials, with the exciting advance of John Eccles making the first intracellular recording from a mammalian neuron—the motoneuron (Brock et al. 1952). In the 1960s, renewed interest in mammalian networks came from the work of Anders Lundberg and colleagues in Sweden who found that treatment of spinalized cats with l-DOPA could allow high-threshold afferents to activate flexors and then extensors in a mutually inhibitory manner (Jankowska et al. 1967a,b). These were the first set of studies to provide evidence in support of Graham-Brown's half-centre hypothesis. This work by Lundberg, his colleagues and trainees led to a renaissance in the field of spinally generated locomotion (Stuart & Hultborn 2008). Around this time, the concept of the CPG first appeared in the literature (Wilson & Wyman 1965; Stuart & Hultborn 2008). The CPG is defined as a set of interneurons that are capable of producing rhythmic output without phasic input from afferent or descending projections. Later on, work using l-DOPA would show that alternating flexor and extensor locomotion could be generated in cats (Grillner & Zangger 1975, 1979). By 1981, a large body of work had been amassed on the operation of networks in cats. This was summarized in a comprehensive review by Grillner (1981). Considering data from work on the cat, Grillner proposed the unit oscillator model of pattern generation (Grillner 1981). A unit oscillator is a simple network controlling movement around a single joint such as the ankle or knee. The unit oscillator model could account for complex behaviours such as walking by connecting multiple unit oscillators through excitatory and inhibitory connections. The advantage of this system is that it is extremely flexible, and different behaviours can be produced by altering the coupling between the sets of unit oscillators. While we have focused on the centrally generated pattern in the above discussion, it is well recognized that sensory input plays a fundamental role in the sculpting the timing and pattern of the rhythm (Rossignol 1996; Whelan 1996).
Around the late 70s and early 80s, there was some agreement in the field that the cat spinal network was too complex to be understood fully with the tools available (Cohen & Wallen 1980). The cat spinal network represented a black box in which inputs were delivered and outputs analysed. While much work had been performed identifying interneurons receiving group I and II afferent input (Jankowska 2001), the problem was how to identify interneurons that comprised the circuit itself. These neurons do not necessarily have to receive afferent input and are not clustered into a nucleus. Lest the cat be discounted, it must be remembered that today work from the cat still provides the most extensive analysis of identified interneurons of any mammalian model (Lundberg 1979; Jankowska 1992).
Around this time, the examination of spinal locomotor networks began to turn to vertebrates that produced locomotor behaviour with fewer classes of interneurons. A useful example is the development of the lamprey as a model preparation where the relatively small number of cells allowed the identification of functional classes of interneurons that comprise the swim CPG (Cohen & Wallen 1980; Poon 1980; Buchanan 1982, 2001; Buchanan & Cohen 1982; Grillner 2003; cf. Parker 2006). This led to a series of studies and new preparations including the turtle swim and scratch CPG (Stein et al. 1998), the Xenopus swim CPG (Roberts et al. 1998, 2008) and the zebrafish escape circuit (Fetcho et al. 2008). The use of these preparations has led to several seminal findings that have advanced the field and that have been ably described in several reviews (Roberts et al. 1998, 2008; Stein et al. 1998; Buchanan 2001; Fetcho et al. 2008). At the same time, CPG work using the cat was not dormant, with interesting findings emerging on the interaction of reflex pathways and the control of stepping (Forssberg et al. 1975, 1980; Grillner & Rossignol 1978; Lundberg 1979; Duysens & Pearson 1980; Barbeau & Rossignol 1987).
Toward the end of the 1980s, there appeared a set of papers from Jack Feldman's laboratory demonstrating the ability of an isolated rat spinal cord preparation to generate locomotor-like behaviour in vitro (Smith & Feldman 1987; Smith et al. 1988). This preparation, which was inspired by earlier work by Suzue (1984), allows ease of access to the spinal cord, manipulation of the extracellular milieu and use of lesioning methodologies, which greatly aided the examination of spinal networks. Yet, despite the subsequent popularity of this preparation, the mammalian CPG remained a ‘black box’, even under in vitro conditions.
Starting in the late 90s, the mouse started to gain popularity as a model preparation for the study of locomotion (Bonnot & Morin 1998; Bonnot et al. 1998; Jiang et al. 1999; Whelan et al. 2000). The adoption of the mouse for studying mammalian network function opened the door to genetic approaches. An important event was the collaboration of developmental neurobiologists with electrophysiologists investigating network function (Kullander et al. 2003; Lanuza et al. 2004; Miles et al. 2004; Gosgnach et al. 2006; Zhang et al. 2008; Crone et al. 2009). This capitalized on the discovery by developmental biologists that classes of motoneurons and interneurons could be defined by expression of progenitors and combinatorial expression of transcription factors (Jessell & Sanes 2000). During the early 2000s, it is fair to say that the entire field was energized by the potential impact of these genetic approaches (Kiehn & Kullander 2004). The potential cannot be overstated since tools now exist that can label interneurons that are candidates to be part of the CPG (Lanuza et al. 2004; Wilson et al. 2005; Gosgnach et al. 2006; Crone et al. 2008; Zhang et al. 2008; Goulding 2009). That said, clearly deciphering the structure of the spinal CPG using genetic approaches is going to be a complex task.
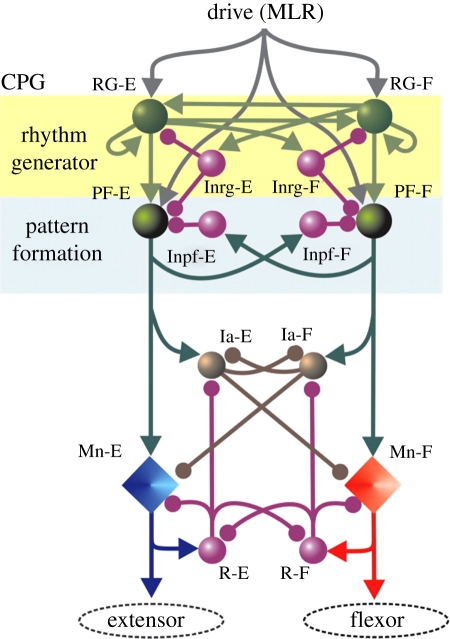
Right now, there are two dominant theories of CPG function. The first, as discussed earlier, is the unit oscillator hypothesis (Grillner 1981). The other is a three-layer model that builds on the original half-centre model (Lafreniere-Roula & McCrea 2005; Rybak et al. 2006a; McCrea & Rybak 2008). The first layer is proposed to consist of a distributed network consisting of excitatory interneurons interconnected which form mutually inhibited half-centres. This first layer is the rhythm generator whose drive is then propagated to the pattern formation layer, which provides the specific drive that is sent to a population of motoneurons innervating a particular muscle (figure 1). The third layer is a set of mutually inhibitory classes of Ia inhibitory interneurons that also receive input from Renshaw cells. These inhibitory Ia interneurons do not contribute to rhythmogenesis but do contribute to the shaping of overall motoneuronal output. There is both experimental and modelling data suggesting that this multiple-layer model can explain many of the behaviours produced (Lafreniere-Roula & McCrea 2005; McCrea & Rybak 2007, 2008; Endo & Kiehn 2008).
Figure 1.
Schematic of model by Rybak & McCrea. The populations of interneurons are indicated by spheres, while the motoneurons are represented by diamonds. This three-layer model consists of a rhythm-generating layer of extensor (RG-E) and flexor (RG-F) interneurons. Both populations have recurrent excitatory connections (see also figure 2). These interneurons in turn receive mutually inhibitory input (Inrg cells). The drive projects to a pattern formation layer (PF), which acts through mutually inhibitory connections (Inpf cells) to sculpt the pattern, which is then output to the extensor and flexor motoneurons. The final output of the motoneurons is modulated by a final layer of Ia inhibitory interneurons (Ia-E, Ia-F) and Renshaw cells (R-E, R-F). Arrows indicate excitatory drive, while the filled circles indicate inhibitory drive. Reproduced with permission.
