Abstract
We outline how principles of optimal foraging developed for diet and food patch selection might be applied to movement behaviour expressed over larger spatial and temporal scales. Our focus is on large mammalian herbivores, capable of carrying global positioning system (GPS) collars operating through the seasonal cycle and dependent on vegetation resources that are fixed in space but seasonally variable in availability and nutritional value. The concept of intermittent movement leads to the recognition of distinct movement modes over a hierarchy of spatio-temporal scales. Over larger scales, periods with relatively low displacement may indicate settlement within foraging areas, habitat units or seasonal ranges. Directed movements connect these patches or places used for other activities. Selection is expressed by switches in movement mode and the intensity of utilization by the settlement period relative to the area covered. The type of benefit obtained during settlement periods may be inferred from movement patterns, local environmental features, or the diel activity schedule. Rates of movement indicate changing costs in time and energy over the seasonal cycle, between years and among regions. GPS telemetry potentially enables large-scale movement responses to changing environmental conditions to be linked to population performance.
Keywords: foraging areas, GPS telemetry, habitat units, movement ecology, seasonal ranges
1. Introduction
Behavioural ecology is concerned with decisions expressed by animals in the form of changes in behaviour, affecting their chances of survival and reproductive success (McFarland 1977; Dale et al. 2005; Gaillard et al. 2010). Foraging theory concerns activities related to the acquisition of food. It addresses decisions regarding (i) where to search, (ii) when to feed, (iii) which food types to consume, and (iv) when to terminate feeding and move on (Schoener 1971; Pyke et al. 1977; Stephens & Krebs 1986). Most applications of this theory have considered only fine-scale behaviour observed over periods of minutes or hours. Nevertheless, foraging behaviour can be expressed over a hierarchy of spatial and associated temporal scales, from bites and steps within food patches to movements generating daily, seasonal or annual home ranges (Senft et al. 1987; Bailey et al. 1996; Owen-Smith 2002; table 1). Global positioning system (GPS) telemetry enables sequential movements to be documented over greater spatio-temporal scales than readily observed directly. Moreover, records are obtained remotely, avoiding disturbance from the human operator generally required for radio tracking using very high frequency (VHF) beacons. The automation of GPS devices furthermore allows the locations of many animals to be recorded simultaneously (Cagnacci et al. 2010). In this paper, we address how aspects of foraging theory developed for short-scale movements might be applied over longer and larger scales.
Table 1.
The hierarchy of temporal and spatial scales associated with foraging behaviour by large mammalian herbivores (adapted from Bailey et al. (1996) and Owen-Smith (2002)).
| temporal scale | spatial scale | defining behaviour | vegetation unit |
|---|---|---|---|
| 1–2 s | bite | plucking, chewing and swallowing | plant part |
| 2 s–2 min | feeding station | moving head, prehending, biting | plant (grass tuft, shrub) |
| 0.5–30 min | food patch | feeding (eating), stepping | clump of plants |
| 1–4 h | foraging area | feeding, walking, standing alert | habitat patch |
| 12–24 h | daily range | foraging, travelling, drinking, ruminating, resting | set of habitats |
| 3–12 months | home range | growth, reproduction, mortality | landscape region |
| several years | lifetime range | survivorship, fecundity, dispersal | geographical region |
Our ecological focus is on terrestrial mammalian herbivores, capable of bearing GPS collars operating through complete seasonal cycles or even multi-year periods. The plant species and parts constituting the food resources for such animals are spatially anchored, although their availability and nutritional value may vary considerably over time. The persistent spatial rigidity of these food resources for terrestrial herbivores contrasts with the fluidity of where food concentrations occur for most marine animals, while the temporal variation in food quality is distinct from the rather constant food quality of the prey consumed by most carnivores. For marine organisms, search strategies for locating feeding opportunities that are unpredictable in space have been emphasized (Bartumeus 2009; Reynolds & Rhodes 2009). For terrestrial herbivores, memory of the places where the most favourable resources are generally found at particular times plays a more important role (Bailey et al. 1996; Van Moorter et al. 2009; Wolf et al. 2009). Intra- and interspecific competition among herbivores arises mainly through the depression of food resources rather than via direct interference with the uptake of this food. Patch choice and movement paths may be influenced additionally by the local risk of predation (Hebblewhite & Merrill 2009).
Optimality assessments based on maximizing short-term rates of gain can be misleading over longer temporal horizons. A dynamic approach considering extended time periods as well as stochastic uncertainty in the conditions likely to be experienced is needed (Clark & Mangel 1999; Houston & McNamara 1999). A core concept is how the current state of the animal in terms of its body reserves, size or other attributes determines its future prospects of survival and reproduction. Dynamic optimization accommodates trade-offs between food gains and predation risks, as well as temporarily effective constraints such as digestive capacity. The optimal action depends additionally on the animal's current body state: individuals that are starving should expose themselves to greater risks of predation than animals in good condition (McNamara & Houston 1987; Sinclair & Arcese 1995). The practical challenge is how to apply optimality analysis in a precise way, given limitations in the information that is available. Nevertheless, behavioural responses to changing circumstances can be used to indicate the circumstances in which changes in population performance are likely to result (Kotler et al. 2007).
We draw on concepts for the emerging field of movement ecology identified by the working group that met recently in Israel (Nathan 2008). Nathan et al. (2008) proposed a unifying paradigm encompassing the interplay between the animal's changing internal state (why move?) and the prevailing state of its environment (where to move?), constrained by individual motion and navigation capabilities. Bartumeus & Levin (2008) suggested that the manifestation of intermittent locomotion by organisms provides an integrating structure across spatio-temporal scales, drawing on concepts developed by O'Brien et al. (1990) and Kramer & McLaughlin (2001). Getz & Saltz (2008) emphasized the integration of fundamental movement elements into canonical activity modes, interacting with the landscape matrix. Fryxell et al. (2008) outlined multiple movement modes expressed over time scales from minutes to years, and spatial scales extending from metres to hundreds of kilometres, as exemplified by one species of large herbivore.
In our contribution, we (i) elaborate on the hierarchical spatio-temporal structure of foraging and other movement behaviour, (ii) evaluate various metrics that have been derived to interpret such behaviour at larger scales, and (iii) consider how these patterns might be evaluated under the optimality paradigm.
2. Hierarchical activity and movement modes
The activity modes emergent at higher levels encompass movement elements distinguished at lower levels (Getz & Saltz 2008). Foraging behaviour is defined by bouts of feeding (biting, chewing and swallowing), interrupted by relocation movements towards places where new feeding opportunities are presented. Herbivores generally encounter food within patches constituted by clusters of plants. One or two steps taken in succession do not interrupt feeding because the time taken per step is approximately equal to the time required to process (i.e. chew and swallow) a bite. Feeding in this concentrated way can continue unbroken for periods of many minutes, particularly for large grazers exploiting the horizontally extended patches constituted by grass swards. Nevertheless, animals may periodically interrupt feeding to stand vigilant with head raised, an activity related to detecting lurking predators or the movements of group companions.
