Abstract
While the mechanistic links between animal movement and population dynamics are ecologically obvious, it is much less clear when knowledge of animal movement is a prerequisite for understanding and predicting population dynamics. GPS and other technologies enable detailed tracking of animal location concurrently with acquisition of landscape data and information on individual physiology. These tools can be used to refine our understanding of the mechanistic links between behaviour and individual condition through ‘spatially informed’ movement models where time allocation to different behaviours affects individual survival and reproduction. For some species, socially informed models that address the movements and average fitness of differently sized groups and how they are affected by fission–fusion processes at relevant temporal scales are required. Furthermore, as most animals revisit some places and avoid others based on their previous experiences, we foresee the incorporation of long-term memory and intention in movement models. The way animals move has important consequences for the degree of mixing that we expect to find both within a population and between individuals of different species. The mixing rate dictates the level of detail required by models to capture the influence of heterogeneity and the dynamics of intra- and interspecific interaction.
Keywords: demography, redistribution kernels, perfect mixing, spatial ecology, dispersal, time budgets
1. Introduction
Many applied ecological questions in population and community ecology are posed in a spatial context. Can viable populations be retained in the areas that we decide to protect? Are populations separated by unsuitable habitat sufficiently well connected? Will restored habitat be colonized? Will reintroduced populations get established? Can populations track the environmental changes brought by global change? To answer these questions, we need to understand the links between movement and population dynamics. Movement responses of individuals to the changing spatial distributions of resources over landscapes will affect their individual performance and, in turn, population-level demography (Gaillard et al. 2010). Capturing these effects using conventional demographic models is difficult, because they are unlikely to anticipate responses to novel landscapes and new environmental conditions.
Recent developments in GPS tracking and remote sensing are increasing our ability to obtain accurate and precise information on individual movement trajectories and the landscape over which they roam (Tomkiewicz et al. 2010; Urbano et al. 2010). Furthermore, biotelemetry devices (Cooke et al. 2004; Wilson et al. 2007, 2008; Burger & Shaffer 2008) are allowing the simultaneous collection of important physiological and behavioural information from free-living animals. This has the potential to improve our understanding of how individual decisions affect population demography parameters and ultimately translate into population dynamics. In this sense, animal movement is the long-sought bridge between behaviour, landscape ecology and population dynamics (Lima & Zollner 1996; Wiens 1997).
Traditional models of population and community dynamics assume well-mixed populations comprising many individuals in which demographic parameters can be defined as functions of overall density (Turchin 2003). Thus, predators and prey will encounter each other in proportion to their average abundance over space; reproductive rates will decrease as global population density increases, and so on. Such ‘mean field’ assumptions can provide good approximations when physical environments are relatively homogeneous and organisms are highly mobile, or when they interact with others over large distances. However, when the external environment or the limited mobility of organisms results in lack of mixing, the conditions experienced by a particular member of a population or community can be quite different from the mean environment (e.g. Lloyd 1967). When local conditions affect per capita vital rates, the observed population and community dynamics can differ markedly from mean field prediction. Even though this is well understood theoretically, its significance is rarely recognized empirically, and it is unclear in what real-world situations ecologists need to account for it.
Spatial structure is now generally seen as an important prerequisite for more accurate ecological predictions (Durrett & Levin 1994; Kareiva & Wennergren 1995; Dieckmann et al. 2000; Hanski & Gaggiotti 2004). The spatial structure of populations can range from classical closed populations to a set of subpopulations with different degrees of interaction (Thomas & Kunin 1999). Poor mixing in populations can occur either through spatio-temporal distance between individuals or social grouping (Matthiopoulos et al. 2005b). The degree to which the fate of an individual affects and is affected by the environment, and by other individuals, clearly depends on its movements.
While many studies of animal movement are motivated by questions on population dynamics, an explicit connection between the two is rarely attained (but see Fryxell et al. 2005; Haydon et al. 2008; Revilla & Wiegand 2008). Here, we examine the link between movement and population dynamics at several scales ranging from small-scale behavioural decisions that affect survival and reproduction to self-organized spatial structures and dynamics that encompass several generations. Ultimately, population dynamics is about births, deaths, immigration and emigration; modern tracking technology together with new statistical models can greatly improve our understanding of these processes. We do not attempt a full review of the subject because the field is quickly growing and touches several disciplines from basic behavioural to sophisticated mathematical, computational and statistical models. Rather, our intention is to draw a thread through these scales and identify research areas that could benefit from increasing availability of movement data and analytical tools.
2. Spatial population models
Early spatial models simplified things greatly for the sake of tractability. One common approach has been to represent landscapes as patches of suitable habitat immersed in an inhospitable matrix which are linked by global dispersal. Such meta-population models (Hanski 1998; Hanski & Gaggiotti 2004) are often tractable analytically, and have yielded a number of important insights regarding effects of space on population demography, including the consequences of habitat loss (Lande 1988; Nee & May 1992; Tilman et al. 1994), and the role of migration in stabilizing and destabilizing population dynamics (e.g. Keeling 2002). An alternative approach is to formulate continuous space models that consider discrete individuals. While challenging mathematically, continuous space models are capable of representing both exogenously imposed and endogenously generated spatial heterogeneity at multiple spatial scales. Furthermore, they link more naturally to models of animal movement and to GPS telemetry data.
(a). Reaction–diffusion
Assuming that individuals move at random over a large and homogeneous area, dying and producing offspring according to rates that depend linearly on local population density, leads to classical reaction–diffusion models such as those used by Fisher (1937) to describe the spread of an advantageous mutation within a population. These models were also used effectively to describe the dynamics of population invasion and range expansion (Skellam 1951; Andow et al. 1990; Shigesada & Kawasaki 1997).
(b). Integro-difference equations
The diffusion equation is justified as a good approximation to the displacement of individuals performing a random walk (figure 1). Even though we know that animal movements are more purposeful than random walks, the diffusion approximation can still be sufficient at certain (usually large) scales and also serves as a yardstick for more complex models (Turchin 1998). Other forms of movement can be considered by formulating spatial population models as integral equations. These have commonly been formulated in discrete time, yielding integro-difference equations where a redistribution kernel (figure 1) that describes the probability that an individual will move from its current location to another one in a given time step is combined with local population growth. A great deal of theoretical and empirical work has explored the consequences of kernel shape, particularly in the tail of the distribution, on invasion speed (Kot et al. 1996; Powell & Zimmermann 2004). Note that the temporal scale of these models usually corresponds to reproductive events so that the redistribution kernel represents successful dispersal rather than regular movements.
Figure 1.
Redistribution kernels. (a) Three different individuals start moving at random from the centre of the plot; the trajectories look different, but they are governed by the same stochastic rules. Given a known starting point, the expected location of an individual at a given time, or a collection of individuals such as those simulated in (b), can be described by a redistribution kernel (c). For simple random walks as those shown here, the redistribution kernel is approximated by the solution of a diffusion equation. That is, a bivariate Gaussian with variance parameters that depend on the rate of movement and time.
(c). Moment closure and other approximations to spatial processes
Reaction–diffusion and integro-difference models are often derived from an underlying process of individual birth, death and movement, but their derivations neglect higher order interaction terms, implying substantial amounts of local scale averaging, and thus work well when there are large numbers of individuals per unit area. In situations when the discrete nature of individuals and the stochastic components of births, deaths and movement are relevant, other approximations are needed. Moment closure (Bolker & Pacala 1997; Law et al. 2003; Murrell et al. 2004) and pair approximations (Matsuda et al. 1992; Ellner 2001; Thomson & Ellner 2003) have been successfully used to analyse spatially realistic models more thoroughly than through simulation alone. These methods describe the probability distribution of a series of a statistical ensemble (i.e. a series of populations starting with the same initial conditions and following the same rules). The dynamics of the average population density (first spatial moment) depends on spatial covariances or pair densities (the second moment). The dynamics of the second spatial moment have terms involving the third moment (triplets) and so on. However, some form of ‘closure’ on higher order moments is performed in order to approximate the dynamics of the system. Different closures have been proposed by Murrell et al. (2004), but it is hard to know a priori how good the approximations are for a particular problem. Recently, Ovaskainen & Cornell (2006) developed a ‘perturbation expansion’ that consists of solving a first-order perturbation around the mean field limit. The advantage of this approach is that it is possible to control for the error in the approximation and that it is asymptotically exact as the mean field model is approached.