4. The dream
The mammalian CPG still remains a black box, but new tools are providing methods to dissect the networks. Before delving into what technology is available, we should pause and review what would be a dream scenario for researchers of spinal cord networks. We would like to have a set of tools that would allow us to test necessity and sufficiency for sets of identified candidate interneurons that may form part of locomotor networks. Ideally, these tools would be specific enough to allow targeting of Renshaw cells, Ia interneurons, commissural interneurons (CCINs) and so on. Under ideal conditions, one can conceive of a switchboard where one could reversibly silence sets of neurons to examine whether the class of interneurons was necessary for network function. At the same time, tests of sufficiency would involve selective activation of the targeted classes to examine whether they alone could elicit episodes of rhythmic activity or influence activity in predictable ways. Ideally, we would be able to identify a single class of conditional pacemaker cells that form the kernel of the network (Marder & Bucher 2001). These pacemaker cells would be responsible for generating the timing of the rhythm and would project to pattern-generating interneurons that sculpt the pattern before in turn projecting onto motoneurons (McCrea & Rybak 2008). Distinct classes of pattern-generating interneurons could be identified and reversibly inactivated to influence the pattern but not the timing of the rhythm. Recent developments indicate that we are getting close to this dream of having a genetic-based switchboard where we can turn on and off selected classes of interneurons (Luo et al. 2008). The outlined scenario is perhaps the most tractable from an experimental point of view. While we still do not know the structure of the mammalian network, it is well known that other models can account for CPG output. Therefore, data collected using new technologies will have to be carefully interpreted with no a priori bias for one model versus another.
5. Current applications of genetic technologies
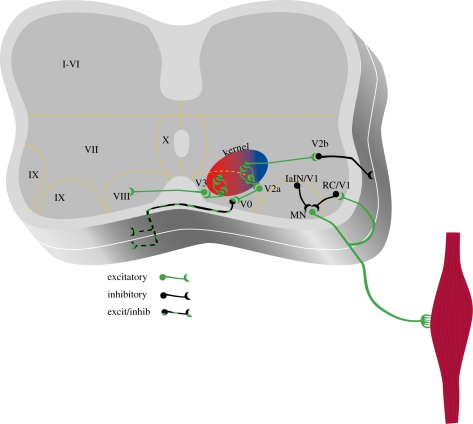
Several years ago, Tom Jessel and colleagues showed that neurons could be clustered into distinct classes based on the expression of transcription factors in progenitor cells (Jessell & Sanes 2000). During early development, 11 progenitor cell classes lead to the production of postmitotic cells by embryonic day 11 in mouse. Six of these classes of interneurons contribute to the formation of the spinal circuitry contributing to CPG function and motoneuronal output (Goulding & Pfaff 2005; Goulding 2009). Each of these early interneuronal classes has unique anatomical and transmitter phenotypes (figure 2). The so-called V0 interneurons project across the ventral commissure and ascend for two to four segments (Lanuza et al. 2004). They are thought to project onto Renshaw cells, Ia inhibitory interneurons, and motoneurons. The V0D are GABAnergic/glycinergic cells that contribute to the control of left–right alternating activity (Lanuza et al. 2004). On the other hand, the V0V class is glutamatergic neurons (Goulding 2009) that do not appear to contribute to locomotor coordination (Lanuza et al. 2004). The V1 class of neurons is restricted to the ipsilateral side of the spinal cord in lamina VII and are inhibitory (Sapir et al. 2004; Alvarez et al. 2005). V2 neurons can be subdivided into V2a and V2b classes (Peng et al. 2007) which are excitatory and inhibitory, respectively (Al-Mosawie et al. 2007; Lundfald et al. 2007). V2 cells do not cross the midline and descend ipsilaterally across several segments. Recent studies indicate that the excitatory V2a interneurons have diverse effects on network output, including left–right coupling, speed, amplitude and regularity of the rhythm (Crone et al. 2008, 2009). Interestingly, their effects on left–right coupling are probably due to projections onto CCINs since V2a cells do not project across the midline (Crone et al. 2008). The role for the V2b class has not been determined, but since the neurons project caudally and are inhibitory, it could contribute to the control of reciprocal inhibition between flexors and extensors (Goulding 2009). The V3 class of interneurons is a mixed class of cells that project both ipsi- and contralaterally (Zhang et al. 2008). However, in contrast to V0 interneurons, they do not appear to contribute to left–right coordination, although they do contribute to the regularity of the rhythm (Zhang et al. 2008). In addition, there is a dorsally identified class of inhibitory interneurons that are derived from dorsally located progenitors termed the dI6 population (Muller et al. 2002). These cells project across the midline and are probably involved in controlling the pattern of locomotion (Goulding 2009).
Figure 2.
Genetic techniques can be used to dissect circuits within the spinal cord. Populations of interneurons derived from progenitor cells can be identified in neonatal mice. Schematic shows different populations of cells that can be identified currently in neonatal mouse preparations. V0 cells are CCINs that consist of both glutamatergic and glycinergic/GABAergic cells. Animals lacking these commissural cells have poor left–right coordination. V1 interneurons are considered to be inhibitory and are a heterogeneous population. At least a portion of them is involved in regulating the speed of the rhythm. Renshaw cells and Ia inhibitory interneurons (IaINs) form part of the V1 population and have been well characterized, but the contribution of individual classes of V1 neurons to rhythm generation has not been well established. V2 cells are also heterogeneous and consist of a population of excitatory interneurons (V2a) that project ipsilaterally and are involved in the regulation of left–right coordination. Another population of inhibitory interneurons possibly projects caudally and regulates flexor–extensor alternation. V3 cells are glutamatergic CCINs that may play a role in coordination of locomotor patterns. Interneurons that belong to the kernel that generates the timing pattern have not been conclusively identified. See text for references.
Each of these classes of interneurons express a unique set of transcription factors that allows the classes to be silenced either permanently or transiently and the effects on locomotion assessed (Lanuza et al. 2004; Gosgnach et al. 2006; Crone et al. 2008, 2009; Zhang et al. 2008). Alternatively, their synaptic and intrinsic properties can be assessed when the neurons are made to express fluorescent proteins (XFPs) by recording from them using electrophysiological or imaging techniques in live slice or isolated spinal cord tissue (Lanuza et al. 2004; Wilson et al. 2007; Dyck & Gosgnach 2009). Different classes of cells can be targeted using different XFPs (Wilson et al. 2007), potentially allowing targeted imaging of circuits as they turn on and off during rhythmic activity. Furthermore, classes of interneurons can be inactivated using pharmacological approaches when non-natively expressed channels are inserted (Lechner et al. 2002; Gosgnach et al. 2006). This overcomes a major obstacle of single-cell recordings that only examine the activity of a single neuron. It also allows tests of necessity and sufficiency to continue across classes of spinal cord interneurons. Therefore, inferences regarding their function can be determined by examining the effects of selective and reversible activation or inactivation of ventral horn interneurons.