Among large ungulates, the proportion of time spent feeding during foraging spells lasting an hour or more is typically 80–90% for grazers, and 65–80% for browsers (Owen-Smith 2002). This difference is an outcome of the contrasting food dispersion of vertically orientated trees and shrubs compared with horizontally extended grass patches. Approximately 5–15% of foraging time is diverted to standing vigilant or other non-foraging actions, leaving 7–20% of time spent moving. Accordingly, a small proportional reduction in the amount of time spent feeding within patches could result in a doubling or even tripling of the overall movement rate while foraging. Hence, a simple measure of foraging performance is the ratio of the time spent feeding to that spent walking (excluding time diverted to vigilance), or inversely the rate of movement while foraging (Owen-Smith 1979). Blesbok (Damaliscus dorcas) foraging during winter in grassland that had been burnt, reducing the amount of food remaining, exhibited a movement rate twice that of animals foraging in unburnt grassland (Novellie 1978). The movement rate of kudus foraging in acacia savanna increased by almost 50 per cent between the early wet season and the dry season, and was twice as great in open thorn savanna than in the hill base ecotone where more woody browse was available (Owen-Smith 1979). More precise measures of foraging benefits would need to take into account the nutritional value of the food consumed, as well as the food intake rate obtained while feeding (Owen-Smith & Novellie 1982; Illius & Gordon 1999).
Movements may also be undertaken towards ends unrelated to feeding. This is evident when animals walk past favoured food types without pausing to feed. For browsing kudus (Tragelaphus strepsiceros), a breakpoint in the frequency distribution of step-sets indicated that there was a reduced probability of movement ending in feeding after animals had walked for longer than 65 s, equivalent to a distance of approximately 60 m (Owen-Smith 1993). For black-tailed deer (Odocoileus hemionus), foraging was regarded as terminated when more than 2 min had elapsed without feeding (Gillingham et al. 1997). Switches from foraging to more extended travel could be directed towards seeking remote sets of food patches, represent motivational shifts towards seeking resting (bedding) sites or other needs, or be related to evading predation. Sets of food patches connected by moves between them during foraging spells lasting a few hours constitute the foraging area.
Movements between food patches within foraging spells generally fall ‘under the radar’ of GPS-based tracking. Although GPS devices could be set to record locations at time intervals of less than an hour, this would generally be at the expense of covering the full annual cycle, based on the current battery power of collars fitted to large ungulates. If the time step between successive GPS locations spans several hours, generally only a single location will be obtained during each foraging spell. Nevertheless, GPS-based locations obtained over time steps of an hour or less can be used to infer activity states, as outlined below.
During the course of a day, foraging spells are interspersed with periods spent resting or engaged in other activities. Resting is indicated by immobility, whether standing or lying (apart from head movements or, in the case of ruminants, chewing the cud). Foraging activity tends to predominate from dawn through the early morning and from late afternoon into the evening (Georgii 1981; Owen-Smith 1998). Resting or bedding activity commonly prevails during the heat of midday, and for much of the night, although ungulates may show greater nocturnal activity when human disturbance occurs during the day (Kamler et al. 2007). Following resting spells, animals may resume foraging in the same area, or move to a new foraging area.
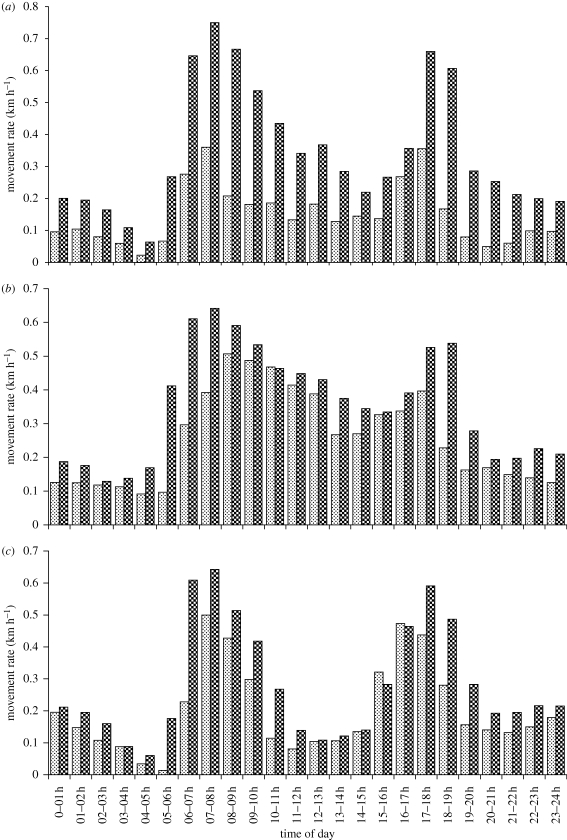
During the African dry season, grazers may need to travel to and from places where surface water remains available every day or two (Western 1975; Gaylard et al. 2003). Browsers are less water-dependent than grazers through being able to secure moisture from the green leaves that persist on evergreen trees and shrubs, but are forced to travel to water when conditions become so dry that few leaves remain. Hence, daily movement ranges become extended by the distance traversed to water sources and back. Journeys to water are commonly undertaken during the morning, before conditions become too hot, displacing some of the time otherwise assigned to foraging. Because of this scheduling of activities, the spatial displacement per time step varies with the time of day, with movement peaks commonly evident around dawn and dusk (figure 1). The daily range encompasses shifts between foraging areas as well as between foraging areas and resting or drinking sites.
Figure 1.
Diel variation in hourly movement rates of three African ungulates obtained from GPS tracking, showing early morning and evening peaks as well as the increase in movement during more stressful conditions in the late dry season. (a) Sable antelope, (b) zebra and (c) buffalo (N. Owen-Smith 2009, unpublished). Dotted bar, early dry; hatched bar, late dry.
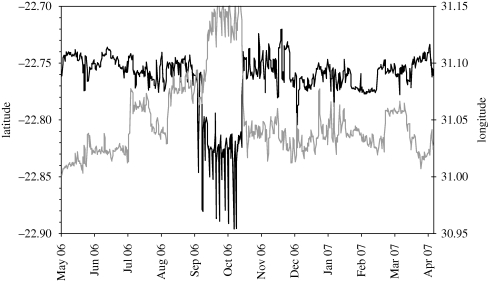
During the growing (summer or wet) season, plants can soon regenerate the parts consumed, allowing herbivores to continue foraging within the same area for periods covering several successive days or even weeks. During winter or the dry season when vegetation becomes a non-renewing resource, edible plant components are progressively depleted, prompting more frequent shifts between foraging areas, unless conditions are so extreme that there is little benefit to be gained by moving. Settlement periods within particular foraging areas can be revealed by plotting the movement trace in latitude and longitude coordinates during morning or evening foraging periods over successive days, revealing how this pattern changes seasonally (figure 2). Also apparent are wider shifts between distinct home ranges occupied during particular periods of the year. An alternative approach that has been used to identify seasonal shifts in home range occupation is based on the overall displacement from some fixed point (Dettki & Ericsson 2008).