Pair approximations and moment closure have been quite useful in promoting our understanding of the interplay between scales of dispersal and competition and how they lead to different population trajectories through their effects on the spatial distribution of individuals (Bolker & Pacala 1997; Law et al. 2003), in clarifying the effect of space in species coexistence (Murrell & Law 2003), and the effects of movement on predator–prey dynamics (Murrell 2005). They have also been used to show how spatial variation in habitat quality (exogenous heterogeneity) can interact with variability generated from dispersal and competition (endogenous heterogeneity), an interaction that might resolve conflicting results from simulation studies of the effects of fragmentation on population viability (Bolker 2003; North & Ovaskainen 2007).
There are many ways to make spatial models more realistic and appropriate for particular species, places and scales of interest. Researchers are increasingly paying attention to the connectivity between subpopulations and to how this results from the interaction between individual phenotype, behaviour and the structure of the landscape. One particular feature of all the above models is that every individual is assumed to move according to the same kernel. However, detailed tracking of individual movements consistently reveals differences among individuals. Below, we discuss possible causes and consequences of different redistribution kernels.
3. Patterns of space use
(a). Redistribution kernel shape and scale
Theoretical and empirical studies have shown how the characteristics of redistribution kernels can depend on differences between individuals (Skalski & Gilliam 2000; Fraser et al. 2001; Morales & Ellner 2002; Delgado & Penteriani 2008), and on the interaction between behaviour and properties of the underlying landscape (Johnson et al. 1992; McIntyre & Wiens 1999; Fahrig 2001; Morales et al. 2004; Mueller & Fagan 2008), including reactions to habitat boundaries (Schultz & Crone 2001; Morales 2002; Schtickzelle & Baguette 2003; Ovaskainen 2004; Haynes & Cronin 2006). In particular, population heterogeneity produces leptokurtic redistribution kernels when a subset of the individuals consistently moves longer distances than others (Skalski & Gilliam 2000; Fraser et al. 2001).
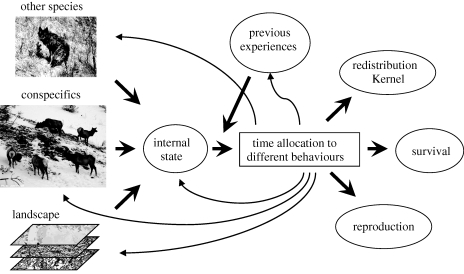
Several factors can explain why two individuals of the same species move differently. They may be experiencing different environments, have different phenotypes (size, condition), different past experiences (e.g. Frair et al. 2007) or even different ‘personalities’ (Fraser et al. 2001; Dall et al. 2004). In a theoretical study, Skalski & Gilliam (2003) modelled fish switching between fast and slow displacements and found that the resulting redistribution kernel depended on the total time spent in each of the movement modes and not on the particular sequence of changes. This result underscores the importance of animals' time budgets in scaling movement processes (figure 2). Individuals might have a small set of movement strategies (Blackwell 1997; Nathan et al. 2008), and the time allocation to these different behaviours might depend on the interaction between their motivations and the landscape where they live (Morales et al. 2004, 2005). The results of Skalski & Gilliam (2003) imply that it might be possible to derive appropriate redistribution kernels if we know the fraction of total time allocated to each behaviour.
Figure 2.
Sketch for developing mechanistic links between animal movement and population dynamics. We consider a catch-all, and usually unobserved, individual internal state that integrates body condition (reserves, reproductive status, etc.). Several factors affect the dynamics of this internal state, including social interactions with conspecifics, trophic or other interaction with other species and surrounding landscape attributes. Internal state dynamics determines the organism's time allocation to different behaviours such as food acquisition, predator avoidance, homing, landscape exploration and so on, but this is also modulated by previous experiences and phenotypic traits such as behavioural predispositions. As these different behaviours imply different movement strategies, the time budget determines the properties of the redistribution kernel that describes space use. Time allocation to different behaviours will also affect individual survival and reproduction and thus overall population dynamics.
Several techniques are being developed to identify and model changes in movement behaviour from trajectory data (reviewed in Patterson et al. 2008; Schick et al. 2008). But this is not an easy task even with detailed GPS tracking data, as several combinations of movement behaviours can lead to very similar trajectories. However, as physiological and other information becomes available through biotelemetry devices, we may gain greater insight into how animals allocate time to different tasks and how this allocation changes in different environments, thus providing a mechanistic way to model redistribution kernels conditional on individual state (figure 2).
Another result from Skalski & Gilliam (2003) is that the mixture of movement processes viewed over a sufficiently long time period can be described as a simple diffusion. The central limit theorem states that the sum of n independent and identically distributed random variables with finite variance will approach a Gaussian distribution as n increases. Thus, if all individuals in a population move according to the same stochastic process, we would expect that at some time after the initiation of movement, the distribution of distance moved becomes Gaussian because the distance travelled is the sum of movement vectors. However, how useful this result is depends on the rate of convergence. This is an important question and is likely to depend in interesting ways on the interaction between individual behaviour and landscape structure (Morales 2002).
(b). Home ranges, territories and groups
Many animals have clearly defined home ranges or territories (Borger et al. 2008) or at least some form of site fidelity and revisitation patterns that are not captured by simple random walks. These animals are likely to spend their reproductive life in a region that is small compared with their movement capabilities. Much progress has been made in developing mechanistic models of animal movement with territorial behaviour (Moorcroft et al. 1999, 2006; Smouse et al. 2010). However, these models typically describe the spatial distribution of space use by particular individuals (or members of a wolf pack for example) rather than an entire population. As a result, they have not yet been linked to models of population demography.
For territorial animals, competition for space can determine the carrying capacity of a landscape. When the environment provides a limited number of essential items such as nest cavities, the maximum number of breeders will be determined from them and surplus individuals would form a population of non-breeders often called floaters (Brown 1969; Penteriani & Delgado 2009). These floaters may become a crucial population reserve for filling empty territories when breeding dispersal or breeder mortality open up previously occupied territories, but floaters can also decrease population growth by interference, conflict or disturbance, and the aggressive behaviour of breeders can also decrease the carrying capacity of the population. Changes in territorial behaviour may have profound implications for population dynamics. For example, Mougeot et al. (2003) demonstrated experimentally the lasting effect of social influences in territorial requirements in red grouse. In particular, they showed that manipulations of testosterone levels in territorial males, whose physiological effect lasted for a few weeks, affected aggressiveness and territorial requirements of both manipulated and un-manipulated individuals for more than 1 year. Such variations in testosterone levels are known to occur naturally as a result of crowding and can be modulated by factors both intrinsic (e.g. kinship between territorial neighbours) and extrinsic (e.g. parasite loads) to the population. Subsequent models by Matthiopoulos et al. (2003, 2005a) established how these documented feedbacks between territorial aggressiveness and population density can explain the observed cyclic dynamics in red grouse.
For many species, space is a more or less a continuous resource and increasing the number of competitors decreases the area that each individual occupies (Adams 2001). Typically, space becomes divided as floaters establish new territories in the boundary zones of established breeders. This can also regulate the population since diminishing territory size must eventually lead to poorer reproduction (Adams 2001). Depending on food availability and the presence of other individuals, the location, size and shape of home ranges can change even on short time scales. In a simulation model, Wang & Grimm (2007) studied the daily dynamics of home ranges in the common shrew (Sorex araneus). In the model, individuals were constantly adapting their home ranges in order to obtain sufficient food resources, and they dispersed when enough resources were not found. These simple rules were able to mimic observed patterns of shrew habitat use, and also showed that home-range size and dispersal are density dependent and therefore likely to have a strong effect on population regulation.
In a theoretical study, Lopez-Sepulcre & Kokko (2005) developed a model where the carrying capacity of a landscape results from the evolution of territorial defence effort and the consequent space use. They found that depending on the balance between fecundity and defence cost, different modes of population regulation in territorial species can be obtained. If fecundity is high or defence is relatively costly, breeding success saturates quickly with territory size and populations would be more likely to be regulated by floaters. On the other hand, low fecundity and a slowly saturating relationship between territory size and reproductive output, together with relatively cheap territory defence is likely to favour regulation by breeders, where territories expand and shrink as populations decline or increase. In this case, floaters are rare since they can always become breeders by squeezing in a territory between existing boundaries. Tracking of floaters together with behavioural observations and territory mapping can lead to quantification of these processes in natural populations but, as far as we are aware, this has not been done to date.