Naturally, there are caveats to the use of these techniques, which limit interpretation of the data. The chief issue is that the changes in the output of the network following silencing of the targeted class of interneurons are often difficult to interpret. For example, recently it was shown by Martyn Goulding's group that the V1 population of inhibitory interneurons was involved in setting the timing of the rhythm. The study was a tour de force, and notably included for the first time in our field a test of necessity. The authors expressed a non-native allatostatin receptor in the V1 class of interneurons. Allatostatin is a peptide that was identified in Drosophila (Birgul et al. 1999) and leads to activation of GIRK channels that hyperpolarizes the neuron (Lechner et al. 2002). When the receptor for allatostatin is introduced into a class of neurons, one can inactivate a defined population of cells by simply bath applying allatostatin (Gosgnach et al. 2006; Zhang et al. 2008). This approach can also be used in adult mice by introducing allatostatin intrathecally and examining changes in the stepping pattern (Zhang et al. 2008). Using this approach, the authors found that inactivation of the V1 inhibitory cells led to a slowing of a rhythm. While this study showed that these cells contributed to the timing of the rhythm, they were not essential (see later discussion of distributed networks). At present, it is difficult to draw conclusions regarding the role of individual classes of V1 interneurons such as IaINs and Renshaw cells. Presumably greater precision will be required to make definitive conclusions regarding the role of the V1 class of interneurons in the timing and patterning of locomotion since at present 75 per cent of the interneurons in this class remain unclassified (Goulding 2009). While there are some ambiguities and difficulties to be resolved, the tools being developed, such as the one discussed (Gosgnach et al. 2006), represent an enormous movement forward for our field.
With the breathtaking progress in the genetic field, network electrophysiologists have numerous new targets to investigate using the mouse. What would make the job easier is more electrophysiological data on afferent connectivity within the spinal cord of the mouse. Most of the electrophysiological classification of interneurons has been accomplished using the adult cat (Jankowska 1992, 2001). We need to examine whether similar connections are made in the neonatal and adult mouse. Indeed, recent evidence in the mouse is starting to turn up interesting differences in connectivity between the mouse and the cat (Mentis et al. 2006).
Apart from the Renshaw cells, which are well characterized in the mouse (Mentis et al. 2005, 2006; Nishimaru et al. 2005, 2006), interneurons in the cat spinal cord were identified by relatively selective activation of classes of afferents (Jankowska 1992, 2001). In developing rodents, it is not as straightforward to selectively activate classes of afferents, and as a consequence, the necessary studies correlating classes of afferent-identified interneurons have not been performed. A problem is that the strategy of using low to high levels of electrical stimulation in the cat to target group I muscle afferents is not very effective in the neonatal mouse or rat. Therefore, it will be necessary to activate muscle spindles (group Ia/II afferents) using piezoelectric vibrators (Iizuka et al. 1997) and miniature muscle pullers (group Ib afferents) to activate Golgi tendon organs. Molecular approaches will probably be developed that allow the selective activation and inactivation of classes of muscle afferents, much as the case for pain afferents (Caterina et al. 1997; Mandadi et al. 2009), but this still remains to be resolved.
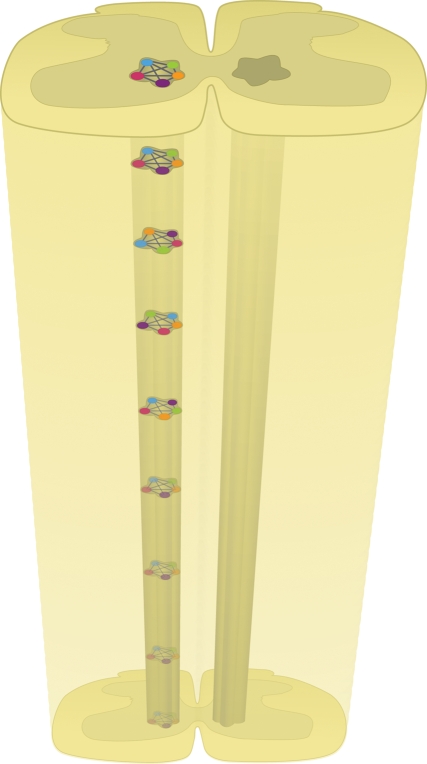
A more serious issue is that these approaches will not work for interneurons that do not receive afferent input and form part of the CPG network. Therefore, these classes of interneurons will need to be activated and inactivated using genetic approaches one class at a time to determine their effects on network output. It is sobering to think of doing this when the structure of the ‘clock’ or kernel of the CPG is probably distributed (figure 3) across multiple segments (Kjaerulff & Kiehn 1996; Cowley & Schmidt 1997; Kremer & Lev-Tov 1997). It is still a matter of debate whether the different patterns are due to multiple unit oscillators or alternatively due to a separate patterning layer (Grillner 1981; Rybak et al. 2006a,b; McCrea & Rybak 2007, 2008). Regardless of the outcome of this debate, the fact remains that multiple neuronal classes probably contribute to the formation of the kernel of the CPG. The closest the field has come to identifying a class of cells that may form part of the kernel of the CPG are the Hb9 interneurons (Hinckley et al. 2005; Wilson et al. 2005). Hb9 is a transcription factor that is expressed in a subclass of ventrally located interneurons that fire in phase with rhythmic activity from the same segments. They also possess conditional pacemaker properties (Wilson et al. 2005; Han et al. 2007) along with other intrinsic properties such as postinhibitory rebound that make them ideal candidates for interneurons that form part of the CPG kernel (Wilson et al. 2005). However, their role in generating locomotion remains controversial for several reasons (Kwan et al. 2009). First, they tend to fire after the onset of motoneuronal activity in L2 segments. Second, they fire few spikes during a bout of locomotion. Third, they are not present in caudal lumbar segments, even though alternating activity can be evoked from isolated caudal lumbosacral segments. Finally, Hb9 interneuronal firing tends to be suppressed when locomotion is evoked by electrical stimulation compared with pharmacological activation. While these observations tend to rule out a role for Hb9 interneurons in forming a kernel of critical pacemaker neurons, they do not rule out a role for Hb9 interneurons being a component of a distributed network of cells that mutually excite each other. Numerous studies show that other cells share conditional oscillatory properties and it would be interesting to examine the firing behaviour of these cells in relation to ventral root bursting behaviour. Ultimately, evidence for a role for Hb9 interneurons awaits tests of necessity and sufficiency.
Figure 3.
The rhythm generator kernel is probably distributed throughout the spinal cord. This diagram shows different classes of mutually excitatory populations of interneurons that form one part of a rhythm generator (RG) half-centre. The different classes comprising the single RG can be interconnected in different ways throughout the network. In this system, deletion of a single class of interneurons would not be expected to completely block the rhythm.