Figure 2.
Temporal trace from GPS telemetry of daily morning (08.00) and evening (20.00) locations of a sable antelope herd in northern Kruger National Park spanning the seasonal cycle. Pattern indicates periods of settlement within foraging areas, the distinct range occupied during October–November at the end of dry season and excursions to the nearest water source every few days during the latter period (N. Owen-Smith 2009, unpublished). Black line, latitude; grey line, longitude.
3. Movement metrics
(a). Movement modes
Foraging activity is commonly identified by ‘area-restricted search’, i.e. a sequence of short moves with frequent large turning angles between them (Benhamou 1992). Franke et al. (2004) applied hidden Markov models to displacement distances and turning angles of woodland caribou (Rangifer tarandus) derived from GPS-based locations 15 min apart to infer changes between feeding, bedding and relocating states. These animals foraged in spells lasting merely 15–30 min and confined foraging to patches encompassing around 18 ha for one or more days. Demarcation criteria, bout durations and activity sequences seemed to vary among individual animals. These authors noted that the 15 min interval was too coarse to reveal the true turning angles between feeding bouts.
Johnson et al. (2002) used a breakpoint in slope of the exponential distribution of movement ‘steps’ between GPS-based locations 3–4 h apart to distinguish intra-patch movements (presumably foraging) from inter-patch travelling for woodland caribou. At this temporal resolution, distinctions were diffuse and inconsistent among individuals. Applied to hourly GPS-based locations for sable antelope (Hippotragus niger), clearer breakpoints emerged between movement rates associated with resting (less than 100 m h−1), foraging (200–600 m h−1), relocating (0.8–1.2 km h−1) or travelling (greater than 1.6 km h−1), allowing for intervening mixes between these movement modes (N. Owen-Smith 2009, unpublished).
An alternative approach employs the ‘first passage time’, representing the time required to pass through a circle of a particular radius from some starting point, to indicate places where animals moved more slowly while foraging (Fauchald & Tveraa 2003). As the radius of the circle is expanded, a rise in the variance of the first passage time indicates the spatial scale at which a switch to longer, more directed moves occurs. Applied to GPS locations of elk (Cervus elaphus canadensis), this approach revealed three nested scales of movement over a 2 h time step: inactive/resting (moves less than 50 m), active/foraging (moves approx. 275 m) and active/relocating (moves approx. 1600 m; Frair et al. 2005). Inherent assumptions underlying this method are that the foraging areas have a constant dimension, and that animals respond to the patch perimeter as a reflecting boundary (Barraquand & Benhamou 2008). A more rigorous approach proposed by Barraquand & Benhamou (2008) uses the residence time in the vicinity of successive path locations to distinguish places where animals slowed their movements to exploit apparently profitable foraging areas. This method accommodates variability in the spatial extent of the feeding areas.
Forester et al. (2007) used a linear state-space model to distinguish movement modes of elk based on the autocorrelation structure of locations obtained at 5 h intervals. Movement tendencies varied with time of day, with greatest movement taking place during the crepuscular hours. Contrary to expectations, these animals did not show slower movements in preferred areas at this temporal scale. Morales et al. (2004) applied a Bayesian state-transition model to location records for elk recently introduced into a new environment obtained at 24 h intervals. They distinguished an ‘encamped’ state, defined by small daily relocation distances and large turning angles from day to day, from ‘exploratory’ states with daily displacements extending over several kilometres and greater directional consistency. For these same elk, Dalziel et al. (2008) applied a different approach based on artificial neural networks to daily locations obtained after the animals had established their home ranges (see also Fryxell et al. 2008). Individual elk concentrated their movements in small areas containing preferred resources for periods of several days to weeks (displacements of 0.23–0.66 km d−1), punctuated by rapid translocations to distant parts of the home range. Often these jumps were to places that had been used previously, indicating an important role of spatial memory in guiding movements.
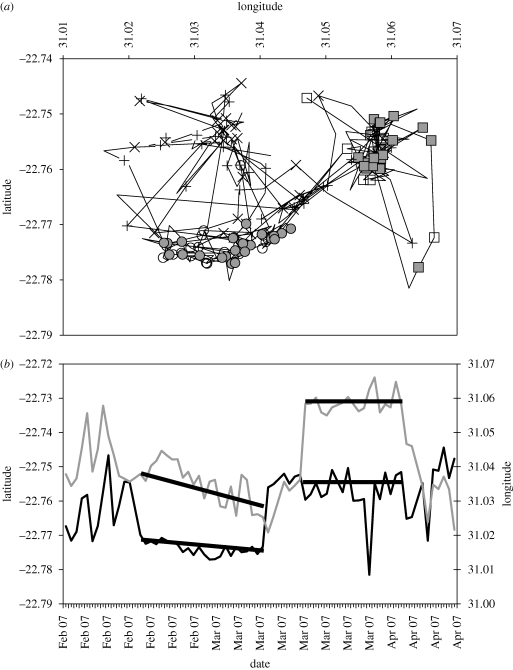
Graphical displays of GPS-based locations obtained on successive days during times when animals were likely to be foraging indicate not only the periods over which the animal remained within the same foraging area, but also the extent of the area exploited during these periods (figure 3). The 24 h iteration excludes temporary relocation from foraging areas to resting sites or places used for other activities at other times of day. For sable antelope, the analysis was restricted to evening locations in order to exclude excursions to drinking places, which displaced foraging activity during the morning on some days (Owen-Smith & Cain 2007). Sable sometimes remained within foraging areas covering 1–3 km2 for periods of two to four weeks, followed by shifts of several kilometres over a few days to new foraging areas (figure 3). The intensity of exploitation of these foraging areas, and hence their effective value, can be obtained from the stay time relative to the patch extent, taking into account the herd size.
Figure 3.
Segmentation of GPS-based locations of sable antelope in Kruger National Park between 1 February and 15 April 2007 showing alternation between settlement within and relocating between foraging areas. (a) Spatial plot connecting sequential locations at 6 h intervals with early morning (08.00) locations marked by open symbols and early evening (20.00) locations by closed symbols within foraging areas, and by plusses and crosses for corresponding locations during relocation periods; circles and squares distinguish two settlement locations. (b) Temporal trace with early evening coordinates plotted as a time sequence, showing one settlement period spanning 24 days followed by a second spanning 19 days and more erratic movements in between (N. Owen-Smith 2009, unpublished). Black line, latitude; grey line, longitude.