(c). Group movement and dynamics
For social animals, understanding the distribution of individuals over landscapes requires scaling-up from individual movement patterns to groups of individuals, and populations of groups (Okubo et al. 2001). Most models of group dynamics focus on relatively short temporal scales (Couzin et al. 2005; Eftimie et al. 2007). However, the interaction between the group structure of a population and the movement of individuals are also relevant at longer time scales (e.g. Fryxell et al. 2007). This would be particularly relevant for reintroduced species, where a balance of spread and coalescence processes will determine the broad-scale distribution of individuals over the landscape. If survival and fecundity are higher in groups, then population persistence may depend on coalescence ultimately dominating and curtailing the spreading process, thereby enabling the establishment of a natural group structure within the designated release area. Haydon et al. (2008) have produced movement models for North American elk (Cervus canadensis), reintroduced to Ontario, emphasizing the social factors that affect the switch between exploratory (large, daily displacements and small turning angles) and encamped behaviour (small daily displacements and frequent reversals in direction). These movement models were then combined with mortality and fecundity analyses to build a spatially explicit, individual-based model for the dynamics of this reintroduced population. Their analysis showed that elk moved further when they were solitary than when they were grouped, and that their mortality rate increased as they moved progressively away from the release area. The simulation model showed how the spatial distribution of individuals and the population rate of increase depended on the balance of fission and fusion processes governing group structure.
4. Decision making and information use
All the above models assume some simple set of possible behaviours such as changes in movement rate in different habitats or in different social context. However, most animals are capable of more sophisticated spatial behaviour that also depends on their condition (nutritional, reproductive state), phenotype (sex, endurance) and experience (memory). Furthermore, we still know little about how animals decide to leave their territory or abandon a group, and how they explore and choose where to establish new territories or home ranges. In the following sections, we discuss how tracking technology can advance our understanding of some of these processes.
(a). Informed dispersal and prospecting
Dispersal involves the attempt to move from a natal or breeding site to another breeding site (Clobert 2000), and is essential for species to persist in changing environments (Ronce 2007). The redistribution models discussed so far represent dispersal as a random process that may be sensitive to the structure of the landscape or the presence of conspecifics. However, there is a great deal of evidence indicating that individuals are capable of sophisticated and informed decision making when choosing a new place to live (Bowler & Benton 2005; Stamps et al. 2005; Stamps 2006; Mabry & Stamps 2008; Clobert et al. 2009). Clobert et al. (2009) recently proposed the concept of ‘informed dispersal’ to convey the idea that individuals gather and exchange information at all three stages of dispersal (departure, transience and settlement). Interestingly, this idea implies that movement involves not only exchange of individuals among habitat patches but also information transfer across the landscape. There are many ways in which animals can acquire information about their environment by ‘looking’ at others' morphology, behaviour or reproductive success (Danchin et al. 2004; Dall et al. 2005). For example, Cote & Clobert (2007) manipulated experimental enclosures of the common lizard (Lacerta vivipara) and quantified emigration rate as a function of whether immigrants to a local population were reared under high or low population densities. They found that the origin of immigrants affected emigration of residents in local populations, providing evidence that immigrants supplied information about surrounding population densities, probably via their phenotype.
Our understanding of how individuals integrate different sources of information in order to make dispersal decisions is rudimentary. Detailed tracking of juvenile movements could shed light on the processes of exploration (transience) and settlement. In particular, movement data can be used to test ideas about search strategies, landscape exploration and the importance of past experience in biasing where animals decide to attempt breeding or remain as floaters. Furthermore, long-term tracking would be needed to study how animals adjust the characteristics of their home ranges/territories and under what conditions they are likely to search for a new home.
(b). Memory
The importance of memory and previous experiences is starting to be explicitly considered in the analysis of movement data (Dalziel et al. 2008; Wolf et al. 2009), and in simulation models of foraging and habitat use (Barraquand et al. 2009; Van Moorter et al. 2009). Smouse et al. (2010) provide a summary of the approaches used to include memory in movement models. This has been largely a theoretical exercise, but the connection with data can surely be accomplished. For example, the approach used to model the effect of scent marking in mechanistic home-range models (Moorcroft & Lewis 2006) could be easily adapted to model memory processes. Less clear is what role memory would play in population dynamics.
5. Individual condition
The fact that the contribution of an individual to the population will be a function of its fitness has historically promoted the development of physiological, age and stage-structured population models (Metz & Diekmann 1986; Caswell 1989; Ellner & Rees 2006). Body condition integrates nutritional intake and demands, affecting both survival and reproduction. For example, recent work on ungulates living in seasonal environments suggests that per cent body fat in early winter is most important in determining whether animals die, live without reproducing or live and reproduce (Coulson et al. 2001; Parker et al. 2009). Also, it is clear that many populations experience ‘carry-over effects’ in which conditions experienced during a period influence vital rates in following periods. Carry-over effects have been identified recently as a form of sequential density dependence that can potentially generate many different population responses (Ratikainen et al. 2008).
(a). Energy balance
Resource uptake and the use of ingested resources for growth and reproduction is at the heart of many aspects of life-history evolution, behavioural ecology and population dynamics. Food acquisition is an important driver of animal movements and some generalization has been made about the scaling of space use and daily distance travelled in relation to body mass and trophic requirements (Jetz et al. 2004; Carbone et al. 2005; Owen-Smith et al. 2010). Developments in biotelemetry (Cooke et al. 2004; Rutz & Hays 2009) promise the possibility of tracking not only animal locations but also a number of relevant physiological data such as heart rate, core temperature, etc. Furthermore, accelerometers can be used for recording energy expenditure, activity budgets (ethograms) and/or rare behavioural events such as prey captures (Wilson et al. 2007, 2008). Combined with detailed environmental maps, these data could lead to empirically based models of animal performance in the wild, linking behavioural decisions with space use, survival and reproduction (figure 2).
(b). Survival and reproduction
Survival analysis can be used to model changes in hazard with time and in relation to covariates such as location, age, body condition, habitat type, etc. Detailed tracking through GPS enables spatial information and survival data to be combined at small temporal scales, leading to an increasingly sophisticated understanding of the determinants of survival (Murray 2006; Haydon et al. 2008). Likewise, changes in movement behaviour can be used to infer reproductive events in some species (Long et al. 2009). However, to take full advantage of these data, new analytic techniques should take into account the sequential nature of individual survival and reproduction. For example, the chance of an animal dying of starvation depends on its history of food encounter and foraging decisions.
(c). Movement and food provision
Animals have to invest more time and energy in food acquisition in poorer habitats (or in good habitats where high population density leads to food shortage), and this is reflected in their movement patterns (e.g. Powell 1994). The effects of increased movement is best documented in central-place foragers such as nesting birds or pinnipeds that forage at sea but breed on land. Many of these animals forage at particular oceanographic features (Boersma & Rebstock 2009) that change in location and quality from year to year. Magellanic penguins (Spheniscus magellanicus) breeding at Punta Tombo, Argentina, showed a decrease in reproductive success with increasing average foraging trip duration (Boersma & Rebstock 2009). Also, penguins stayed longer times at feeding sites in more distant than closer foraging areas, presumably to feed themselves and recover from the increased cost of swimming (Boersma & Rebstock 2009). Thus, the spatial distribution of food at sea influences offspring feeding frequency (Pinaud et al. 2005), reproductive success (Inchausti et al. 2003) and adult energy balance (Shaffer et al. 2003). In the case of seabirds sharing incubation and provisioning of chicks, long trip distances and durations may also affect the fasting mate's condition, eventually causing abandonment of the nest (Yorio & Boersma 1994; Numata et al. 2000; Tveraa & Christensen 2002). In this way, GPS technology has allowed a better understanding of the interplay between landscape or seascape variability and breeding success.
6. Encounter rates
A key component of population models that include trophic interactions is the functional response, which describes the rate of prey consumption by individual predators as a function of prey density (Holling 1959). The shape and dimensionality of this function is crucial in determining the dynamics and persistence of interacting populations (Turchin 2003). The functional response depends of course on encounter rates. A useful null model for encounter rates is one where individuals move randomly and independently of each other. Maxwell (1860) calculated the expected rates of molecular collisions of an ideal gas as a function of density, particle size and speed (assuming independent movements in any direction and with normally distributed velocities). This model has been used and rediscovered in many ways, including Lotka's justification of predator–prey encounters being proportional to predator speed and size and to predator and prey densities.