Rather than a single pacemaker class within the spinal cord, a more likely scenario is that we will discover that multiple classes of genetically identified interneurons form the kernel of the CPG (figure 3). Indeed, the literature already shows evidence for conditional oscillations being more the norm rather than the exception in ventrally located interneurons in lamina VIII and VII in the rodent (Hochman et al. 1994; Han et al. 2007; Tazerart et al. 2007). A distributed network is not great news for experimenters since no one class of interneurons can be reversibly inactivated and found to be necessary for the rhythm. More likely, what would occur is graceful degradation of the rhythm. Graceful degradation is a term commonly used in the design of distributed computer networks (Randell et al. 1978). It refers to the ability of the network to maintain limited functionality even if large portions of it are destroyed or rendered inactive. In essence, it is a design to prevent catastrophic failure if elements of the network fail. Perhaps the best known implementation of this system is the worldwide web. This analogy is useful when thinking about a network as important as the locomotor network. The prediction would be that the rhythm would slow down or tend to become irregular as elements of the distributed network were silenced. This is often what is observed when lesion studies or split-bath techniques are used to inactivate areas of the spinal cord (Kjaerulff & Kiehn 1996; Cowley & Schmidt 1997; Kremer & Lev-Tov 1997). To test this idea using genetics, multiple classes of interneurons will have to be turned off independently of each other. For the sake of argument, let us say that there are eight classes of cells that we hypothesize form the kernel of the CPG. If class 1 + class 2 are turned off and the rhythm stops then it would argue that both of these classes are necessary for rhythmogenesis. However, if class 5 + class 8 are inactivated, or any other combination, and the rhythm is blocked, then it suggests that a distributed network exists. It can be appreciated that to test all combinations would be a time-consuming task. A key concern is carefully controlling for a false negative. Let us say we find evidence that both classes 1 and 2 are necessary for rhythmogenesis. However, this may be simply due to a reduction in the net excitability of the network owing to the removal of these elements. In a distributed network, it should be possible to increase the excitability of the network by increasing the concentration of rhythmogenic drugs (Kjaerulff & Kiehn 1996; Kremer & Lev-Tov 1997) or by increasing the electrical stimulation intensity. Another feature of distributed networks is that they often exhibit network homeostasis (Maffei & Fontanini 2009). Network homeostasis has been observed in several species including the spinal cord of the embryonic chick (Chub & O'Donovan 1998; Tabak et al. 2001). Therefore, even after the rhythm is blocked, compensation within the network can result in the return of rhythmicity. What this means is that appropriate controls that allow for extended silencing of each interneuronal population should be completed.
So far we have discussed the genetic manipulation of classes of interneurons and the subsequent modification of network function. In all likelihood, these bottom-up approaches (reverse genetic screen) will need to be married with a top-down or forward screening strategy that targets spontaneous mutations, some of which produce interesting behavioural phenotypes. Essentially, this approach works in the opposite direction. The mutation that produces the behaviour needs to be mapped to a particular locus on the genome that corresponds to a gene or a set of genes. Then these genes need to be ablated in normal mice to see if the same behaviour can be reproduced. An example of this approach is the use of mice lacking a receptor EphA4 or its ligand EphB3 that normally functions to repress certain classes of developing excitatory interneuronal axons from crossing the midline (Kullander et al. 2003). These mice exhibit a hopping-like locomotor behaviour instead of normal alternating stepping. This behaviour was thought to be caused by excitatory glutamatergic interneurons that normally innervate neurons ipsilaterally, which instead made aberrant connections with the contralateral network. The additional excitatory commissural drive appeared to overwhelm the drive from inhibitory CCINs. However, recent evidence suggests that the other possibility, namely that inhibitory CCINs are reduced, could also be responsible (Restrepo et al. 2009a).
Similar types of approaches have been used with success in other species, such as the zebrafish (McLean & Fetcho 2008), to identify candidate interneurons that contribute to rhythmogenesis. An excellent example of this approach was published recently whereby a screening approach was used to isolate a particular cell type (Wyart et al. 2009). The authors used an optogenetic approach whereby they inserted a light activated glutamate subunit into the cells they wished to examine. Using this approach, they were able to verify that photoactivation of a specific class of cells was sufficient to induce locomotion. Furthermore, they were able to selectively reduce synaptic transmission from these classes of cells and correlate this to a marked reduction in the frequency of locomotion. These results are exciting since to the best of my knowledge it represents the first time that genetic techniques have been used to test sufficiency and necessity in a homogeneous class of vertebrate spinal cord neurons.
6. Future applications of technology in spinal cord systems
Assuming the CPG kernel is distributed, I suspect that genetic approaches will be most beneficial when used to examine the pattern-generating layer. A good example is the CCINs. These CCINs connect networks on each half of the spinal cord and are responsible for coordinating left–right and flexor–extensor limb coordination (Kiehn 2006). Several classes of CCINs have been identified using both genetic (Lanuza et al. 2004; Zhang et al. 2008; Goulding 2009) and anatomical approaches (Butt et al. 2002; Stokke et al. 2002; Bannatyne et al. 2003; Butt & Kiehn 2003; Kiehn & Butt 2003; Jankowska et al. 2005), and it is already clear that their projections, along with their intrinsic and synaptic properties can differ between and within classes (Kiehn 2006). These classes of interneurons are not necessary for rhythmicity since it is possible to elicit an alternating flexor–extensor rhythm from each side of a split spinal cord (Whelan et al. 2000). Nevertheless, CCINs represent attractive targets since they can be identified using electrophysiological and genetic approaches. An interesting anatomical class of CCINs cross the midline and descend to innervate flexor and extensor motoneurons along with Renshaw cells and Ia inhibitory interneurons (Butt & Kiehn 2003). These descending CCINs are heterogeneous and are composed of both excitatory and inhibitory cells (Restrepo et al. 2009b). It would be interesting to label these circuits since they represent a tractable target for examining pattern generation. One possibility is the use of transneuronal markers to label these classes of CCINs (Standish et al. 1995). In the past, this has been accomplished by injecting the transneuronal pseudorabies virus tracer into muscles (Lanuza et al. 2004). After 1 day, the marker is expressed in premotoneuronal neurons, and since it is packaged with EGFP, the neurons can be identified. However, these methods suffer from several problems. Since the virus tends to propagate to neurons where there are more synaptic projections, it is difficult to discern weak monosynaptic from strong indirect connections (Luo et al. 2008). Another approach is to identify classes of neurons using approaches discussed earlier and ensure that they express Cre recombinase. Retrograde virus, such as pseudorabies virus, can then be manufactured so that they will start to replicate only when they encounter Cre expressed in the targeted class of interneurons (DeFalco et al. 2001). Once the replication stop is excised by Cre, PRV is free to infect other cells that project onto the targeted class of interneurons. Another technique is to use monosynaptic trans-synaptic labelling (Wickersham et al. 2007). Rabies is expressed in cells that one wishes to target, and a special construct is developed which when combined with the rabies virus allows it to trans-synaptically infect the neighbouring presynaptic cell (Wickersham et al. 2007). However, since the presynaptic cell lacks rabies the reaction stops there and cannot go any further.
The ability to dissect spinal cord circuits is difficult owing to the heterogenous nature of the spinal cord. In this regard, the development of ‘brainbow’ mice offers an attractive technique to identify neurons (Livet et al. 2007). Brainbow mice essentially mix multiple XFPs together in such a way that over 90 different hues can be identified in neurons. What this means is that single neurons and their processes can be differentiated. Potentially this approach can be used to target individual classes of cells within the spinal cord (Wilson et al. 2007). The attractive feature is that the colours are maintained throughout the neurons soma, dendrites and axons. Therefore, it is possible to identify multiple presynaptic inputs onto a cell and determine whether they come from different neurons or from the same cell. Presumably, use of brainbow mice should also make paired intracellular recording easier since one can trace the processes of the presynaptic cell onto the target cell since the colour will remain the same. Currently, the technique is still under development. It relies on the expression of Thy1, which does not label all classes of neurons. Furthermore, synaptic connectivity cannot be unambiguously resolved (Luo et al. 2008). That said, this will be a useful technique for tracing projections to and from candidate CPG interneurons within the spinal cord. As I mentioned at the beginning, the use of these techniques is best suited to examining defined circuits rather than a distributed network. Circuits that would be especially amenable would be CIN or Renshaw cell circuits.