(b). Habitat patch occupation
Selection of food patches can be expressed in terms of the frequency with which food types (plant species and parts) are accepted for feeding when encountered, the duration of feeding before patch departure occurs and the orientation of search paths towards regions where favoured food types are more likely to be encountered (Owen-Smith & Cooper 1987a,b; Owen-Smith 1994). Similarly, habitat patch selection is indicated by switches in movement mode from travelling/relocating to settlement in a foraging area. Habitat conditions rejected are shown by places where animals were recorded but did not stay, as distinct from regions that animals never entered for whatever reason. The activity associated with the location can be identified from movement patterns as described above, if the time step for GPS-based locations is sufficiently short (an hour or less), or alternatively inferred from the time of day relative to the activity schedule. Stay periods within particular areas, controlled for the extent of the area traversed, indicate the favourability of the habitat conditions there for the activity performed. As outlined by Moorcroft & Barnett (2008), measures of the relative intensity of space use obviate the need to define available habitat (see also Beyer et al. 2010). GPS-based tracking showed how habitats were favoured by elk through frequent return visits rather than prolonged stays after entry (Anderson et al. 2008; see also Forester et al. 2007).
Maps of habitat types based on vegetation patterns and topographic features evident in the most commonly used satellite imagery are generally somewhat crude compared with the accuracy and frequency of GPS-based locations (Frair et al. 2010). Although satellite images with sub-metre resolution can be obtained (Urbano et al. 2010), this is at the expense of temporal replication, so that seasonal changes in salient features cannot be followed at fine spatial scales. Moreover, the features to which the animals respond may not be revealed in these images. Nevertheless, GPS-based locations allow specific places where animals were present for particular activities to be visited, and micro-habitat features in terms of vegetation structure and composition recorded. The habitat attributes can in turn be related to food availability and security from predation. Problems associated with estimates of habitat preference are addressed more comprehensively by Beyer et al. (2010).
In Yellowstone National Park, elk favoured open grassland and flat terrain in the absence of wolves (Canis lupus), but shifted their habitat use towards conifer forests in high wolf-use areas (Fortin et al. 2005). Disturbance by humans can alter the activity schedule as well as habitat features selected. Red deer subject to high hunting pressure in Norway remained inactive in forest cover for most of the day, moving into pastures to forage at night (Godvik et al. 2009). Influences of predation on prey movements are discussed in more detail by Merrill et al. (2010).
(c). Seasonal home ranges
Home ranges mapped from locations determined using VHF telemetry are subject to sampling biases towards the particular times of the day when tracking was conducted, as well as lack of independence among locations obtained close together in time (Kernohan et al. 2001). GPS-based tracking provides a uniform sampling regime (Cagnacci et al. 2010), thereby avoiding over-weighting of day versus night locations and particular seasons. Furthermore, the temporal relationship between successive moves is informative, revealing the movement mode and hence the types of activity performed in particular places. This enables the utilization pattern to be associated with the needs provided by particular regions of the home range at that time (Kie et al. 2010). GPS-based records are sufficiently intensive to expose gaps in the area traversed as well as the places visited. These gaps as well as sharp bounds to the range tend to be obscured by parametric kernel smoothing, which extend the apparently used area somewhat beyond actually recorded locations. The local nearest-neighbour convex-hull method outlined by Getz & Wilmers (2004) is more appropriate for mapping the fine-scale utilization patterns documented by GPS-based tracking. Benhamou & Cornelis (in press) showed further how uncertainty in the path followed between successive GPS locations could be represented and taken into account statistically. Another relevant feature for interpretation is the time between return visits to particular localities, potentially related to the time required for resources to recover following local exploitation (Bar-David et al. 2009). A comprehensive review of various approaches relating movement patterns to landscape features within a hierarchical framework was presented by Schick et al. (2008). Another approach, outlined by McRae et al. (2008), used circuit theory to analyse connectivity among habitat patches exploited within complex landscapes. Analogues of resistance, current and voltage represent the tendency of animals to move along particular corridors.
Mitchell & Powell (2004) developed a mechanistic home range model relating the use of spatially distributed resources to resource benefits discounted for travel costs. This model was developed with carnivores in mind and thus represented movements orientated around a central den site (see also Moorcroft et al. 1999). In this simulation, resource depression through consumption or as a result of increased vigilance of prey lowers the value of exploited patches and prompts the redistribution of animals across the landscape. Although the movements of grazing ungulates may be constrained around surface water points during the dry season, journeys to and from water are best interpreted as excursions beyond the foraging range because the time spent at water is generally brief.
Mueller & Fagan (2008) suggested that the primary influence on the home range occupation patterns of large herbivores was the spatio-temporal availability of resources. Resources that are abundant, spatially concentrated and predictable through time promote small home ranges inhabited year-round, and hence high local population densities. Home ranges become larger if resources are more patchily distributed so that some regions offer little or no food benefit. If resources are seasonally variable but spatially predictable, home ranges should contract during the adverse season, but when the location of food resources is unpredictable (e.g. influenced by local rainfall events), home ranges should expand. Resources that are predictably found in different places at particular times of the year promote seasonally disparate home ranges, or even migration between widely separated ranges, as exemplified by red deer (Albon & Langvatn 1992), elk (Hebblewhite et al. 2008) and wildebeest (Connochaetes taurinus; Boone et al. 2006; Holdo et al. 2009). If resources are both temporally and spatially unpredictable, animals are likely to occupy different regions in the same season in different years, so that their movements appear nomadic (e.g. gazelles in both Africa and Asia; Fryxell et al. 2004, 2005; Mueller et al. 2008). When food or water runs out in adverse years (e.g. during severe droughts), animals may wander beyond their usual home range limits.
Different regions within seasonal home ranges may be exploited at particular times. Both wildebeest and Thomson's gazelle (Gazella thomsoni) covered large wet season ranges in the Serengeti ecosystem to take advantage of erratic rain showers governing grass growth (Fryxell et al. 2004, 2005; Boone et al. 2006; Holdo et al. 2009). Elephants (Loxodonta africana) moved over vast areas in northern Botswana in response to rainfall events three to four weeks earlier (Cushman et al. 2005). GPS-based locations can establish corridors of movement between seasonal home ranges as well as the consistency of the places occupied in different years (Thirgood et al. 2004; Dettki & Ericsson 2008; Sawyer et al. 2009). If habitat types included within the home range are highly productive, return times should be short, and thus the extent of the home range relatively small. When habitat productivity declines, or becomes less predictable, resource exploitation is extended over a wider area. Hence, home range extents indicate the suitability of local habitats at a landscape scale. The larger the home ranges required, the lower the regional population density supported, taking into account range overlap and the size of the groups sharing a common home range.
Social relationships governing space utilization could be studied by simultaneously tracking the movements of animals occupying overlapping or neighbouring home ranges. Animals of the same species could either (i) move independently in overlapping home ranges, (ii) move largely independently but share a common home range, (iii) move as a cohesive herd within a shared home range, overlapping or largely separate from the ranges of other herds, (iv) occupy exclusive home ranges as individuals or groups through aggressive inhibition of the movements of neighbours, or (v) aggregate in common areas. Haydon et al. (2008) showed that attraction to conspecifics was a key factor influencing the movements of newly introduced elk, with gregarious animals moving more slowly and surviving better than solitary individuals. Moorcroft & Barnett (2008) included deterrence by neighbouring groups as an influential factor in their home range model. A comprehensive review of home range concepts and assessments is provided by Kie et al. (2010).