Mosimann (1958) used the ideal gas model to estimate the probability of a female encountering no males during the breeding season in low-density populations, quantifying the suggestion of Allee (1931) that populations below some minimum density would decline owing to the difficulty of finding mates. For a more recent example, the scaling of home ranges with body size derived by Jetz et al. (2004) assume that the proportion of resources lost to neighbours is related to encounter rate that was calculated assuming the ideal gas model given known scaling relationships of speed, population density and detection distance.
The movements of animals almost certainly deviate from the assumptions of Maxwell's model, and it would be interesting to use information about the characteristics of the movement paths of real animals in order to derive better predictions of encounter rates, or in the case of carnivores, kill rates (Merrill et al. 2010). The thorough review by Hutchinson & Waser (2007) shows many more examples of the application of Maxwell's model plus several refinements, including different assumptions about detection, speed and density.
Environmental heterogeneity can also be an important determinant in encounter rates and group dynamics. For example, Flierl et al. (1999) used individual-based models of fish groups to study the interplay between the forces acting on the individuals and the transport induced by water motion and found that flows often enhanced grouping by increasing the encounter rate among groups and thereby promoting merger into larger groups (although the effect breaks down for strong flows). We expect that habitat structure in general will affect encounter rates between individuals of the same species but also between predators and prey and so on.
Encounter rates and population dynamics are also altered when predators and/or prey form social groups. Fryxell et al. (2007) developed simple models of group-dependent functional responses and applied them to the Serengeti ecosystem. They found that grouping strongly stabilizes interactions between lions and wildebeest, suggesting that social groups rather than individuals were the basic building blocks for these predator–prey interactions.
As GPS tracking devices come down in cost, and larger numbers of individuals can be tracked in the same study areas, we can expect to learn more about these forms of interactions. Furthermore, combinations with other technologies can make this more feasible. For example Prange et al. (2006) used proximity detectors in collars fitted to free-living racoons and were able to obtain accurate information in terms of detection range, duration of contact and contacted collar identification. Animal-borne video systems also may help identify social interactions and foraging events for a focal individual (Hooker et al. 2008; Moll et al. 2009). Hence, the study of encounters offers significant opportunities for marrying theory with data and to greatly improve our understanding of spatial dynamics.
7. Large-scale dynamics
(a). Invasion and range expansion
Important questions at large spatial scales are how fast an invading species can spread (Kot et al. 1996; Clark et al. 2003; Powell & Zimmermann 2004; Phillips et al. 2008), and whether species would be able to track favourable environments under climate change scenarios (Parmesan 2006; Petit et al. 2008; Morin & Thuiller 2009). Also, because many species have been reduced to small, fragmented populations and eliminated from much of their historical range, their recovery depends not only on increased population size but also on recolonization of the species' former range (Tinker et al. 2008).
Biological invasions in natural environments have received attention for decades (e.g. Elton 1958), and their economic and conservation implications are increasingly being recognized (Clavero & Garcia-Berthou 2005). Thus, it is important to disentangle the processes underlying invasion success and rates of spread (Hastings et al. 2005). Theoretical work has highlighted that long-distance dispersal events, even when rare, can have a large influence on the rate of range expansion (Mollison 1977; Kot et al. 1996). Meanwhile, empirical work is producing detailed accounts of the role of spatial heterogeneity, temporal variability, heterospecifics and evolution. The study of range expansion of invasive species provides numerous opportunities to confront theory with data and to discern which are the important underlying processes that control spread rates.
There is increasing awareness (and evidence) that evolution can act at time scales usually considered relevant to population dynamics (Hairston et al. 2005; Pelletier et al. 2009). In the case of invading populations, we expect that individuals with better dispersal abilities will increasingly dominate the expanding front and that selection would favour traits that increase dispersal. For example, cane toads (Bufo marinus) invading tropical Australia showed a fivefold increase in their rate of spread in about 50 generations. Alford et al. (2009) studied radio-tracking data from cane toads, spanning 15 years, and found dramatic shifts in behavioural traits associated with the rapid acceleration of toad invasion.
Contrary to these observations, some authors have argued that the spread of invading species might be regulated, with the change in invasion speed negatively related to current speed (Arim et al. 2006). However, De Valpine et al. (2008) and Starrfelt & Kokko (2008) show theoretically how apparent regulation can be a spurious effect of data collection, highlighting the importance of the scale of observation. This calls for more sophisticated analyses of the factors, such as Allee effects (Johnson et al. 2006; Tobin et al. 2007), that might regulate invasion speed. At low densities, dispersers might struggle to find mates and establish new breeding units. Recent model developments have attempted to include this effect in spatial population dynamics (Hurford et al. 2006; Tinker et al. 2008; Jerde et al. 2009), but they still make some of the assumptions of the perfect mixing model. We expect animals to be more efficient at finding each other than are randomly moving particles, but it might be a conservative feature of invasion models.
(b). Migration
Conserving migratory species requires mapping migratory routes (Thirgood et al. 2004; Wilcove & Wikelski 2008; Sawyer et al. 2009; Strandberg et al. 2009), and understanding the role of individual and environmental drivers of migration patterns (Alerstam 2006; Bolger et al. 2008). The combination of remotely sensed resource availability with GPS movement data has been very useful in this regard (Leimgruber et al. 2001; Boone et al. 2006; Hebblewhite & Merrill 2009; Holdo et al. 2009; Hebblewhite & Haydon 2010).
8. Conclusion
The way animals move has important consequences for the degree of mixing that we expect to find in a population. This dictates how much detail must be included when building dynamical population models. How much of the small-scale, individual-level detail can be ignored without impoverishing the accuracy of population-level predictions? How do local dynamics translate into large-scale patterns? Conversely, how do larger (and slower) features of the environment constrain local processes? These questions call for models and data for developing our understanding of how individual behaviours are linked to the spatial and temporal dynamics of populations and communities.
There are good reasons to believe that the study of animal movements is going through a renaissance as GPS and other technologies are enabling detailed tracking of animal location concurrently with acquisition of landscape data and information on individual physiology (Cagnacci et al. 2010). Global databases such as Movebank are facilitating the exchange of data and methodology. Furthermore, the statistical machinery needed to make full use of these data is slowly but steadily catching up (Patterson et al. 2008; Schick et al. 2008). However, many of the processes we discuss in this article involve the interaction of a focal individual with conspecifics, heterospecifics and changing environmental conditions (figure 2). Furthermore, our capacity to map and represent relevant landscape features for particular species could be limited (Hebblewhite & Haydon 2010). These types of studies will be challenging and expensive with existing technologies, but should become easier in the future.
The availability of better tracking and bio-logging technologies is challenging researchers' ability to make sense of the resulting data, but also creating exiting opportunities to test and develop theory (Cagnacci et al. 2010). Here, we have highlighted some areas where these data could improve our understanding of population dynamics in changing landscapes. We anticipate a future in which computer-intensive, statistically robust approaches are developed to draw population-level inferences from sampled individuals. In the future, we can expect to see much progress in the understanding of the drivers of movement and on the relationship between movement decisions and fitness, ultimately providing the link between behaviour, landscape ecology and population dynamics.
Acknowledgements
JMM was funded by CONICET and PIP 114-200801-00276. M. M. Delgado and R. R. Dunn provided useful comments on earlier versions of the manuscript. This paper originated from stimulating discussions at the workshop ‘GPS-Telemetry data: challenges and opportunities for behavioural ecology studies’, organized by the Edmund Mach Foundation (FEM) in September 2008 and held in Viote del Monte Bondone, Trento, Italy. Funding of the workshop by the Autonomous Province of Trento is gratefully acknowledged.
Footnotes
One contribution of 15 to a Theme Issue ‘Challenges and opportunities of using GPS-based location data in animal ecology’.