While powerful, these anatomical mapping strategies represent a static representation of network connectivity. Ultimately, functional connectivity and how this alters during actual network activity need to be measured. How can this be accomplished? One possibility is intracellular recordings from pairs of neurons (Jonas et al. 1998; Hinckley & Ziskind-Conhaim 2006). This allows the experimenter to directly examine connectivity between the two putative elements in the circuits. However, this approach is hard and the reality is that the number of sampled pairs will be unacceptably low. Ideally, it would be useful to examine connectivity between an identified neuron and the multiple neurons it connects to. In principle, this can be accomplished by recording intracellularly from the first neuron, eliciting a spike, and then recording a spike-triggered average from multiple neurons using voltage-sensitive dye imaging. The advantages of voltage-sensitive dye recordings are that subthreshold depolarizations can be detected and the temporal resolution is excellent (O'Donovan et al. 2008; Homma et al. 2009). Unfortunately, the spatial resolution of this approach is low since a photodiode array is used to sum the faint changes in fluorescence produced. Another option is to use calcium imaging approaches. Since there are a large number of high-threshold Ca2+ channels in cells, Ca2+ indicators that are bulk loaded provide a measure of the degree of extracellular spiking in a population of cells (O'Donovan et al. 1993, 1994). Since stimulation of a single cell rarely evokes spiking in target neurons, activation of a class of interneurons would need to be performed to detect connectivity using Ca2+ imaging. It is possible to do this using either glutamate uncaging (Callaway & Katz 1993) or light-activated channels, such as channel 2 rhodopsin (Nagel et al. 2003). The response of the target interneurons could then be measured by loading these cells with a calcium indicator, such as Fura2-AM (Zhang et al. 2007).
A key advantage of using light over other approaches (e.g. allatostain) is that the laser can be pulsed at different frequencies, therefore, activation of classes of cells can be graded. For example, expression of channel rhodopsin 2 (ChR2) in classes of interneurons allows the investigator to activate interneurons that incorporate ChR2 simply by pulsing a blue laser onto the area of interest (Zhang et al. 2006). ChR2 is a microbial light-activated channel that conducts cationic currents with fast kinetics similar to AMPA conductances (Nagel et al. 2003). On the other hand, another opsin, halorhodopsin can be expressed that rapidly hyperpolarizes the neurons it is inserted into when yellow light illuminates the population. Halorhodopsin works by activating a chloride pump that rapidly increases the intracellular chloride concentration (Zhao et al. 2008). Since halorhodopsin is activated by pulses of yellow laser light, it is possible to optically activate and inactivate populations of interneurons by using yellow and blue laser pulses, making the task of sufficiency and necessity tests tractable. In fact, pure optical stimulation and recording experiments can be constructed. These types of strategies have been used to dissect out cortical microcircuits that produce oscillatory activity (Sohal et al. 2009), which points to the potential of optogenetics as a tool for understanding the CPG function. One of the drawbacks of using this approach in the spinal cord is that the neurons comprising the CPG are distributed rostrocaudally across multiple segments. This limits the utility of this approach since it may not be possible to completely illuminate all segments of the cord equally. However, it may be ideal for examining connectivity in pattern-generating circuits that are located within a segment. On the other hand, pharmacological approaches, such as the use of allatostatin (Lechner et al. 2002; Gosgnach et al. 2006; Zhang et al. 2008; Goulding 2009), are of greater use when examining functionality within a distributed network since bath application affects all the tissue. Use of light versus pharmacological approaches needs to be carefully considered depending on the requirement for fast (ms) activation and inactivation or the need for widespread but slower (min) inactivation.
7. conclusions
There is little doubt that genetic approaches have provided a plethora of new tools that allow investigators to dissect genetically spinal cord networks. These tools have led to interesting applications of technologies that allow identification and reversible activation of classes of interneurons. In principle, we have at our disposal tools that allow us to test whether a class of interneurons is necessary for rhythmogenesis and furthermore whether activation of that class is sufficient to produce rhythmic drive to the pattern-generating layer and onto motoneurons. Nearly 100 years following the seminal work of Graham-Brown, we are finally getting a peek inside the black box that encompasses the neural network that produces locomotion. Not surprisingly, this work is raising more questions than it is answering. It is unlikely that we will discover a single ‘magic bullet’ class of interneurons that generates the locomotor rhythm. On the other hand, a comparatively easier task would be to examine connectivity within the pattern-generating layer. For example, selectively inactivating CCINs that coordinate left–right and flexor–extensor locomotion is one example.
In closing, it was not that long ago that the possibility of dissecting specific classes of interneurons that form part of the mammalian CPG would have been considered improbable. We have now entered a phase where the field as a whole needs to consider how to interpret this important data. As a field, we need to clarify what are the most tractable targets using genetic and other techniques.
Acknowledgements
Dr Patrick Whelan is a Senior Scholar of the Alberta Heritage Foundation for Medical Research. Support from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research is gratefully acknowledged.
Footnotes
One contribution of 8 to a Theme Issue ‘Neuronal network analyses: progress, problems, and uncertainties’.
References
- Airan R. D., Hu E. S., Vijaykumar R., Roy M., Meltzer L. A., Deisseroth K.2007Integration of light-controlled neuronal firing and fast circuit imaging. Curr. Opin. Neurobiol. 17, 587–592 (doi:10.1016/j.conb.2007.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mosawie A., Wilson J. M., Brownstone R. M.2007Heterogeneity of V2-derived interneurons in the adult mouse spinal cord. Eur. J. Neurosci. 26, 3003–3015 (doi:10.1111/j.1460-9568.2007.05907.x) [DOI] [PubMed] [Google Scholar]
- Alvarez F. J., Jonas P. C., Sapir T., Hartley R., Berrocal M. C., Geiman E. J., Todd A. J., Goulding M.2005Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 493, 177–192 (doi:10.1002/cne.20711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne B. A., Edgley S. A., Hammar I., Jankowska E., Maxwell D. J.2003Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur. J. Neurosci. 18, 2273–2284 (doi:10.1046/j.1460-9568.2003.02973.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H., Rossignol S.1987Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 412, 84–95 (doi:10.1016/0006-8993(87)91442-9) [DOI] [PubMed] [Google Scholar]
- Birgul N., Weise C., Kreienkamp H. J., Richter D.1999Reverse physiology in Drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J 18, 5892–5900 (doi:10.1093/emboj/18.21.5892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnot A., Morin D.1998Hemisegmental localisation of rhythmic networks in the lumbosacral spinal cord of neonate mouse. Brain Res. 793, 136–148 (doi:10.1016/S0006-8993(98)00153-X) [DOI] [PubMed] [Google Scholar]