4. Optimality assessments
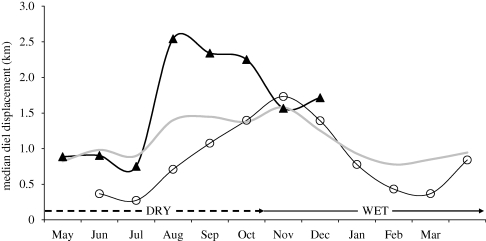
Optimality assessments relate benefits obtained to the associated costs. A crucial first step is to identify the type of activity performed in particular places at specific times, and hence the kind of benefit derived (e.g. food resources versus shelter from environmental extremes). As noted above, the activity state can be identified from the movement mode if the time step between GPS-based locations is sufficiently short (less than or equal to 1 h), otherwise it can be inferred from the time of day when particular activities predominate. Movement rates associated with particular activities reflect costs incurred in terms of time and energy expenditures. Accordingly, diel displacement distances are expected to be greater during the adverse (winter or dry) season and in bad years (e.g. following low wet season rainfall) than under benign conditions, as exemplified by sable antelope (N. Owen-Smith 2009, unpublished; figure 4). Changes in movement patterns can be used as behavioural indicators of stressful conditions before the consequences for survival and reproduction are manifested (Owen-Smith & Cain 2007).
Figure 4.
Monthly variation in diel (24 h) displacement distances of a sable antelope herd in northern Kruger National Park, comparing seasonal pattern in a benign year with that the following adverse year with low wet season rainfall; grey line indicates average pattern over all years and study areas (N. Owen-Smith 2009, unpublished). Open circle, 2006/2007; filled triangle, 2007.
More precise estimates of food benefits derived by herbivores require records of the vegetation components consumed and nutritional contents thereof, as well as the rates of food intake achieved (Owen-Smith & Novellie 1982). The close-range observations required for such estimates are possible for free-ranging animals tracked by VHF telemetry only if these animals have been habituated to the close approach of a human observer (e.g. Owen-Smith & Cooper 1989). GPS tags allow animals to be located remotely with sufficient precision for the specific sites where they were feeding to be identified, and hence the plant species eaten or rejected to be established (V. A. Macandza 2009, unpublished). Furthermore, faecal samples can be collected, enabling the plant species consumed over some prior period to be determined from leaf epidermal fragments, and even the diet quality established from faecal nitrogen contents. In this way, nutritional benefits could be related to the movement costs incurred in different places, seasons and years. As yet few researchers have taken advantage of such opportunities.
Fero et al. (2008) reviewed the advantages of dynamic state-dependent models projecting optimal annual routines over the seasonal cycle as a class of behaviour-based models (see also McNamara & Houston 2008). This approach could be applied to seasonally varying habitat selection, taking into account various trade-offs and carry-over effects, subject to the limitations of the data available. Noonberg et al. (2007) developed an individual-based model of elk movements and patch choice as a dynamic game, with the output projecting habitat use as well as aggregation and survival patterns. They emphasized that models treating single movement decisions in isolation from the seasonal sequence of decisions were insufficient to capture landscape-scale behaviour. New perspectives opened by circuit theory (McRae et al. 2008) could prove productive by directing attention towards spatial connections as well as the underlying energizing needs.
The concept of a competitive equilibrium represented by the ‘ideal free’ distribution (Fretwell 1972; Van der Meer & Ens 1997) is not readily applicable to large herbivores in the short term because of the pulsed rather than continuous production of vegetation resources. Ungulates of the same or different species commonly aggregate in regions offering highest forage quality over periods of several days or weeks, with spacing patterns reflecting the spatial dispersion of this food (Wilmshurst et al. 1999). This concept may be more appropriately applied to spacing among home ranges, allowing for group sharing. However, home ranges occupied by roe deer in France did not fit the ‘ideal free’ expectation because individual range locations depended mainly on initial dispersal opportunities (McLoughlin et al. 2007).
5. Summary and conclusions
GPS technology enables the sequential locations of sets of animals to be recorded remotely, without disturbing the ongoing activity of the animals. Rates of movement can be used to infer activity states and hence the kinds of benefit derived from particular habitat types or regions of the home range. Movement patterns of large herbivores can be documented with a temporal resolution of an hour over annual or multi-annual time frames. The spatial precision opens opportunities to establish the micro-habitat features selected, and even the food types eaten and rejected, through supporting field observations. Rates of displacement within and between days can serve as behavioural indicators of the times and places when or where stressful conditions were experienced. Assessments can be extended to address optimal annual routines of behaviour and to show how animal responses vary between good and bad years. Influences of changing predation pressure as well as food availability can potentially be established. Behavioural responses at larger spatio-temporal scales are more appropriately related to the consequences of environmental variation for survival and reproduction than fine-scale foraging behaviour.
Theoretical concepts for understanding and interpreting behavioural responses in terms of their adaptive value at these scales remain at an early stage of development. The conceptual framework outlined by Nathan et al. (2008) in terms of the interplay between an animal's internal state and the state of its environment, taking into account what is possible given the animal's movement and navigation capacities, offers a helpful unifying framework. Also useful for cross-scale integration is the notion of intermittent locomotion expressed at various spatio-temporal scales and corresponding hierarchical activity modes (Fryxell et al. 2008; Getz & Saltz 2008).
What strikes us as we reflect back on our review is how many of the papers we cite were published in the past few years. Interpretations of the movement patterns revealed by GPS-based tracking are clearly in a state of flux as new ideas, data and analytical approaches enter the scientific market place. Nevertheless, for large herbivores the literature remains dominated by studies on caribou, elk and moose undertaken in northern temperate latitudes. A wider sample of herbivore species needs to be encompassed to establish the broader generality of the patterns shown, including influences of predators besides wolves. Links between movement behaviour and population performance still need consolidation (but see Morales et al. 2010). Our contribution has outlined how concepts of adaptive behaviour in response to changing environments could help establish a cohesive scientific framework for movement ecology.
Acknowledgements
We thank the Edmund Mach Foundation for sponsoring the GPS Telemetry Workshop held in Viote del Monte Bondone, Trento, Italy, in September 2008, which spurred us to develop these ideas, and Francesca Cagnacci for her dedicated management of the publication process. Helpful comments on previous drafts of our manuscript were provided by Mike Mitchell, Daniel Fortin, Simon Benhamou, Luigi Boitani and an anonymous reviewer. We acknowledge support from the following agencies for funding to attend the workshop: NSERC and the South African National Research Foundation.
Footnotes
One contribution of 15 to a Theme Issue ‘Challenges and opportunities of using GPS-based location data in animal ecology’.