References
- Adams E. S.2001Approaches to the study of territory size and shape. Ann. Rev. Ecol. Syst. 32, 277–303 (doi:10.1146/annurev.ecolsys.32.081501.114034) [Google Scholar]
- Alerstam T.2006Conflicting evidence about long-distance animal navigation. Science 313, 791–794 (doi:10.1126/science.1129048) [DOI] [PubMed] [Google Scholar]
- Alford R. A., Brown G. P., Schwarzkopf L., Phillips B. L., Shine R.2009Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildl. Res. 36, 23–28 (doi:10.1071/wr08021) [Google Scholar]
- Allee W. C.1931Animal aggregations: a study in general sociology. Chicago, IL: Chicago University Press [Google Scholar]
- Andow D. A., Kareiva P. M., Levin S. A., Okubo A.1990Spread of invading organisms. Land Ecol. 4, 177–188 (doi:10.1007/BF00132860) [Google Scholar]
- Arim M., Kareiva P. M., Levin S. M., Okubo A.2006Spread dynamics of invasive species. Proc. Natl Acad. Sci. USA 103, 374–378 (doi:10.1073/pnas.0504272102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraquand F., Inchausti P., Bretagnolle V.2009Cognitive abilities of a central place forager interact with prey spatial aggregation in their effect on intake rate. Anim. Behav. 78, 505–514 (doi:10.1016/j.anbehav.2009.06.008) [Google Scholar]
- Blackwell P. G.1997Random diffusion models for animal movement. Ecol. Model. 100, 87–102 (doi:10.1016/S0304-3800(97)00153-1) [Google Scholar]
- Boersma P. D., Rebstock G. A.2009Foraging distance affects reproductive success in magellanic penguins. Mar. Ecol. Progr. Ser. 375, 263–275 (doi:10.3354/meps07753) [Google Scholar]
- Bolger D. T., et al. 2008The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77 [DOI] [PubMed] [Google Scholar]
- Bolker B. M.2003Combining endogenous and exogenous spatial variability in analytical population models. Theor. Popul. Biol. 64, 255–270 (doi:10.1016/s0040-5809(03)00090-x) [DOI] [PubMed] [Google Scholar]
- Bolker B., Pacala S. W.1997Using moment equations to understand stochastically driven spatial pattern formation in ecological systems. Theor. Popul. Biol. 52, 179–197 (doi:10.1006/tpbi.1997.1331) [DOI] [PubMed] [Google Scholar]
- Boone R. B., Thirgood S. J., Hopcraft J. G. C.2006Serengeti wildebeest migratory patterns modeled from rainfall and new vegetation growth. Ecology 87, 1987–1994 (doi:10.1890/0012-9658(2006)87[1987:SWMPMF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Borger L., Dalziel B. D., Fryxell J. M.2008Are there general mechanisms of animal home range behaviour? A review and prospects for future research. Ecol. Lett. 11, 637–650 (doi:10.1111/j.1461-0248.2008.01182.x) [DOI] [PubMed] [Google Scholar]
- Bowler D. E., Benton T. G.2005Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. (Camb.) 80, 205–225 (doi:10.1017/s1464793104006645) [DOI] [PubMed] [Google Scholar]
- Brown J. L.1969Territorial behavior and population regulation in birds. Wilson Bull. 81, 293–329 [Google Scholar]
- Burger A. E., Shaffer S. A.2008Application of tracking and data-logging technology in research and conservation of seabirds. Auk 125, 253–264 (doi:10.1525/auk.2008.1408) [Google Scholar]
- Cagnacci F., Boitani L., Powell R. A., Boyce M. S.2010Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Phil. Trans. R. Soc. B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone C., Cowlishaw G., Isaac N. J. B., Rowcliffe J. M.2005How far do animals go? Determinants of day range in mammals. Am. Nat. 165, 290–297 (doi:10.1086/426790) [DOI] [PubMed] [Google Scholar]
- Caswell H.1989Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates [Google Scholar]
- Clark J. S., Lewis M., McLachlan J. S., HilleRisLambers J.2003Estimating population spread: what can we forecast and how well? Ecology 84, 1979–1988 (doi:10.1890/01-0618) [Google Scholar]
- Clavero M., Garcia-Berthou E.2005Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 20, 110–110 (doi:10.1016/j.tree.2005.01.003) [DOI] [PubMed] [Google Scholar]
- Clobert J.2000Dispersal New York, NY: Oxford University Press [Google Scholar]
- Clobert J., Le Galliard J.-F., Cote J., Meylan S., Massot M.2009Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209 (doi:10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- Cooke S. J., Hinch S. G., Wikelski M., Andrews R. D., Kuchel L. J., Wolcott T. G., Butler P. J.2004Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334–343 (doi:10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- Cote J., Clobert J.2007Social information and emigration: lessons from immigrants. Ecol. Lett. 10, 411–417 (doi:10.1111/j.1461.0248.2007.01032.x) [DOI] [PubMed] [Google Scholar]
- Coulson T., Catchpole E. A., Albon S. D., Morgan B. J. T., Pemberton J. M., Clutton-Brock T. H., Crawley M. J., Grenfell B. T.2001Age, sex, density, winter weather, and population crashes in Soay sheep. Science 292, 1528–1531 (doi:10.1126/science.292.5521.1528) [DOI] [PubMed] [Google Scholar]
- Couzin I. D., Couzin I. D., Krause J., Franks N. R., Levin S. A.2005Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (doi:10.1038/nature03236) [DOI] [PubMed] [Google Scholar]
- Dall S. R. X., Houston A. I., McNamara J. M.2004The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol. Lett. 7, 734–739 (doi:10.1111/j.1461-0248.2004.00618.x) [Google Scholar]
- Dall S. R. X., Giraldeaub L.-A., Olssonc O., McNamara J. M., Stephens D. W.2005Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- Dalziel B. D., Morales J. M., Fryxell J. M.2008Fitting probability distributions to animal movement trajectories: using artificial neural networks to link distance, resources, and memory. Am. Nat. 172, 248–258 (doi:10.1086/589448) [DOI] [PubMed] [Google Scholar]
- Danchin E., Giraldeau L.-A., Valone T. J., Wagner R. H.2004Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- Delgado M. D., Penteriani V.2008Behavioral states help translate dispersal movements into spatial distribution patterns of floaters. Am. Nat. 172, 475–485 (doi:10.1086/590964) [DOI] [PubMed] [Google Scholar]
- De Valpine P., Cuddington K., Hoopes M. F., Lockwood J. L.2008Is spread of invasive species regulated? Using ecological theory to interpret statistical analysis. Ecology 89, 2377–2383 (doi:10.1890/07-0090.1) [DOI] [PubMed] [Google Scholar]
- Dieckmann U., Law R., Metz J. A. J.2000The geometry of ecological interactions: simplifying spatial complexity, Cambridge Studies in Adaptive Dynamics Cambridge, UK: IIASA/Cambridge University Press [Google Scholar]
- Durrett R., Levin S.1994The importance of being discrete (and spatial). Theor. Popul. Biol. 46, 363–394 (doi:10.1006/tpbi.1994.1032) [Google Scholar]
- Eftimie R., de Vries G., Lewis M. A., Lutscher F.2007Modeling group formation and activity patterns in self-organizing collectives of individuals. Bull. Math. Biol. 69, 1537–1565 (doi:10.1007/s11538-006-9175-8) [DOI] [PubMed] [Google Scholar]
- Ellner S. P.2001Pair approximation for lattice models with multiple interaction scales. J. Theor. Biol. 210, 435–447 (doi:10.1006/jtbi.2001.2322) [DOI] [PubMed] [Google Scholar]
- Ellner S. P., Rees M.2006Integral projection models for species with complex demography. Am. Nat. 167, 410–428 (doi:10.1086/499438) [DOI] [PubMed] [Google Scholar]
- Elton C. S.1958The ecology of invasions by animals and plants. London, UK: Methuen [Google Scholar]
- Fahrig L.2001How much habitat is enough? Biol. Conserv. 100, 65–74 (doi:10.1016/S0006-3207(00)00208-1) [Google Scholar]
- Fisher R. A.1937The wave of advance of advantageous genes. Ann. Eugenics 7, 255–369 [Google Scholar]