- Bonnot A., Morin D., Viala D.1998Genesis of spontaneous rhythmic motor patterns in the lumbosacral spinal cord of neonate mouse. Brain Res. Dev. Brain Res. 108, 89–99 (doi:10.1016/S0165-3806(98)00033-9) [DOI] [PubMed] [Google Scholar]
- Brock L. G., Coombs J. S., Eccles J. C.1952The recording of potentials from motoneurones with an intracellular electrode. J. Physiol. 117, 431–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan J. T.1982Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. J. Neurophysiol. 47, 961–975 [DOI] [PubMed] [Google Scholar]
- Buchanan J. T.2001Contributions of identifiable neurons and neuron classes to lamprey vertebrate neurobiology. Prog. Neurobiol. 63, 441–466 (doi:10.1016/S0301-0082(00)00050-2) [DOI] [PubMed] [Google Scholar]
- Buchanan J. T., Cohen A. H.1982Activities of identified interneurons, motoneurons, and muscle fibers during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J. Neurophysiol. 47, 948–960 [DOI] [PubMed] [Google Scholar]
- Butt S. J., Kiehn O.2003Functional identification of interneurons responsible for left–right coordination of hindlimbs in mammals. Neuron 38, 953–963 (doi:10.1016/S0896-6273(03)00353-2) [DOI] [PubMed] [Google Scholar]
- Butt S. J., Harris-Warrick R. M., Kiehn O.2002Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J. Neurosci. 22, 9961–9971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. M., Katz L. C.1993Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc. Natl Acad. Sci. USA 90, 7661–7665 (doi:10.1073/pnas.90.16.7661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D.1997The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (doi:10.1038/39807) [DOI] [PubMed] [Google Scholar]
- Chub N., O'donovan M. J.1998Blockade and recovery of spontaneous rhythmic activity after application of neurotransmitter antagonists to spinal networks of the chick embryo. J. Neurosci. 18, 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. H., Wallen P.1980The neuronal correlate of locomotion in fish. ‘Fictive swimming’ induced in an in vitro preparation of the lamprey spinal cord. Exp. Brain. Res. 41, 11–18 [DOI] [PubMed] [Google Scholar]
- Cowley K. C., Schmidt B. J.1997Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J. Neurophysiol. 77, 247–259 [DOI] [PubMed] [Google Scholar]
- Crone S. A., et al. 2008Genetic ablation of V2a ipsilateral interneurons disrupts left–right locomotor coordination in mammalian spinal cord. Neuron 60, 70–83 (doi:10.1016/j.neuron.2008.08.009) [DOI] [PubMed] [Google Scholar]
- Crone S. A., Zhong G., Harris-Warrick R., Sharma K.2009In mice lacking V2a interneurons, gait depends on speed of locomotion. J. Neurosci. 29, 7098–7109 (doi:10.1523/JNEUROSCI.1206-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defalco J., Tomishima M., Liu H., Zhao C., Cai X., Marth J. D., Enquist L., Friedman J. M.2001Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science 291, 2608–2613 (doi:10.1126/science.1056602) [DOI] [PubMed] [Google Scholar]
- Deisseroth K., Feng G., Majewska A. K., Miesenbock G., Ting A., Schnitzer M. J.2006Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 26, 10 380–10 386 (doi:10.1523/JNEUROSCI.3863-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J., Pearson K. G.1980Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 187, 321–332 (doi:10.1016/0006-8993(80)90206-1) [DOI] [PubMed] [Google Scholar]
- Dyck J., Gosgnach S.2009Whole cell recordings from visualized neurons in the inner laminae of the functionally intact spinal cord. J. Neurophysiol. 102, 590–597 (doi:10.1152/jn.00212.2009) [DOI] [PubMed] [Google Scholar]
- Endo T., Kiehn O.2008Asymmetric operation of the locomotor central pattern generator in the neonatal mouse spinal cord. J. Neurophysiol. 100, 3043–3054 (doi:10.1152/jn.90729.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho J. R., Higashijima S., Mclean D. L.2008Zebrafish and motor control over the last decade. Brain Res. Rev. 57, 86–93 (doi:10.1016/j.brainresrev.2007.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S.1975Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 85, 103–107 (doi:10.1016/0006-8993(75)91013-6) [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Halbertsma J., Rossignol S.1980The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol. Scand. 108, 283–295 (doi:10.1111/j.1748-1716.1980.tb06534.x) [DOI] [PubMed] [Google Scholar]
- Getting P. A.1989Emerging principles governing the operation of neural networks. Annu. Rev. Neurosci. 12, 185–204 (doi:10.1146/annurev.ne.12.030189.001153) [DOI] [PubMed] [Google Scholar]
- Gordon I. T., Whelan P. J.2006Deciphering the organization and modulation of spinal locomotor central pattern generators. J. Exp. Biol. 209, 2007–2014 (doi:10.1242/jeb.02213) [DOI] [PubMed] [Google Scholar]
- Gosgnach S., et al. 2006V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440, 215–219 (doi:10.1038/nature04545) [DOI] [PubMed] [Google Scholar]
- Goulding M.2009Circuits controlling vertebrate locomotion: moving in a new direction. Nat. Rev. Neurosci. 10, 507–518 (doi:10.1038/nrn2608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M., Pfaff S. L.2005Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr. Opin. Neurobiol. 15, 14–20 (doi:10.1016/j.conb.2005.01.017) [DOI] [PubMed] [Google Scholar]
- Graham-Brown T.1911The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. B 84, 308–319 (doi:10.1098/rspb.1911.0077) [Google Scholar]
- Grillner S.1981Control of locomotion in bipeds, tetrapods and fish. In Handbook of physiology, sect. 1. The nervous system II. Motor control. (ed. Brooks V. B.), pp. 1179–1236 Bethesda, MD: American Physiological Society, Waverly Press [Google Scholar]
- Grillner S.2003The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586 (doi:10.1038/nrn1137) [DOI] [PubMed] [Google Scholar]
- Grillner S., Rossignol S.1978On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 146, 269–277 (doi:10.1016/0006-8993(78)90973-3) [DOI] [PubMed] [Google Scholar]
- Grillner S., Zangger P.1975How detailed is the central pattern generation for locomotion? Brain Res. 88, 367–371 (doi:10.1016/0006-8993(75)90401-1) [DOI] [PubMed] [Google Scholar]
- Grillner S., Zangger P.1979On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 34, 241–261 (doi:10.1007/BF00235671) [DOI] [PubMed] [Google Scholar]
- Han P., Nakanishi S. T., Tran M. A., Whelan P. J.2007Dopaminergic modulation of spinal neuronal excitability. J. Neurosci. 27, 13 192–13 204 (doi:10.1523/JNEUROSCI.1279-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley C. A., Ziskind-Conhaim L.2006Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J. Neurosci. 26, 8477–8483 (doi:10.1523/JNEUROSCI.0395-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley C. A., Hartley R., Wu L., Todd A., Ziskind-Conhaim L. H.2005Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J. Neurophysiol. 93, 1439–1449 (doi:10.1152/jn.00647.2004) [DOI] [PubMed] [Google Scholar]
- Hochman S., Jordan L. M., Schmidt B. J.1994TTX-resistant NMDA receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J. Neurophysiol. 72, 2559–2562 [DOI] [PubMed] [Google Scholar]
- Homma R., et al. 2009Wide-field and two-photon imaging of brain activity with voltage- and calcium-sensitive dyes. Methods Mol. Biol. 489, 43–79 (doi:10.1007/978-1-59745-543-5_3) [DOI] [PubMed] [Google Scholar]
- Iizuka M., Kiehn O., Kudo N.1997Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Exp. Brain Res. 114, 193–204 (doi:10.1007/PL00005628) [DOI] [PubMed] [Google Scholar]
- Jankowska E.1992Interneuronal relay in spinal pathways from proprioceptors. Prog. Neurobiol. 38, 335–378 (doi:10.1016/0301-0082(92)90024-9) [DOI] [PubMed] [Google Scholar]
- Jankowska E.2001Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J. Physiol. 533, 31–40 (doi:10.1111/j.1469-7793.2001.0031b.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A.1967aThe effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol. Scand. 70, 369–388 (doi:10.1111/j.1748-1716.1967.tb03636.x) [DOI] [PubMed] [Google Scholar]