References
- Albon S. D., Langvatn R.1992Plant phenology and the benefits of migration in a temperate ungulate. Oikos 65, 502–513 (doi:10.2307/3545568) [Google Scholar]
- Anderson D. P., Forester J. D., Turner M. G.2008When to slow down: elk residency rates on a heterogeneous landscape. J. Mammal. 89, 105–114 (doi:10.1644/07-MAMM-A-035.1) [Google Scholar]
- Bailey D. W., Gross J. E., Laca E. A., Rittenhouse L. R., Coughenour M. B., Swift D. M., Sims P. L.1996Mechanisms that result in large herbivore grazing distribution patterns. J. Range Manag. 49, 386–400 (doi:10.2307/4002919) [Google Scholar]
- Bar-David S., Bar-David I., Cross P. C., Ryan S. J., Knechtel C. U., Getz W. M.2009Methods for assessing movement path recursion with application to African buffalo in South Africa. Ecology 90, 2467–2479 (doi:10.1890/08-1532.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraquand F., Benhamou S.2008Animal movements in heterogeneous landscapes: identifying profitable places and homogeneous movement bouts. Ecology 89, 3336–3348 (doi:10.1890/08-0162.1) [DOI] [PubMed] [Google Scholar]
- Bartumeus F.2009Behavioral intermittence, Levy patterns, and randomness in animal movement. Oikos 118, 488–494 [Google Scholar]
- Bartumeus F., Levin S. A.2008Fractal variation of clocks: linking animal behavior to statistical patterns of search. Proc. Natl Acad. Sci. USA 105, 19 072–19 077 (doi:10.1073/pnas.0801926105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou S.1992Efficiency of area-restricted searching behaviour in a continuous patchy environment. J. Theor. Biol. 159, 67–81 (doi:10.1016/S0022-5193(05)80768-4) [Google Scholar]
- Benhamou S., Cornelis D.In press Incorporating movement behavior and barriers to improve biological relevance of kernel home range space use estimates. J. Wildl. Manag [Google Scholar]
- Beyer H. L., Haydon D. T., Morales J. M., Frair J. L., Hebblewhite M., Mitchell M., Matthiopoulos J.2010The interpretation of habitat preference metrics under use-availability designs. Phil. Trans. R. Soc. B 365, 2245–2254 (doi:10.1098/rstb.2010.0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone R. B., Thirgood S. J., Hopcraft J. G. C.2006Serengeti wildebeest migratory patterns modeled from rainfall and new vegetation growth. Ecology 87, 1987–1994 (doi:10.1890/0012-9658(2006)87[1987:SWMPMF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Cagnacci F., Boitani L., Powell R. A., Boyce M. S.2010Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Phil. Trans. R. Soc. B 365, 2157–2162 (doi:10.1098/rstb.2010.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. W., Mangel M.1999Dynamic state variable models in ecology. Oxford, UK: Oxford University Press [Google Scholar]
- Cushman S. A., Chase M., Griffin C.2005Elephants in space and time. Oikos 109, 331–341 (doi:10.1111/j.0030-1299.2005.13538.x) [Google Scholar]
- Dale S. R., Giraldeau L.-A., Olsson O., McNamara J. M., Stephens D. W.2005Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 [DOI] [PubMed] [Google Scholar]
- Dalziel B. D., Morales J. M., Fryxell J. M.2008Fitting probability distributions to animal movement trajectories: using artificial neural networks to link distances, resources and memory. Am. Nat. 172, 248–258 (doi:10.1086/589448) [DOI] [PubMed] [Google Scholar]
- Dettki H., Ericsson G.2008Screening radiolocation datasets for movement strategies with time series segmentation. J. Wildl. Manag. 72, 535–542 (doi:10.2193/2006-363) [Google Scholar]
- Fauchald P., Tveraa T.2003Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84, 282–288 (doi:10.1890/0012-9658(2003)084[0282:UFPTIT]2.0.CO;2) [Google Scholar]
- Fero O., Stephens P. A., Barta Z., McNamara J. M., Houston A. I.2008Optimal annual routines: new tools for conservation biology? Ecol. Appl. 18, 1563–1577 (doi:10.1890/07-1012.1) [DOI] [PubMed] [Google Scholar]
- Forester J. D., Ives A. R., Turner M. G., Anderson D. P., Fortin D., Beyer H. L., Smith D. W., Boyce M. S.2007State-space models link elk movement patterns to landscape characteristics in Yellowstone National Park. Ecol. Monogr. 77, 285–299 (doi:10.1890/06-0534) [Google Scholar]
- Fortin D., Beyer H. L., Boyce M. S., Smith D. W., Duchesne T., Mao J. S.2005Wolves influence elk movements: behaviour shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320–1330 (doi:10.1890/04-0953) [Google Scholar]
- Frair J. L., Merrill E. H., Visscher D. R., Fortin D., Beyer H. L., Morales J. M.2005Scales of movement by elk in response to heterogeneity in forage resources and predation risk. Landscape Ecol. 20, 273–287 (doi:10.1007/s10980-005-2075-8) [Google Scholar]
- Frair J. L., Fieberg J., Hebblewhite M., Cagnacci F., DeCesare N. J., Pedrotti L.2010Resolving issues of imprecise and habitat-biased locations in ecological analyses using GPS telemetry data. Phil. Trans. R. Soc. B 365, 2187–2200 (doi:10.1098/rstb.2010.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A., Caelli T., Hudson R. J.2004Analysis of movements and behaviour of caribou using hidden Markov models. Ecol. Model. 173, 259–270 (doi:10.1016/j.ecolmodel.2003.06.004) [Google Scholar]
- Fretwell S. D.1972Populations in a seasonal environment. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- Fryxell J. M., Wilmshurst J. F., Sinclair A. R. E.2004Predictive models of movement of Serengeti grazers. Ecology 85, 2429–2435 (doi:10.1890/04-0147) [Google Scholar]
- Fryxell J. M., Wilmshurst J. F., Sinclair A. R. E., Haydon D. T., Holt R. D., Abrams P. A.2005Landscape scale, heterogeneity, and the viability of Serengeti grazers. Ecol. Lett. 8, 328–335 (doi:10.1111/j.1461-0248.2005.00727.x) [Google Scholar]
- Fryxell J., Hazell M., Borger L., Dalziel B. D., Haydon D. T., Morales J. M., McIntosh T., Rosatte R. C.2008Multiple movement modes by large herbivores at multiple spatiotemporal scales. Proc. Natl Acad. Sci. USA 105, 19 114–19 119 (doi:10.1073/pnas.0801737105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J.-M., Hebblewhite M., Loison A., Fuller M., Powell R., Basille M., Van Moorter B.2010Habitat-performance relationships: finding the right metric at a given spatial scale. Phil. Trans. R. Soc. B 365, 2255–2265 (doi:10.1098/rstb.2010.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylard A., Owen-Smith N., Redfern J.2003Surface water availability: implications for heterogeneity and ecosystem processes. In The Kruger experience: ecology and management of savanna heterogeneity (eds du Toit J. T., Rogers K. H., Biggs H. C.), pp. 171–188 Washington, DC: Island Press [Google Scholar]