- Flierl G., Grünbaumb D., Levinsc S., Olson D.1999From individuals to aggregations: the interplay between behavior and physics. J. Theor. Biol. 196, 397–454 (doi:10.1006/jtbi.1998.0842) [DOI] [PubMed] [Google Scholar]
- Frair J. L., Merrill E. H., Allen J. R., Boyce M. S.2007Know thy enemy: experience affects elk translocation success in risky landscapes. J. Wildl. Manage. 71, 541–554 (doi:10.2193/2006-141) [Google Scholar]
- Fraser D. E., Gilliam J. F., Skalski G. T.2001Explaining leptokurtic movement distributions: intrapopulation variation in boldness and exploration. Am. Nat. 158, 124–135 (doi:10.1086/321307) [DOI] [PubMed] [Google Scholar]
- Fryxell J. M., Wilmshurst J. F., Sinclair A. R. E., Haydon D. T., Holt R. D., Abrams P. A.2005Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecol. Lett. 8, 328–335 (doi:10.1111/j.1461-0248.2005.00727.x) [Google Scholar]
- Fryxell J. M., Mosser A., Sinclair A. R. E., Packer C.2007Group formation stabilizes predator–prey dynamics. Nature 449, 1041–1044 (doi:10.1038/nature06177) [DOI] [PubMed] [Google Scholar]
- Gaillard J.-M., Hebblewhite M., Loison A., Fuller M., Powell R., Basille M., Van Moorter B.2010Habitat-performance relationships: finding the right metric at a given spatial scale. Phil. Trans. R. Soc. B 365, 2255–2265 (doi:10.1098/rstb.2010.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A.2005Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 (doi:10.1111/j.1461-0248.2005.00812.x) [Google Scholar]
- Hanski I.1998Metapopulation dynamics. Nature 396, 41–49 (doi:10.1038/23876) [Google Scholar]
- Hanski I., Gaggiotti O. E.2004Ecology, genetics, and evolution of metapopulations. Burlington, MA: Elsevier [Google Scholar]
- Hastings A., et al. 2005The spatial spread of invasions: new developments in theory and evidence. Ecol. Lett. 8, 91–101 (doi:10.1111/j.1461-0248.2004.00687.x) [Google Scholar]
- Haydon D. T., Morales J. M., Yott A., Jenkins D. A., Rosatte R., Fryxell J. M.2008Socially informed random walks: incorporating group dynamics into models of population spread and growth. Proc. R. Soc. B 275, 1101–1109 (doi:10.1098/rspb.2007.1688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes K. J., Cronin J. T.2006Interpatch movement and edge effects: the role of behavioral responses to the landscape matrix. Oikos 113, 43–54 (doi:10.1111/j.0030-1299.2006.13977.x) [Google Scholar]
- Hebblewhite M., Haydon D. T.2010Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303–2312 (doi:10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebblewhite M., Merrill E. H.2009Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454 (doi:10.1890/08-2090.1) [DOI] [PubMed] [Google Scholar]
- Holdo R. M., Holt R. D., Fryxell J. M.2009Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. Am. Nat. 173, 431–445 (doi:10.1086/597229) [DOI] [PubMed] [Google Scholar]
- Holling C. S.1959The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 91, 293–320 (doi:10.4039/Ent91293-5) [Google Scholar]
- Hooker S. K., Heaslip S. G., Matthiopoulos J., Cox O., Boyd I. L.2008Data sampling options for animal-borne video cameras: considerations based on deployments with Antarctic fur seals. Mar. Technol. Soc. J. 42, 65–75 (doi:10.4031/002533208786829179) [Google Scholar]
- Hurford A., Hebblewhite M., Lewis M. A.2006A spatially explicit model for an allee effect: why wolves recolonize so slowly in greater yellowstone. Theor. Popul. Biol. 70, 244–254 (doi:10.1016/j.tpb.2006.06.009) [DOI] [PubMed] [Google Scholar]
- Hutchinson J. M. C., Waser P. M.2007Use, misuse and extensions of ‘ideal gas’ models of animal encounter. Biol. Rev. 82, 335–359 (doi:10.1111/j.1469-185X.2007.00014.x) [DOI] [PubMed] [Google Scholar]
- Inchausti P., Guinet C., Koudil M., Durbec J.-P., Barbraud C., Weimerskirch H., Cherel Y., Jouventin P.2003Inter-annual variability in the breeding performance of seabirds in relation to oceanographic anomalies that affect the crozet and the kerguelen sectors of the southern ocean. J. Avian Biol. 34, 170–176 (doi:10.1034/j.1600-048X.2003.03031.x) [Google Scholar]
- Jerde C. L., Bampfylde C. J., Lewis M. A.2009Chance establishment for sexual, semelparous species: overcoming the allee effect. Am. Nat. 173, 734–746 (doi:10.1086/598496) [DOI] [PubMed] [Google Scholar]
- Jetz W., Jetz W., Carbone C., Fulford J., Brown J. H.2004The scaling of animal space use. Science 306, 266–268 (doi:10.1126/science.1102138) [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Wiens J. A., Milne B. T., Crist T. O.1992Animal movement patterns and population dynamics in heterogeneous landscapes. Landscape Ecol. 7, 63–75 (doi:10.1007/BF02573958) [Google Scholar]
- Johnson D. M., Johnson D. M., Liebhold A. M., Tobin P. C., Bjørnstad O. N.2006Allee effects and pulsed invasion by the gypsy moth. Nature 444, 361–363 (doi:10.1038/nature05242) [DOI] [PubMed] [Google Scholar]
- Kareiva P., Wennergren U.1995Connecting landscape patterns to ecosystem and population processes. Nature 373, 299–302 (doi:10.1038/373299a0) [Google Scholar]
- Keeling M. J.2002Using individual-based simulations to test the levins metapopulation paradigm. J. Anim. Ecol. 71, 270–279 (doi:10.1046/j.1365-2656.2002.00594.x) [Google Scholar]
- Kot M., Lewis M. A., van den Driessche P.1996Dispersal data and the spread of invading organisms. Ecology 77, 2027–2042 (doi:10.2307/2265698) [Google Scholar]
- Lande R.1988Demographic-models of the northern spotted owl (Strix occidentalis caurina). Oecologia 75, 601–607 (doi:10.1007/BF00776426) [DOI] [PubMed] [Google Scholar]
- Law R., Murrell D. J., Dieckmann U.2003Population growth in space and time: spatial logistic equations. Ecology 84, 252–262 (doi:10.1890/0012-9658(2003)084[0252:PGISAT]2.0.CO;2) [Google Scholar]
- Leimgruber P., McSheaa W. J., Brookesb C. J., Bolor-Erdeneb L., Wemmera C., Larsona C.2001Spatial patterns in relative primary productivity and gazelle migration in the Eastern Steppes of Mongolia. Biol. Conserv. 102, 205–212 (doi:10.1016/S0006-3207(01)00041-6) [Google Scholar]
- Lima S. L., Zollner P. A.1996Towards a behavioral ecology of ecological landscapes. Trends Ecol. Evol. 11, 131–135 (doi:10.1016/0169-5347(96)81094-9) [DOI] [PubMed] [Google Scholar]
- Lloyd M.1967Mean crowding. J. Anim. Ecol. 36, 1–30 (doi:10.2307/3012) [Google Scholar]
- Long R. A., Kie J. G., Bowyer R. T., Hurley M. A.2009Resource selection and movements by female mule deer Odocoileus hemionus: effects of reproductive stage. Wildl. Biol. 15, 288–298 (doi:10.2981/09-003) [Google Scholar]
- Lopez-Sepulcre A., Kokko H.2005Territorial defense, territory size, and population regulation. Am. Nat. 166, 317–329 (doi:10.1086/432560) [DOI] [PubMed] [Google Scholar]
- Mabry K. E., Stamps J. A.2008Searching for a new home: decision making by dispersing brush mice. Am. Nat. 172, 625–634 (doi:10.1086/591682) [DOI] [PubMed] [Google Scholar]
- Matsuda H., Ogita N., Sasaki A., Sato K.1992Statistical-mechanics of population—the lattice Lotka–Volterra model. Progr. Theor. Phys. 88, 1035–1049 (doi:10.1143/PTP.88.1035) [Google Scholar]
- Matthiopoulos J., Moss R., Mougeot F., Lambin X., Redpath S. M.2003Territorial behaviour and population dynamics in red grouse Lagopus lagopus scoticus. II. Population models. J. Anim. Ecol. 72, 1083–1096 (doi:10.1046/j.1365-2656.2003.00780.x) [Google Scholar]
- Matthiopoulos J., Halley J. M., Moss R.2005aSocially induced red grouse population cycles need abrupt transitions between tolerance and aggression. Ecology 86, 1883–1893 (doi:10.1890/04-0253) [Google Scholar]
- Matthiopoulos J., Harwood J., Thomas L.2005bMetapopulation consequences of site fidelity for colonially breeding mammals and birds. J. Anim. Ecol. 74, 716–727 (doi:10.1111/j.1365-2656.2005.00970.x) [Google Scholar]