- Jankowska E., Jukes M. G., Lund S., Lundberg A.1967bThe effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol. Scand. 70, 389–402 (doi:10.1111/j.1748-1716.1967.tb03637.x) [DOI] [PubMed] [Google Scholar]
- Jankowska E., Edgley S. A., Krutki P., Hammar I.2005Functional differentiation and organization of feline midlumbar commissural interneurones. J. Physiol. 565, 645–658 (doi:10.1113/jphysiol.2005.083014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Sanes J. R.2000Development. The decade of the developing brain. Curr. Opin. Neurobiol. 10, 599–611 (doi:10.1016/S0959-4388(00)00136-7) [DOI] [PubMed] [Google Scholar]
- Jiang Z., Carlin K. P., Brownstone R. M.1999An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 816, 493–499 (doi:10.1016/S0006-8993(98)01199-8) [DOI] [PubMed] [Google Scholar]
- Jonas P., Bischofberger J., Sandkuhler J.1998Corelease of two fast neurotransmitters at a central synapse. Science 281, 419–424 (doi:10.1126/science.281.5375.419) [DOI] [PubMed] [Google Scholar]
- Kiehn O.2006Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (doi:10.1146/annurev.neuro.29.051605.112910) [DOI] [PubMed] [Google Scholar]
- Kiehn O., Butt S. J.2003Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog. Neurobiol. 70, 347–361 (doi:10.1016/S0301-0082(03)00091-1) [DOI] [PubMed] [Google Scholar]
- Kiehn O., Kullander K.2004Central pattern generators deciphered by molecular genetics. Neuron 41, 317–321 (doi:10.1016/S0896-6273(04)00042-X) [DOI] [PubMed] [Google Scholar]
- Kjaerulff O., Kiehn O.1996Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J. Neurosci. 16, 5777–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E., Lev-Tov A.1997Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J. Neurophysiol. 77, 1155–1170 [DOI] [PubMed] [Google Scholar]
- Kullander K., Butt S. J., Lebret J. M., Lundfald L., Restrepo C. E., Rydstrom A., Klein R., Kiehn O.2003Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299, 1889–1892 (doi:10.1126/science.1079641) [DOI] [PubMed] [Google Scholar]
- Kwan A. C., Dietz S. B., Webb W. W., Harris-Warrick R. M.2009Activity of Hb9 interneurons during fictive locomotion in mouse spinal cord. J. Neurosci. 29, 11 601–11 613 (doi:10.1523/JNEUROSCI.1612-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafreniere-Roula M., Mccrea D. A.2005Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J. Neurophysiol. 94, 1120–1132 (doi:10.1152/jn.00216.2005) [DOI] [PubMed] [Google Scholar]
- Lanuza G. M., Gosgnach S., Pierani A., Jessell T. M., Goulding M.2004Genetic identification of spinal interneurons that coordinate left–right locomotor activity necessary for walking movements. Neuron 42, 375–386 (doi:10.1016/S0896-6273(04)00249-1) [DOI] [PubMed] [Google Scholar]
- Lechner H. A., Lein E. S., Callaway E. M.2002A genetic method for selective and quickly reversible silencing of mammalian neurons. J. Neurosci. 22, 5287–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J., Weissman T. A., Kang H., Draft R. W., Lu J., Bennis R. A., Sanes J. R., Lichtman J. W.2007Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (doi:10.1038/nature06293) [DOI] [PubMed] [Google Scholar]
- Lundberg A.1979Multisensory control of spinal reflex pathways. Prog. Brain. Res. 50, 11–28 (doi:10.1016/S0079-6123(08)60803-1) [DOI] [PubMed] [Google Scholar]
- Lundfald L., Restrepo C. E., Butt S. J., Peng C. Y., Droho S., Endo T., Zeilhofer H. U., Sharma K., Kiehn O.2007Phenotype of V2-derived interneurons and their relationship to the axon guidance molecule EphA4 in the developing mouse spinal cord. Eur. J. Neurosci. 26, 2989–3002 (doi:10.1111/j.1460-9568.2007.05906.x) [DOI] [PubMed] [Google Scholar]
- Luo L., Callaway E. M., Svoboda K.2008Genetic dissection of neural circuits. Neuron 57, 634–660 (doi:10.1016/j.neuron.2008.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A., Fontanini A.2009Network homeostasis: a matter of coordination. Curr. Opin. Neurobiol. 19, 168–173 (doi:10.1016/j.conb.2009.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S., Nakanishi S. T., Takashima Y., Dhaka A., Patapoutian A., Mckemy D. D., Whelan P. J.2009Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience 162, 1377–1397 (doi:10.1016/j.neuroscience.2009.05.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E., Bucher D.2001Central pattern generators and the control of rhythmic movements. Curr. Biol. 11, R986–R996 (doi:10.1016/S0960-9822(01)00581-4) [DOI] [PubMed] [Google Scholar]
- McCrea D. A., Rybak I. A.2007Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog. Brain Res. 165, 235–253 (doi:10.1016/S0079-6123(06)65015-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea D. A., Rybak I. A.2008Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 57, 134–146 (doi:10.1016/j.brainresrev.2007.08.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean D. L., Fetcho J. R.2008Using imaging and genetics in zebrafish to study developing spinal circuits in vivo. Dev. Neurobiol. 68, 817–834 (doi:10.1002/dneu.20617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis G. Z., Alvarez F. J., Bonnot A., Richards D. S., Gonzalez-Forero D., Zerda R., O'Donovan M. J.2005Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc. Natl Acad. Sci. USA 102, 7344–7349 (doi:10.1073/pnas.0502788102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis G. Z., Siembab V. C., Zerda R., O'donovan M. J., Alvarez F. J.2006Primary afferent synapses on developing and adult Renshaw cells. J. Neurosci. 26, 13 297–13 310 (doi:10.1523/JNEUROSCI.2945-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles G. B., Yohn D. C., Wichterle H., Jessell T. M., Rafuse V. F., Brownstone R. M.2004Functional properties of motoneurons derived from mouse embryonic stem cells. J. Neurosci. 24, 7848–7858 (doi:10.1523/JNEUROSCI.1972-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T., Brohmann H., Pierani A., Heppenstall P. A., Lewin G. R., Jessell T. M., Birchmeier C.2002The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 34, 551–562 (doi:10.1016/S0896-6273(02)00689-X) [DOI] [PubMed] [Google Scholar]
- Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E.2003Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13 940–13 945 (doi:10.1073/pnas.1936192100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H., Restrepo C. E., Ryge J., Yanagawa Y., Kiehn O.2005Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc. Natl Acad. Sci. USA 102, 5245–5249 (doi:10.1073/pnas.0501331102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H., Restrepo C. E., Kiehn O.2006Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J. Neurosci. 26, 5320–5328 (doi:10.1523/JNEUROSCI.5127-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan M. J., Ho S., Sholomenko G., Yee W.1993Real-time imaging of neurons retrogradely and anterogradely labelled with calcium-sensitive dyes. J. Neurosci. Methods 46, 91–106 (doi:10.1016/0165-0270(93)90145-H) [DOI] [PubMed] [Google Scholar]
- O'Donovan M., Ho S., Yee W.1994Calcium imaging of rhythmic network activity in the developing spinal cord of the chick embryo. J. Neurosci. 14, 6354–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan M. J., Bonnot A., Mentis G. Z., Arai Y., Chub N., Shneider N. A., Wenner P.2008Imaging the spatiotemporal organization of neural activity in the developing spinal cord. Dev. Neurobiol. 68, 788–803 (doi:10.1002/dneu.20620) [DOI] [PubMed] [Google Scholar]