- Georgii B.1981Activity patterns of female red deer in the Alps. Oecologia 49, 127–136 (doi:10.1007/BF00376910) [DOI] [PubMed] [Google Scholar]
- Getz W. M., Saltz D.2008A framework for generalizing and analyzing movement paths on ecological landscapes. Proc. Natl Acad. Sci. USA 105, 19 066–19 071 (doi:10.1073/pnas.0801732105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz W. M., Wilmers C. C.2004A local nearest-neighbor convex-hull construction of home ranges and utilization distributions. Ecography 27, 489–505 (doi:10.1111/j.0906-7590.2004.03835.x) [Google Scholar]
- Gillingham M. P., Parker K. L., Hanley T. A.1997Forage intake by black-tailed deer in a natural environment: bout dynamics. Can. J. Zool. 75, 1118–1128 (doi:10.1139/z97-134) [Google Scholar]
- Godvik I. M. R., Loe L. E., Vik J. O., Veiberg V., Langvatn R., Mysterud A.2009Temporal scales, trade-offs, and functional responses in red deer habitat selection. Ecology 90, 699–710 (doi:10.1890/08-0576.1) [DOI] [PubMed] [Google Scholar]
- Haydon D. T., Morales J. M., Yott A., Jenkins D. A., Rosatte R., Fryxell J. M.2008Socially informed random walks: incorporating group dynamics into models of population spread and growth. Proc. R. Soc. B 275, 1101–1109 (doi:10.1098/rspb.2007.1688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebblewhite M., Merrill E. H.2009Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454 (doi:10.1890/08-2090.1) [DOI] [PubMed] [Google Scholar]
- Hebblewhite M., Merrill E. H., McDermid E.2008A multi-scale test of the forage maturation hypothesis in a partially migratory ungulate population. Ecol. Monogr. 78, 141–166 (doi:10.1890/06-1708.1) [Google Scholar]
- Holdo R. M., Holt R. D., Fryxell J. M.2009Opposing rainfall and plant nutritional gradients explain the wildebeest migration in the Serengeti. Am. Nat. 173, 431–445 (doi:10.1086/597229) [DOI] [PubMed] [Google Scholar]
- Houston A. J., McNamara J. M.1999Models of adaptive behaviour. An approach based on state. Cambridge, UK: Cambridge University Press [Google Scholar]
- Illius A. W., Gordon I. J.1999Scaling up from functional response to numerical response in vertebrate herbivores. In Herbivores: between plants and predators (eds Olff H., Brown V. K., Drent R. H.), pp. 397–427 Oxford, UK: Blackwell [Google Scholar]
- Johnson C. J., Parker K. L., Heard D. C., Gillingham M. P.2002Movement parameters of ungulates and scale-specific responses to the environment. J. Anim. Ecol. 71, 225–235 (doi:10.1046/j.1365-2656.2002.00595.x) [Google Scholar]
- Kamler J. F., Jedrzejewska B., Jedrzejewski W.2007Activity patterns of red deer in Bialowieza National Park, Poland. J. Mammal. 88, 508–514 (doi:10.1644/06-MAMM-A-169R.1) [Google Scholar]
- Kernohan B. J., Gitzen R. A., Millspaugh J. J.2001Analysis of animal space use and movements. In Radiotracking and animal populations (eds Millspaugh J. J., Marzluff J. M.), pp. 126–166 New York, NY: Academic Press [Google Scholar]
- Kie J. G., Matthiopoulos J., Fieberg J., Powell R. A., Cagnacci F., Mitchell M. S., Gaillard J.-M., Moorcroft P. R.2010The home-range concept: are traditional estimators still relevant with modern telemetry technology? Phil. Trans. R. Soc. B 365, 2221–2231 (doi:10.1098/rstb.2010.0093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler B. P., Morris D. W., Brown J. S.2007Behavioral indicators and conservation: wielding ‘the biologist's tricorder’. Israel J. Ecol. Evol. 53, 237–244 (doi:10.1560/IJEE.53.3.237) [Google Scholar]
- Kramer D. L., McLaughlin R. L.2001The behavioral ecology of intermittent locomotion. Am. Zool. 41, 137–153 (doi:10.1668/0003-1569(2001)041[0137:TBEOIL]2.0.CO;2) [Google Scholar]
- McFarland D. J.1977Decision making in animals. Nature 269, 15–21 (doi:10.1038/269015a0) [Google Scholar]
- McLoughlin P. D., et al. 2007Lifetime reproductive success and composition of the home range in a large herbivore. Ecology 88, 3192–3201 (doi:10.1890/06-1974.1) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Houston A. I.1987Starvation and predation as factors limiting population size. Ecology 68, 1515–1519 (doi:10.2307/1939235) [Google Scholar]
- McNamara J. M., Houston A. I.2008Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B 363, 301–319 (doi:10.1098/rstb.2007.2141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae B. H., Dickson B. G., Keitt T. H., Shoh V. B.2008Using circuit theory to model connectivity in ecology, evolution and conservation. Ecology 89, 2712–2724 (doi:10.1890/07-1861.1) [DOI] [PubMed] [Google Scholar]
- Merrill E., Sand H., Zimmermann B., McPhee H., Webb N., Hebblewhite M., Wabakken P., Frair J. L.2010Building a mechanistic understanding of predation with GPS-based movement data. Phil. Trans. R. Soc. B 365, 2279–2288 (doi:10.1098/rstb.2010.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. S., Powell R. A.2004A mechanistic home range model for optimal use of spatially distributed resources. Ecol. Model. 177, 209–232 (doi:10.1016/j.ecolmodel.2004.01.015) [Google Scholar]
- Moorcroft P. R., Barnett A.2008Mechanistic home range models and resource selection analysis: a reconciliation and unification. Ecology 89, 1112–1119 (doi:10.1890/06-1985.1) [DOI] [PubMed] [Google Scholar]
- Moorcroft P. R., Lewis M. A., Crabtree R. L.1999Analysis of coyote home ranges using a mechanistic home range model. Ecology 80, 1656–1665 (doi:10.1890/0012-9658(1999)080[1656:HRAUAM]2.0.CO;2) [Google Scholar]
- Morales J. M., Haydon D. T., Frair J., Holsinger K. E., Fryxell J. M.2004Extracting more out of relocation data: building movement models as mixtures of random walks. Ecology 85, 2436–2445 (doi:10.1890/03-0269) [Google Scholar]