- Maxwell J. C.1860Illustrations of the dynamical theory of gases. Part I. On the motions and collisions of perfectly elastic spheres. Phil. Mag. 19, 19–32 [Google Scholar]
- McIntyre N. E., Wiens J. A.1999How does habitat patch size affect animal movement? An experiment with darkling beetles. Ecology 80, 2261–2270 (doi:10.1890/0012-9658(1999)080[2261:HDHPSA]2.0.CO;2) [Google Scholar]
- Merrill E., Sand H., Zimmermann B., McPhee H., Webb N., Hebblewhite M., Wabakken P., Frair J. L.2010Building a mechanistic understanding of predation with GPS-based movement data. Phil. Trans. R. Soc. B 365, 2279–2288 (doi:10.1098/rstb.2010.0077). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J. A. J., Diekmann O.1986The dynamics of physiologically structured populations. Lecture Notes in Biomathematics, no 68 Berlin, Germany: Springer-Verlag [Google Scholar]
- Moll R. J., Millspaugha J. J., Beringerb J., Sartwellb J., Hec Z., Eggertc J. A., Zhao X.2009A terrestrial animal-borne video system for large mammals. Comp. Electr. Agric. 66, 133–139 (doi:10.1016/j.compag.2009.01.001) [Google Scholar]
- Mollison D.1977Spatial contact models for ecological and epidemic spread. J. R. Stat. Soc. Ser. B (Stat. Meth.) 39, 283–326 [Google Scholar]
- Moorcroft P., Lewis M.2006Mechanistic home range analysis Monographs in Population Biology, no. 43 Princeton, NJ: Princeton University Press [Google Scholar]
- Moorcroft P. R., Lewis M. A., Crabtree R. L.1999Home range analysis using a mechanistic home range model. Ecology 80, 1656–1665 (doi:10.1890/0012-9658(1999)080[1656:HRAUAM]2.0.CO;2) [Google Scholar]
- Moorcroft P. R., Lewis M. A., Crabtree R. L.2006Mechanistic home range models capture spatial patterns and dynamics of coyote territories in yellowstone. Proc. R. Soc. B 273, 1651–1659 (doi:10.1098/rspb.2005.3439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J. M.2002Behavior at habitat boundaries can produce leptokurtic movement distributions. Am. Nat. 160, 531–538 (doi:10.1086/342076) [DOI] [PubMed] [Google Scholar]
- Morales J. M., Ellner S. P.2002Scaling up animal movements in heterogeneous landscapes: the importance of behavior. Ecology 83, 2240–2247 (doi:10.1890/0012-9658(2002)083[2240:SUAMIH]2.0.CO;2) [Google Scholar]
- Morales J. M., Morales J. M., Haydon D. T., Frair J., Holsinger K. E., Fryxell J. M.2004Extracting more out of relocation data: building movement models as mixtures of random walks. Ecology 85, 2436–2445 (doi:10.1890/03-0269) [Google Scholar]
- Morales J. M., Fortin D., Frair J. L., Merrill E. H.2005Adaptive models for large herbivore movements in heterogeneous landscapes. Landscape Ecol. 20, 301–316 (doi:10.1007/s10980-005-0061-9) [Google Scholar]
- Morin X., Thuiller W.2009Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90, 1301–1313 (doi:10.1890/08-0134.1) [DOI] [PubMed] [Google Scholar]
- Mosimann J. E.1958The evolutionary significance of rare matings in animal populations. Evolution 12, 246–261 (doi:10.2307/2406035) [Google Scholar]
- Mougeot F., Mougeot F., Redpath S. M., Moss R., Matthiopoulos J., Hudson P. J.2003Territorial behaviour and population dynamics in red grouse Lagopus lagopus scoticus. I. Population experiments. J. Anim. Ecol. 72, 1073–1082 (doi:10.1046/j.1365-2656.2003.00781.x) [Google Scholar]
- Mueller T., Fagan W. F.2008Search and navigation in dynamic environments—from individual behaviors to population distributions. Oikos 117, 654–664 (doi:10.1111/j.2008.0030-1299.16291.x) [Google Scholar]
- Murray D. L.2006On improving telemetry-based survival estimation. J. Wildl. Manage. 70, 1530–1543 (doi:10.2193/0022-541X(2006)70[1530:OITSE]2.0.CO;2) [Google Scholar]
- Murrell D. J.2005Local spatial structure and predator–prey dynamics: counterintuitive effects of prey enrichment. Am. Nat. 166, 354–367 (doi:10.1086/432035) [DOI] [PubMed] [Google Scholar]
- Murrell D. J., Law R.2003Heteromyopia and the spatial coexistence of similar competitors. Ecol. Lett. 6, 48–59 (doi:10.1046/j.1461-0248.2003.00397.x) [Google Scholar]
- Murrell D. J., Dieckmann U., Law R.2004On moment closures for population dynamics in continuous space. J. Theor. Biol. 229, 421–432 (doi:10.1016/j.jtbi.2004.04.013) [DOI] [PubMed] [Google Scholar]
- Nathan R., Getzb W. M., Revillac E., Holyoakd M., Kadmona R., Saltze D., Smousef P. E.2008A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. USA 105, 19 052–19 059 (doi:10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee S., May R. M.1992Dynamics of metapopulations—habitat destruction and competitive coexistence. J. Anim. Ecol. 61, 37–40 (doi:10.2307/5506) [Google Scholar]
- North A., Ovaskainen O.2007Interactions between dispersal, competition, and landscape heterogeneity. Oikos 116, 1106–1119 (doi:10.1111/j.2007.0030-1299.15366.x) [Google Scholar]
- Numata M., Davis L. S., Renner M.2000Prolonged foraging trips and egg desertion in little penguins (Eudyptula minor). N Z J. Zool. 27, 277–289 [Google Scholar]
- Okubo A., Grünbaum D., Edelstein-Keshet L.2001The dynamics of animal grouping. In Diffusion and ecological problems: modern perspectives (eds Okubo A., Levin S. A.). New York, NY: Springer [Google Scholar]
- Ovaskainen O.2004Habitat-specific movement parameters estimated using mark-recapture data and a diffusion model. Ecology 85, 242–257 (doi:10.1890/02-0706) [Google Scholar]
- Ovaskainen O., Cornell S. J.2006Asymptotically exact analysis of stochastic metapopulation dynamics with explicit spatial structure. Theor. Popul. Biol. 69, 13–33 (doi:10.1016/j.tpb.2005.05.005) [DOI] [PubMed] [Google Scholar]
- Owen-Smith N., Fryxell J. M., Merrill E. H.2010Foraging theory upscaled: the behavioural ecology of herbivore movement. Phil. Trans. R. Soc. B 365, 2267–2278 (doi:10.1098/rstb.2010.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. L., Barboza P. S., Gillingham M. P.2009Nutrition integrates environmental responses of ungulates. Funct. Ecol. 23, 57–69 (doi:10.1111/j.1365-2435.2009.01528.x) [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Patterson T. A., Patterson T. A., Thomas L., Wilcox C., Ovaskainen O., Matthiopoulos J.2008State–space models of individual animal movement. Trends Ecol. Evol. 23, 87–94 (doi:10.1016/j.tree.2007.10.009) [DOI] [PubMed] [Google Scholar]
- Pelletier F., Garant D., Hendry A. P.2009Eco-evolutionary dynamics introduction. Phil. Trans. R. Soc. B 364, 1483–1489 (doi:10.1098/rstb.2009.0027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penteriani V., Delgado M. D.2009Thoughts on natal dispersal. J. Raptor. Res. 43, 90–98 (doi:10.3356/JRR-08-39.1) [Google Scholar]
- Petit R. J., Hu F. S., Dick C. W.2008Forests of the past: a window to future changes. Science 320, 1450–1452 (doi:10.1126/science.1155457) [DOI] [PubMed] [Google Scholar]
- Phillips B. L., Brown G. P., Travis J. M. J., Shine R.2008Reid's paradox revisited: the evolution of dispersal kernels during range expansion. Am. Nat. 172, S34–S48 (doi:10.1086/588255) [DOI] [PubMed] [Google Scholar]
- Pinaud D., Cherel Y., Weimerskirch H.2005Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in yellow-nosed albatrosses. Mar. Ecol. Progr. Ser. 298, 295–304 (doi:10.3354/meps298295) [Google Scholar]
- Powell R. A.1994Effects of scale on habitat selection and foraging behavior of fishers in winter. J. Mamm. 75, 349–356 (doi:10.2307/1382553) [Google Scholar]
- Powell J. A., Zimmermann N. E.2004Multiscale analysis of active seed dispersal contributes to resolving Reid's paradox. Ecology 85, 490–506 (doi:10.1890/02-0535) [Google Scholar]