- Parker D.2006Complexities and uncertainties of neuronal network function. Phil. Trans. R. Soc. B 361, 81–99 (doi:10.1098/rstb.2005.1779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D.2010Neuronal network analyses: premises, promises, and uncertainties. Phil. Trans. R. Soc. B 365, 2315–2328 (doi:10.1098/rstb.2010.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. Y., Yajima H., Burns C. E., Zon L. I., Sisodia S. S., Pfaff S. L., Sharma K.2007Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron 53, 813–827 (doi:10.1016/j.neuron.2007.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon M.1980Induction of swimming in lamprey by l-DOPA and amino acids. J. Comp. Physiol. 136, 337–344 (doi:10.1007/BF00657354) [Google Scholar]
- Prinz A. A.2010Computational approaches to neuronal network analysis. Phil. Trans. R. Soc. B 365, 2397–2405 (doi:10.1098/2010.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz A. A., Bucher D., Marder E.2004Similar network activity from disparate circuit parameters. Nat. Neurosci. 7, 1345–1352 (doi:10.1038/nn1352) [DOI] [PubMed] [Google Scholar]
- Randell B., Lee P. A., Treleaven P. C.1978Reliability issues in computing system design. Comput. Surv. 10, 123–165 (doi:10.1145/356725.356729) [Google Scholar]
- Restrepo C. E., Lundfald L., Borgius L., Kiehn O.2009aNeurotransmitter phenotype of crossing neurons in the hopping EphA4 knock-out mice Chicago, IL: Society for Neuroscience [Google Scholar]
- Restrepo C. E., Lundfald L., Szabo G., Erdelyi F., Zeilhofer H. U., Glover J. C., Kiehn O.2009bTransmitter-phenotypes of commissural interneurons in the lumbar spinal cord of newborn mice. J. Comp. Neurol. 517, 177–192 (doi:10.1002/cne.22144) [DOI] [PubMed] [Google Scholar]
- Roberts A.2000Early functional organization of spinal neurons in developing lower vertebrates. Brain Res. Bull. 53, 585–593 (doi:10.1016/S0361-9230(00)00392-0) [DOI] [PubMed] [Google Scholar]
- Roberts A., Soffe S. R., Wolf E. S., Yoshida M., Zhao F. Y.1998Central circuits controlling locomotion in young frog tadpoles. Ann. N Y Acad. Sci. 860, 19–34 (doi:10.1111/j.1749-6632.1998.tb09036.x) [DOI] [PubMed] [Google Scholar]
- Roberts A., Li W. C., Soffe S. R., Wolf E.2008Origin of excitatory drive to a spinal locomotor network. Brain Res. Rev. 57, 22–28 (doi:10.1016/j.brainresrev.2007.06.015) [DOI] [PubMed] [Google Scholar]
- Rossignol S.1996Neural control of stereotypical limb movements. In Handbook of physiology, section 12. Exercise: regulation and integration of multiple systems (eds Rowell L. B., Shepard J. T.), pp. 173–216 Oxford, UK: American Physiological Society [Google Scholar]
- Rybak I. A., Shevtsova N. A., Lafreniere-Roula M., Mccrea D. A.2006aModelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J. Physiol. 577, 617–639 (doi:10.1113/jphysiol.2006.118703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak I. A., Stecina K., Shevtsova N. A., Mccrea D. A.2006bModelling spinal circuitry involved in locomotor pattern generation: insights from the effects of afferent stimulation. J. Physiol. 577, 641–658 (doi:10.1113/jphysiol.2006.118711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir T., Geiman E. J., Wang Z., Velasquez T., Mitsui S., Yoshihara Y., Frank E., Alvarez F. J., Goulding M.2004Pax6 and engrailed 1 regulate two distinct aspects of Renshaw cell development. J. Neurosci. 24, 1255–1264 (doi:10.1523/JNEUROSCI.3187-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston A. I.2005A neural infrastructure for rhythmic motor patterns. Cell. Mol. Neurobiol. 25, 223–244 (doi:10.1007/s10571-005-3154-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston A. I.2010Invertebrate central pattern generator circuits. Phil. Trans. R. Soc. B 365, 2329–2345 (doi:10.1098/rstb.2009.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Feldman J. L.1987In vitro brainstem–spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J. Neurosci. Methods 21, 321–333 (doi:10.1016/0165-0270(87)90126-9) [DOI] [PubMed] [Google Scholar]
- Smith J. C., Feldman J. L., Schmidt B. J.1988Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 2, 2283–2288 [DOI] [PubMed] [Google Scholar]
- Sohal V. S., Zhang F., Yizhar O., Deisseroth K.2009Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (doi:10.1038/nature07991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standish A., Enquist L. W., Escardo J. A., Schwaber J. S.1995Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J. Neurosci. 15, 1998–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P. S., Mccullough M. L., Currie S. N.1998Spinal motor patterns in the turtle. Ann. N. Y. Acad. Sci. 860, 142–154 (doi:10.1111/j.1749-6632.1998.tb09045.x) [DOI] [PubMed] [Google Scholar]
- Stokke M. F., Nissen U. V., Glover J. C., Kiehn O.2002Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J. Comp. Neurol. 446, 349–359 (doi:10.1002/cne.10211) [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Hultborn H.2008Thomas Graham-Brown (1882–1965), Anders Lundberg (1920–), and the neural control of stepping. Brain Res. Rev. 59, 74–95 (doi:10.1016/j.brainresrev.2008.06.001) [DOI] [PubMed] [Google Scholar]
- Suzue T.1984Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J. Physiol. 354, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak J., Rinzel J., O'donovan M. J.2001The role of activity-dependent network depression in the expression and self-regulation of spontaneous activity in the developing spinal cord. J. Neurosci. 21, 8966–8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazerart S., Viemari J. C., Darbon P., Vinay L., Brocard F.2007Contribution of persistent sodium current to locomotor pattern generation in neonatal rats. J. Neurophysiol 98, 613–628 [DOI] [PubMed] [Google Scholar]
- Whelan P. J.1996Control of locomotion in the decerebrate cat. Prog. Neurobiol. 49, 481–515 (doi:10.1016/0301-0082(96)00028-7) [DOI] [PubMed] [Google Scholar]
- Whelan P., Bonnot A., O'donovan M. J.2000Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J. Neurophysiol. 84, 2821–2833 [DOI] [PubMed] [Google Scholar]
- Wickersham I. R., Lyon D. C., Barnard R. J., Mori T., Finke S., Conzelmann K. K., Young J. A., Callaway E. M.2007Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (doi:10.1016/j.neuron.2007.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Wyman R. J.1965Motor output patterns during random and rhythmic stimulation of locust thoracic ganglia. Biophys. J. 5, 121–143 (doi:10.1016/S0006-3495(65)86706-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Hartley R., Maxwell D. J., Todd A. J., Lieberam I., Kaltschmidt J. A., Yoshida Y., Jessell T. M., Brownstone R. M.2005Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J. Neurosci. 25, 5710–5719 (doi:10.1523/JNEUROSCI.0274-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Dombeck D. A., Diaz-rios M., Harris-Warrick R. M., Brownstone R. M.2007Two-photon calcium imaging of network activity in XFP-expressing neurons in the mouse. J. Neurophysiol. 97, 3118–3125 (doi:10.1152/jn.01207.2006) [DOI] [PubMed] [Google Scholar]
- Wyart C., Del Bene F., Warp E., Scott E. K., Trauner D., Baier H., Isacoff E. Y.2009Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature 461, 407–410 (doi:10.1038/nature08323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R., Maclean J. N., Smith J., Lansner A.2005The cortex as a central pattern generator. Nat. Rev. Neurosci. 6, 477–483 (doi:10.1038/nrn1686) [DOI] [PubMed] [Google Scholar]
- Zhang F., Wang L. P., Boyden E. S., Deisseroth K.2006Channelrhodopsin-2 and optical control of excitable cells. Nat. Methods 3, 785–792 (doi:10.1038/nmeth936) [DOI] [PubMed] [Google Scholar]
- Zhang F., et al. 2007Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (doi:10.1038/nature05744) [DOI] [PubMed] [Google Scholar]
- Zhang Y., et al. 2008V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron 60, 84–96 (doi:10.1016/j.neuron.2008.09.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Cunha C., Zhang F., Liu Q., Gloss B., Deisseroth K., Augustine G. J., Feng G.2008Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 36, 141–154 (doi:10.1007/s11068-008-9034-7) [DOI] [PMC free article] [PubMed] [Google Scholar]