- Morales J. M., Moorcroft P. R., Matthiopoulos J., Frair J. L., Kie J. G., Powell R. A., Merrill E. H., Haydon D. T.2010Building the bridge between animal movement and population dynamics. Phil. Trans. R. Soc. B 365, 2289–2301 (doi:10.1098/rstb.2010.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T., Fagan W. F.2008Search and navigation in dynamics environments—from individual behaviors to population distributions. Oikos 117, 654–664 (doi:10.1111/j.0030-1299.2008.16291.x) [Google Scholar]
- Mueller T., Olson K. A., Fuller T. D., Schaller G. B., Murray M. G., Leimgruber P.2008In search of forage: predicting dynamic habitats of Mongolian gazelles using satellite-based estimates of vegetation productivity. J. Appl. Ecol. 45, 649–658 (doi:10.1111/j.1365-2664.2007.01371.x) [Google Scholar]
- Nathan R.2008An emerging movement ecology paradigm. Proc. Natl Acad. Sci. USA 105, 19 050–19 051 (doi:10.1073/pnas.0808918105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R., Getz W. M., Revilla E., Holyoak M., Kadmon R., Saltz D., Smouse P. E.2008A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059 (doi:10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonberg E. G., Newman L. A., Lewis M., Crabtree R. L., Potapov A. B.2007Sequential decision-making in a variable environment: modeling elk movement in Yellowstone National Park as a dynamic game. Theor. Popul. Biol. 71, 182–195 [DOI] [PubMed] [Google Scholar]
- Novellie P. A.1978Comparison of the foraging strategies of blesbok and springbok on the Transvaal highveld. S. Afr. J. Wildl. Res. 8, 137–144 [Google Scholar]
- O'Brien W. J., Browman H. I., Evans B. I.1990Search strategies for foraging animals. Am. Scientist 78, 152–160 [Google Scholar]
- Owen-Smith N.1979Assessing the forage efficiency of a large herbivore, the kudu. S. Afr. J. Wildl. Res. 9, 102–110 [Google Scholar]
- Owen-Smith N.1993Evaluating optimal diet models for an African browsing ruminant, the kudu: how constraining are the assumed constraints? Evol. Ecol. 7, 499–524 (doi:10.1007/BF01237644) [Google Scholar]
- Owen-Smith N.1994Foraging responses of kudus to seasonal changes in food resources: elasticity in constraints. Ecology 75, 1050–1062 (doi:10.2307/1939429) [Google Scholar]
- Owen-Smith N.1998How high ambient temperature affects the daily activity and foraging time of a subtropical ungulate, the greater kudu. J. Zool. 246, 183–192 (doi:10.1111/j.1469-7998.1998.tb00147.x) [Google Scholar]
- Owen-Smith N.2002Adaptive herbivore ecology: from resources to populations in variable environments. Cambridge, UK: Cambridge University Press [Google Scholar]
- Owen-Smith N., Cain J. W., III2007Indicators of adaptive responses in home range utilization and movement patterns by a large mammalian herbivore. Israel J. Ecol. Evol. 53, 423–439 (doi:10.1560/IJEE.53.3.423) [Google Scholar]
- Owen-Smith N., Cooper S. M.1987aAcceptability indices for assessing the food preferences of ungulates. J. Wildl. Manag. 51, 372–378 [Google Scholar]
- Owen-Smith N., Cooper S. M.1987bPalatability of woody plants to browsing ungulates in a South African savanna. Ecology 68, 319–331 (doi:10.2307/1939263) [Google Scholar]
- Owen-Smith N., Cooper S. M.1989Nutritional ecology of a browsing ruminant, the kudu, through the seasonal cycle. J. Zool. 219, 29–43 (doi:10.1111/j.1469-7998.1989.tb02563.x) [Google Scholar]
- Owen-Smith N., Novellie P.1982What should a clever ungulate eat? Am. Nat. 119, 151–178 (doi:10.1086/283902) [Google Scholar]
- Pyke G. H., Pulliam H. R., Charnov E. L.1977Optimal foraging: a selective review of theory and tests. Q. Rev. Biol. 52, 137–153 (doi:10.1086/409852) [Google Scholar]
- Reynolds A. M., Rhodes C. J.2009The Levy flight paradigm: random search patterns and mechanisms. Ecology 90, 877–887 (doi:10.1890/08-0153.1) [DOI] [PubMed] [Google Scholar]
- Sawyer H., Kauffman M. J., Nielson R. M., Horne J. S.2009Identifying and prioritizing migration routes for landscape-level conservation. Ecol. Appl. 19, 2016–2025 (doi:10.1890/08-2034.1) [DOI] [PubMed] [Google Scholar]
- Schick R. S., et al. 2008Understanding movement data and movement processes: current and emerging directions. Ecol. Lett. 11, 1338–1350 (doi:10.1111/j.1461-0248.2008.01249.x) [DOI] [PubMed] [Google Scholar]
- Schoener T. W.1971Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404 (doi:10.1146/annurev.es.02.110171.002101) [Google Scholar]
- Senft R. L., Coughenour M. B., Bailey D. W., Rittenhouse L. R., Sala O. E., Swift D. M.1987Large herbivore foraging and ecological hierarchies. BioScience 37, 789–799 (doi:10.2307/1310545) [Google Scholar]
- Sinclair A. R. E., Arcese P.1995Population consequences of predation—sensitive foraging: the Serengeti wildebeest. Ecology 76, 882–891 (doi:10.2307/1939353) [Google Scholar]
- Stephens D. W., Krebs J. R.1986Foraging theory. Princeton, NJ: Princeton University Press [Google Scholar]
- Thirgood S., et al. 2004Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. 7, 113–120 (doi:10.1017/S1367943004001404) [Google Scholar]
- Urbano F., Cagnacci F., Calenge C., Dettki H., Cameron A., Neteler M.2010Wildlife tracking data management: a new vision. Phil. Trans. R. Soc. B 365, 2177–2185 (doi:10.1098/rstb.2010.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer J., Ens B. J.1997Models of interference and their consequences for the spatial distribution of ideal and free predators. J. Anim. Ecol. 66, 846–858 [Google Scholar]
- Van Moorter B., Visscher D., Benhamou S., Börger L., Boyce M., Gaillard J. M.2009Memory keeps you at home: a mechanistic model for home range emergence. Oikos 118, 641–652 (doi:10.1111/j.1600-0706.2008.17003.x) [Google Scholar]
- Western D.1975Water availability and its influence on the structure and dynamics of a savannah large mammal community. E. Afr. Wildl. J. 13, 265–286 [Google Scholar]
- Wilmshurst J. F., Fryxell J. M., Farm B. P., Sinclair A. R. E., Henschel C. P.1999Spatial distribution of Serengeti wildebeest in relation to resources. Can. J. Zool. 77, 1223–1232 (doi:10.1139/cjz-77-8-1223) [Google Scholar]
- Wolf M., Frair J., Merrill E., Turchin P.2009The attraction of the known: the importance of spatial familiarity in habitat selection in wapiti Cervus elaphus. Ecography 32, 401–410 (doi:10.1111/j.1600-0587.2008.05626.x) [Google Scholar]