- Prange S., Jordan T., Hunter C., Gehrt S. D.2006New radiocollars for the detection of proximity among individuals. Wildl. Soc. Bull. 34, 1333–1344 (doi:10.2193/0091-7648(2006)34[1333:NRFTDO]2.0.CO;2) [Google Scholar]
- Ratikainen I. I., Gill J. A., Gunnarsson T. G., Sutherland W. J., Hanna K.2008When density dependence is not instantaneous: theoretical developments and management implications. Ecol. Lett. 11, 184–198 (doi:10.1111/j.1461-0248.2008.01165.x) [DOI] [PubMed] [Google Scholar]
- Revilla E., Wiegand T.2008Individual movement behavior, matrix heterogeneity, and the dynamics of spatially structured populations. Proc. Natl Acad. Sci. USA 105, 19 120–19 125 (doi:10.1073/pnas.0801725105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronce O.2007How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Ann. Rev. Ecol. Evol. Syst. 38, 231–253 (doi:10.1146/annurev.ecolsys.38.091206.095611) [Google Scholar]
- Rutz C., Hays G. C.2009New frontiers in biologging science. Biol. Lett. 5, 289–292 (doi:10.1098/rsbl.2009.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer H., Sawyer H., Kauffman M. J., Nielson R. M., Horne J. S.2009Identifying and prioritizing ungulate migration routes for landscape-level conservation. Ecol. Appl. 19, 2016–2025 (doi:10.1890/08-2034.1) [DOI] [PubMed] [Google Scholar]
- Schick R. S., et al. 2008Understanding movement data and movement processes: current and emerging directions. Ecol. Lett. 11, 1338–1350 (doi:10.1111/j.1461-0248.2008.01249.x) [DOI] [PubMed] [Google Scholar]
- Schtickzelle N., Baguette M.2003Behavioural responses to habitat patch boundaries restrict dispersal and generate emigration-patch area relationships in fragmented landscapes. J. Anim. Ecol. 72, 533–545 (doi:10.1046/j.1365-2656.2003.00723.x) [DOI] [PubMed] [Google Scholar]
- Schultz C. B., Crone E. E.2001Edge-mediated dispersal behavior in a prairie butterfly. Ecology 82, 1879–1892 (doi:10.1890/0012-9658(2001)082[1879:EMDBIA]2.0.CO;2) [Google Scholar]
- Shaffer S. A., Costa D. P., Weimerskirch H.2003Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct. Ecol. 17, 66–74 (doi:10.1046/j.1365-2435.2003.00705.x) [Google Scholar]
- Shigesada N., Kawasaki K.1997Biological invasions: theory and practice. New York, NY: Oxford University Press [Google Scholar]
- Skalski G. T., Gilliam J. F.2000Modeling diffusive spread in a heterogeneous population: a movement study with stream fish. Ecology 81, 1685–1700 [Google Scholar]
- Skalski G. T., Gilliam J. F.2003A diffusion-based theory of organism dispersal in heterogeneous populations. Am. Nat. 161, 441–458 (doi:10.1086/367592) [DOI] [PubMed] [Google Scholar]
- Skellam J. G.1951Random dispersal in theoretical populations. Biometrika 38, 196–218 (doi:10.1093/biomet/38.1-2.196) [PubMed] [Google Scholar]
- Smouse P. E., Focardi S., Moorcroft P. R., Kie J. G., Forester J. D., Morales J. M.2010Stochastic modelling of animal movement. Phil. Trans. R. Soc. B 365, 2201–2211 (doi:10.1098/rstb.2010.0078). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps J. A.2006The silver spoon effect and habitat selection by natal dispersers. Ecol. Lett. 9, 1179–1185 (doi:10.1111/j.1461-0248.2006.00972.x) [DOI] [PubMed] [Google Scholar]
- Stamps J. A., Krishnan V. V., Reid M. L.2005Search costs and habitat selection by dispersers. Ecology 86, 510–518 (doi:10.1890/04-0516) [Google Scholar]
- Starrfelt J., Kokko H.2008Are the speeds of species invasions regulated? The importance of null models. Oikos 117, 370–375 (doi:10.1111/j.2007.0030-1299.16164.x) [Google Scholar]
- Strandberg R., Klaassen R. H. G., Hake M., Olofsson P., Alerstam T.2009Converging migration routes of Eurasian hobbies Falco subbuteo crossing the African equatorial rain forest. Proc R. Soc. B 276, 727–733 (doi:10.1098/rspb.2008.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirgood S., et al. 2004Can parks protect migratory ungulates? The case of the serengeti wildebeest. Anim. Conserv. 7, 113–120 (doi:10.1017/s1367943004001404) [Google Scholar]
- Thomas C. D., Kunin W. E.1999The spatial structure of populations. J. Anim. Ecol. 68, 647–657 (doi:10.1046/j.1365-2656.1999.00330.x) [Google Scholar]
- Thomson N. A., Ellner S. P.2003Pair-edge approximation for heterogeneous lattice population models. Theor. Popul. Biol. 64, 271–280 (doi:10.1016/s0040-5809(03)00088-1) [DOI] [PubMed] [Google Scholar]
- Tilman D., Tilman D., May R. M., Lehman C. L., Nowak M. A.1994Habitat destruction and the extinction debt. Nature 371, 65–66 (doi:10.1038/371065a0) [Google Scholar]
- Tinker M. T., Doak D. F., Estes J. A.2008Using demography and movement behavior to predict range expansion of the southern sea otter. Ecol. Appl. 18, 1781–1794 (doi:10.1890/07-0735.1) [DOI] [PubMed] [Google Scholar]
- Tobin P. C., Whitmire S. L., Johnson D. M., Bjørnstad O. N., Liebhold A. M.2007Invasion speed is affected by geographical variation in the strength of allee effects. Ecol. Lett. 10, 36–43 (doi:10.1111/j.1461-0248.2006.00991.x) [DOI] [PubMed] [Google Scholar]
- Tomkiewicz S. M., Fuller M. R., Kie J. G., Bates K. K.2010Global positioning system and associated technologies in animal behaviour and ecological research. Phil. Trans. R. Soc. B 365, 2163–2176 (doi:10.1098/rstb.2010.0090). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchin P.1998Quantitative analysis of movement: measuring and modeling population redistribution in animals and plants. Sunderland, MA: Sinauer Associates [Google Scholar]
- Turchin P.2003Complex population dynamics: a theoretical/empirical synthesis, Monographs in Population Biology, no. 35 Princeton, NJ: Princeton University Press [Google Scholar]
- Tveraa T., Christensen G. N.2002Body condition and parental decisions in the snow petrel (Pagodroma nivea). Auk 119, 266–270 (doi:10.1642/0004-8038(2002)119[0266:BCAPDI]2.0.CO;2) [Google Scholar]
- Urbano F., Cagnacci F., Calenge C., Dettki H., Cameron A., Neteler M.2010Wildlife tracking data management: a new vision. Phil. Trans. R. Soc. B 365, 2177–2185 (doi:10.1098/rstb.2010.0081). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Moorter B., Visscher D., Benhamou S., Börger L., Boyce M. S., Gaillard J.-M.2009Memory keeps you at home: a mechanistic model for home range emergence. Oikos 118, 641–652 (doi:10.1111/j.1600-0706.2008.17003.x) [Google Scholar]
- Wang M., Grimm V.2007Home range dynamics and population regulation: an individual-based model of the common shrew Sorex araneus. Ecol. Model. 205, 397–409 (doi:10.1016/j.ecolmodel.2007.03.003) [Google Scholar]
- Wiens J. A.1997Metapopulation dynamics and landscape ecology. In Metapopulation biology (eds Hanski I., Gilpin M. E.), pp. 43–62 San Diego, CA: Academic Press [Google Scholar]
- Wilcove D. S., Wikelski M.2008Going, going, gone: is animal migration disappearing? PLoS Biol. 6, 1361–1364 (doi:10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. P., et al. 2007All at sea with animal tracks; methodological and analytical solutions for the resolution of movement. Deep-Sea Res. II 54, 193–210 (doi:10.1016/j.dsr2.2006.11.017) [Google Scholar]
- Wilson R. P., Shepard E. L. C., Liebsch N.2008Prying into the intimate details of animal lives: use of a daily diary on animals. Endang. Species Res. 4, 123–137 (doi:doi:10.3354/esr00064) [Google Scholar]
- Wolf M., Frair J., Merrill E., Turchin P.2009The attraction of the known: the importance of spatial familiarity in habitat selection in Wapiti cervus elaphus. Ecography 32, 401–410 (doi:10.1111/j.1600-0587.2008.05626.x) [Google Scholar]
- Yorio P., Boersma P. D.1994Causes of nest desertion during incubation in the magellanic penguin (Spheniscus magellanicus). Condor 96, 1076–1083 (doi:10.2307/1369116) [Google Scholar]